GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION



- Slides: 43

GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION PETRUCCI HERRING MADURA BISSONNETTE The Periodic Table and Some Atomic Properties 9 PHILIP DUTTON UNIVERSITY OF WINDSOR DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY Slide 9 - 1 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

The Periodic Table and Some Atomic Properties Slide 9 - 2 CONTENTS 9 -1 Classifying the Elements: The Periodic Law and the Periodic Table 9 -2 Metals and Nonmetals and Their Ions 9 -3 Sizes of Atoms and Ions 9 -4 Ionization Energy 9 -5 Electron Affinity 9 -6 Magnetic Properties 9 -7 Polarizability General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

9 -1 Classifying the Elements: The Periodic Law and the Periodic Table 1869 Dimitri Mendeleev Lothar Meyer When the elements are arranged in order of increasing atomic mass, certain sets of properties recur periodically. Dimitri Mendeleev (1834 -1907) Slide 9 - 3 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

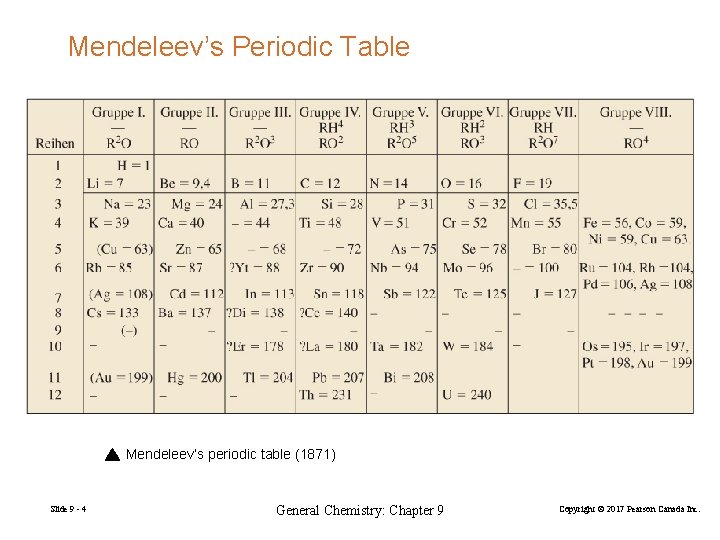
Mendeleev’s Periodic Table Mendeleev’s periodic table (1871) Slide 9 - 4 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

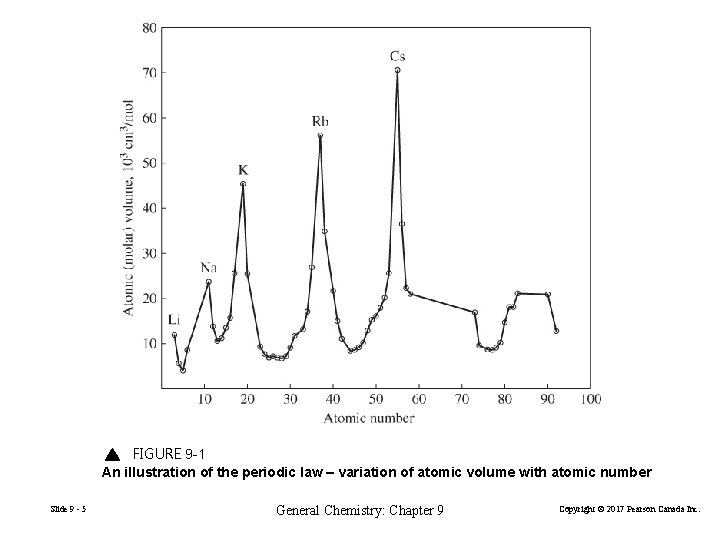
FIGURE 9 -1 An illustration of the periodic law – variation of atomic volume with atomic number Slide 9 - 5 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

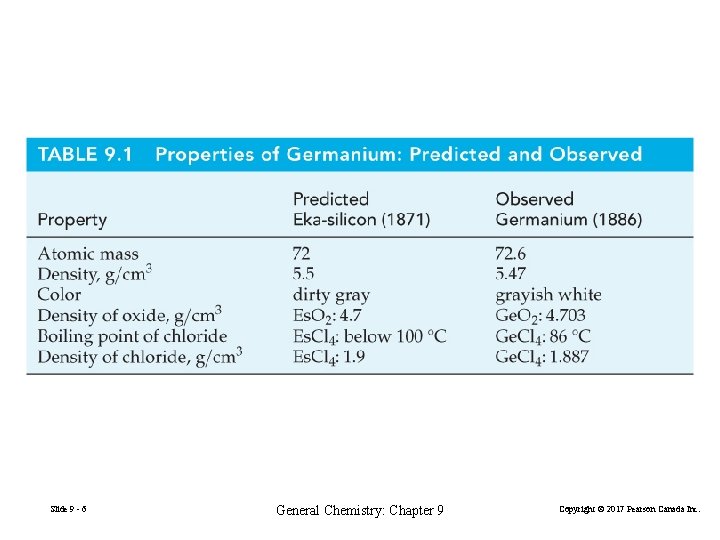
Slide 9 - 6 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

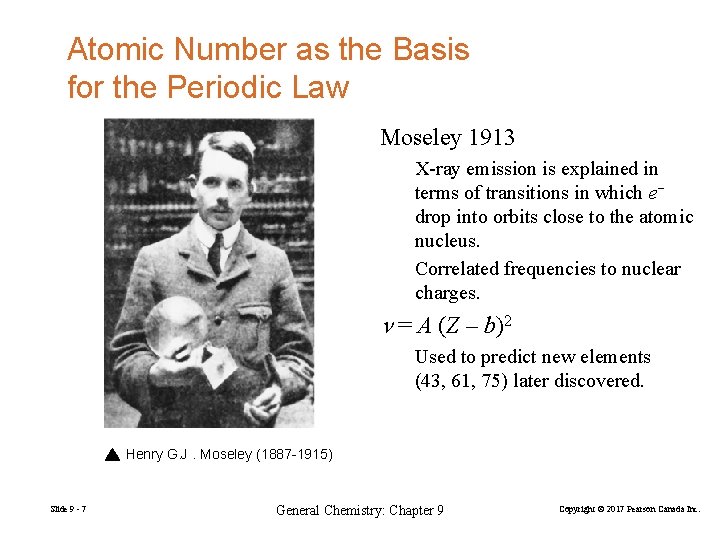
Atomic Number as the Basis for the Periodic Law Moseley 1913 X-ray emission is explained in terms of transitions in which e− drop into orbits close to the atomic nucleus. Correlated frequencies to nuclear charges. = A (Z – b)2 Used to predict new elements (43, 61, 75) later discovered. Henry G. J. Moseley (1887 -1915) Slide 9 - 7 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

