GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION






- Slides: 46

GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION PETRUCCI HERRING MADURA Nuclear Chemistry BISSONNETTE 25 PHILIP DUTTON UNIVERSITY OF WINDSOR DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY Slide 25 - 1 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

Nuclear Chemistry Slide 25 - 2 CONTENTS 25 -1 Radioactivity 25 -2 Naturally Occurring Radioactive Isotopes 25 -3 Nuclear Reactions and Artificially Induced Radioactivity 25 -4 Transuranium Elements 25 -5 Rate of Radioactive Decay 25 -6 Energetics of Nuclear Reactions 25 -7 Nuclear Stability 25 -8 Nuclear Fission General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

Nuclear Chemistry Slide 25 - 3 CONTENTS 25 -9 Nuclear Fission 25 -10 Effect of Radiation on Matter 25 -11 Applications of Radioisotopes General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

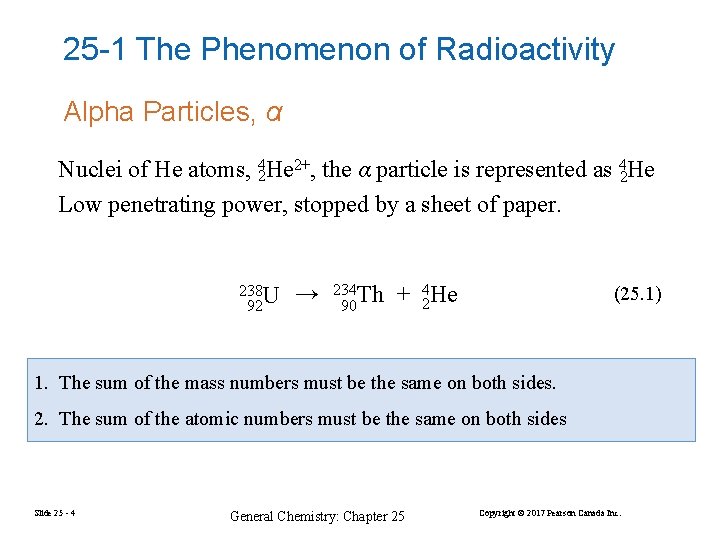
25 -1 The Phenomenon of Radioactivity Alpha Particles, α Nuclei of He atoms, 24 He 2+, the α particle is represented as 42 He Low penetrating power, stopped by a sheet of paper. 238 U 92 → 234 Th 90 + 24 He (25. 1) 1. The sum of the mass numbers must be the same on both sides. 2. The sum of the atomic numbers must be the same on both sides Slide 25 - 4 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

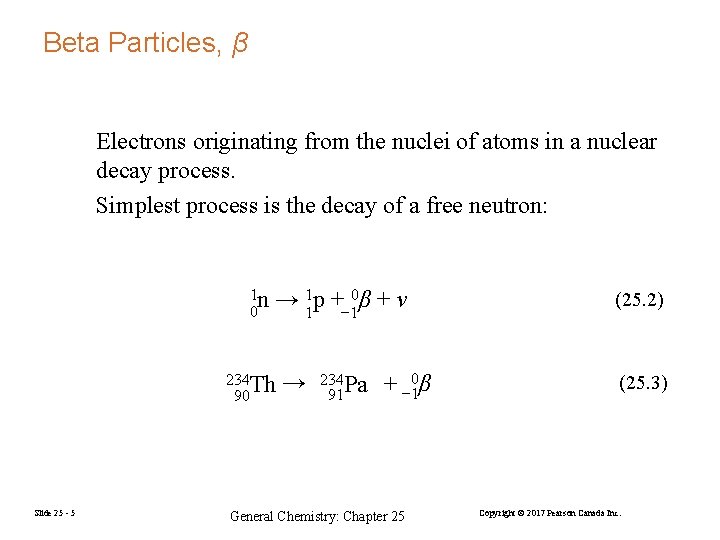
Beta Particles, β Electrons originating from the nuclei of atoms in a nuclear decay process. Simplest process is the decay of a free neutron: 1 n 0 → 11 p +− 10β + ν 234 Th 90 Slide 25 - 5 → 234 Pa 91 + − 10β General Chemistry: Chapter 25 (25. 2) (25. 3) Copyright © 2017 Pearson Canada Inc.

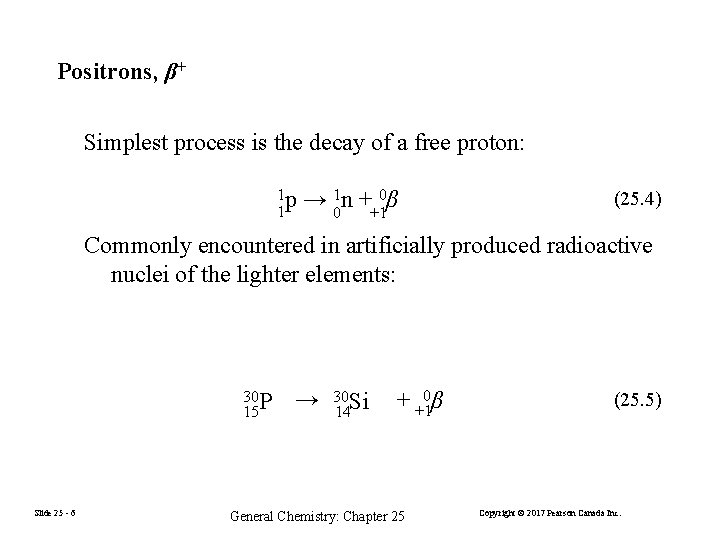
Positrons, β+ Simplest process is the decay of a free proton: 1 p 1 → 01 n ++10β (25. 4) Commonly encountered in artificially produced radioactive nuclei of the lighter elements: 30 P 15 Slide 25 - 6 → 30 Si 14 + +10β General Chemistry: Chapter 25 (25. 5) Copyright © 2017 Pearson Canada Inc.

A colorized cloud chamber photograph. Slide 25 - 7 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

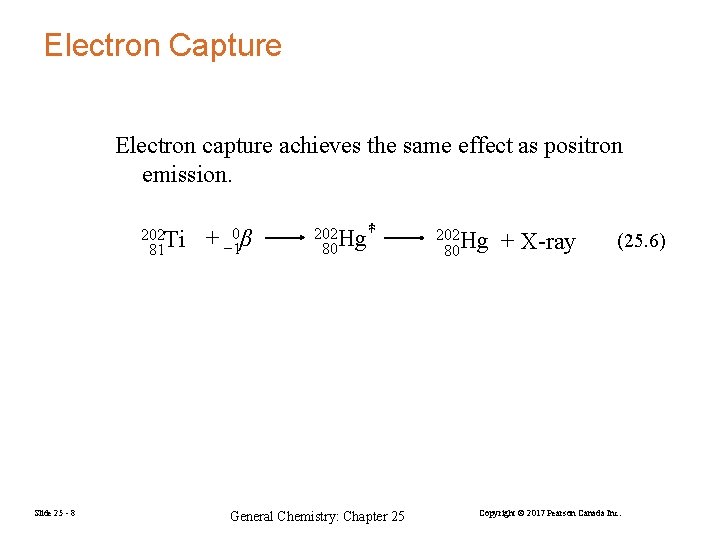
Electron Capture Electron capture achieves the same effect as positron emission. 202 Ti 81 Slide 25 - 8 + − 10β 202 Hg ‡ 80 General Chemistry: Chapter 25 202 Hg 80 + X-ray (25. 6) Copyright © 2017 Pearson Canada Inc.

