GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION





- Slides: 53

GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION PETRUCCI HERRING MADURA Chemical Bonding I: Basic Concepts BISSONNETTE 10 PHILIP DUTTON UNIVERSITY OF WINDSOR DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY Slide 10 - 1 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

Chemical Bonding I: Basic Concepts Slide 10 - 2 CONTENTS 10 -1 Lewis Theory: An Overview 10 -2 Covalent Bonding: An Introduction 10 -3 Polar Covalent Bonds and Electrostatic Potential Maps 10 -4 Writing Lewis Structures 10 -5 Resonance 10 -6 Exceptions to the Octet Rule 10 -7 Shapes of Molecules 10 -8 Bond Order and Bond Length 10 -9 Bond Energies General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

10 -1 Lewis Theory: An Overview 1. Valence e− play a fundamental role in chemical bonding. 2. e– transfer leads to ionic bonds. 3. Sharing of e– leads to a covalent bond. 4. e– are transferred or shared to give each atom a noble gas configuration, the octet. Gilbert Newton Lewis (1875 -1946) Slide 10 - 3 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

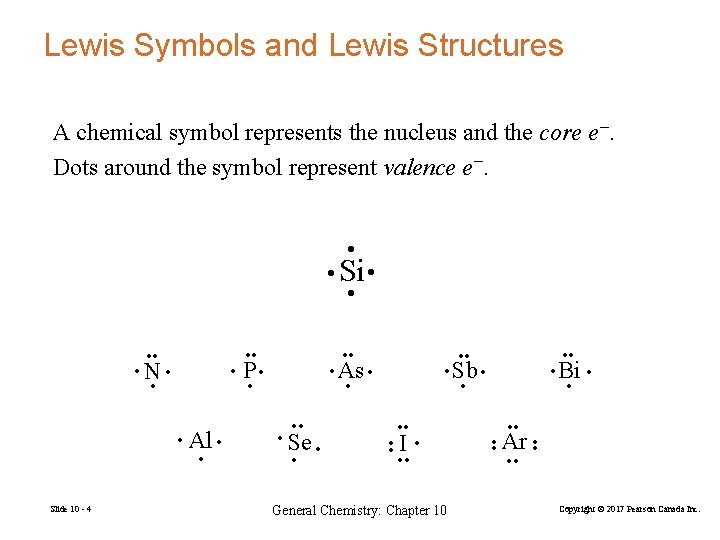
Lewis Symbols and Lewis Structures A chemical symbol represents the nucleus and the core e−. Dots around the symbol represent valence e−. • • Si • • • Al • • Slide 10 - 4 • As • • P • • • Se • • • Bi • • • Sb • • • • I • • • General Chemistry: Chapter 10 • • Ar • • • N • • • • Copyright © 2017 Pearson Canada Inc.

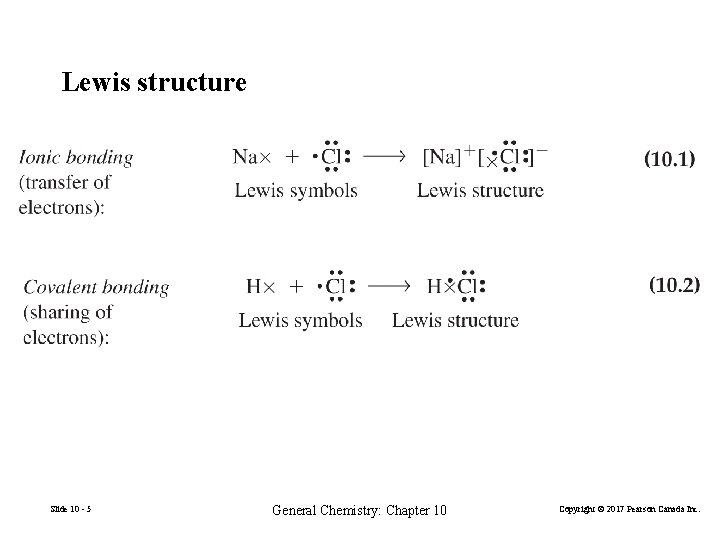
Lewis structure Slide 10 - 5 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

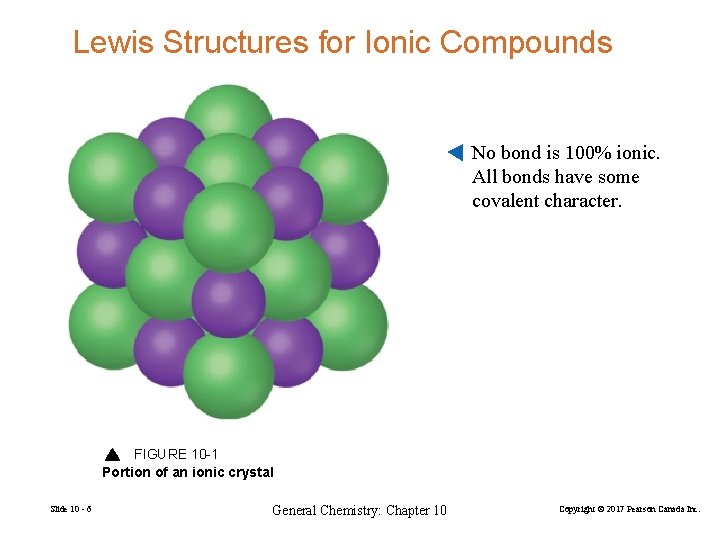
Lewis Structures for Ionic Compounds No bond is 100% ionic. All bonds have some covalent character. FIGURE 10 -1 Portion of an ionic crystal Slide 10 - 6 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

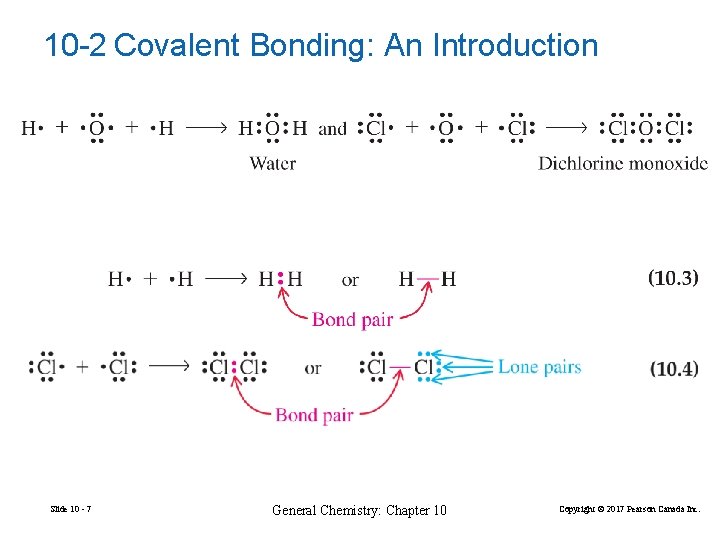
10 -2 Covalent Bonding: An Introduction Slide 10 - 7 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

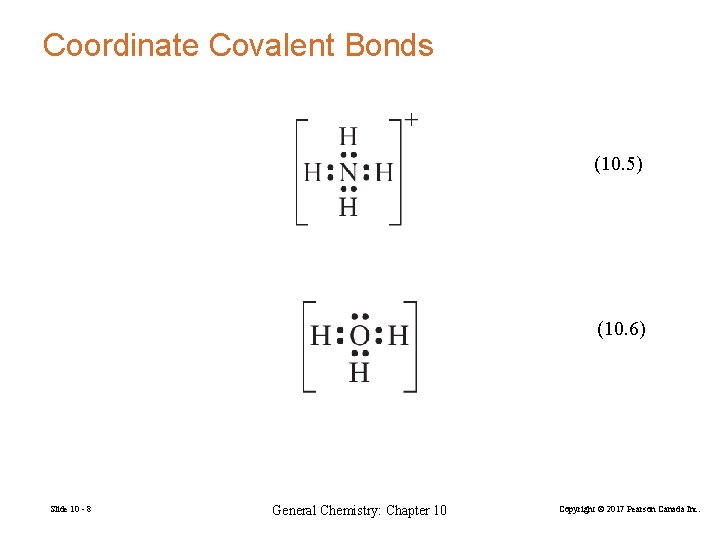
Coordinate Covalent Bonds (10. 5) (10. 6) Slide 10 - 8 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

