GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION





- Slides: 71

GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION PETRUCCI HERRING MADURA Electrochemistry BISSONNETTE 19 PHILIP DUTTON UNIVERSITY OF WINDSOR DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY Slide 19 - 1 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

Electrochemistry Slide 19 - 2 CONTENTS 19 -1 Electrode Potentials and Their Measurement 19 -2 Standard Electrode Potentials 19 -3 Ecell, ΔG, and K 19 -4 Ecell as a Function of Concentrations 19 -5 Batteries: Producing Electricity Through Chemical Reactions 19 -6 Corrosion: Unwanted Voltaic Cells 19 -7 Electrolysis: Causing Nonspontaneous Reactions to Occur 19 -8 Industrial Electrolysis Processes General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

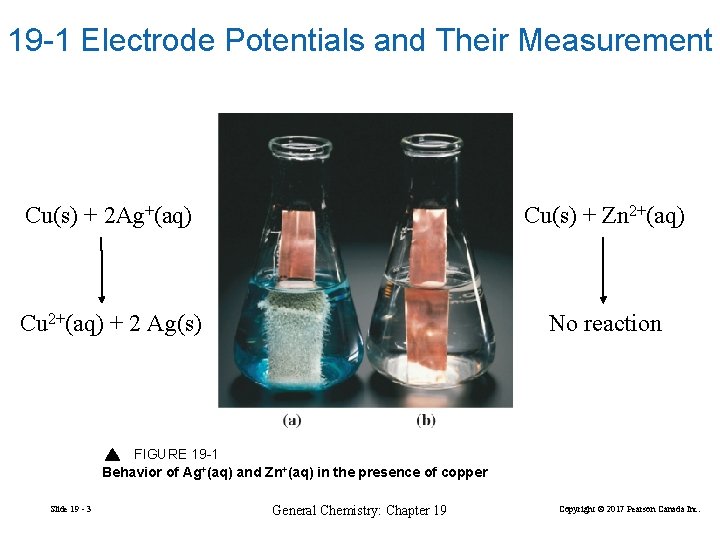
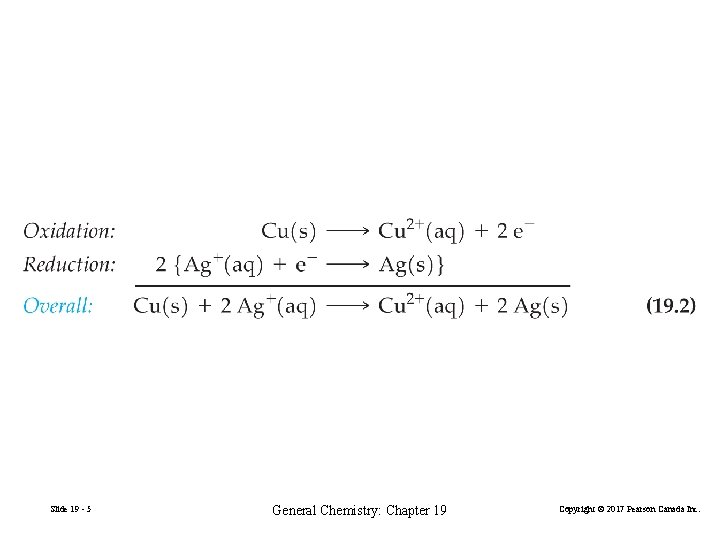
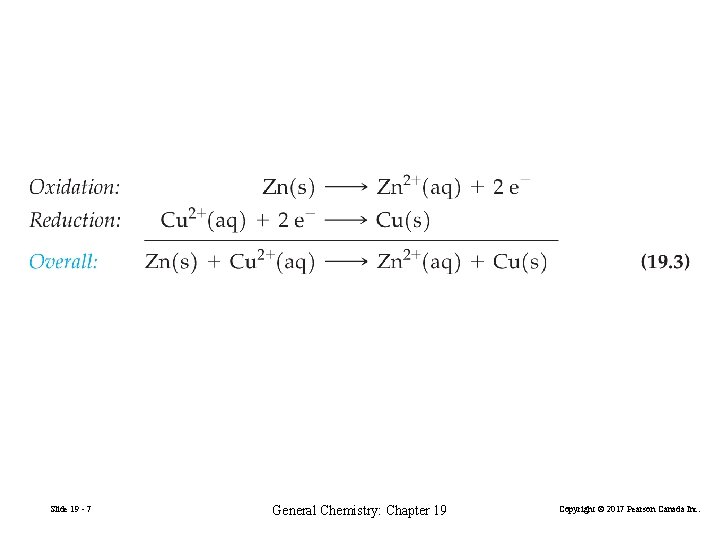
19 -1 Electrode Potentials and Their Measurement Cu(s) + 2 Ag+(aq) Cu(s) + Zn 2+(aq) Cu 2+(aq) + 2 Ag(s) No reaction FIGURE 19 -1 Behavior of Ag+(aq) and Zn+(aq) in the presence of copper Slide 19 - 3 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

