GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION














- Slides: 50

GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION PETRUCCI HERRING MADURA BISSONNETTE 6 Gases PHILIP DUTTON UNIVERSITY OF WINDSOR DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY Slide 6 - 1 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

Gases CONTENTS Slide 6 - 2 6 -1 Properties of Gases: Gas Pressure 6 -2 The Simple Gas Laws 6 -3 Combining the Gas Laws: The Ideal Gas Equation and The General Gas Equation 6 -4 Applications of the Ideal Gas Equation 6 -5 Gases in Chemical Reactions 6 -6 Mixtures of Gases 6 -7 Kinetic—Molecular Theory of Gases 6 -8 Gas Properties Relating to the Kinetic —Molecular Theory 6 -9 Nonideal (Real) Gases General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

FIGURE 6 -1 The gaseous state of three halogens (group 17) Slide 6 - 3 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

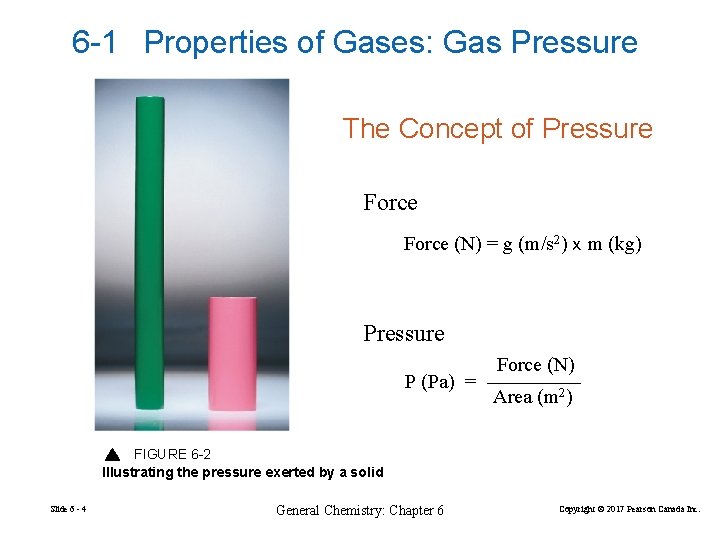
6 -1 Properties of Gases: Gas Pressure The Concept of Pressure Force (N) = g (m/s 2) x m (kg) Pressure P (Pa) = Force (N) Area (m 2) FIGURE 6 -2 Illustrating the pressure exerted by a solid Slide 6 - 4 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

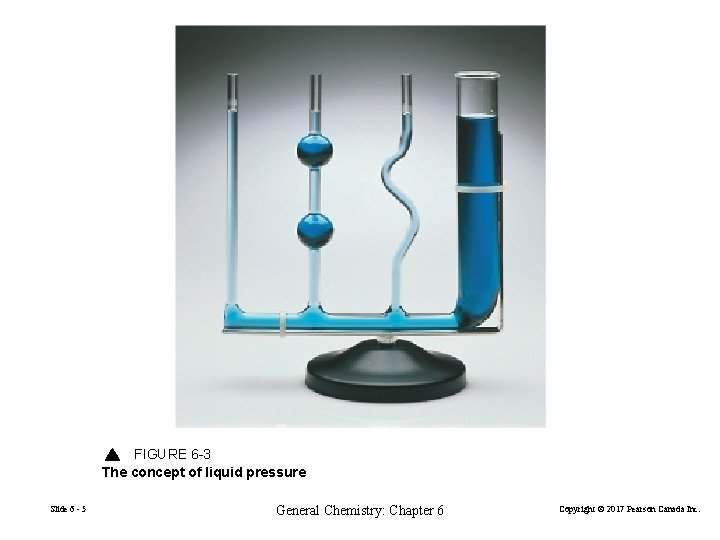
FIGURE 6 -3 The concept of liquid pressure Slide 6 - 5 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

Liquid Pressure P (Pa) = F = W = A A gxm = gx. Vxd = gxhx. Axd A A A = gxhxd liquid pressure is directly proportional to the liquid density and the height of the liquid column Slide 6 - 6 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

Barometric Pressure FIGURE 6 -4 Measurement of atmospheric pressure with a mercury barometer Slide 6 - 7 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

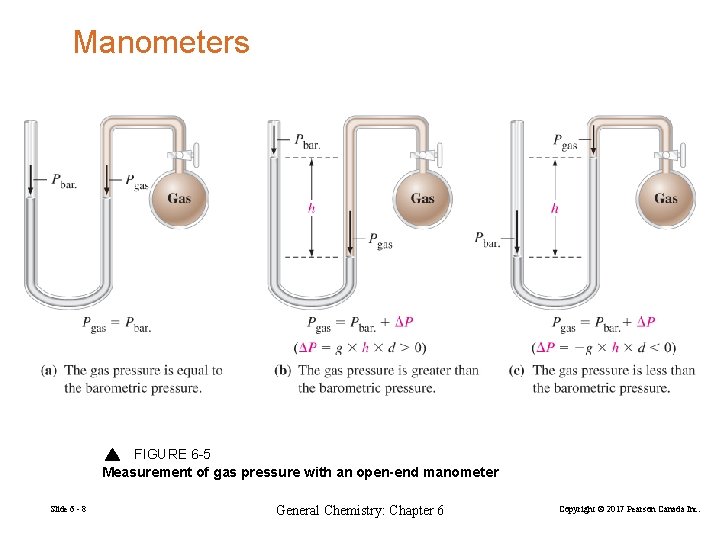
Manometers FIGURE 6 -5 Measurement of gas pressure with an open-end manometer Slide 6 - 8 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

Units of Pressure: A Summary Slide 6 - 9 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

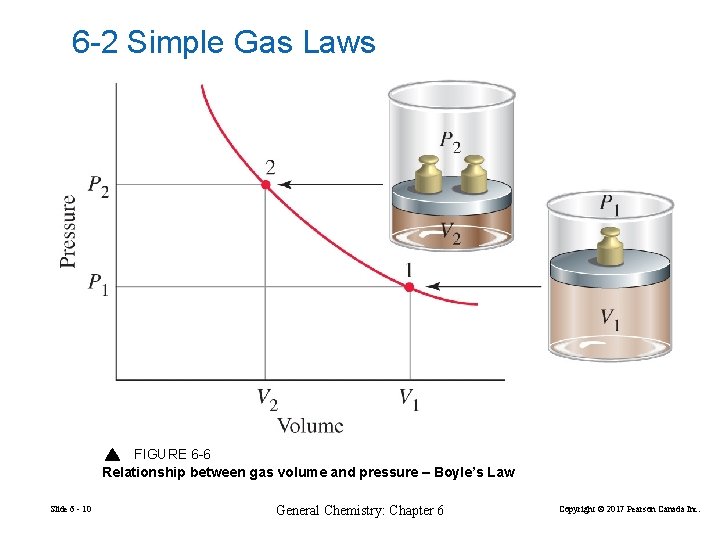
6 -2 Simple Gas Laws FIGURE 6 -6 Relationship between gas volume and pressure – Boyle’s Law Slide 6 - 10 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