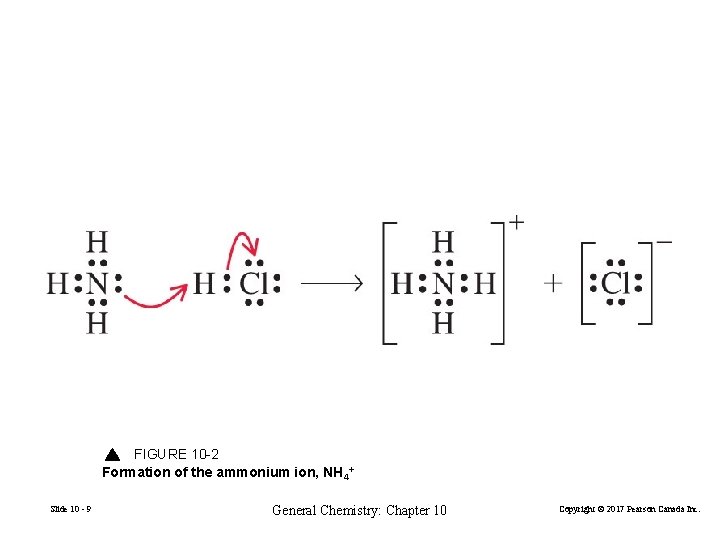
FIGURE 10 -2 Formation of the ammonium ion, NH 4+ Slide 10 - 9 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

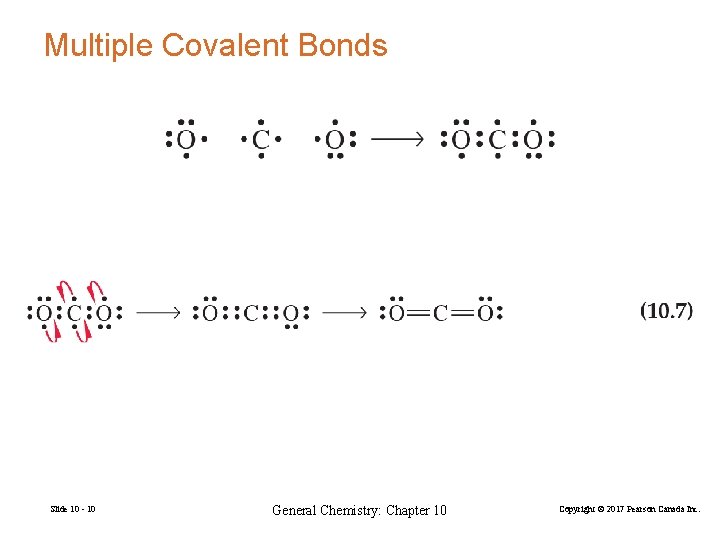
Multiple Covalent Bonds Slide 10 - 10 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

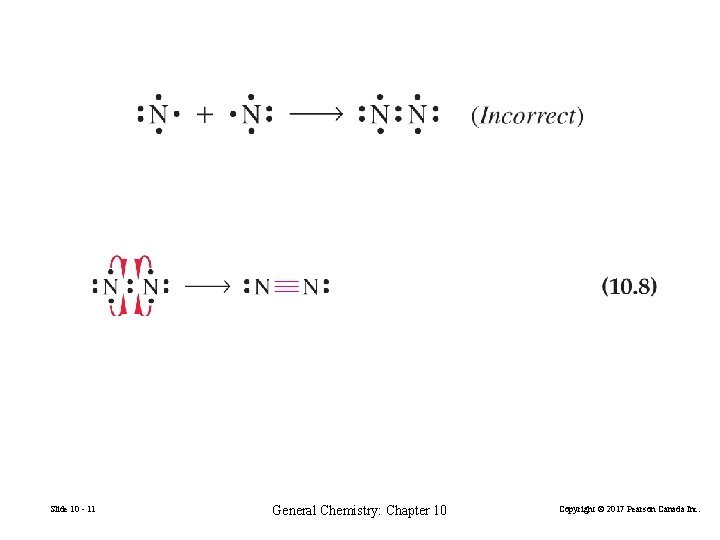
Slide 10 - 11 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

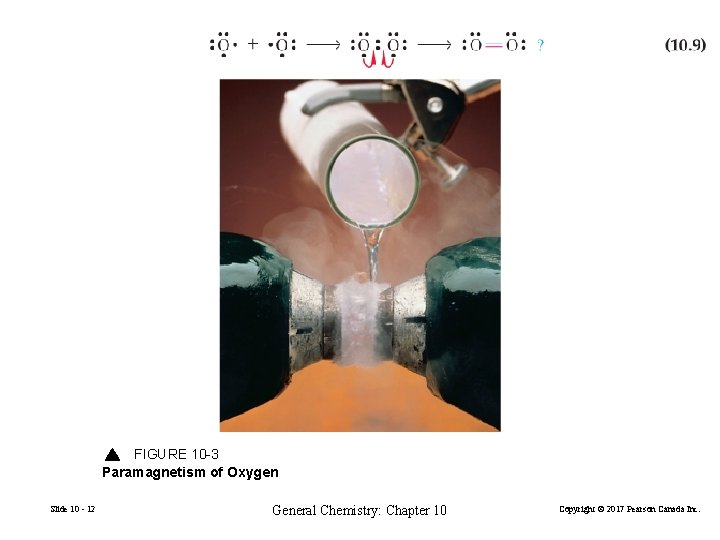
FIGURE 10 -3 Paramagnetism of Oxygen Slide 10 - 12 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

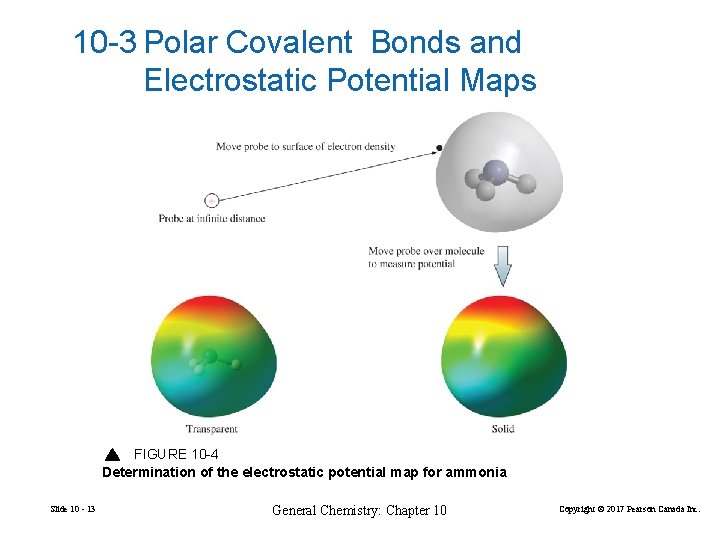
10 -3 Polar Covalent Bonds and Electrostatic Potential Maps FIGURE 10 -4 Determination of the electrostatic potential map for ammonia Slide 10 - 13 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

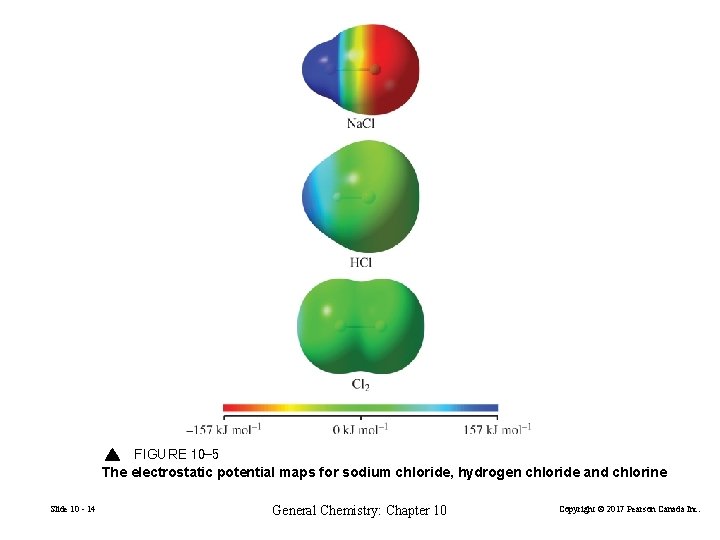
FIGURE 10 -5 The electrostatic potential maps for sodium chloride, hydrogen chloride and chlorine Slide 10 - 14 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