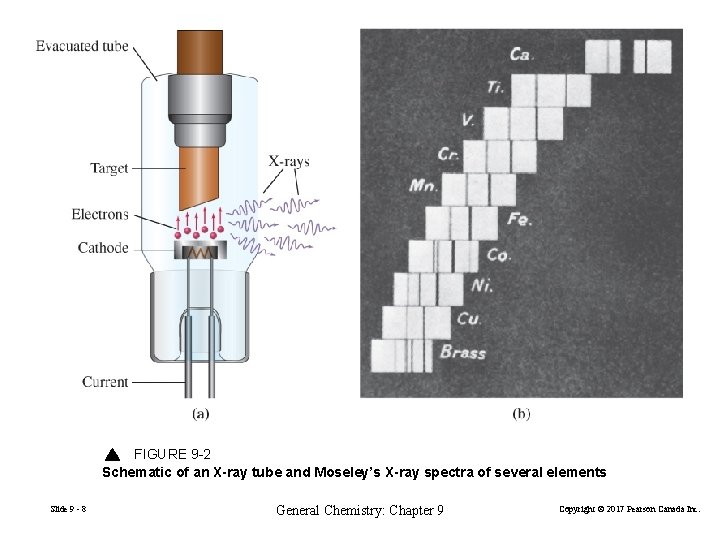
FIGURE 9 -2 Schematic of an X-ray tube and Moseley’s X-ray spectra of several elements Slide 9 - 8 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

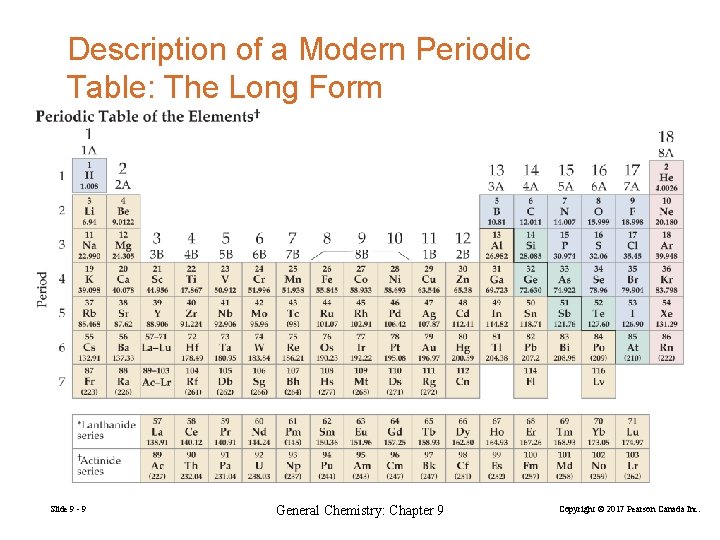
Description of a Modern Periodic Table: The Long Form Slide 9 - 9 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

9 -2 Metals and Nonmetals and Their Ions Metals Good conductors of heat and electricity. Malleable and ductile. Moderate to high melting points. Nonmetals Nonconductors of heat and electricity. Brittle solids. Some are gases at room temperature. Metalloids Metallic and non-metallic properties Slide 9 - 10 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

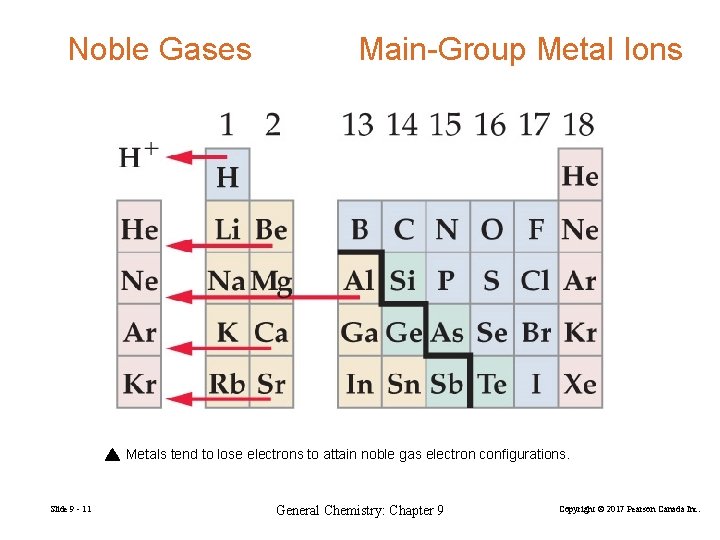
Noble Gases Main-Group Metal Ions Metals tend to lose electrons to attain noble gas electron configurations. Slide 9 - 11 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

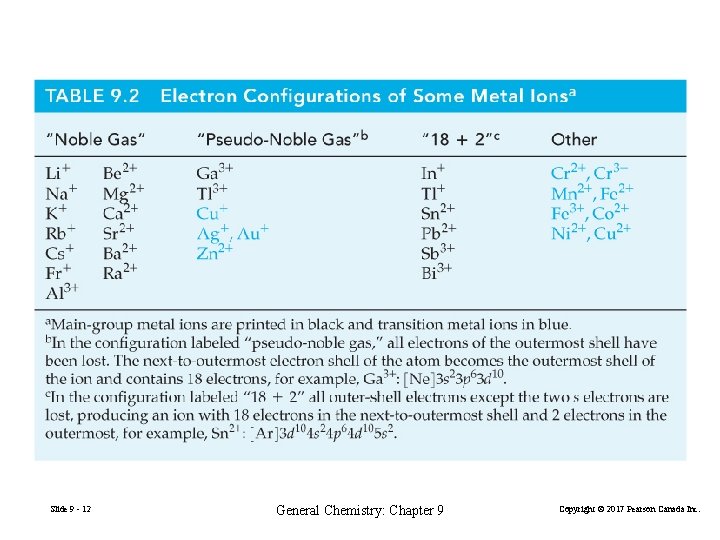
Slide 9 - 12 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