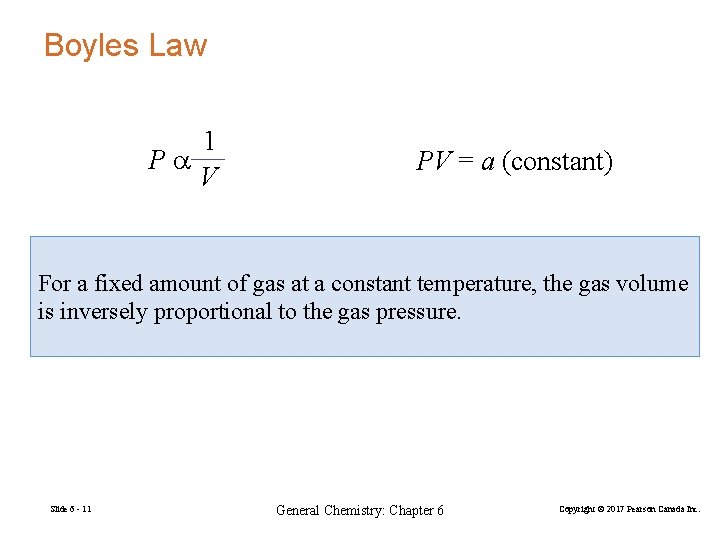
Boyles Law 1 Pa V PV = a (constant) For a fixed amount of gas at a constant temperature, the gas volume is inversely proportional to the gas pressure. Slide 6 - 11 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

FIGURE 6 -7 Gas volume as a function of temperature Slide 6 - 12 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

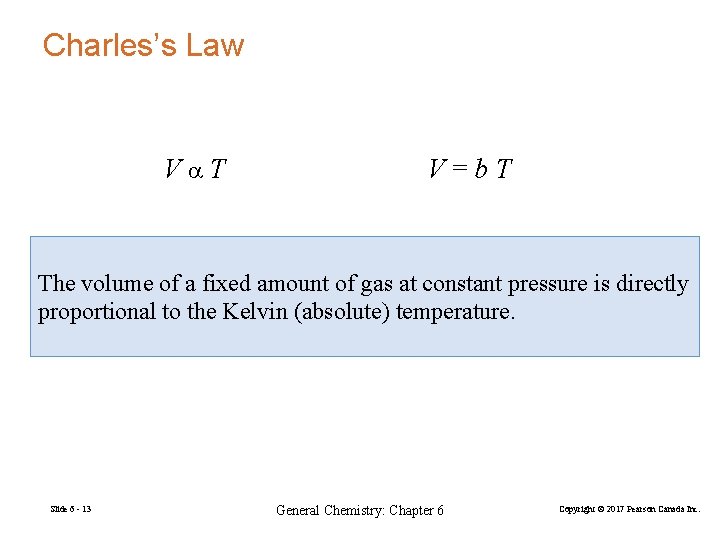
Charles’s Law Va. T V=b. T The volume of a fixed amount of gas at constant pressure is directly proportional to the Kelvin (absolute) temperature. Slide 6 - 13 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

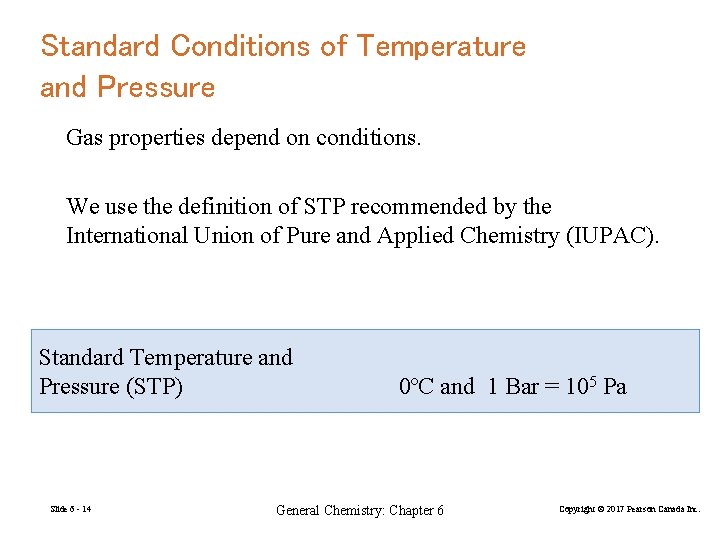
Standard Conditions of Temperature and Pressure Gas properties depend on conditions. We use the definition of STP recommended by the International Union of Pure and Applied Chemistry (IUPAC). Standard Temperature and Pressure (STP) Slide 6 - 14 0ºC and 1 Bar = 105 Pa General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

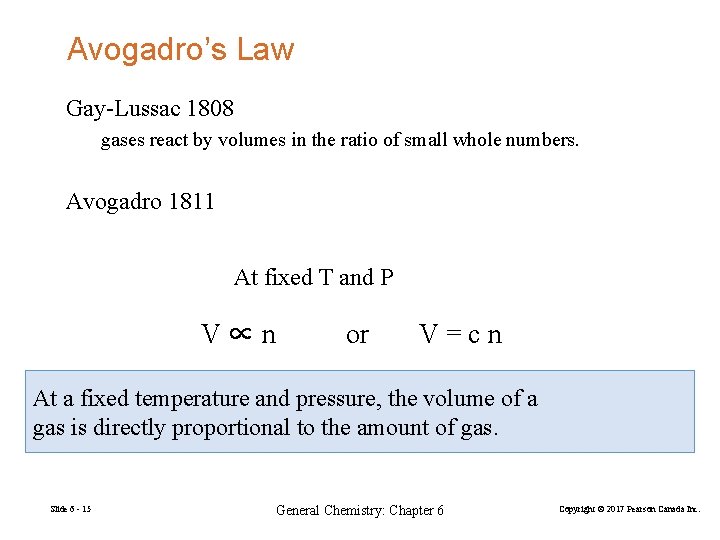
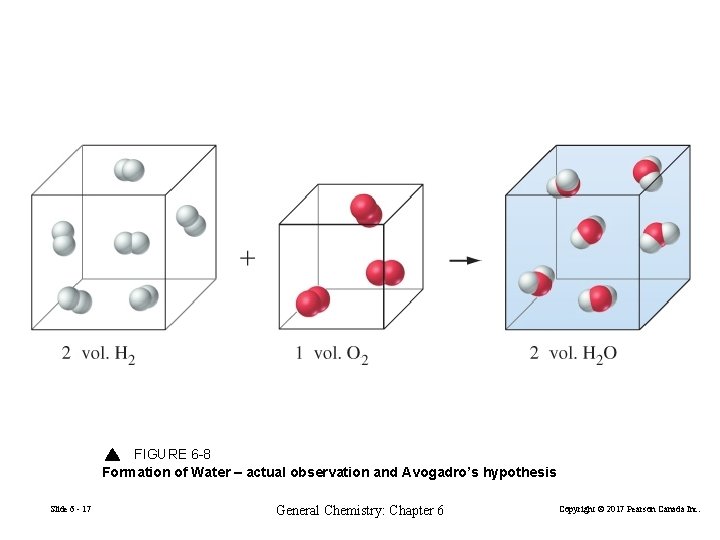
Avogadro’s Law Gay-Lussac 1808 gases react by volumes in the ratio of small whole numbers. Avogadro 1811 At fixed T and P V∝n or V=cn At a fixed temperature and pressure, the volume of a gas is directly proportional to the amount of gas. Slide 6 - 15 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