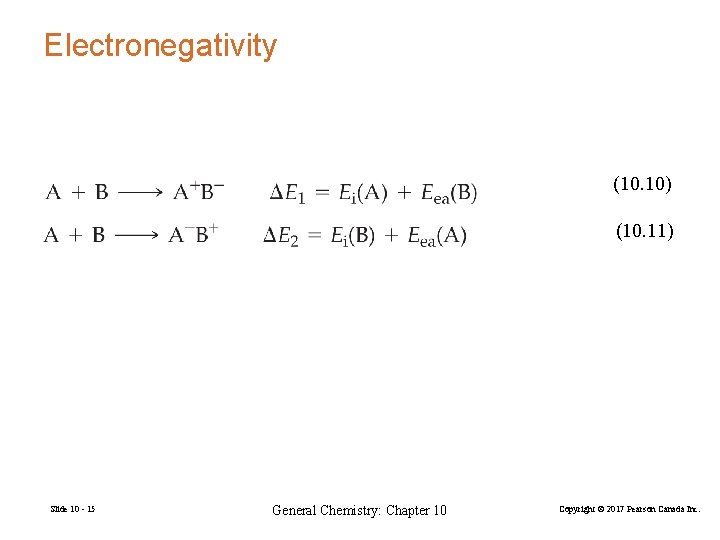
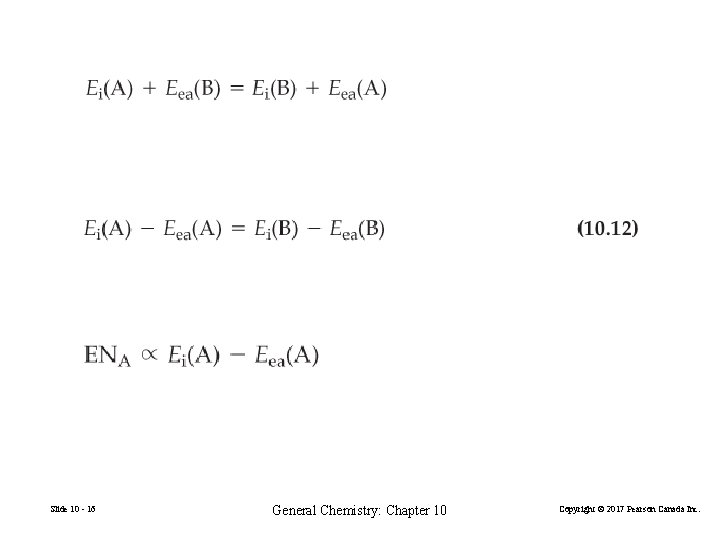
Electronegativity (10. 10) (10. 11) Slide 10 - 15 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

Slide 10 - 16 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

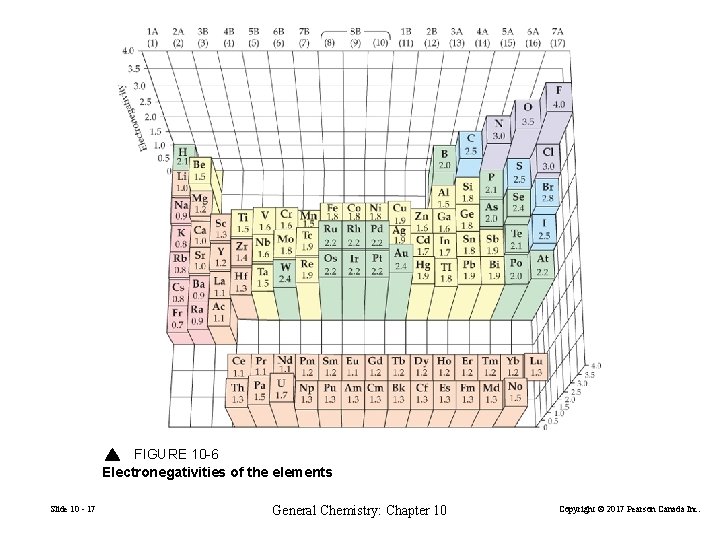
FIGURE 10 -6 Electronegativities of the elements Slide 10 - 17 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

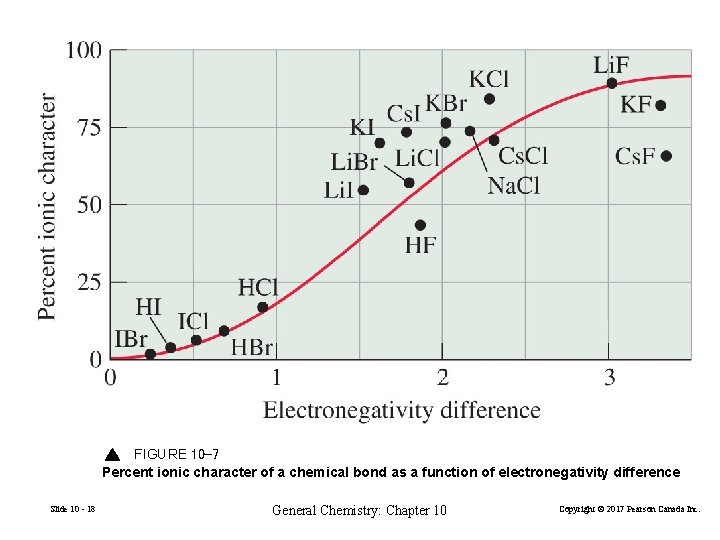
FIGURE 10 -7 Percent ionic character of a chemical bond as a function of electronegativity difference Slide 10 - 18 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

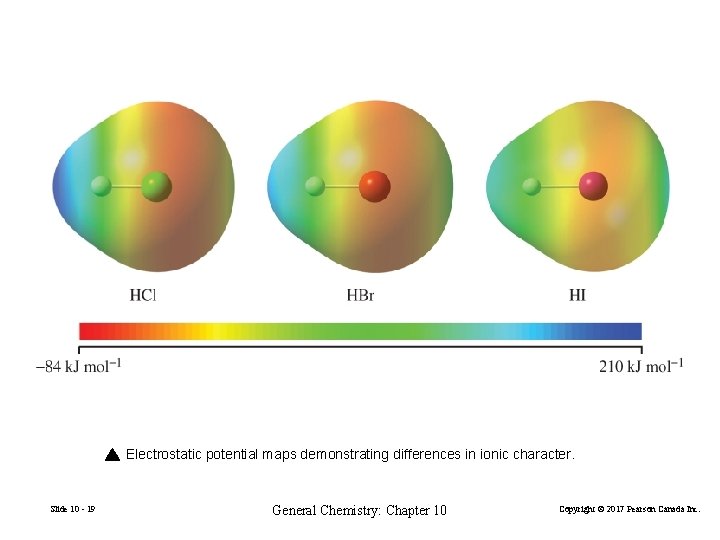
Electrostatic potential maps demonstrating differences in ionic character. Slide 10 - 19 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

10 -4 Writing Lewis Structures • All the valence e– of atoms must appear in the structure. • Usually, all the e– are paired. • Usually, each atom requires an outer-shell octet of e–. • H only requires 2 e–. • Sometimes, multiple bonds may be needed. • Readily formed by C, N, O, S, and P. Slide 10 - 20 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

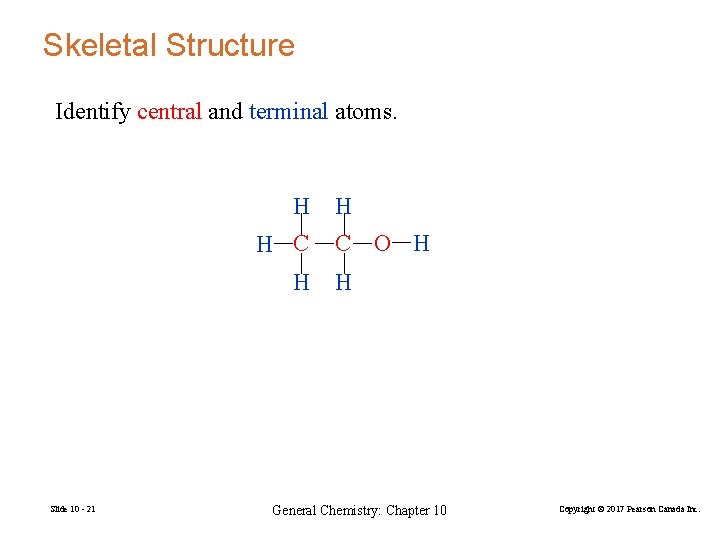
Skeletal Structure Identify central and terminal atoms. H H C H Slide 10 - 21 H C O H H General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

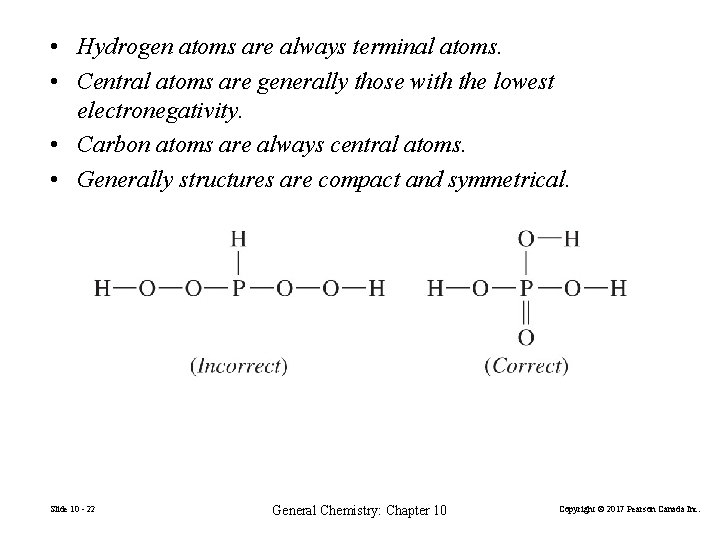
• Hydrogen atoms are always terminal atoms. • Central atoms are generally those with the lowest electronegativity. • Carbon atoms are always central atoms. • Generally structures are compact and symmetrical. Slide 10 - 22 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