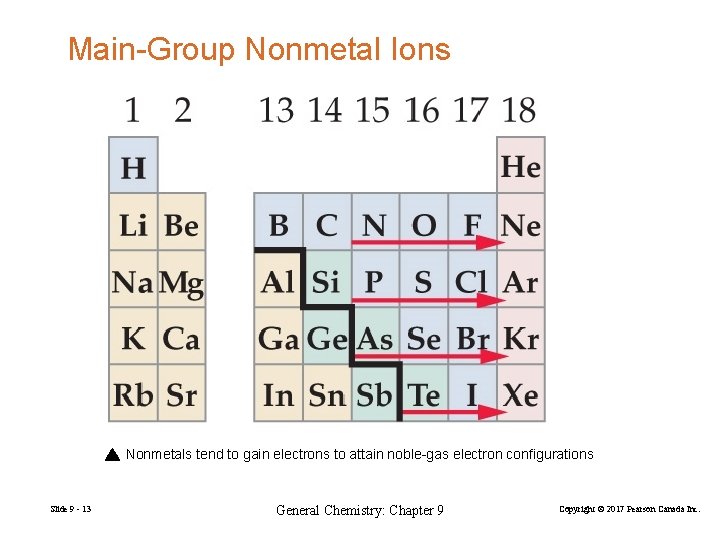
Main-Group Nonmetal Ions Nonmetals tend to gain electrons to attain noble-gas electron configurations Slide 9 - 13 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

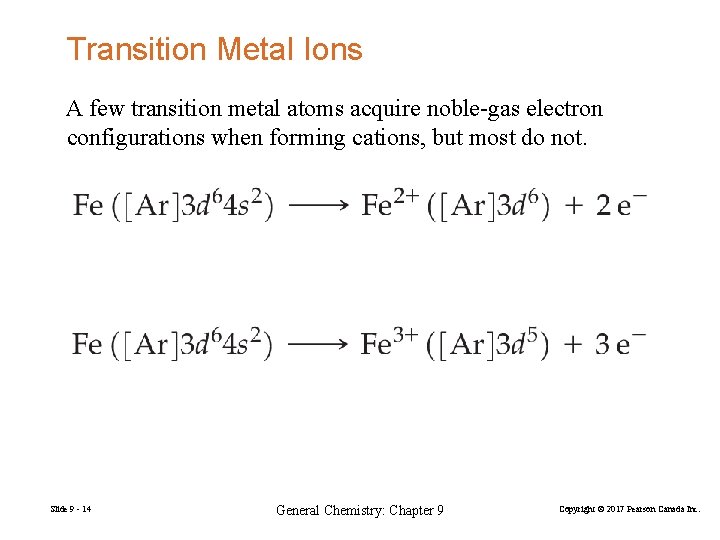
Transition Metal Ions A few transition metal atoms acquire noble-gas electron configurations when forming cations, but most do not. Slide 9 - 14 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

Hydrogen H atom (1 s 1) Usually loses one electron, but can gain one, to attain a noble gas configuration. Is often placed in Group 1, but sometimes in Group 17. H 2 is a reducing agent (compare to F 2, or Cl 2) Slide 9 - 15 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

9 -3 Sizes of Atoms and Ions Atomic Radius Covalent radius is one half of the distance between the nuclei of two identical atoms joined by a single covalent bond. Ionic radius is based on the distance between the nuclei of ions joined by an ionic bond. (properly apportioned by assigning r(O 22–) = 140 pm) Metallic radius is one-half the distance between the nuclei of two atoms in contact in the crystalline solid metal. van der Waals radius is similar to metallic radius except is for solid samples of noble gases. Slide 9 - 16 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

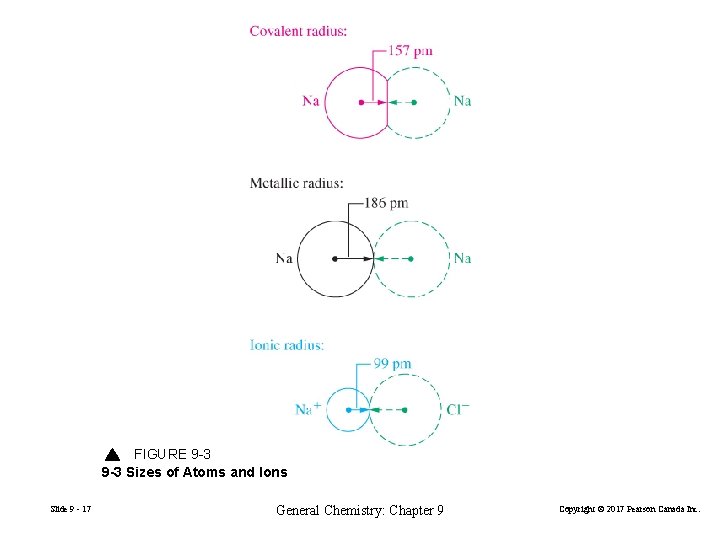
FIGURE 9 -3 Sizes of Atoms and Ions Slide 9 - 17 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