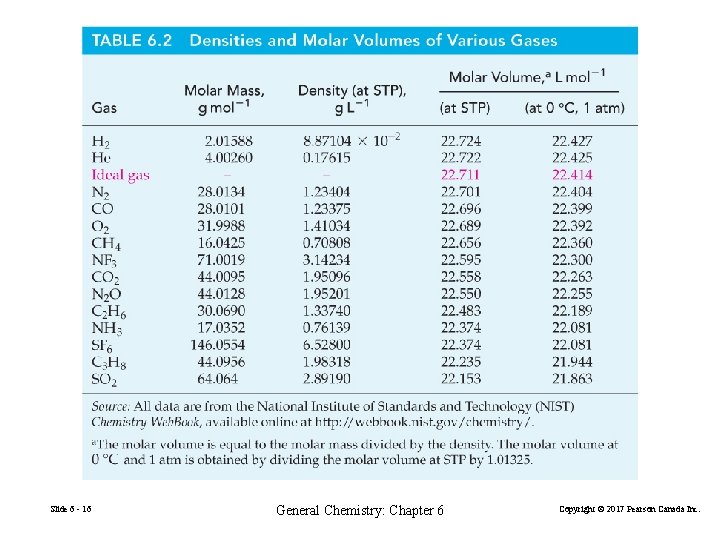
Slide 6 - 16 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

FIGURE 6 -8 Formation of Water – actual observation and Avogadro’s hypothesis Slide 6 - 17 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

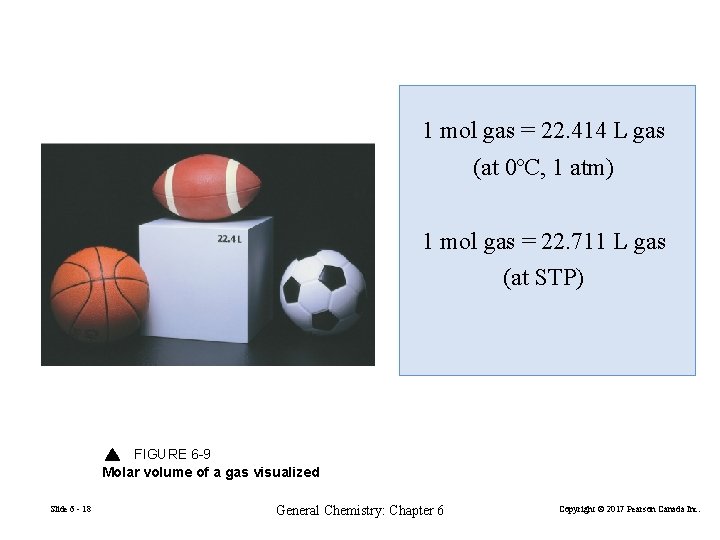
1 mol gas = 22. 414 L gas (at 0ºC, 1 atm) 1 mol gas = 22. 711 L gas (at STP) FIGURE 6 -9 Molar volume of a gas visualized Slide 6 - 18 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

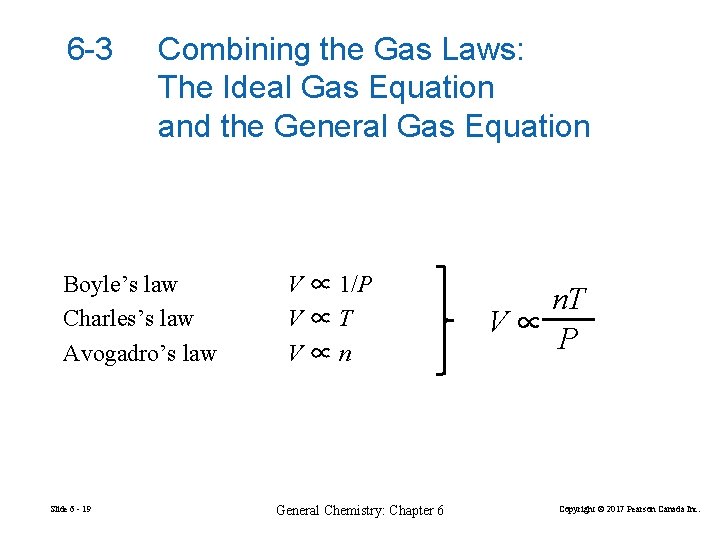
6 -3 Combining the Gas Laws: The Ideal Gas Equation and the General Gas Equation Boyle’s law Charles’s law Avogadro’s law Slide 6 - 19 V ∝ 1/P V∝T V∝n General Chemistry: Chapter 6 n. T V∝ P Copyright © 2017 Pearson Canada Inc.

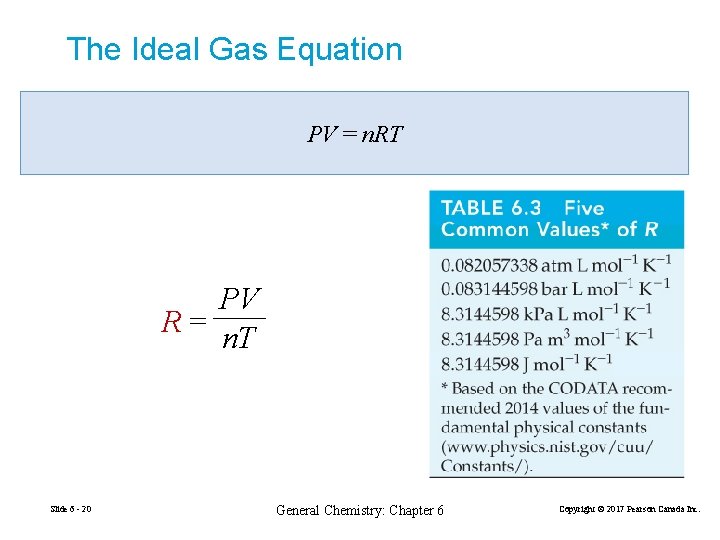
The Ideal Gas Equation PV = n. RT PV R= n. T Slide 6 - 20 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