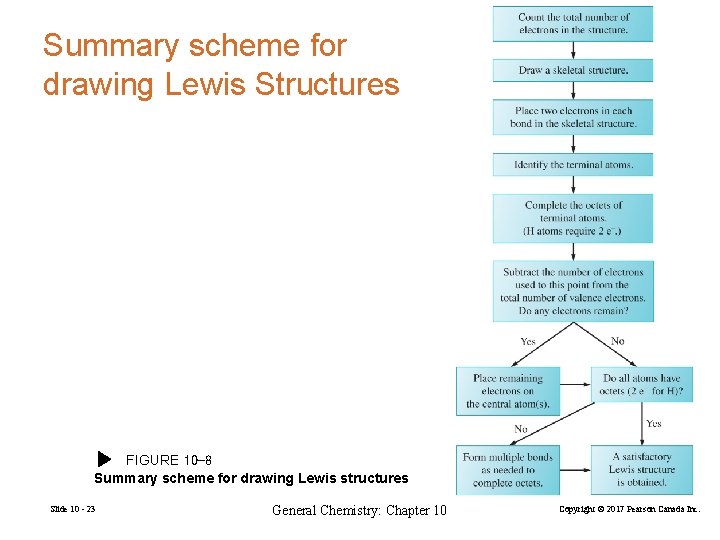
Summary scheme for drawing Lewis Structures FIGURE 10 -8 Summary scheme for drawing Lewis structures Slide 10 - 23 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

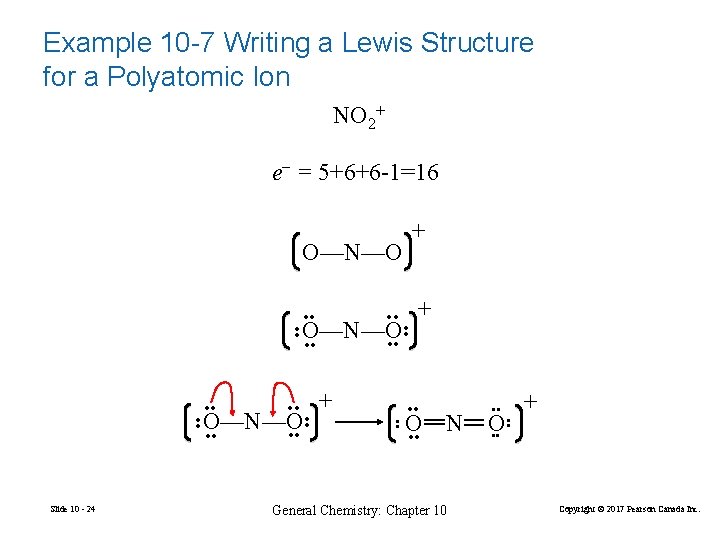
Example 10 -7 Writing a Lewis Structure for a Polyatomic Ion NO 2+ e– = 5+6+6 -1=16 + O—N—O + • • • • Slide 10 - 24 • • O—N—O • • + • • O • • N General Chemistry: Chapter 10 • • O • • + • • • • O—N—O • • Copyright © 2017 Pearson Canada Inc.

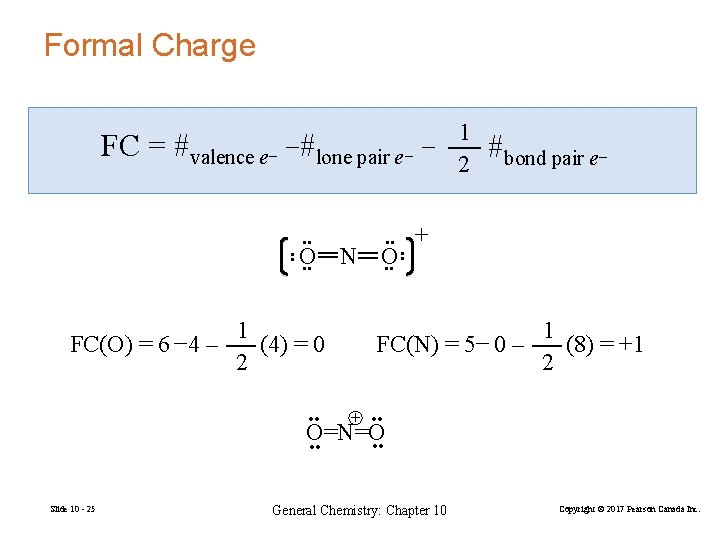
Formal Charge • • O • • FC(O) = 6 − 4 – 1 (4) = 0 2 • • N • • O • • #bond pair e− + • • FC = 1 #valence e− −#lone pair e− − 2 FC(N) = 5− 0 – 1 (8) = +1 2 + • • O=N=O • • Slide 10 - 25 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

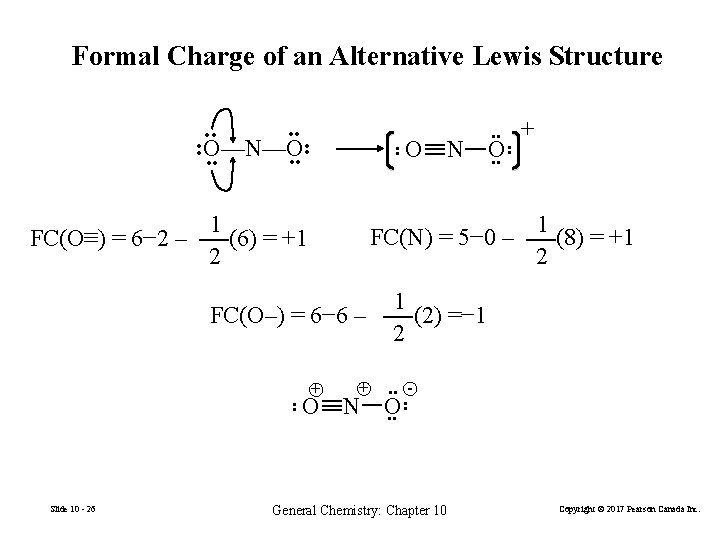
Formal Charge of an Alternative Lewis Structure O • • O—N—O • • FC(O–) = 6− 6 – + O • • O • • + 1 FC(N) = 5− 0 – (8) = +1 2 1 FC(O≡) = 6− 2 – (6) = +1 2 Slide 10 - 26 N • • + N 1 (2) =− 1 2 • • O • • - • • General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

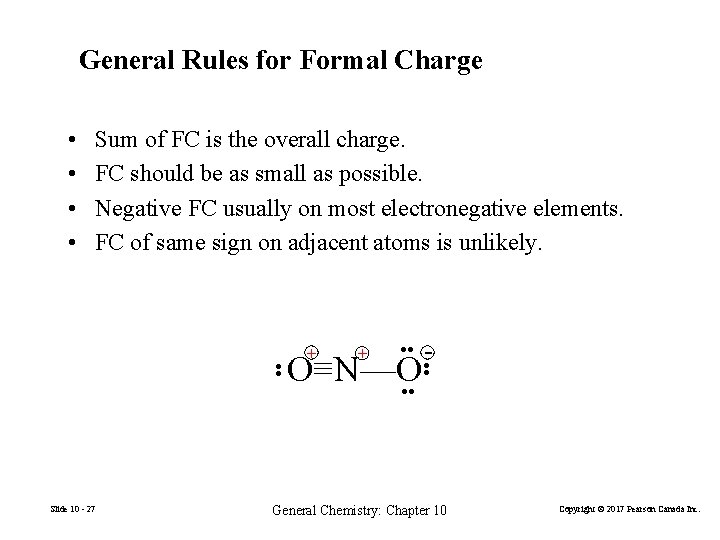
General Rules for Formal Charge • • Sum of FC is the overall charge. FC should be as small as possible. Negative FC usually on most electronegative elements. FC of same sign on adjacent atoms is unlikely. + + • • Slide 10 - 27 • • O≡N—O • • - General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