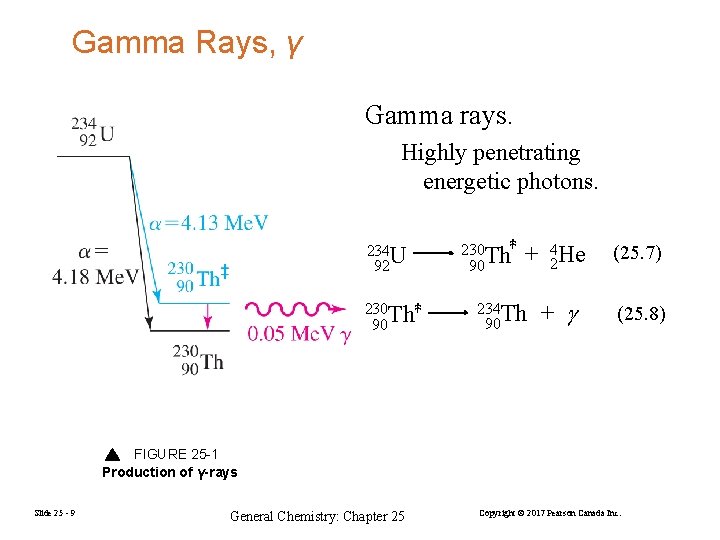
Gamma Rays, γ Gamma rays. Highly penetrating energetic photons. 234 U 92 230 Th‡ 90 + 42 He (25. 7) + (25. 8) 234 Th 90 FIGURE 25 -1 Production of γ-rays Slide 25 - 9 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

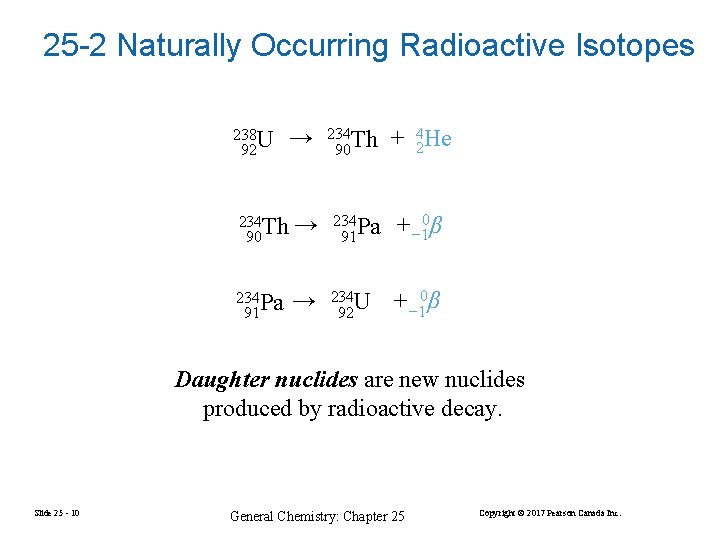
25 -2 Naturally Occurring Radioactive Isotopes 238 U 92 → 234 Th 90 + 24 He 234 Th 90 → 234 Pa 91 + − 10β 234 Pa 91 → 234 U 92 +− 10β Daughter nuclides are new nuclides produced by radioactive decay. Slide 25 - 10 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

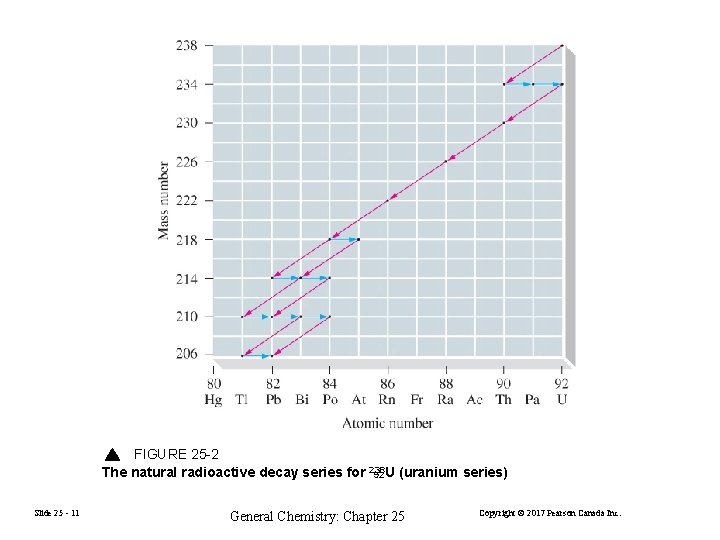
FIGURE 25 -2 The natural radioactive decay series for 238 92 U (uranium series) Slide 25 - 11 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

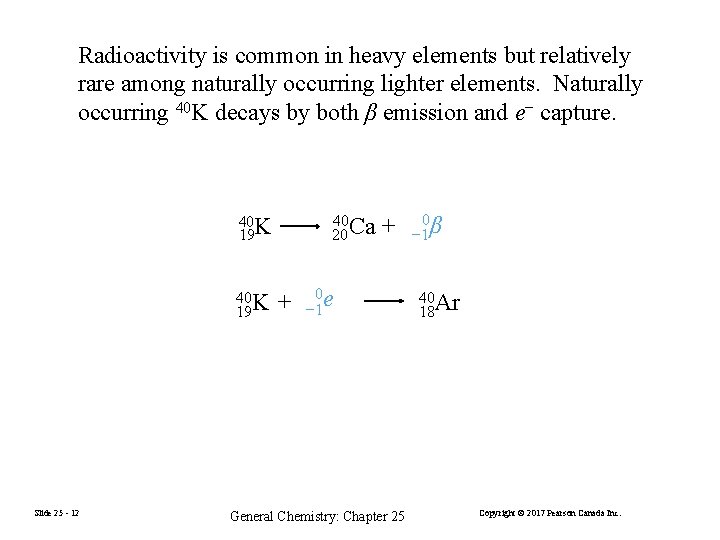
Radioactivity is common in heavy elements but relatively rare among naturally occurring lighter elements. Naturally occurring 40 K decays by both β emission and e− capture. 40 20 Ca 40 K 19 Slide 25 - 12 + + 0 e − 1 General Chemistry: Chapter 25 0 − 1 β 40 Ar 18 Copyright © 2017 Pearson Canada Inc.