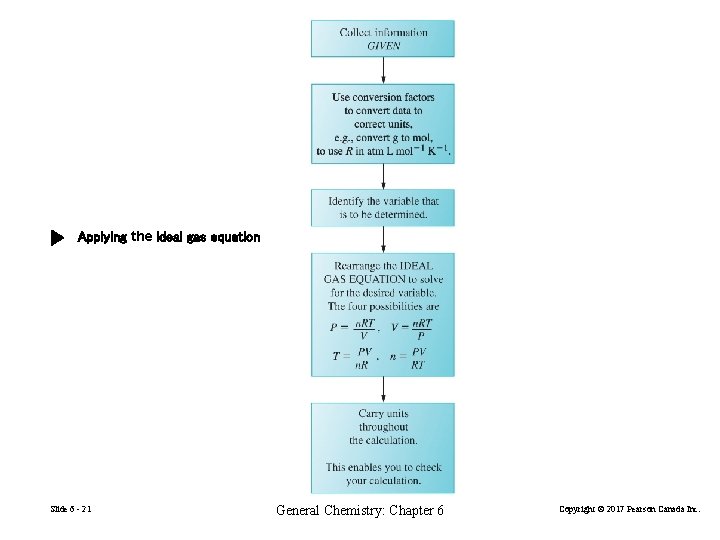
Applying the ideal gas equation Slide 6 - 21 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

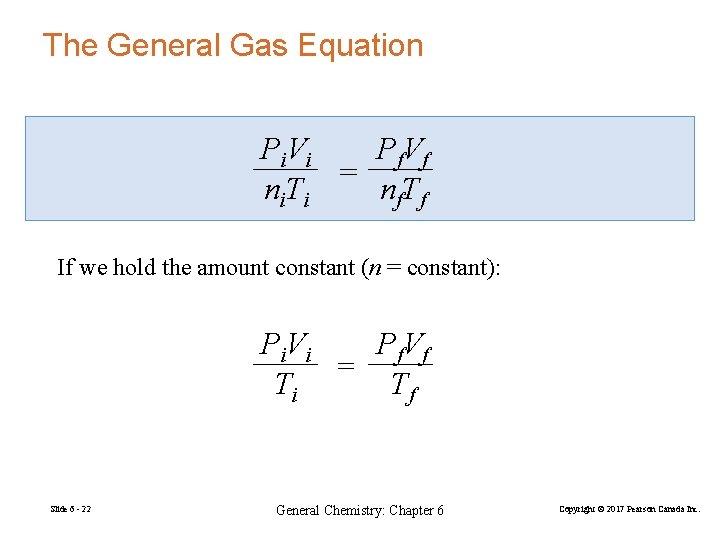
The General Gas Equation P i. V i P f. V f = ni. Ti nf. Tf If we hold the amount constant (n = constant): P i. V i P f. V f = Ti Tf Slide 6 - 22 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

6 -4 Applications of the Ideal Gas Equation Molar Mass Determination PV = n. RT and n= m M m RT PV = M m RT M= PV Slide 6 - 23 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

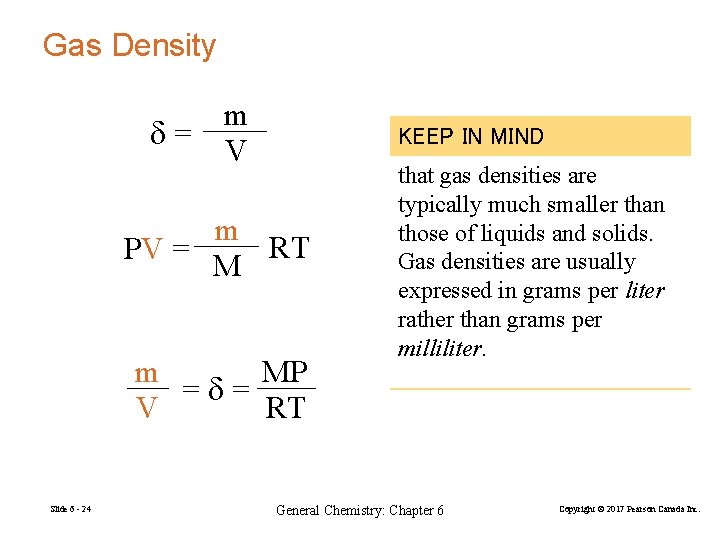
Gas Density m d= V KEEP IN MIND m RT PV = M m MP =d= V RT Slide 6 - 24 that gas densities are typically much smaller than those of liquids and solids. Gas densities are usually expressed in grams per liter rather than grams per milliliter. General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

6 -5 Gases in Chemical Reactions Stoichiometric factors relate gas quantities to quantities of other reactants or products. Ideal gas equation relates the amount of a gas to volume, temperature and pressure. Law of Combining Volumes can be developed using the gas law. Slide 6 - 25 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

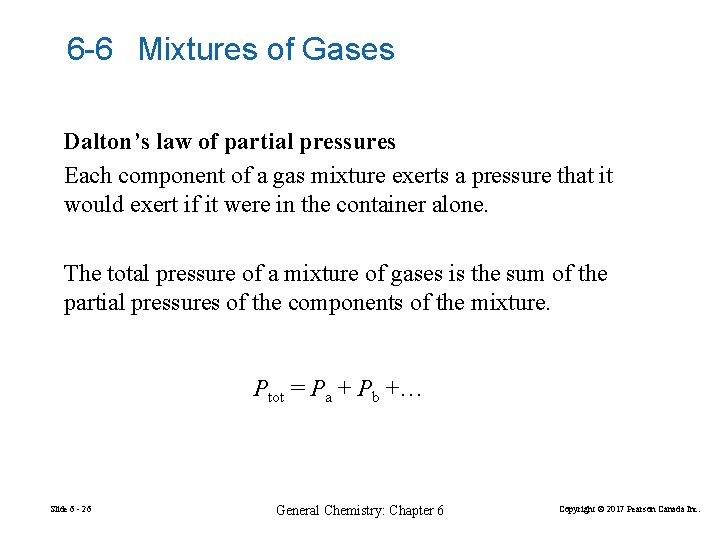
6 -6 Mixtures of Gases Dalton’s law of partial pressures Each component of a gas mixture exerts a pressure that it would exert if it were in the container alone. The total pressure of a mixture of gases is the sum of the partial pressures of the components of the mixture. Ptot = Pa + Pb +… Slide 6 - 26 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

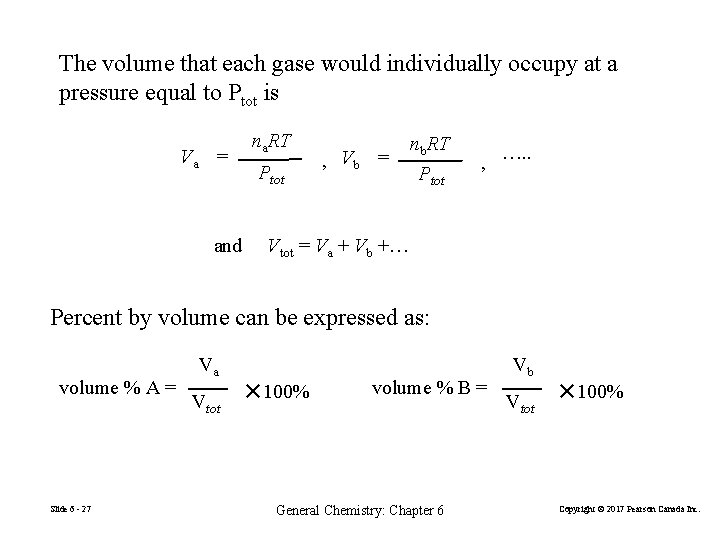
The volume that each gase would individually occupy at a pressure equal to Ptot is Va = and na. RT Ptot , Vb = nb. RT Ptot , …. . Vtot = Va + Vb +… Percent by volume can be expressed as: volume % A = Slide 6 - 27 Va Vtot ✕ 100% volume % B = General Chemistry: Chapter 6 Vb Vtot ✕ 100% Copyright © 2017 Pearson Canada Inc.