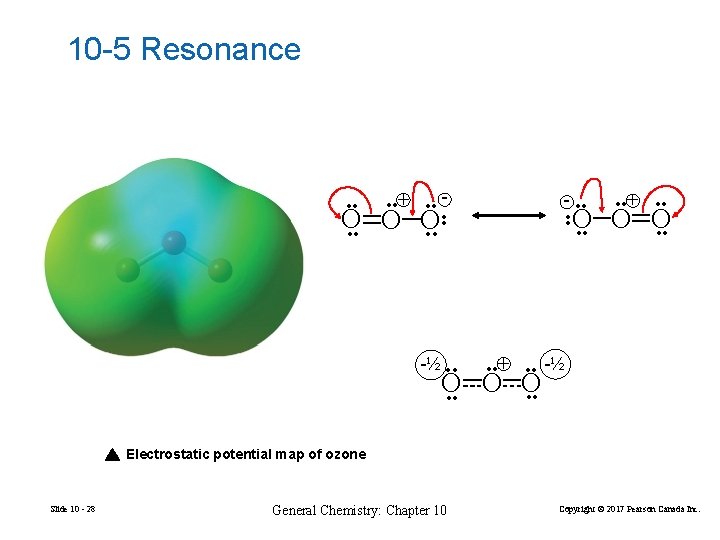
10 -5 Resonance • • + • • - - • • O O O • • -½ • • + • • -½ O O O • • Electrostatic potential map of ozone Slide 10 - 28 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

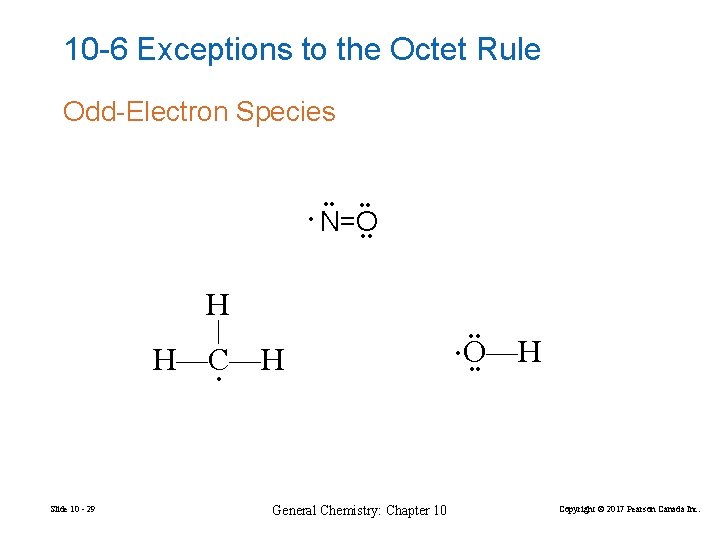
10 -6 Exceptions to the Octet Rule Odd-Electron Species • • • N=O • • H • Slide 10 - 29 General Chemistry: Chapter 10 O—H • • • H—C—H • • Copyright © 2017 Pearson Canada Inc.

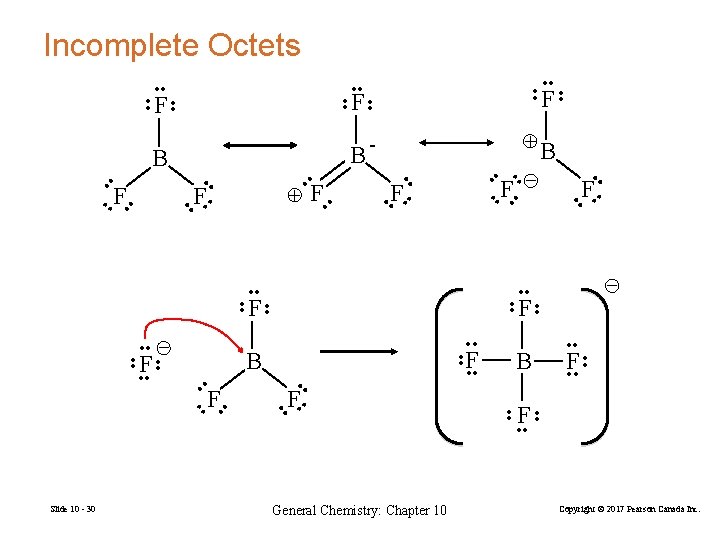
Incomplete Octets • • • • • • F • • F • • F B • • F • • • • F B F – • • F • • F F • • – B • • F • • + • • F • • B B + - • • F F F • • • • Slide 10 - 30 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

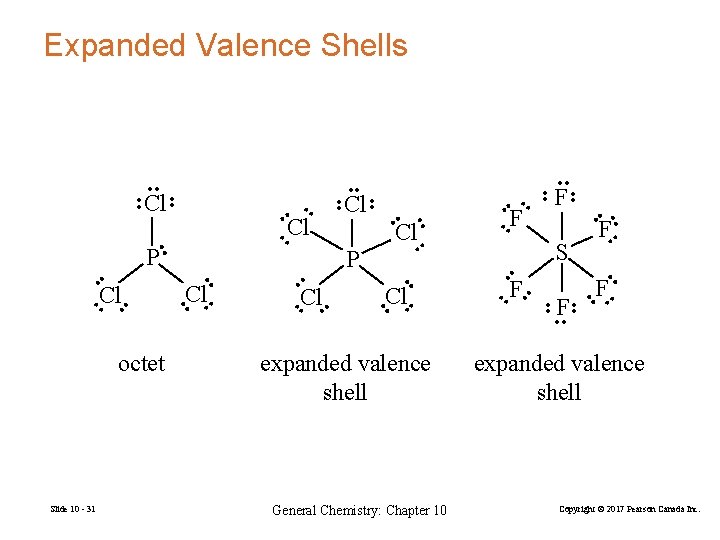
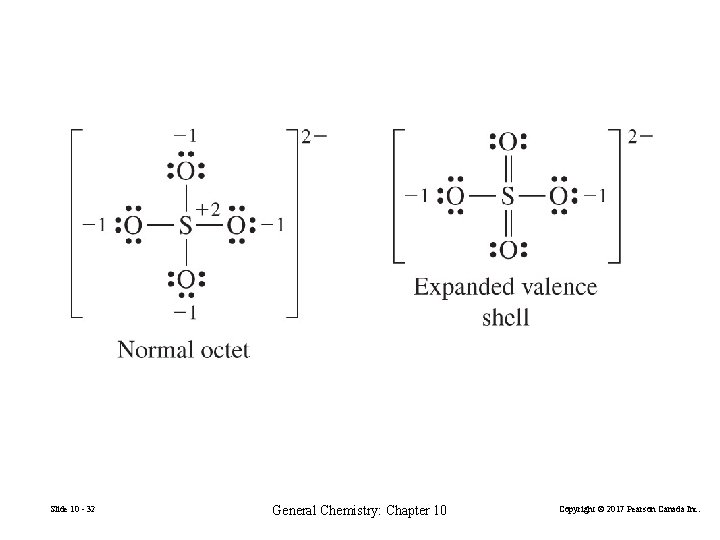
Expanded Valence Shells • • expanded valence shell General Chemistry: Chapter 10 • • F • • • • • F • • F • • • Cl • • • • • S • • • F F • • Cl • • • Slide 10 - 31 • • • Cl P • • octet Cl • Cl • • Cl P • • Cl • • • • • expanded valence shell Copyright © 2017 Pearson Canada Inc.

Slide 10 - 32 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

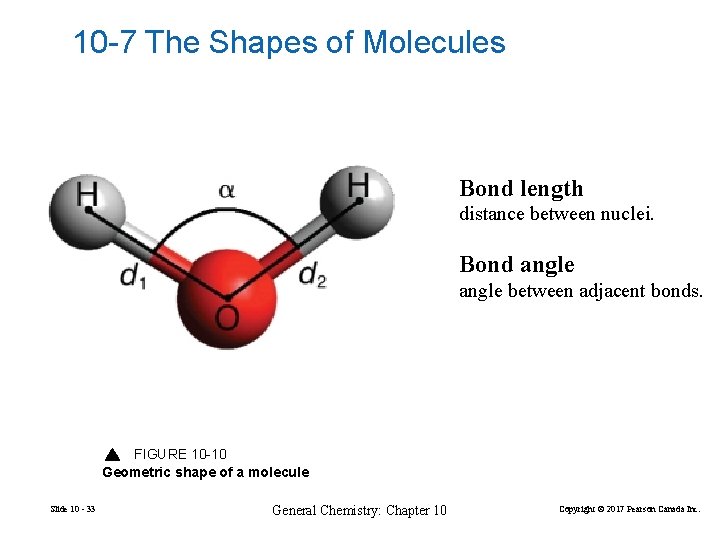
10 -7 The Shapes of Molecules Bond length distance between nuclei. Bond angle between adjacent bonds. FIGURE 10 -10 Geometric shape of a molecule Slide 10 - 33 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

Valence-Shell Electron Pair Repulsion (VSEPR) Theory Electron pairs repel each other whether they are in chemical bonds (bond pairs) or unshared (lone pairs). Electron pairs assume orientations about an atom to minimize repulsions. Electron group geometry – distribution of e− pairs. Molecular geometry – distribution of nuclei. Slide 10 - 34 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