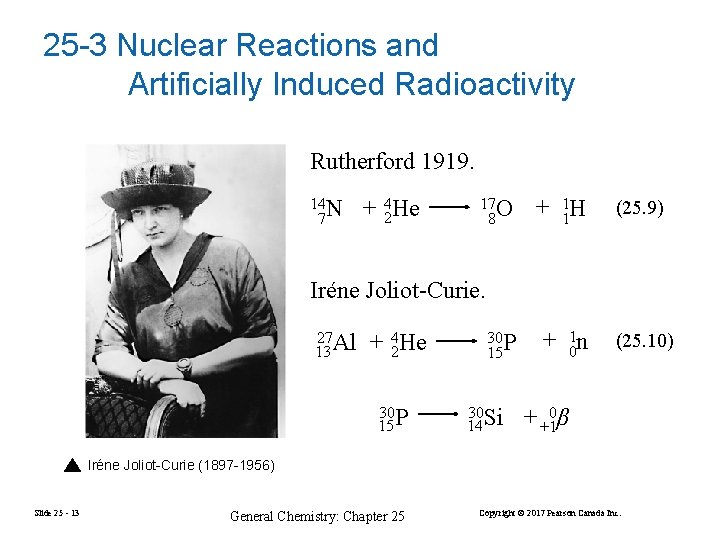
25 -3 Nuclear Reactions and Artificially Induced Radioactivity Rutherford 1919. 14 N 7 + 42 He 17 O 8 + 11 H (25. 9) + 10 n (25. 10) Iréne Joliot-Curie. 27 Al 13 + 42 He 30 15 P 30 P 15 30 14 Si + +10β Iréne Joliot-Curie (1897 -1956) Slide 25 - 13 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

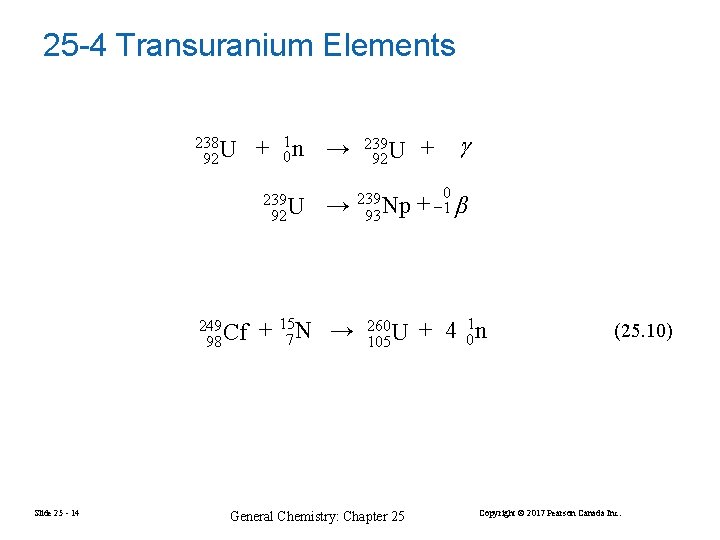
25 -4 Transuranium Elements 238 92 U + 01 n → → 239 93 Np + 157 N → 260 105 U 239 92 U 249 98 Cf Slide 25 - 14 239 92 U General Chemistry: Chapter 25 + 0 + − 1 β + 4 01 n (25. 10) Copyright © 2017 Pearson Canada Inc.

Stanford Linear Accelerator Centre (SLAC 0 PEP-II collider) Slide 25 - 15 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

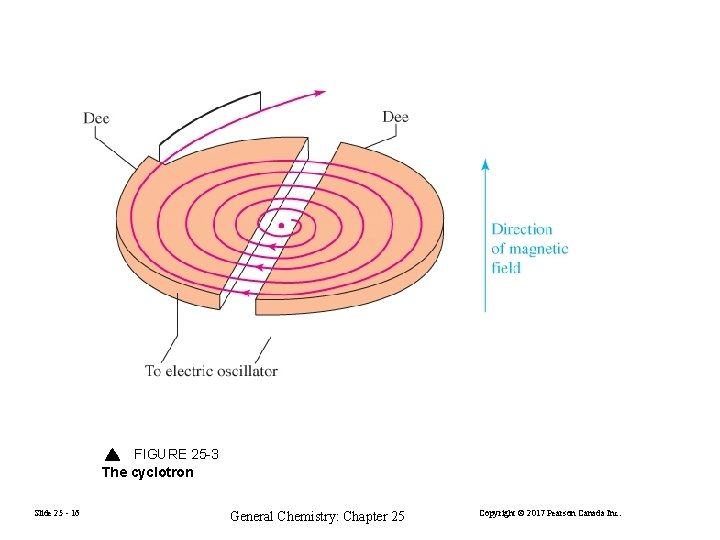
FIGURE 25 -3 The cyclotron Slide 25 - 16 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

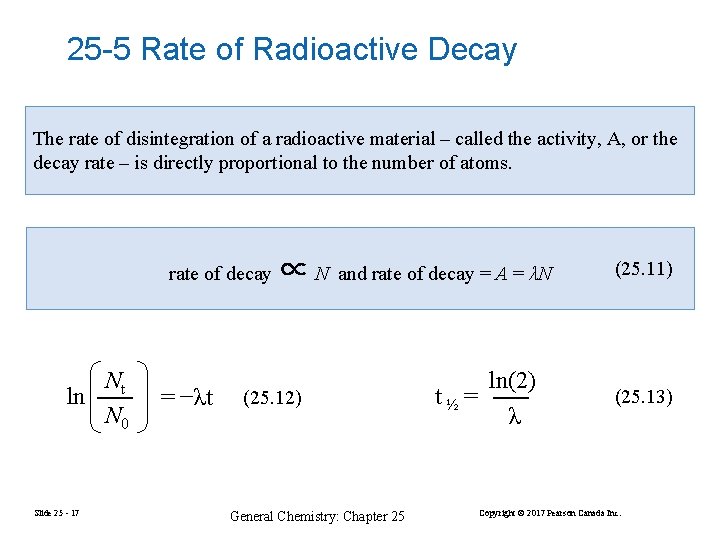
25 -5 Rate of Radioactive Decay The rate of disintegration of a radioactive material – called the activity, A, or the decay rate – is directly proportional to the number of atoms. rate of decay Nt ln N 0 Slide 25 - 17 = −λt ∝N and rate of decay = A = λN (25. 12) General Chemistry: Chapter 25 ln(2) t½ = λ (25. 11) (25. 13) Copyright © 2017 Pearson Canada Inc.