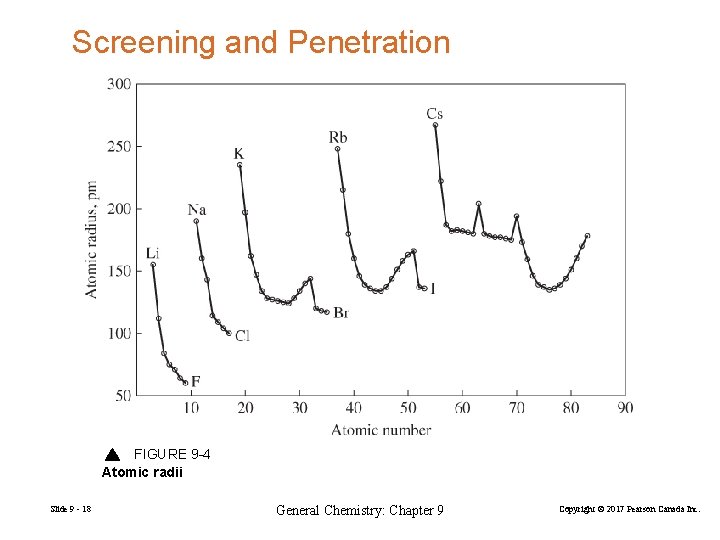
Screening and Penetration FIGURE 9 -4 Atomic radii Slide 9 - 18 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

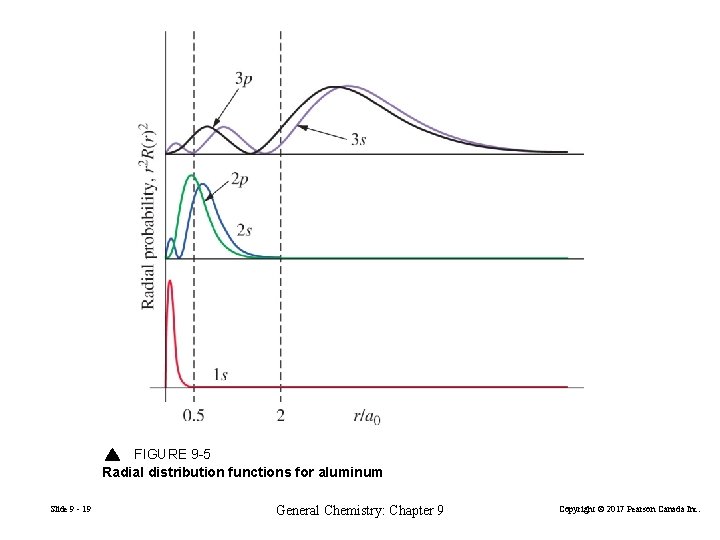
FIGURE 9 -5 Radial distribution functions for aluminum Slide 9 - 19 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

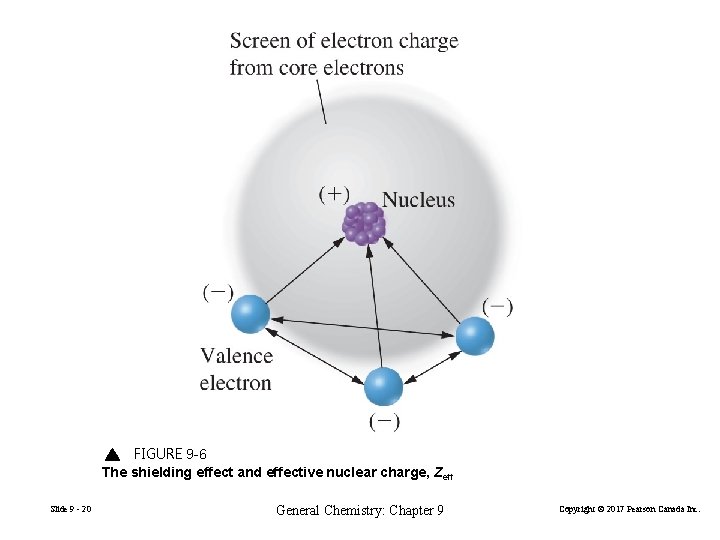
FIGURE 9 -6 The shielding effect and effective nuclear charge, Zeff Slide 9 - 20 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

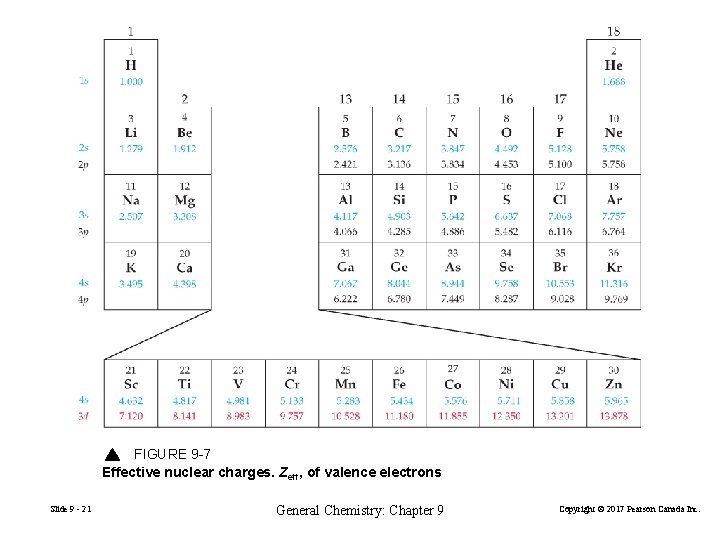
FIGURE 9 -7 Effective nuclear charges. Zeff, of valence electrons Slide 9 - 21 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

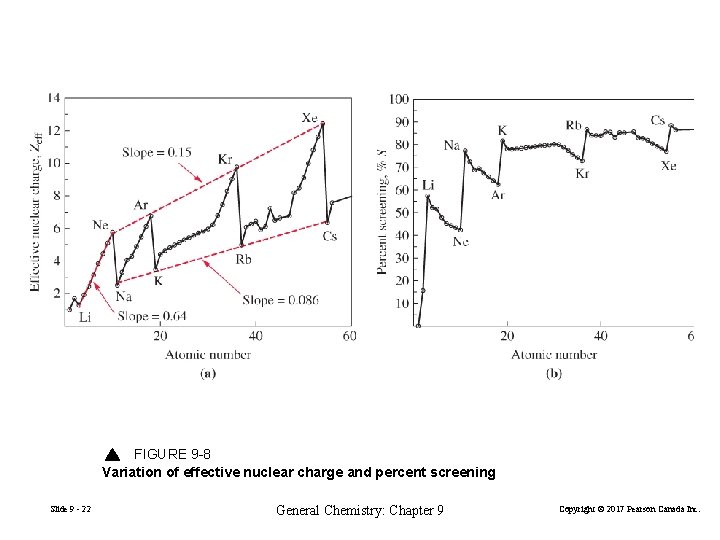
FIGURE 9 -8 Variation of effective nuclear charge and percent screening Slide 9 - 22 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