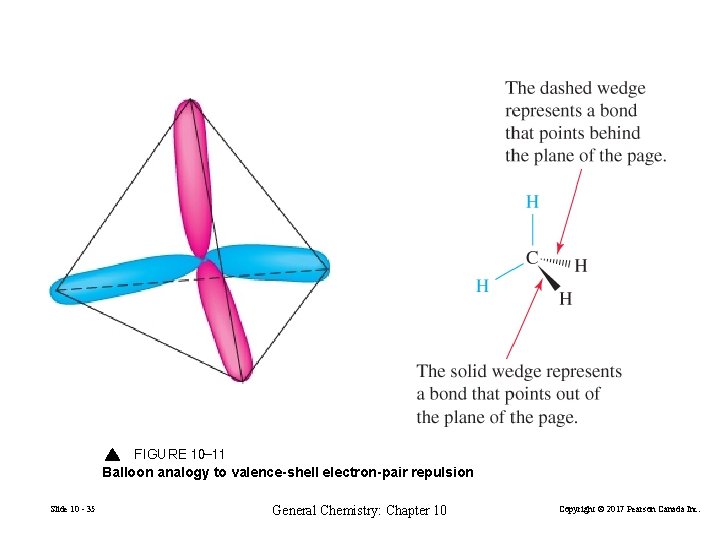
FIGURE 10 -11 Balloon analogy to valence-shell electron-pair repulsion Slide 10 - 35 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

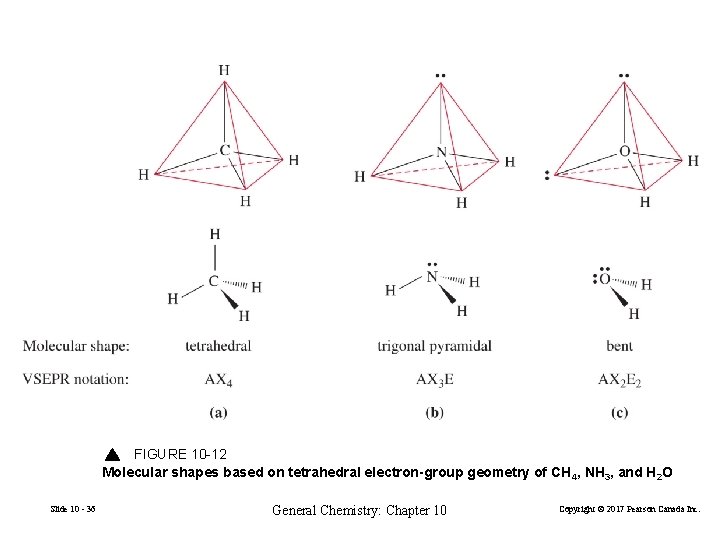
FIGURE 10 -12 Molecular shapes based on tetrahedral electron-group geometry of CH 4, NH 3, and H 2 O Slide 10 - 36 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

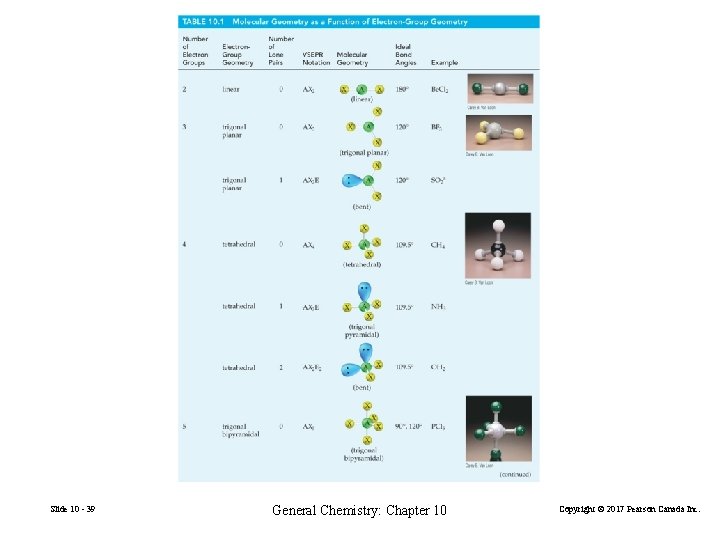
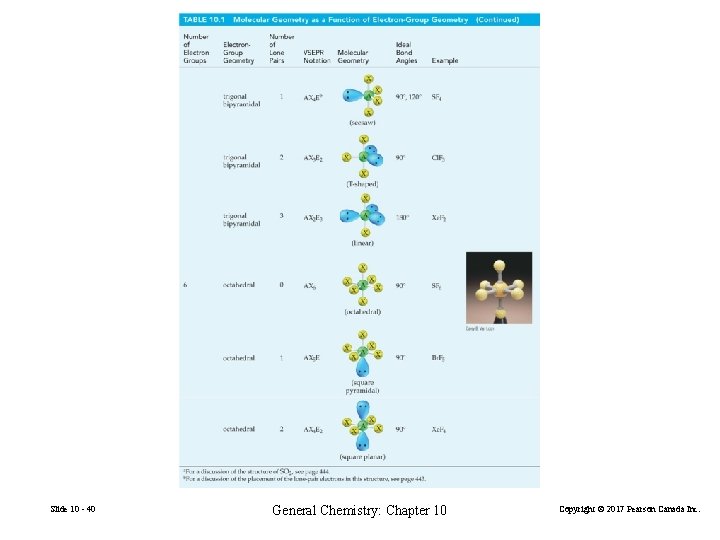
Possibilities for Electron-Group Distributions Electron-group geometries • • • Slide 10 - 37 two electron groups: linear three electron groups: trigonal planar four electron groups: tetrahedral five electron groups: trigonal bipyramidal six electron groups: octahedral General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

FIGURE 10 -13 Several electron-group geometries illustrated Slide 10 - 38 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

Slide 10 - 39 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

Slide 10 - 40 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

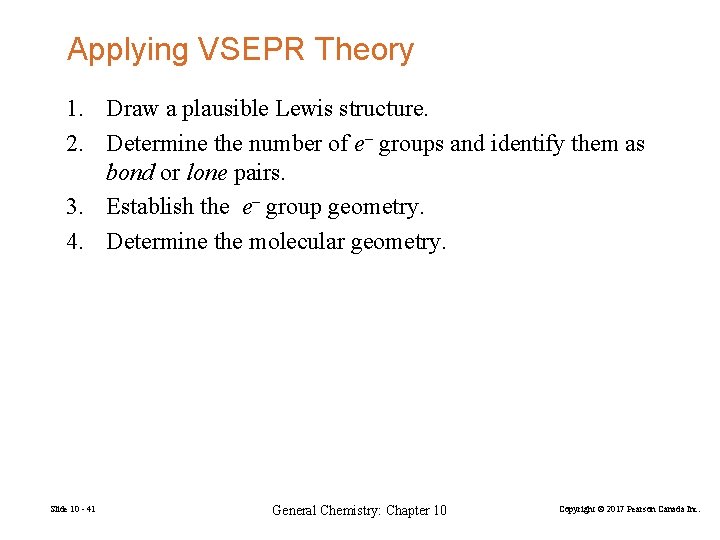
Applying VSEPR Theory 1. Draw a plausible Lewis structure. 2. Determine the number of e– groups and identify them as bond or lone pairs. 3. Establish the e– group geometry. 4. Determine the molecular geometry. Slide 10 - 41 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

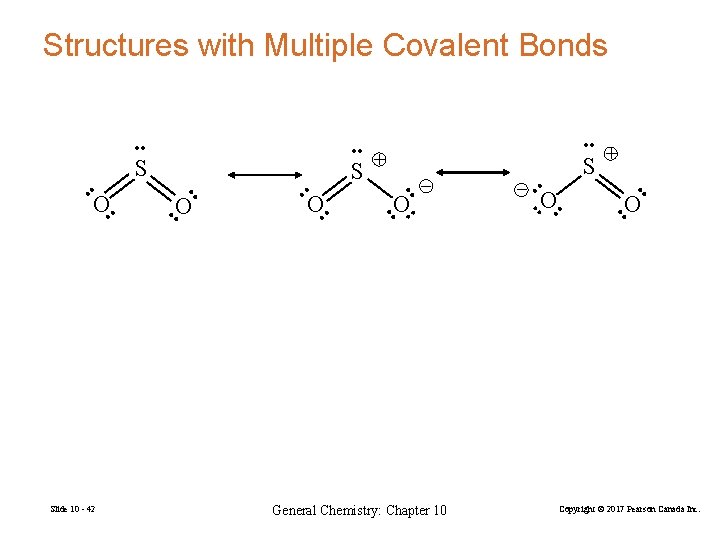
Structures with Multiple Covalent Bonds • • • • O • • General Chemistry: Chapter 10 – S • • O – • • O • • Slide 10 - 42 S • • S O • • + Copyright © 2017 Pearson Canada Inc.