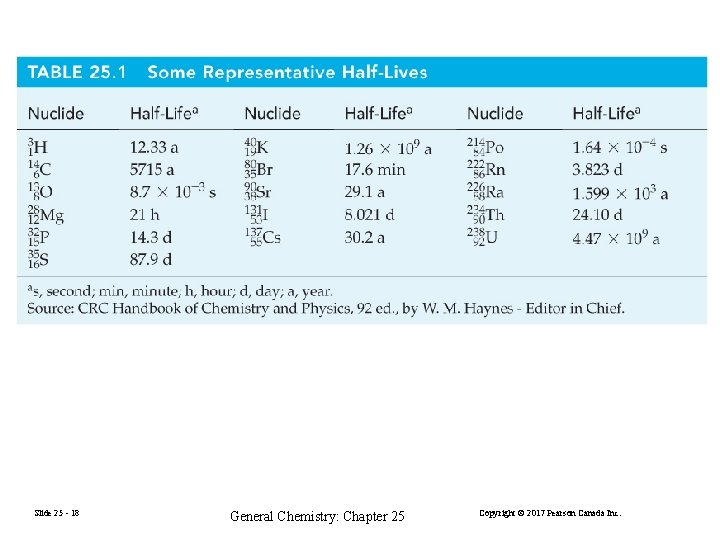
Slide 25 - 18 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

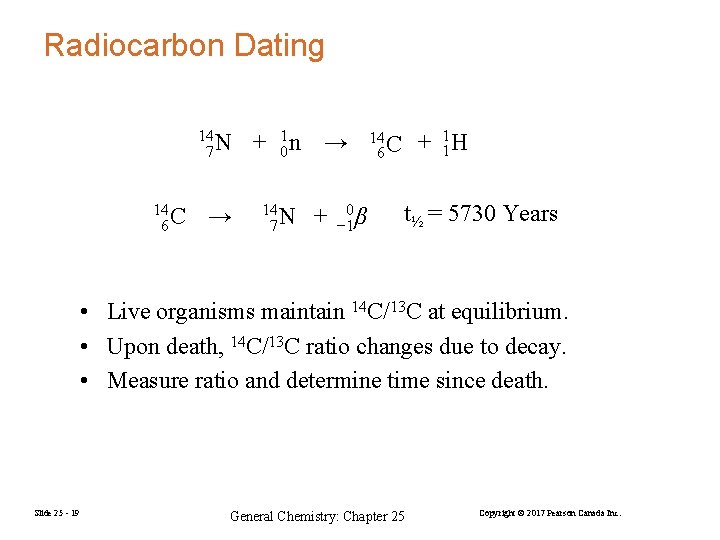
Radiocarbon Dating 14 7 N 14 6 C → + 01 n → 14 7 N + 0 − 1 β + 11 H 14 6 C t½ = 5730 Years • Live organisms maintain 14 C/13 C at equilibrium. • Upon death, 14 C/13 C ratio changes due to decay. • Measure ratio and determine time since death. Slide 25 - 19 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

The Age of Earth 238 U 92 14 steps 206 Pb 82 + 8 24 He + 6− 10β A lunar rock that has been radiometrically dated to be about 4. 6 billion years old. Slide 25 - 20 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

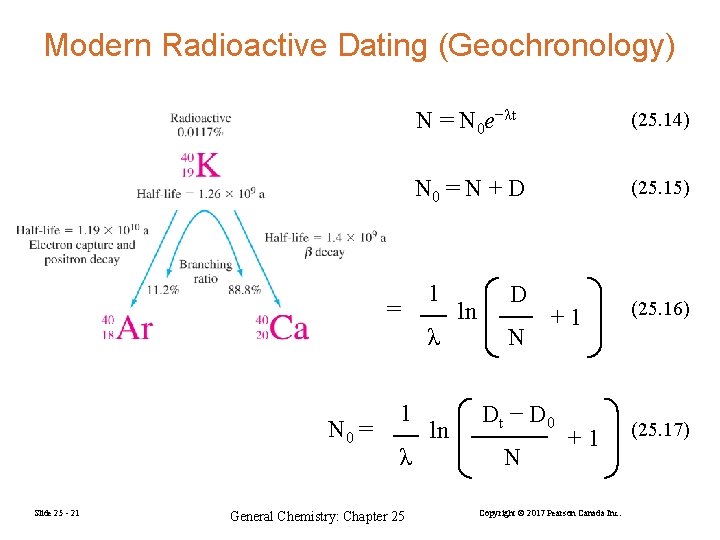
Modern Radioactive Dating (Geochronology) N 0 = Slide 25 - 21 1 λ General Chemistry: Chapter 25 N = N 0 e−λt (25. 14) N 0 = N + D (25. 15) 1 λ ln ln D N +1 Dt − D 0 N +1 Copyright © 2017 Pearson Canada Inc. (25. 16) (25. 17)

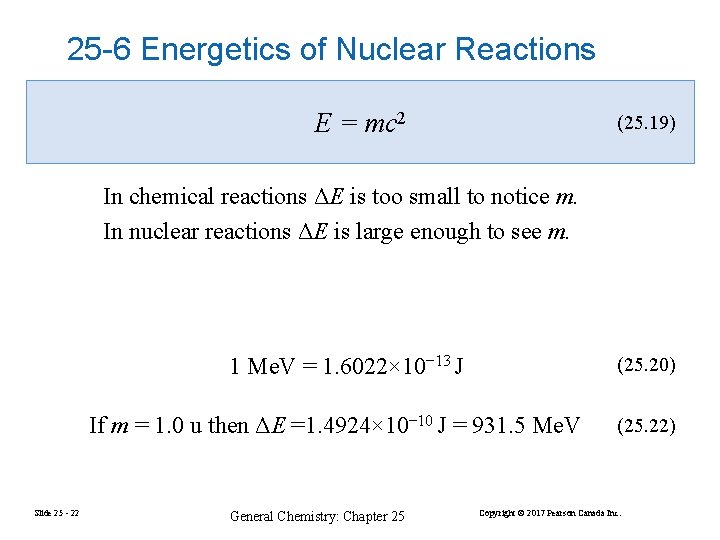
25 -6 Energetics of Nuclear Reactions E = mc 2 (25. 19) In chemical reactions ΔE is too small to notice m. In nuclear reactions ΔE is large enough to see m. 1 Me. V = 1. 6022× 10− 13 J (25. 20) If m = 1. 0 u then ΔE =1. 4924× 10− 10 J = 931. 5 Me. V Slide 25 - 22 General Chemistry: Chapter 25 (25. 22) Copyright © 2017 Pearson Canada Inc.

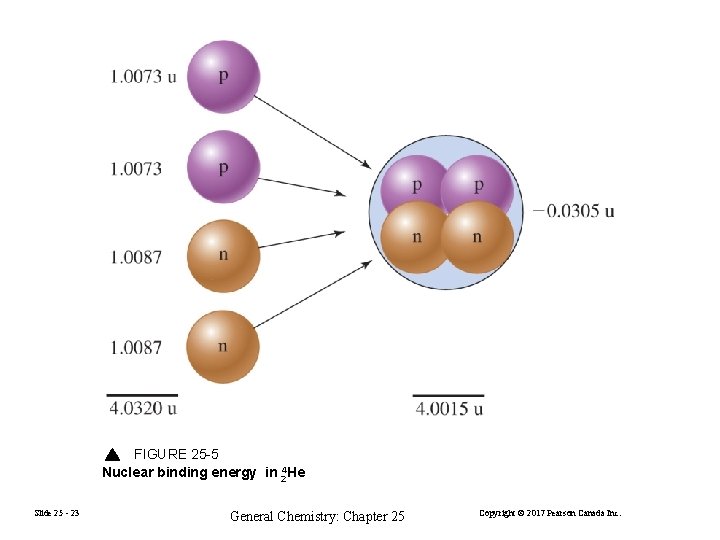
FIGURE 25 -5 Nuclear binding energy in 24 He Slide 25 - 23 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