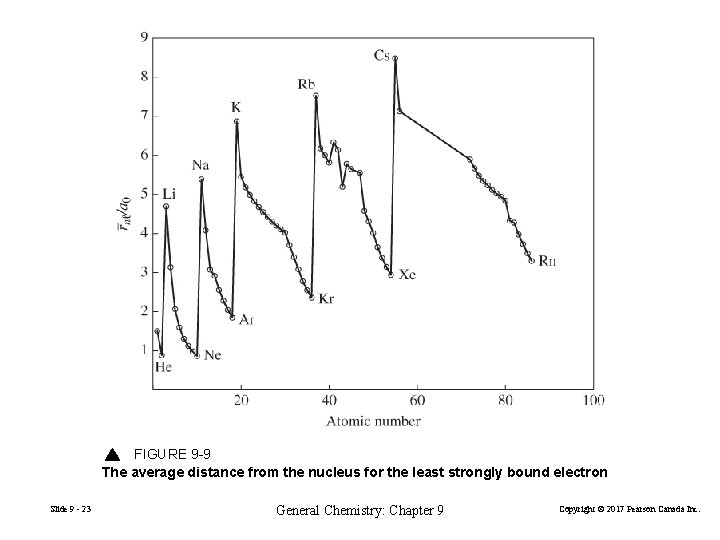
FIGURE 9 -9 The average distance from the nucleus for the least strongly bound electron Slide 9 - 23 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

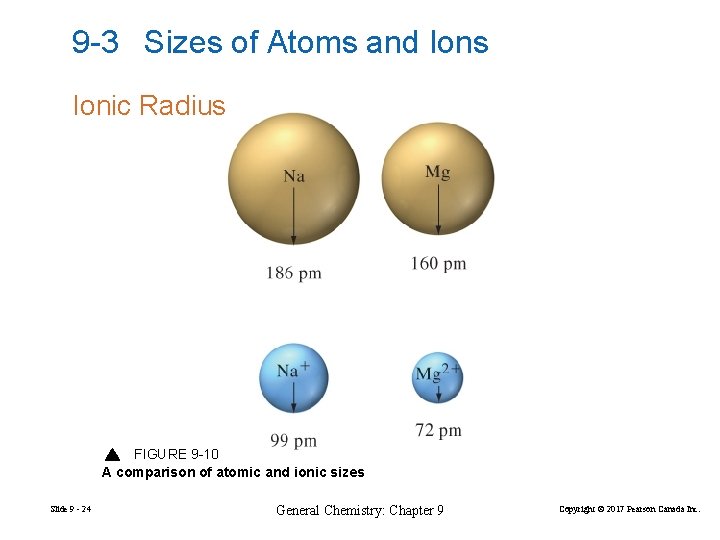
9 -3 Sizes of Atoms and Ions Ionic Radius FIGURE 9 -10 A comparison of atomic and ionic sizes Slide 9 - 24 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

Ionic Radius Cations are smaller than the atoms from which they are formed. For isoelectronic cations, the more positive the ionic charge, the smaller the ionic radius. Anions are larger than the atoms from which they are formed. For isoelectronic anions, the more negative the charge, the larger the ionic radius. Slide 9 - 25 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

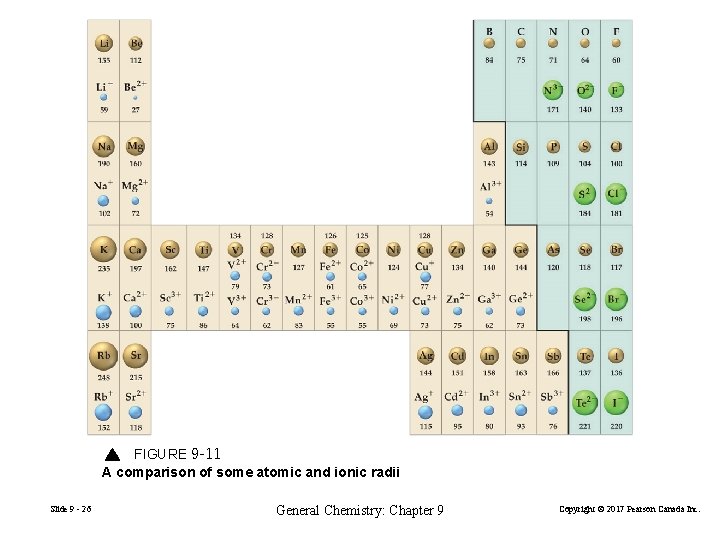
FIGURE 9 -11 A comparison of some atomic and ionic radii Slide 9 - 26 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

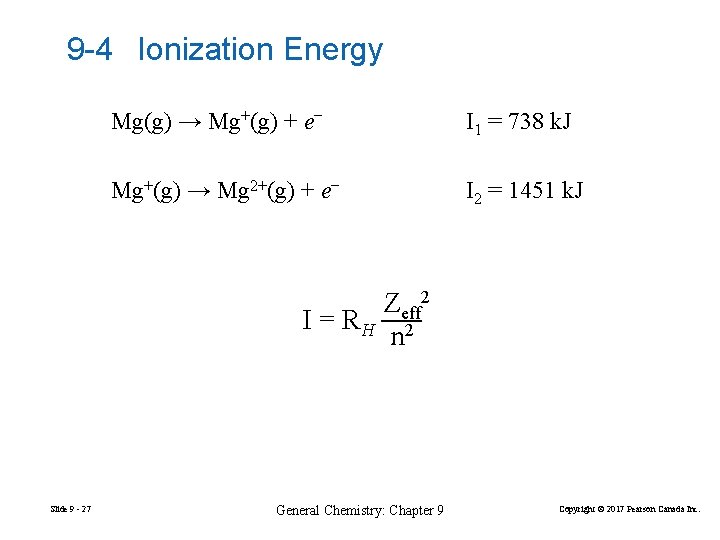
9 -4 Ionization Energy Mg(g) → Mg+(g) + e− I 1 = 738 k. J Mg+(g) → Mg 2+(g) + e− I 2 = 1451 k. J Zeff 2 I = RH 2 n Slide 9 - 27 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