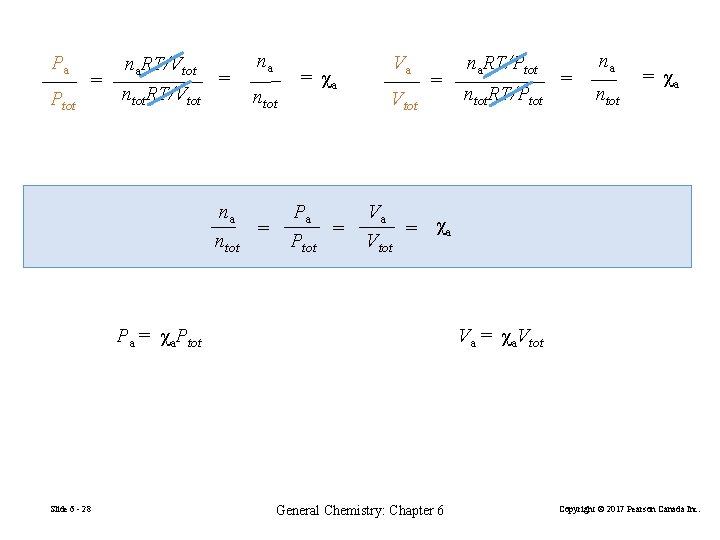
Pa Ptot = na. RT/Vtot ntot. RT/Vtot = na ntot na = ntot = χa Pa = Ptot Va Vtot = ntot. RT/Ptot = na ntot = χa Va = χa Vtot Va = χa. Vtot Pa = χa. Ptot Slide 6 - 28 na. RT/Ptot General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

FIGURE 6 -12 Dalton’s law of partial pressures illustrated Slide 6 - 29 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

FIGURE 6 -13 Collecting a gas over water Slide 6 - 30 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

Ptot = Pbar = Pgas + PH 2 O Pgas = Pbar − PH 2 O Slide 6 - 31 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

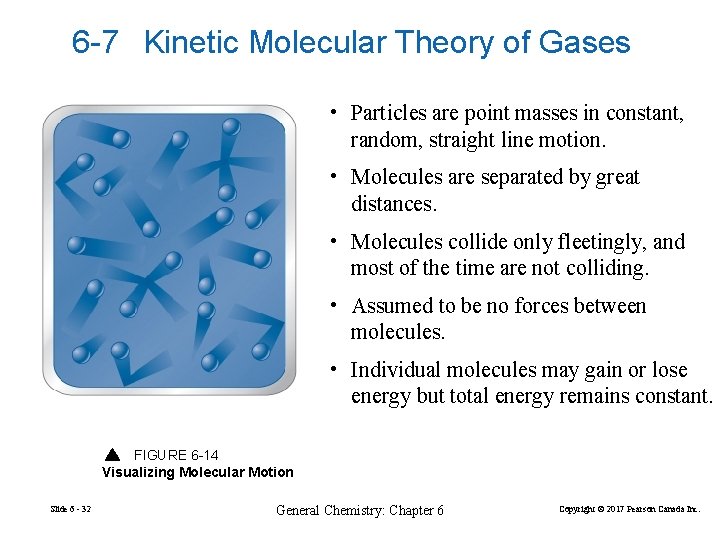
6 -7 Kinetic Molecular Theory of Gases • Particles are point masses in constant, random, straight line motion. • Molecules are separated by great distances. • Molecules collide only fleetingly, and most of the time are not colliding. • Assumed to be no forces between molecules. • Individual molecules may gain or lose energy but total energy remains constant. FIGURE 6 -14 Visualizing Molecular Motion Slide 6 - 32 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

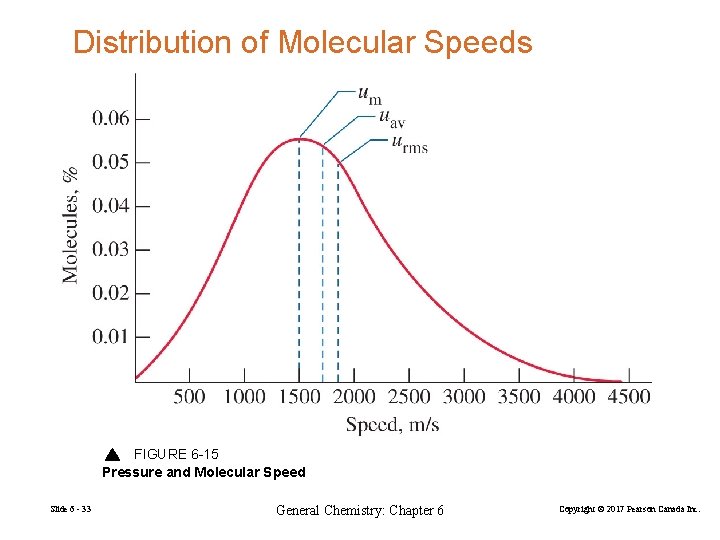
Distribution of Molecular Speeds FIGURE 6 -15 Pressure and Molecular Speed Slide 6 - 33 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

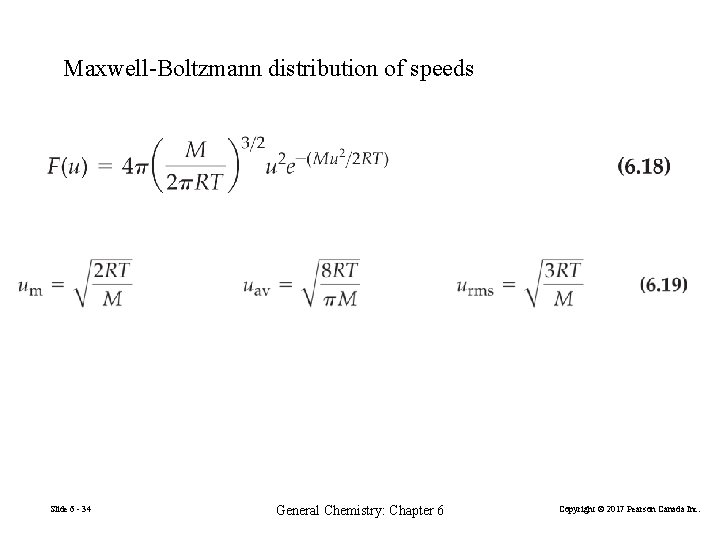
Maxwell-Boltzmann distribution of speeds Slide 6 - 34 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