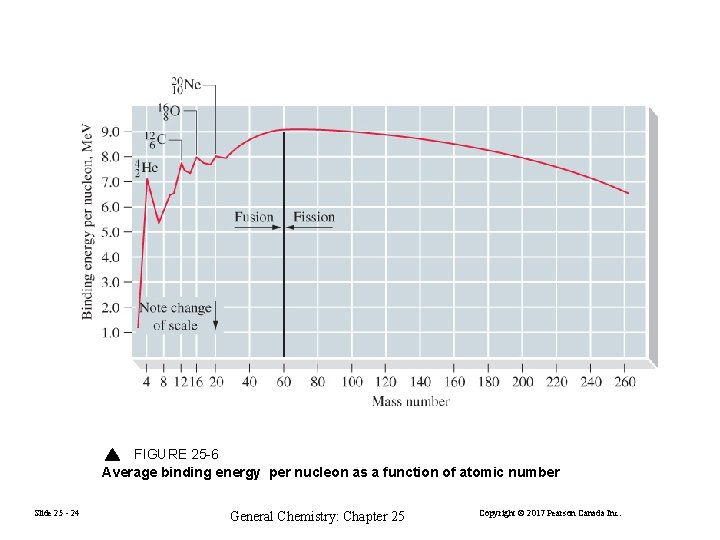
FIGURE 25 -6 Average binding energy per nucleon as a function of atomic number Slide 25 - 24 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

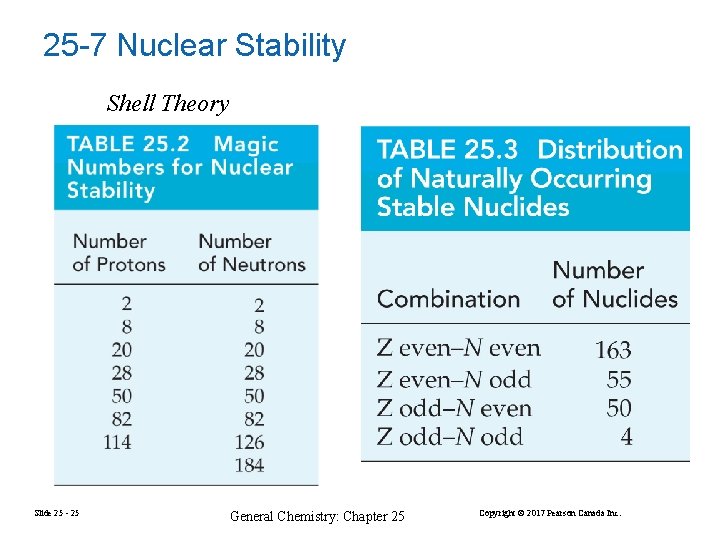
25 -7 Nuclear Stability Shell Theory Slide 25 - 25 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

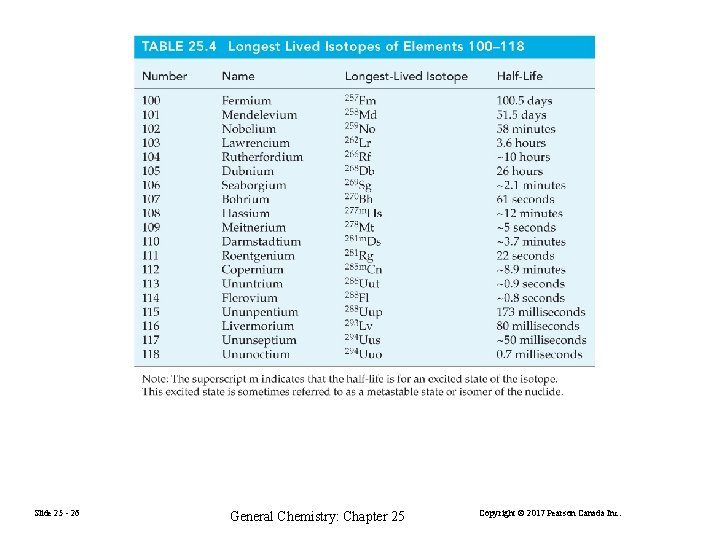
Slide 25 - 26 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

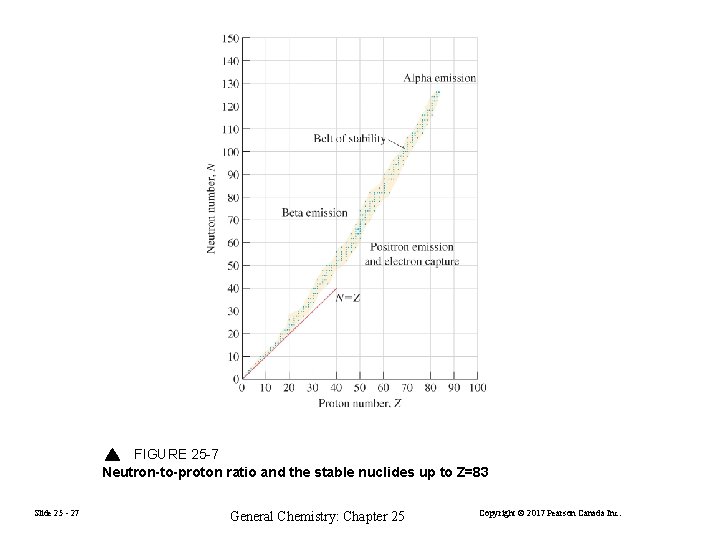
FIGURE 25 -7 Neutron-to-proton ratio and the stable nuclides up to Z=83 Slide 25 - 27 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

25 -8 Nuclear Fission 1934 Enrico Fermi. Search for transuranium elements U was bombarded with neutrons. β emission was observed. 1938 Otto Hahn, Lise Meitner, and Fritz Stassman. Z was not greater than 92. Ra, Ac, Th and Pa were found. The atom had been split. Slide 25 - 28 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

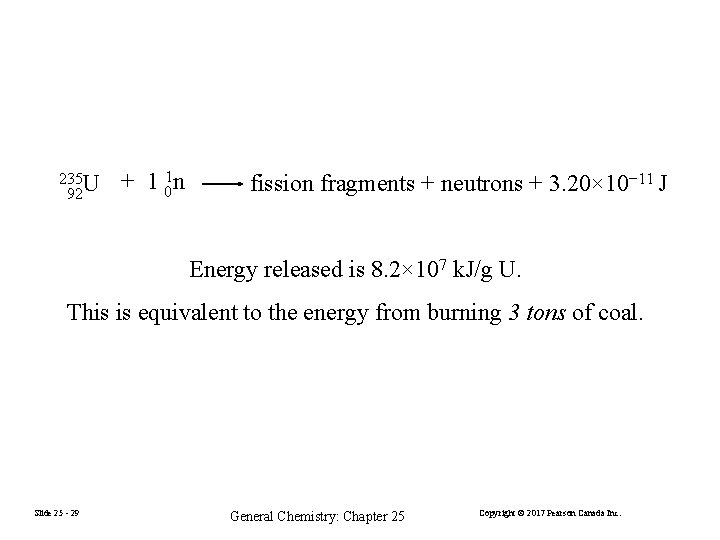
235 U 92 + 1 01 n fission fragments + neutrons + 3. 20× 10− 11 J Energy released is 8. 2× 107 k. J/g U. This is equivalent to the energy from burning 3 tons of coal. Slide 25 - 29 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