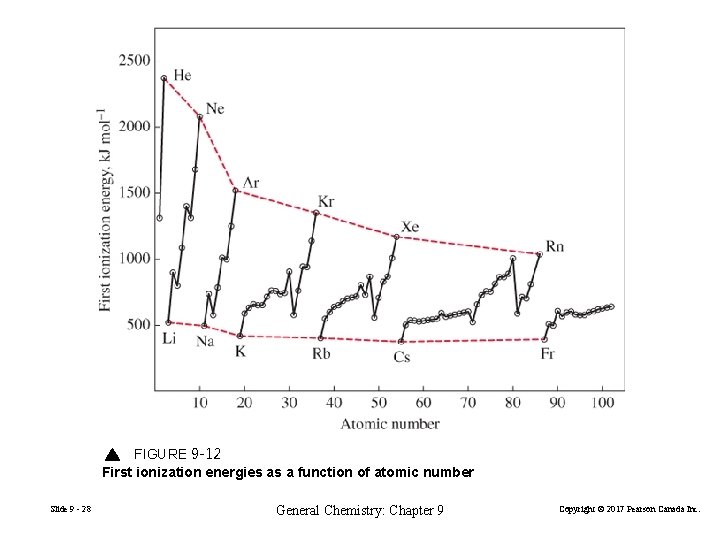
FIGURE 9 -12 First ionization energies as a function of atomic number Slide 9 - 28 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

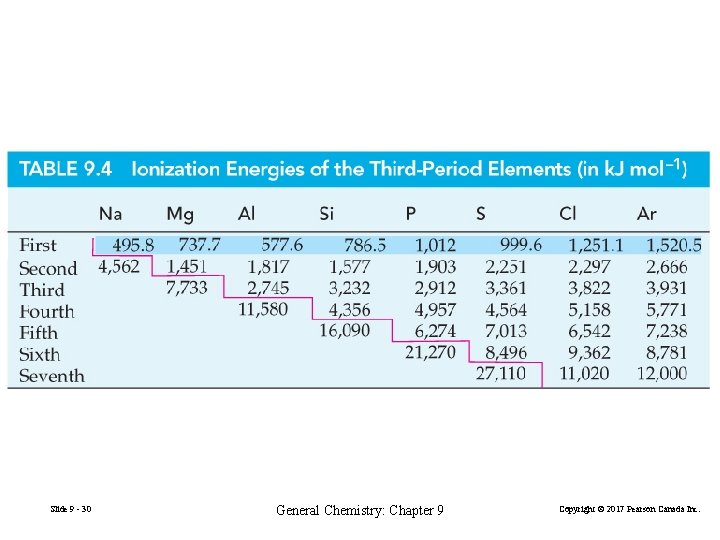
With relatively few exceptions, ionization energies increase from left to right across a period and decrease from top to bottom within a group. Ionization energies decrease as atomic radii increase. Slide 9 - 29 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

Slide 9 - 30 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

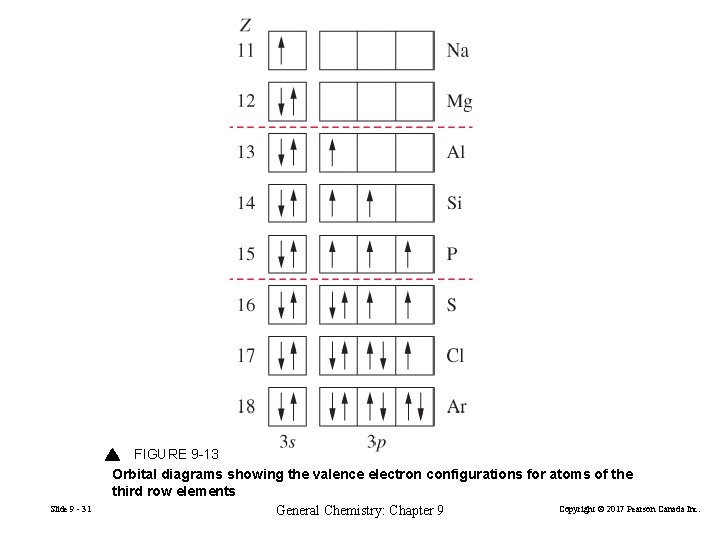
Slide 9 - 31 FIGURE 9 -13 Orbital diagrams showing the valence electron configurations for atoms of the third row elements Copyright © 2017 Pearson Canada Inc. General Chemistry: Chapter 9

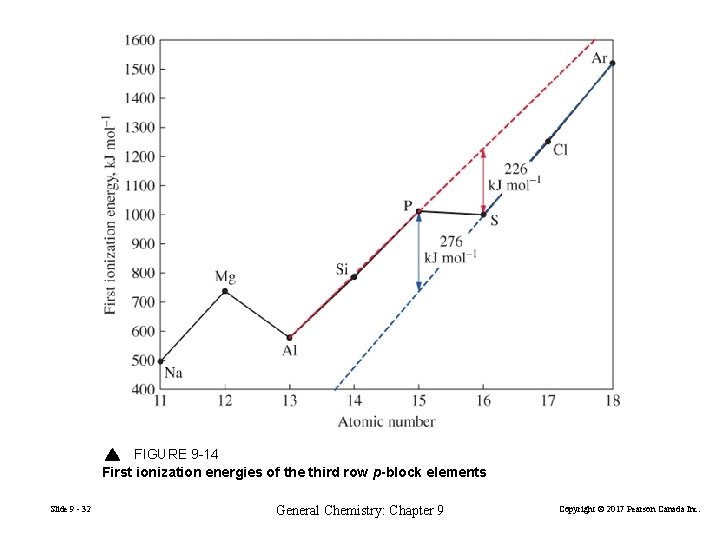
FIGURE 9 -14 First ionization energies of the third row p-block elements Slide 9 - 32 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