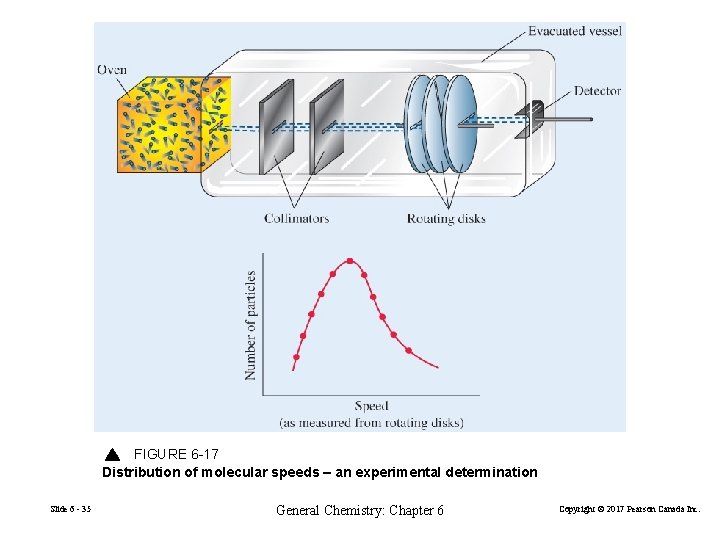
FIGURE 6 -17 Distribution of molecular speeds – an experimental determination Slide 6 - 35 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

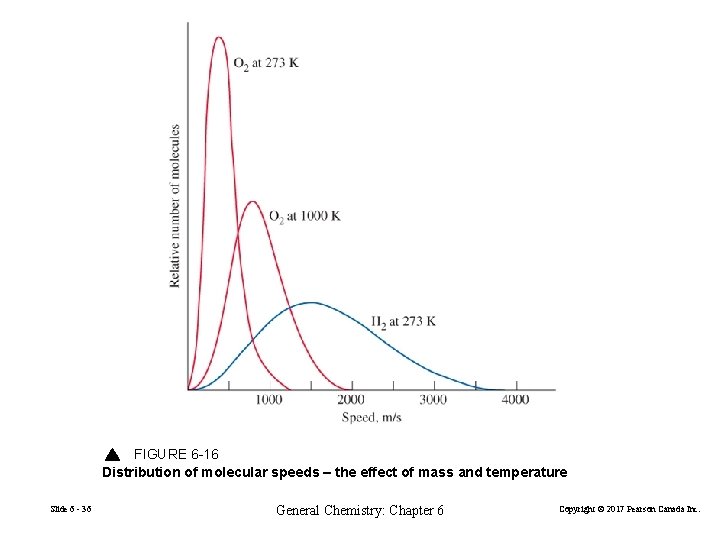
FIGURE 6 -16 Distribution of molecular speeds – the effect of mass and temperature Slide 6 - 36 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

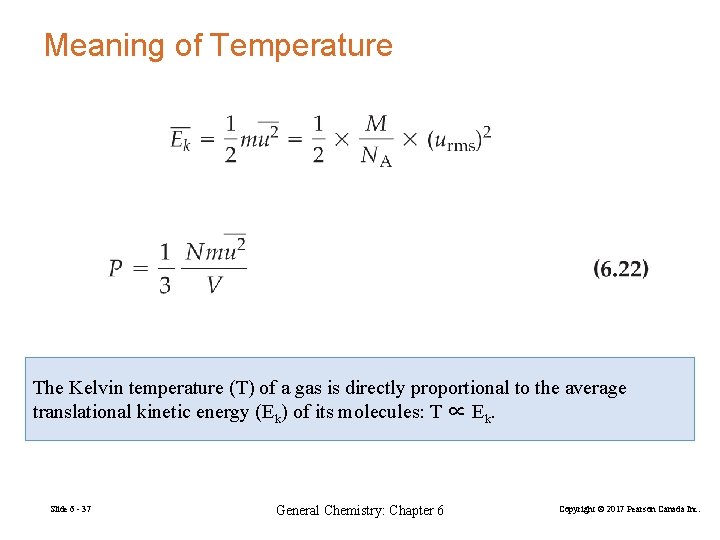
Meaning of Temperature The Kelvin temperature (T) of a gas is directly proportional to the average translational kinetic energy (Ek) of its molecules: T ∝ Ek. Slide 6 - 37 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

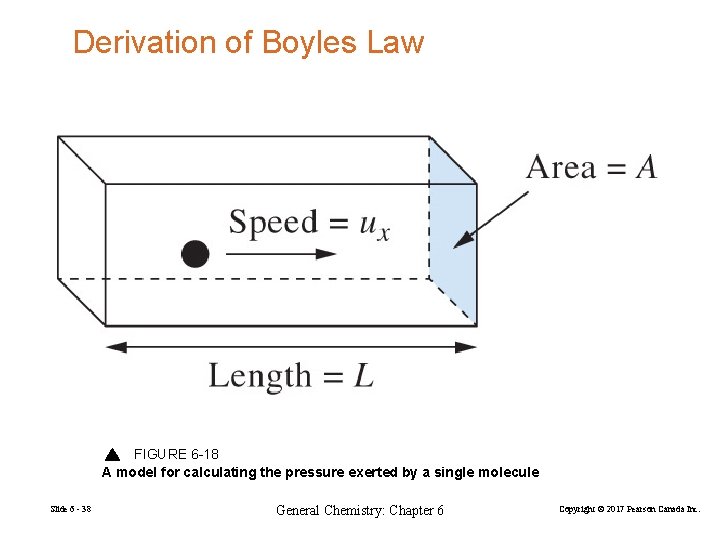
Derivation of Boyles Law FIGURE 6 -18 A model for calculating the pressure exerted by a single molecule Slide 6 - 38 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

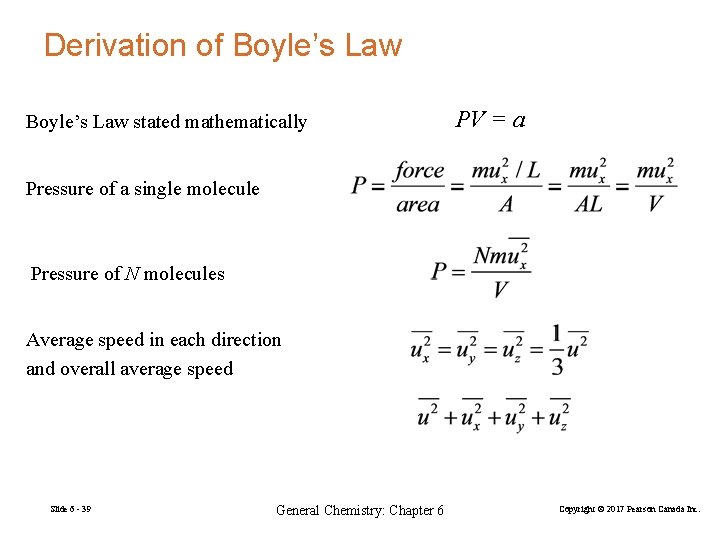
Derivation of Boyle’s Law stated mathematically PV = a Pressure of a single molecule Pressure of N molecules Average speed in each direction and overall average speed Slide 6 - 39 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