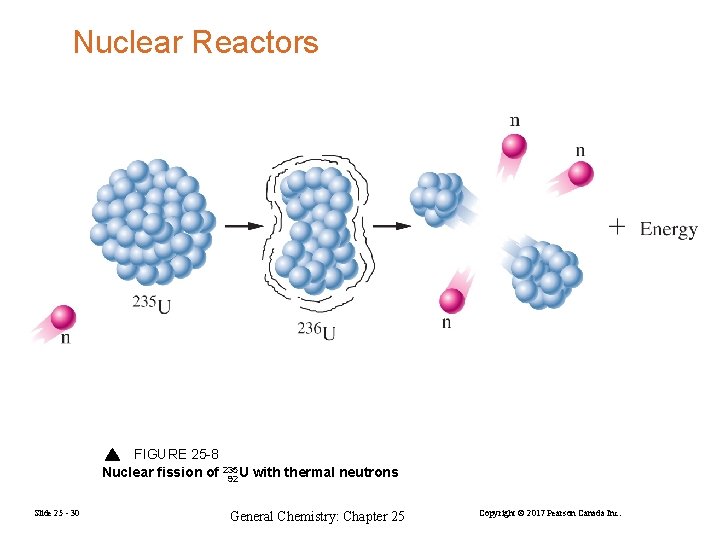
Nuclear Reactors FIGURE 25 -8 Nuclear fission of 235 U with thermal neutrons 92 Slide 25 - 30 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

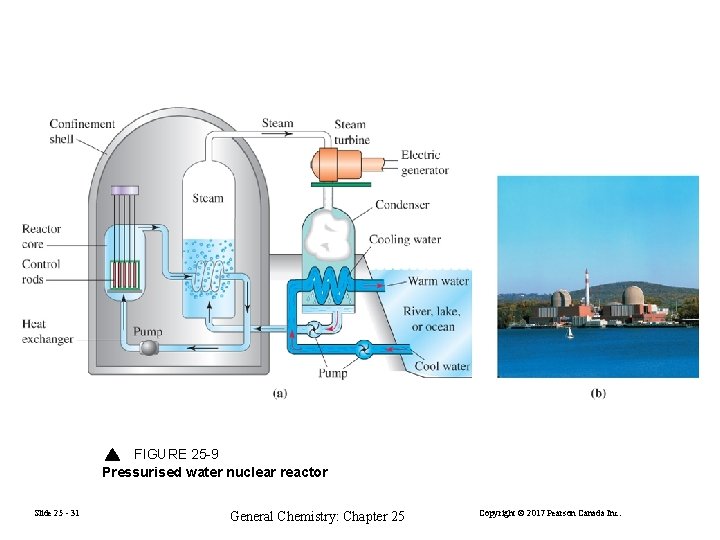
FIGURE 25 -9 Pressurised water nuclear reactor Slide 25 - 31 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

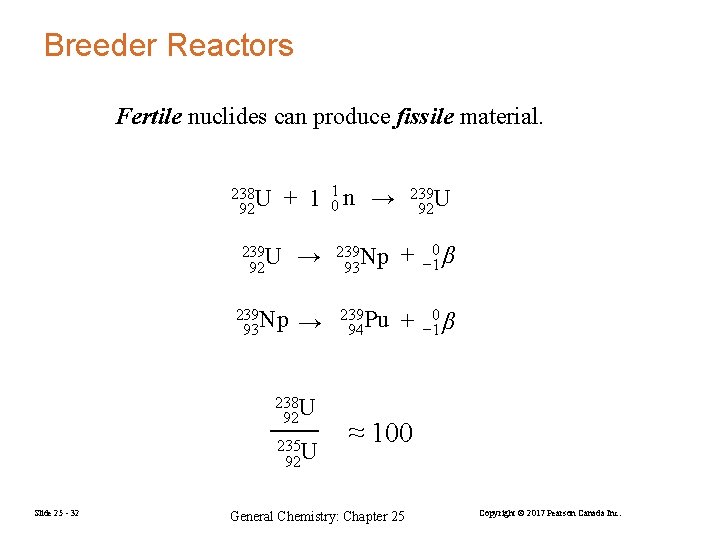
Breeder Reactors Fertile nuclides can produce fissile material. + 1 01 n → 238 U 92 239 U 92 → 239 Np 93 + 0 − 1 β 239 Np 93 → 239 Pu 94 + 0 − 1 β 238 U 92 235 U 92 Slide 25 - 32 239 U 92 ≈ 100 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

Disadvantages of Breeder Reactors Liquid-metal-cooled fast breeder reactor (LMFBR). • Sodium becomes highly radioactive in the reactor. • Heat and neutron production are high, so materials deteriorate more rapidly. • Radioactive waste and plutonium recovery. Plutonium is highly poisonous and has a long half life (24, 000 years). Slide 25 - 33 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

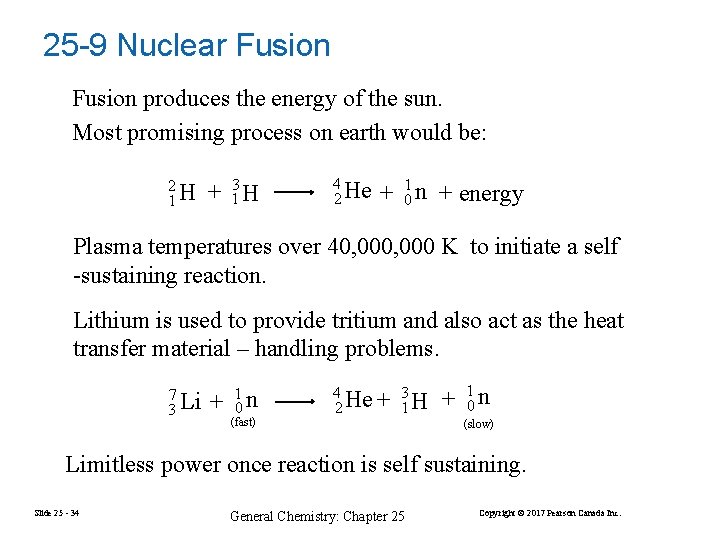
25 -9 Nuclear Fusion produces the energy of the sun. Most promising process on earth would be: 2 1 H + 13 H 4 2 He + 10 n + energy Plasma temperatures over 40, 000 K to initiate a self -sustaining reaction. Lithium is used to provide tritium and also act as the heat transfer material – handling problems. 7 3 Li + 1 0 n (fast) 3 4 He + 1 H 2 + 10 n (slow) Limitless power once reaction is self sustaining. Slide 25 - 34 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