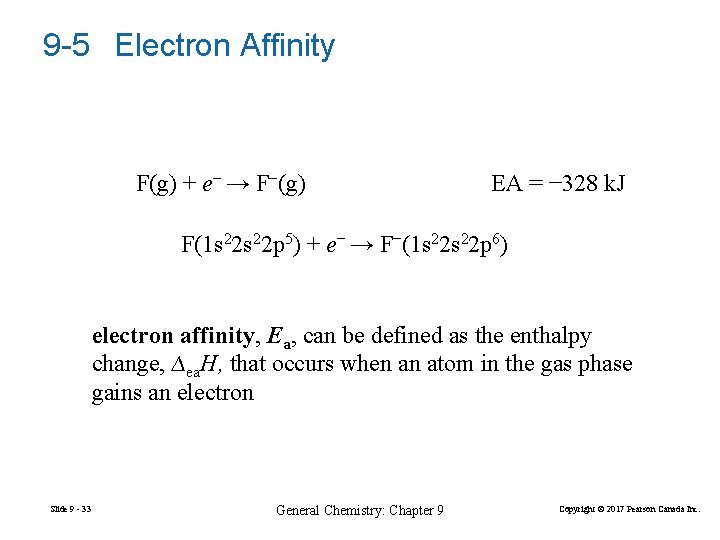
9 -5 Electron Affinity F(g) + e− → F−(g) EA = − 328 k. J F(1 s 22 p 5) + e− → F−(1 s 22 p 6) electron affinity, Ea, can be defined as the enthalpy change, ∆ea. H, that occurs when an atom in the gas phase gains an electron Slide 9 - 33 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

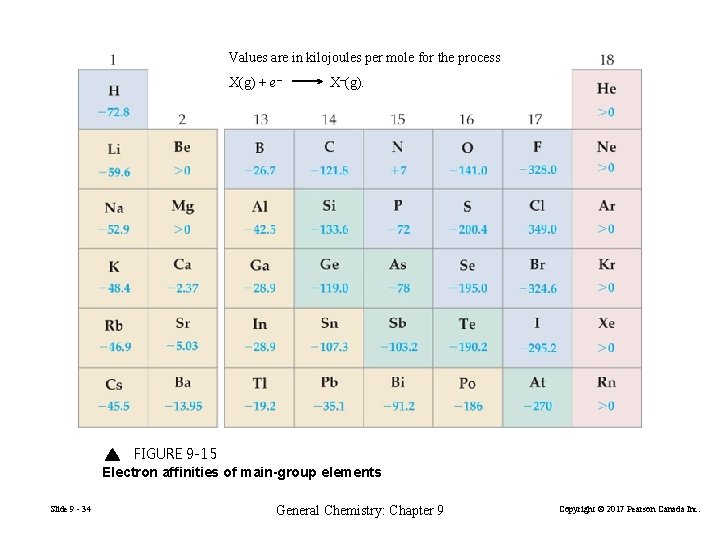
Values are in kilojoules per mole for the process X(g) + e– X–(g). FIGURE 9 -15 Electron affinities of main-group elements Slide 9 - 34 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

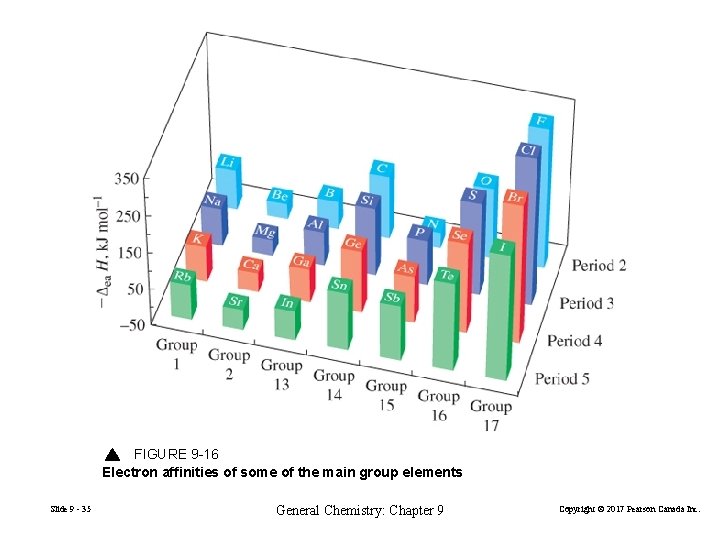
FIGURE 9 -16 Electron affinities of some of the main group elements Slide 9 - 35 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

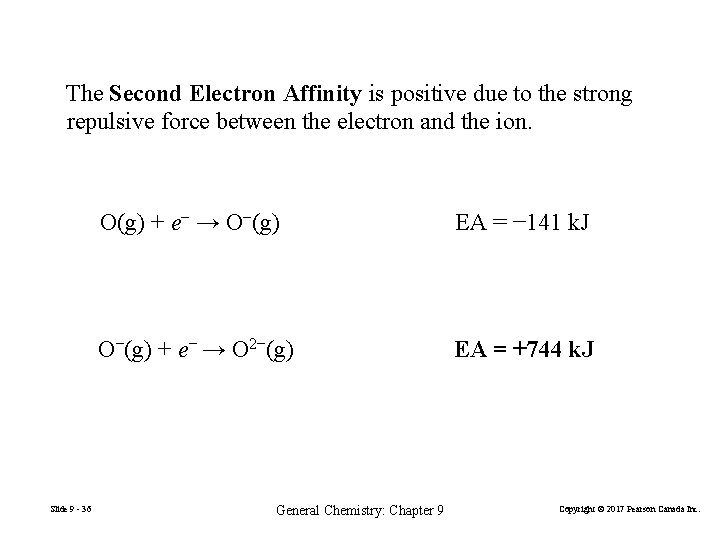
The Second Electron Affinity is positive due to the strong repulsive force between the electron and the ion. Slide 9 - 36 O(g) + e− → O−(g) EA = − 141 k. J O−(g) + e− → O 2−(g) EA = +744 k. J General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