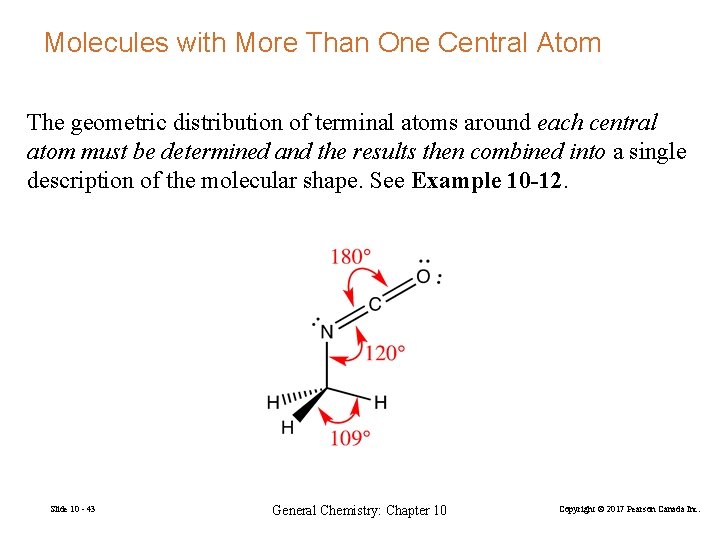
Molecules with More Than One Central Atom The geometric distribution of terminal atoms around each central atom must be determined and the results then combined into a single description of the molecular shape. See Example 10 -12. Slide 10 - 43 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

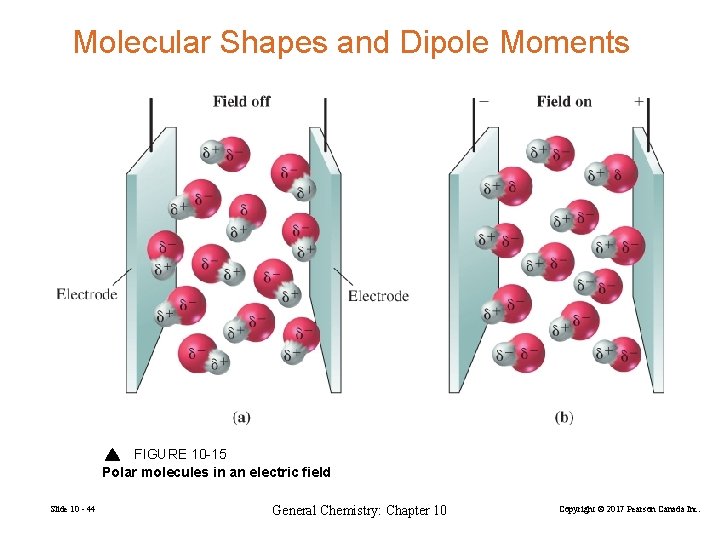
Molecular Shapes and Dipole Moments FIGURE 10 -15 Polar molecules in an electric field Slide 10 - 44 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

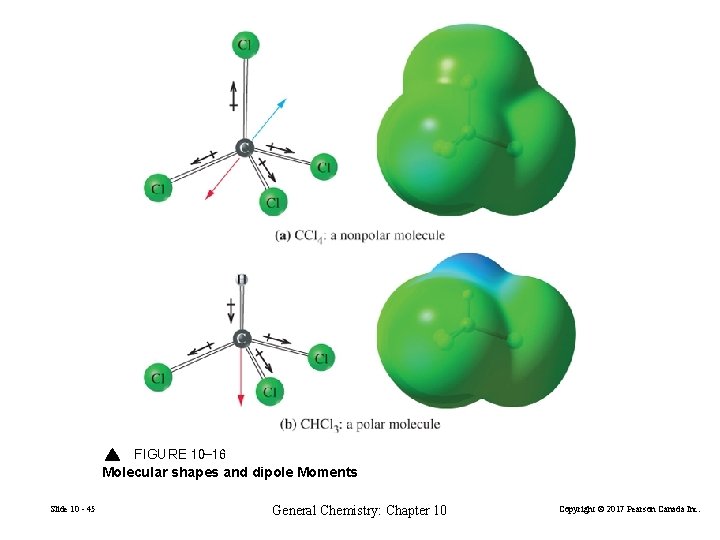
FIGURE 10 -16 Molecular shapes and dipole Moments Slide 10 - 45 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

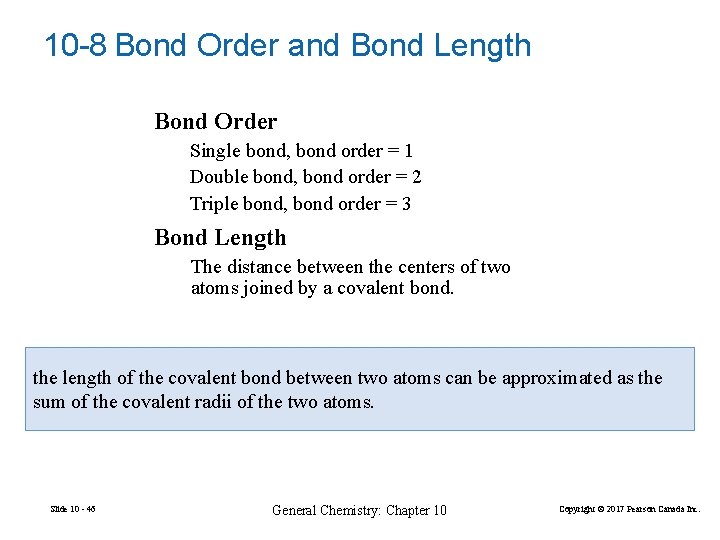
10 -8 Bond Order and Bond Length Bond Order Single bond, bond order = 1 Double bond, bond order = 2 Triple bond, bond order = 3 Bond Length The distance between the centers of two atoms joined by a covalent bond. the length of the covalent bond between two atoms can be approximated as the sum of the covalent radii of the two atoms. Slide 10 - 46 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

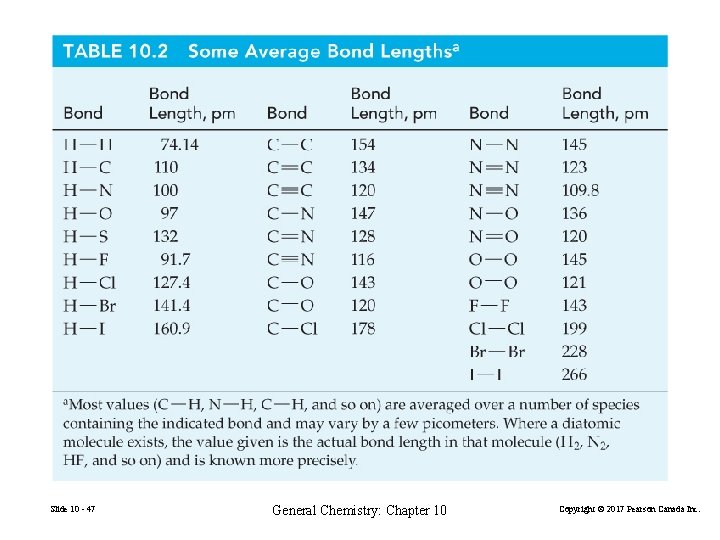
Slide 10 - 47 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

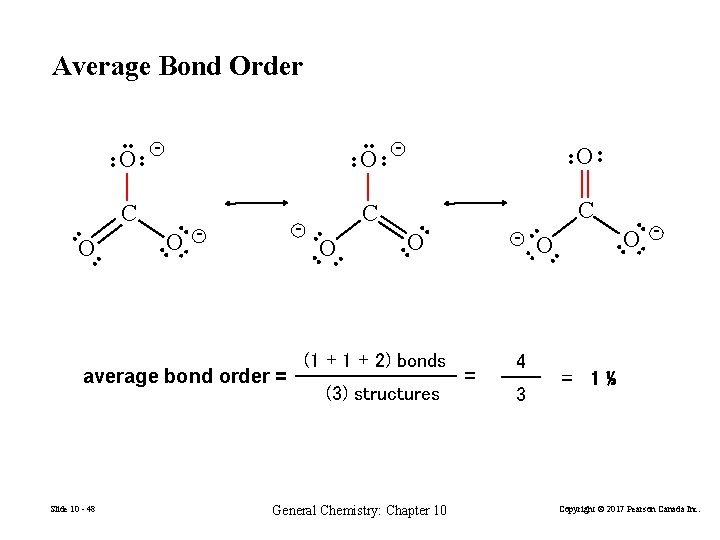
Average Bond Order • • General Chemistry: Chapter 10 • • (3) structures • • (1 + 2) bonds O = 4 3 O • - • • Slide 10 - 48 O - O • • average bond order = C C • • • O • • O O • - - - • • C • • O • • - • • O • = 1⅓ Copyright © 2017 Pearson Canada Inc.