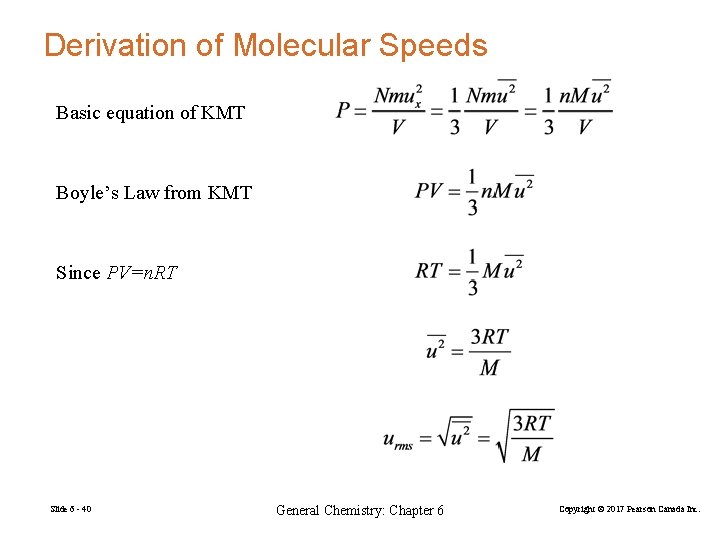
Derivation of Molecular Speeds Basic equation of KMT Boyle’s Law from KMT Since PV=n. RT Slide 6 - 40 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

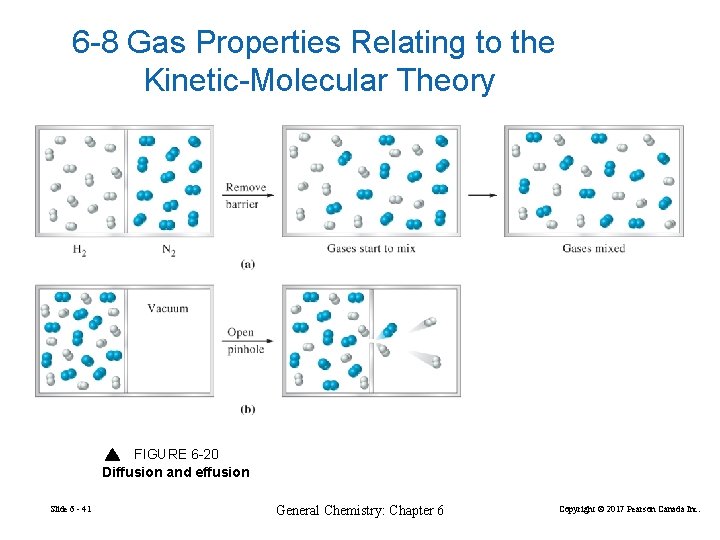
6 -8 Gas Properties Relating to the Kinetic-Molecular Theory FIGURE 6 -20 Diffusion and effusion Slide 6 - 41 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

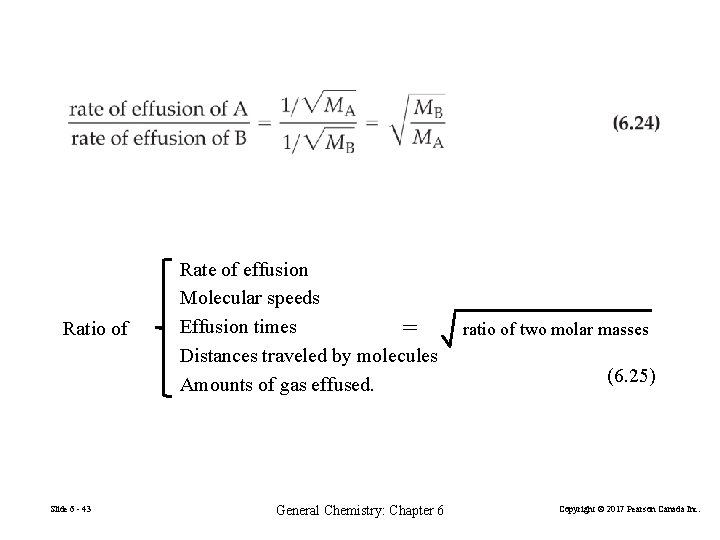
Graham’s Law The rate of effusion of a gas is inversely proportional to the square root of its molar mass. Rateeffusion 1 MW Only for gases at low pressure (natural escape, not a jet). Tiny orifice (no collisions) Does not apply to diffusion. Slide 6 - 42 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

Ratio of Slide 6 - 43 Rate of effusion Molecular speeds Effusion times = Distances traveled by molecules Amounts of gas effused. General Chemistry: Chapter 6 ratio of two molar masses (6. 25) Copyright © 2017 Pearson Canada Inc.

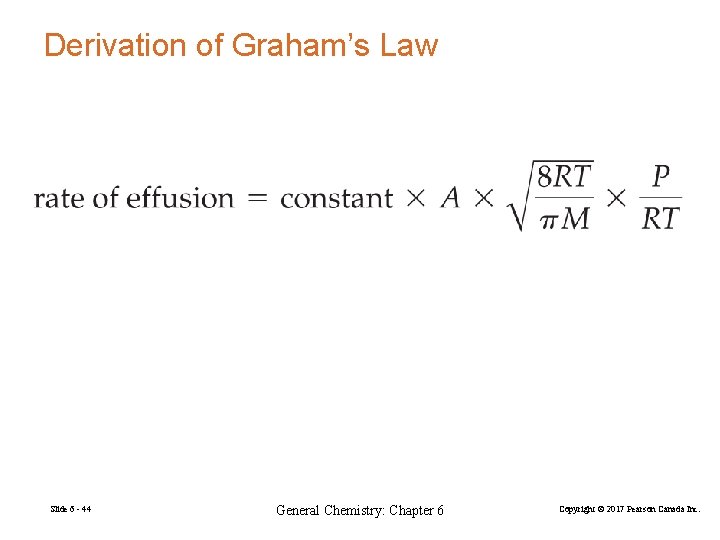
Derivation of Graham’s Law Slide 6 - 44 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

6. 9 Nonideal (Real) Gases FIGURE 6 -21 The behavior of real gases – compressibility factor as a function of pressure at 0ºC Slide 6 - 45 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