The plasma chamber of a fusion reactor of the magnetic confinement type (tokamak). Slide 25 - 35 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

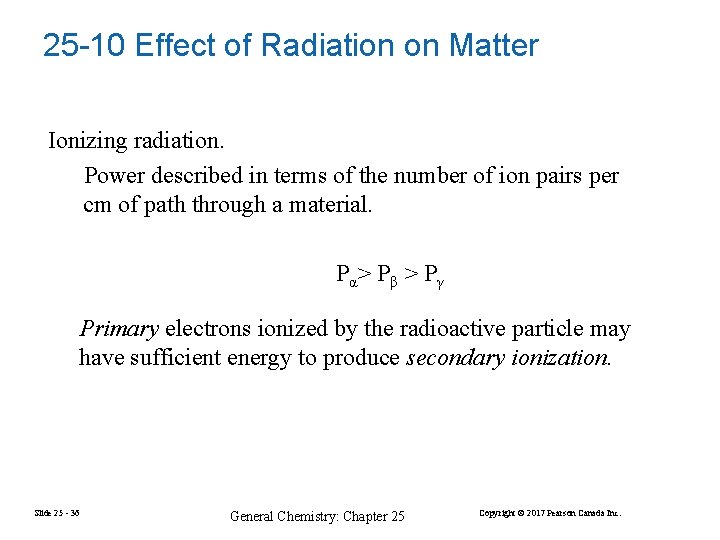
25 -10 Effect of Radiation on Matter Ionizing radiation. Power described in terms of the number of ion pairs per cm of path through a material. Pα > P β > P γ Primary electrons ionized by the radioactive particle may have sufficient energy to produce secondary ionization. Slide 25 - 36 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

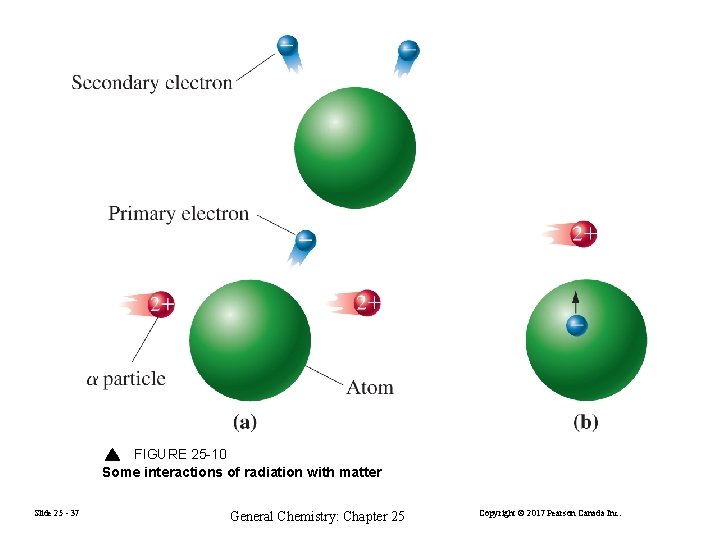
FIGURE 25 -10 Some interactions of radiation with matter Slide 25 - 37 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

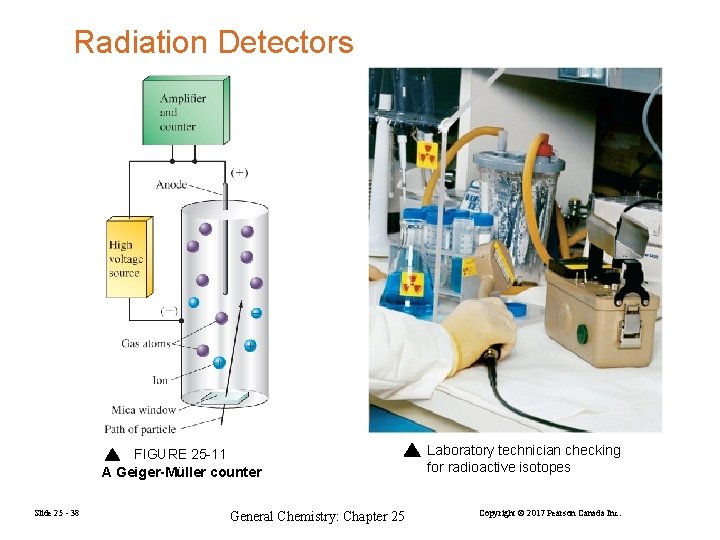
Radiation Detectors FIGURE 25 -11 A Geiger-Müller counter Slide 25 - 38 General Chemistry: Chapter 25 Laboratory technician checking for radioactive isotopes Copyright © 2017 Pearson Canada Inc.

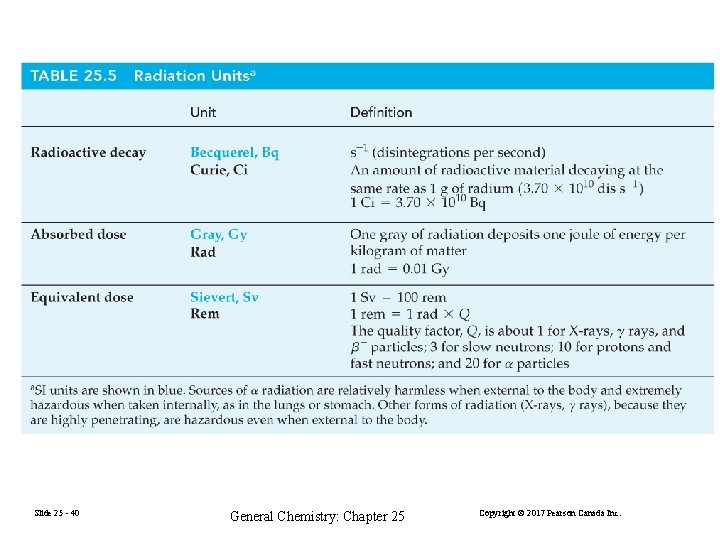
Effect of Ionizing Radiation on Living Matter Effect is the same as on other forms of matter, ionization, excitation, and dissociation of molecules. Even small doses cause changes in cell chromosomes. Radiation Dosage 1 rad (radiation absorbed dose) = 0. 001 J/kg matter 1 rem (radiation equivalent for man) = rad×Q Q = relative biological effectiveness Slide 25 - 39 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

Slide 25 - 40 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

25 -11 Applications of Radioisotopes Cancer Therapy In low doses, ionizing radiation induces cancer. In high doses it destroys cells. Cancer cells are dividing quickly and are more susceptible to ionizing radiation than normal cells. The same is true of chemotherapeutic approaches. Slide 25 - 41 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