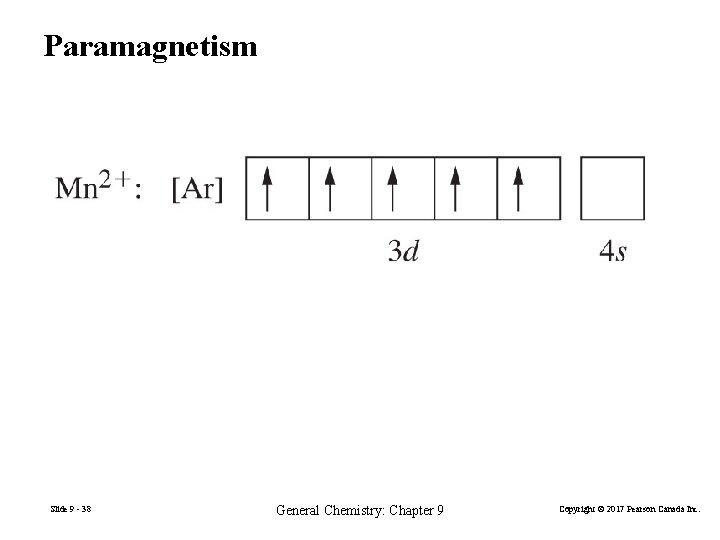
9 -6 Magnetic Properties Diamagnetic atoms or ions: All e− are paired. Weakly repelled by a magnetic field. Paramagnetic atoms or ions: Unpaired e −. Attracted to an external magnetic field. Slide 9 - 37 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

Paramagnetism Slide 9 - 38 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

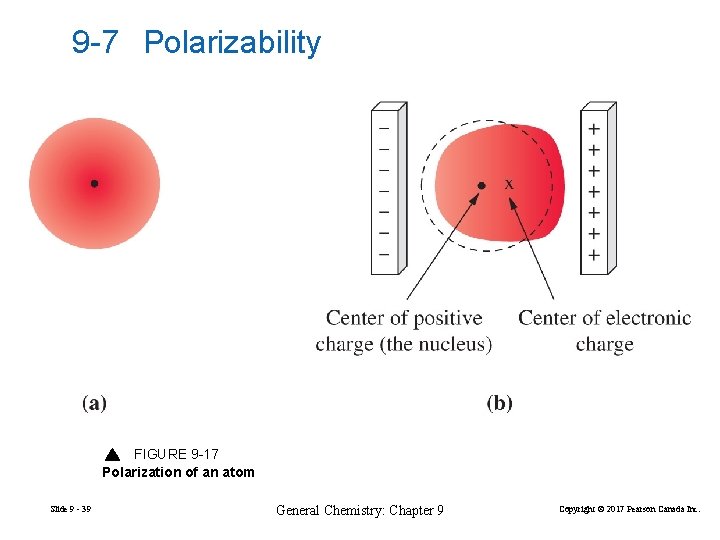
9 -7 Polarizability FIGURE 9 -17 Polarization of an atom Slide 9 - 39 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

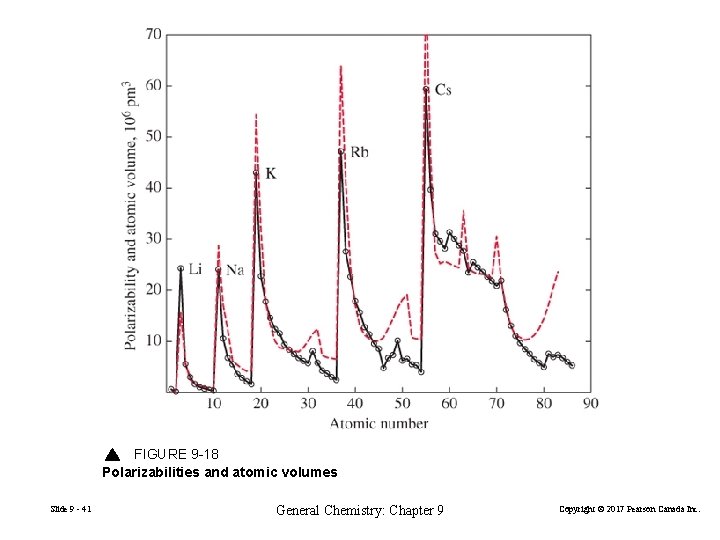
Polarizability increases with the size of the atom. Slide 9 - 40 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

FIGURE 9 -18 Polarizabilities and atomic volumes Slide 9 - 41 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

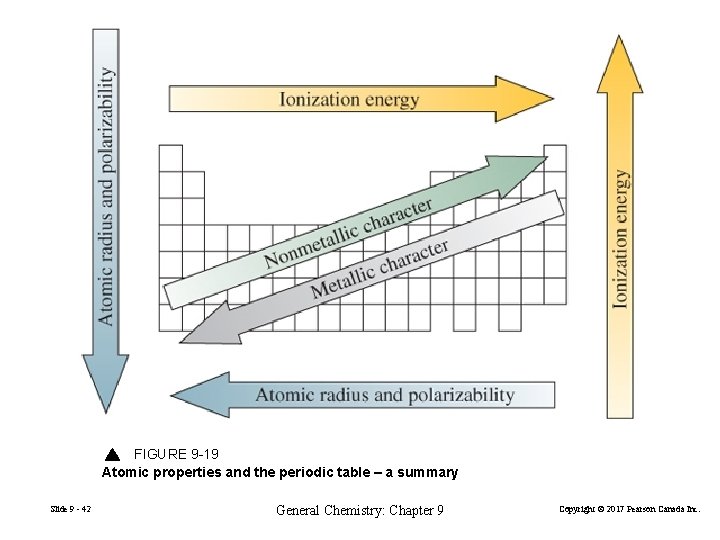
FIGURE 9 -19 Atomic properties and the periodic table – a summary Slide 9 - 42 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.

End of Chapter Slide 9 - 43 General Chemistry: Chapter 9 Copyright © 2017 Pearson Canada Inc.