The van der Waals Equation n 2 a P+ V 2 V – nb = n. RT The van der Waals equation reproduces the observed behavior of gases with moderate accuracy. It is most accurate for gases comprising approximately spherical molecules that have small dipole moments. Slide 6 - 46 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

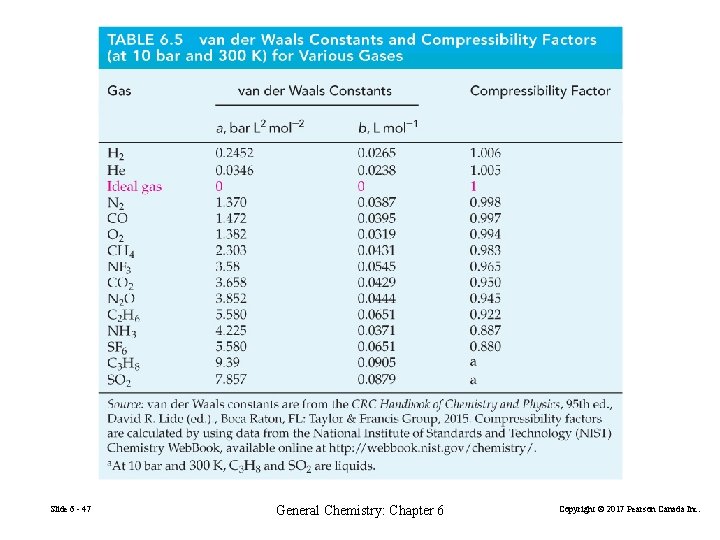
Slide 6 - 47 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

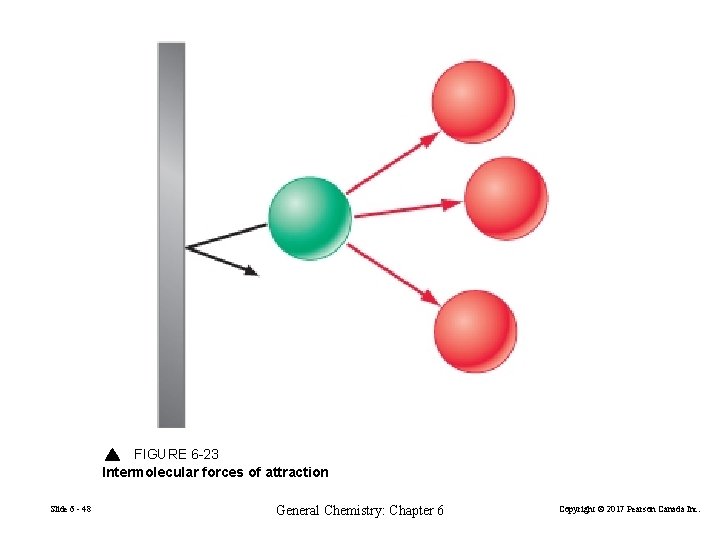
FIGURE 6 -23 Intermolecular forces of attraction Slide 6 - 48 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

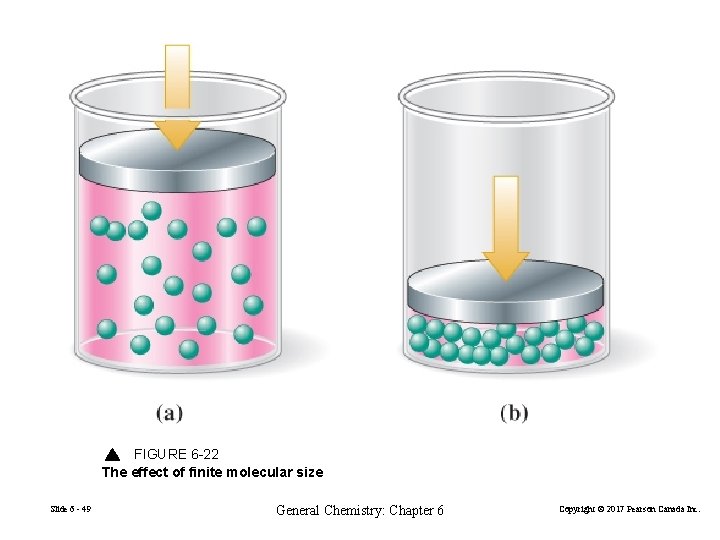
FIGURE 6 -22 The effect of finite molecular size Slide 6 - 49 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.

End of Chapter Slide 6 - 50 General Chemistry: Chapter 6 Copyright © 2017 Pearson Canada Inc.
 Management eleventh edition
Management eleventh edition Eleventh edition management
Eleventh edition management Management eleventh edition
Management eleventh edition Management eleventh edition stephen p robbins
Management eleventh edition stephen p robbins General chemistry
General chemistry Chadha committee
Chadha committee Eleventh 5 year plan
Eleventh 5 year plan Thfive
Thfive For his eleventh birthday elvis presley
For his eleventh birthday elvis presley Introduction to genetic analysis tenth edition
Introduction to genetic analysis tenth edition Fluid mechanics fundamentals and applications
Fluid mechanics fundamentals and applications Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Modern systems analysis and design 7th edition
Modern systems analysis and design 7th edition A computer programming team has 13 members
A computer programming team has 13 members Using mis (10th edition) 10th edition
Using mis (10th edition) 10th edition Mis
Mis Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Principles and applications of electrical engineering
Principles and applications of electrical engineering Learning principles and applications
Learning principles and applications Applications of nuclear chemistry
Applications of nuclear chemistry Irradiated food
Irradiated food Modern labor economics 12th edition solution
Modern labor economics 12th edition solution Modern labor economics 12th edition pdf
Modern labor economics 12th edition pdf Modern real estate practice in pennsylvania 14th edition
Modern real estate practice in pennsylvania 14th edition Modern database management 12th edition ppt
Modern database management 12th edition ppt Modern database management
Modern database management Modern operating systems 3rd edition
Modern operating systems 3rd edition Structured computer organization
Structured computer organization Modern database management 8th edition
Modern database management 8th edition University physics with modern physics fifteenth edition
University physics with modern physics fifteenth edition Modern database management 11th edition
Modern database management 11th edition Modern labor economics 12th edition
Modern labor economics 12th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Expert systems: principles and programming, fourth edition
Expert systems: principles and programming, fourth edition Transition state energy diagram
Transition state energy diagram Thermodynamic vs kinetic control
Thermodynamic vs kinetic control Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein Introductory chemistry 4th edition
Introductory chemistry 4th edition Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Chapter 1 chapter assessment the central science
Chapter 1 chapter assessment the central science Organic chemistry (3rd) edition chapter 1 problem 20s
Organic chemistry (3rd) edition chapter 1 problem 20s Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Ap chemistry notes zumdahl
Ap chemistry notes zumdahl Organic chemistry third edition david klein
Organic chemistry third edition david klein Drop in molecular views answer key
Drop in molecular views answer key Chemistry by raymond chang 10th edition
Chemistry by raymond chang 10th edition Democritus atomic model diagram
Democritus atomic model diagram