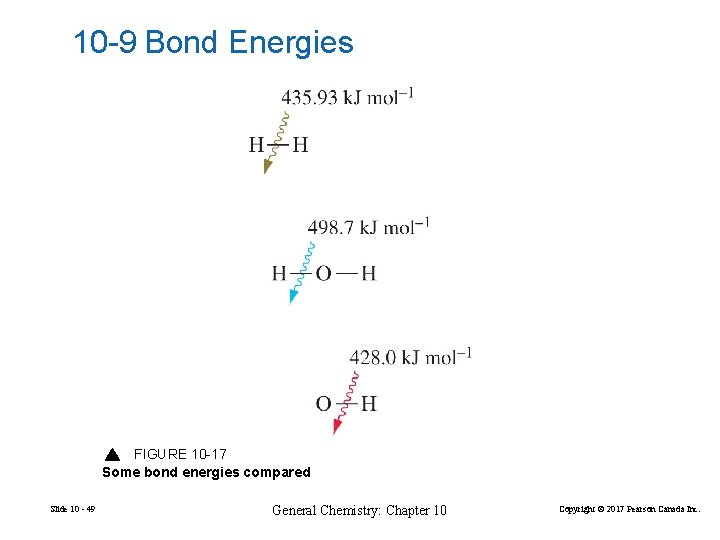
10 -9 Bond Energies FIGURE 10 -17 Some bond energies compared Slide 10 - 49 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

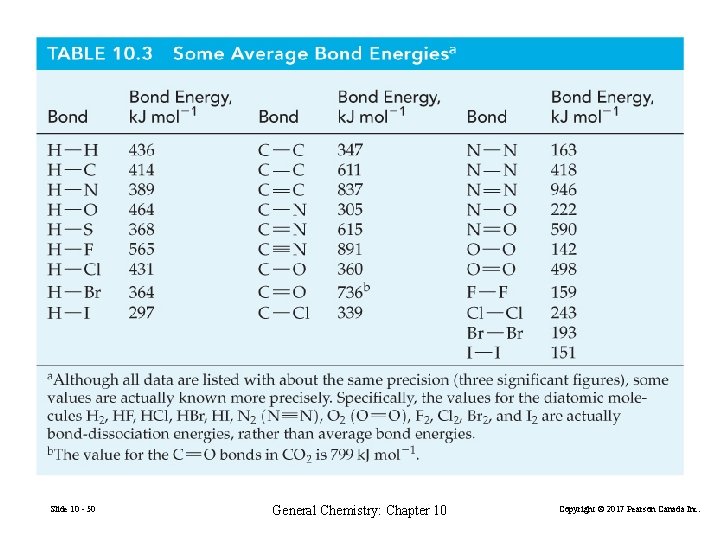
Slide 10 - 50 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

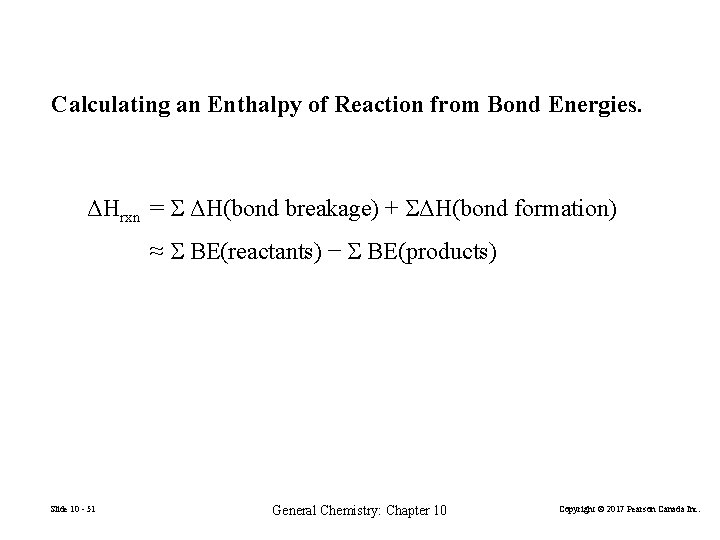
Calculating an Enthalpy of Reaction from Bond Energies. ΔHrxn = ΔH(bond breakage) + ΔH(bond formation) ≈ BE(reactants) − BE(products) Slide 10 - 51 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

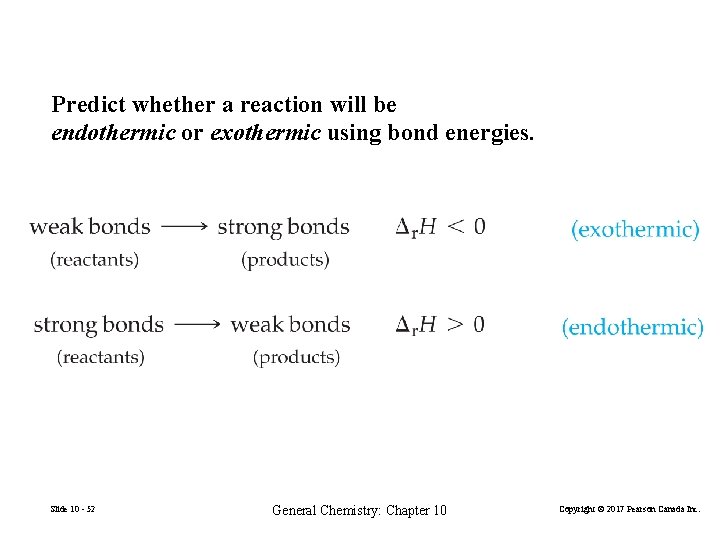
Predict whether a reaction will be endothermic or exothermic using bond energies. Slide 10 - 52 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.

End of Chapter Slide 10 - 53 General Chemistry: Chapter 10 Copyright © 2017 Pearson Canada Inc.
 Management eleventh edition stephen p robbins
Management eleventh edition stephen p robbins Management eleventh edition stephen p robbins
Management eleventh edition stephen p robbins Management eleventh edition
Management eleventh edition Management eleventh edition stephen p robbins
Management eleventh edition stephen p robbins General chemistry 11th edition
General chemistry 11th edition Eleventh 5 year plan
Eleventh 5 year plan Eleventh 5 year plan
Eleventh 5 year plan Thfive
Thfive For his eleventh birthday elvis presley
For his eleventh birthday elvis presley Human genetics concepts and applications 10th edition
Human genetics concepts and applications 10th edition Fluid mechanics fundamentals and applications
Fluid mechanics fundamentals and applications Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Modern systems analysis and design 7th edition
Modern systems analysis and design 7th edition Susanna s epp
Susanna s epp Using mis (10th edition) 10th edition
Using mis (10th edition) 10th edition Using mis 10th edition
Using mis 10th edition Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Electrical engineering
Electrical engineering Learning principles and applications
Learning principles and applications 25 m/s
25 m/s Applications of nuclear chemistry
Applications of nuclear chemistry Modern labor economics 12th edition solution
Modern labor economics 12th edition solution Modern labor economics 12th edition
Modern labor economics 12th edition Modern real estate practice in pennsylvania
Modern real estate practice in pennsylvania Modern database management 12th edition ppt
Modern database management 12th edition ppt Modern database management 10th edition
Modern database management 10th edition Modern operating systems 3rd edition
Modern operating systems 3rd edition Tanenbaum structured computer organization
Tanenbaum structured computer organization Modern database management 8th edition
Modern database management 8th edition University physics with modern physics fifteenth edition
University physics with modern physics fifteenth edition Transaction cannot be subdivided
Transaction cannot be subdivided Modern labor economics 12th edition
Modern labor economics 12th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Expert systems: principles and programming, fourth edition
Expert systems: principles and programming, fourth edition Rearranged most stable carbocation is
Rearranged most stable carbocation is Pericyclic
Pericyclic Is alkane an organic compound
Is alkane an organic compound Introductory chemistry 4th edition
Introductory chemistry 4th edition Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro The central science 14th edition
The central science 14th edition Reaction of grignard reagent with acid chloride
Reaction of grignard reagent with acid chloride David klein organic chemistry
David klein organic chemistry Ap chemistry notes zumdahl
Ap chemistry notes zumdahl Organic chemistry third edition david klein
Organic chemistry third edition david klein Lesson 81 drop in molecular views answer key
Lesson 81 drop in molecular views answer key Chemistry by raymond chang 10th edition
Chemistry by raymond chang 10th edition Fifth edition chemistry a molecular approach
Fifth edition chemistry a molecular approach Halohydrin
Halohydrin Thermodynamic control
Thermodynamic control Organic chemistry
Organic chemistry