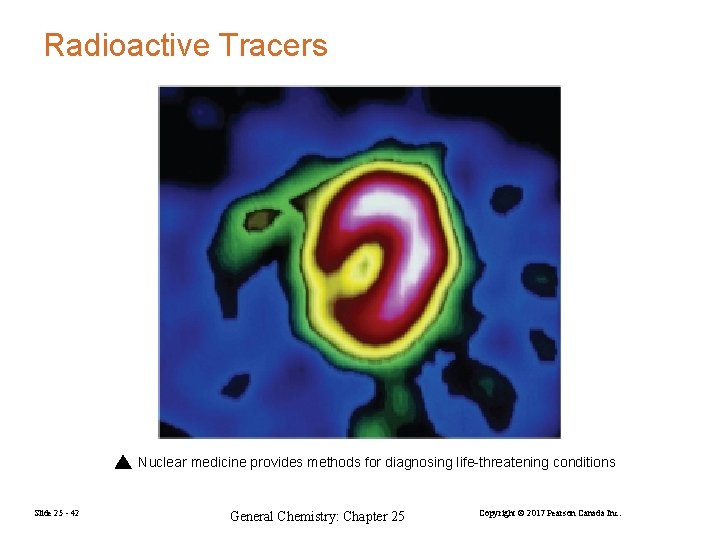
Radioactive Tracers Nuclear medicine provides methods for diagnosing life-threatening conditions Slide 25 - 42 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

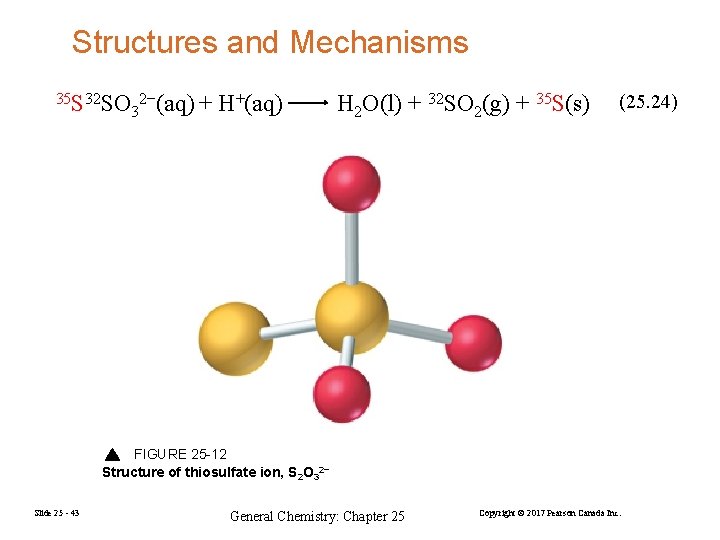
Structures and Mechanisms 35 S 32 SO 2−(aq) + 3 H+(aq) H 2 O(l) + 32 SO 2(g) + 35 S(s) (25. 24) FIGURE 25 -12 Structure of thiosulfate ion, S 2 O 32− Slide 25 - 43 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

Analytical Chemistry Precipitate ions and weigh them to get a mass of material. Incorporate radioactive ions in the precipitating mixture and measure the radioactivity. Neutron activation analysis. Induce radioactivity with neutron bombardment. Measure in trace quantities, down to ppb or less. Non-destructive and any state of matter can be probed. Slide 25 - 44 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

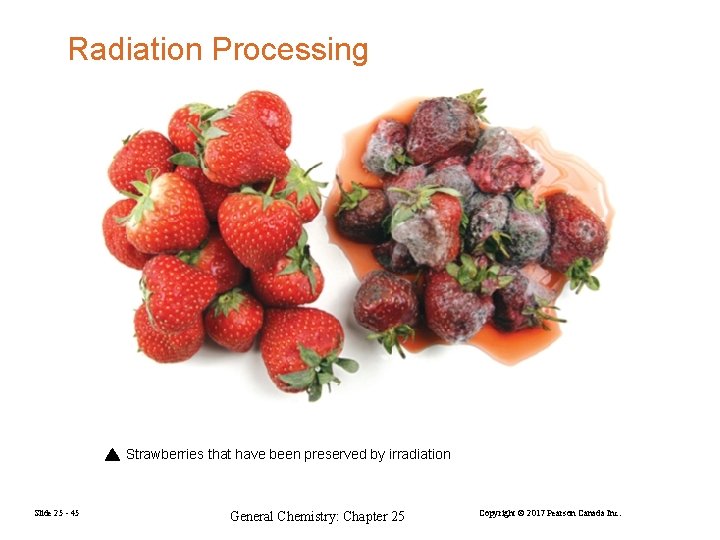
Radiation Processing Strawberries that have been preserved by irradiation Slide 25 - 45 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.

End of Chapter Slide 25 - 46 General Chemistry: Chapter 25 Copyright © 2017 Pearson Canada Inc.
 Management eleventh edition stephen p robbins
Management eleventh edition stephen p robbins Management 11th edition by stephen p robbins
Management 11th edition by stephen p robbins Management eleventh edition
Management eleventh edition Management eleventh edition stephen p robbins
Management eleventh edition stephen p robbins General chemistry
General chemistry Bhore committee
Bhore committee Eleventh 5 year plan
Eleventh 5 year plan 11th five year plan
11th five year plan For his eleventh birthday elvis presley
For his eleventh birthday elvis presley Human genetics concepts and applications 10th edition
Human genetics concepts and applications 10th edition Fluid mechanics fundamentals and applications
Fluid mechanics fundamentals and applications Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Modern systems analysis and design 7th edition
Modern systems analysis and design 7th edition A computer programming team has 13 members
A computer programming team has 13 members Using mis (10th edition) 10th edition
Using mis (10th edition) 10th edition Report
Report Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Principles and applications of electrical engineering
Principles and applications of electrical engineering Learning principles and applications
Learning principles and applications 25 m/s
25 m/s Irradiated food
Irradiated food Modern labor economics 12th edition solution
Modern labor economics 12th edition solution Modern labor economics 12th edition
Modern labor economics 12th edition Modern real estate practice in pennsylvania
Modern real estate practice in pennsylvania Modern database management 12th edition ppt
Modern database management 12th edition ppt Modern database management 10th edition
Modern database management 10th edition Modern operating systems 3rd edition
Modern operating systems 3rd edition Modern operating systems tanenbaum 5th edition
Modern operating systems tanenbaum 5th edition Modern database management 8th edition
Modern database management 8th edition University physics with modern physics fifteenth edition
University physics with modern physics fifteenth edition Modern database management solutions
Modern database management solutions Modern labor economics 12th edition
Modern labor economics 12th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Expert systems: principles and programming, fourth edition
Expert systems: principles and programming, fourth edition Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Introductory chemistry 4th edition
Introductory chemistry 4th edition Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Chapter 1 chapter assessment the central science
Chapter 1 chapter assessment the central science Organic chemistry (3rd) edition chapter 1 problem 20s
Organic chemistry (3rd) edition chapter 1 problem 20s Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Zumdahl chemistry, 9th edition notes
Zumdahl chemistry, 9th edition notes