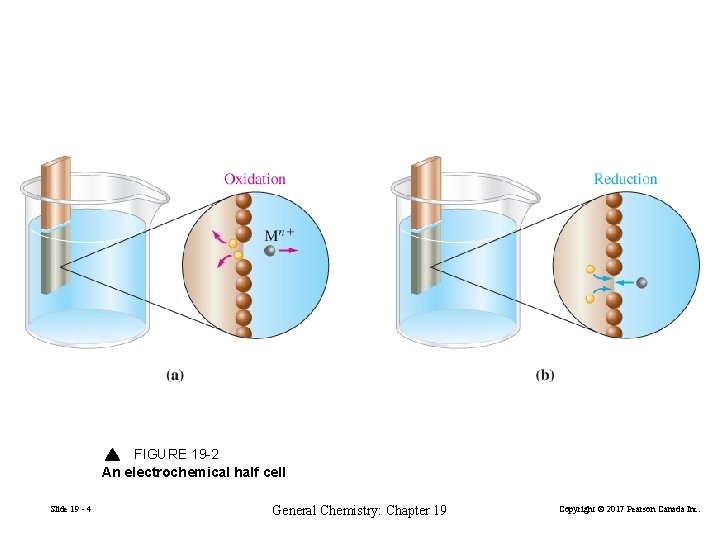
Cathode FIGURE 19 -2 An electrochemical half cell Slide 19 - 4 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

Slide 19 - 5 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

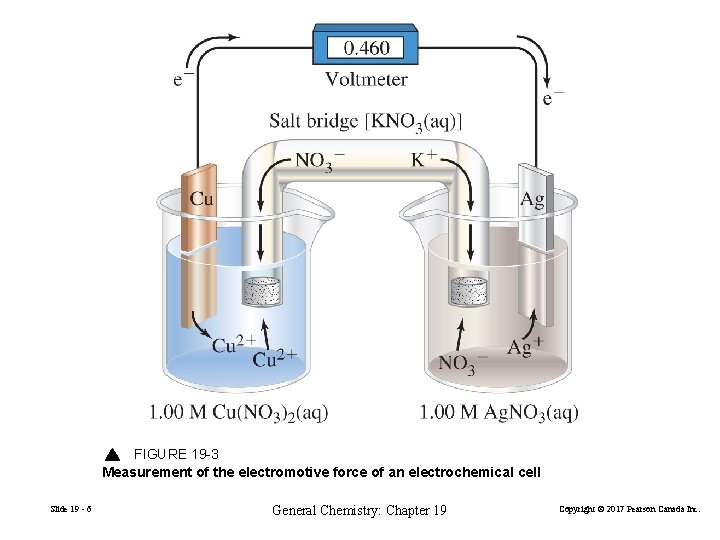
FIGURE 19 -3 Measurement of the electromotive force of an electrochemical cell Slide 19 - 6 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

Slide 19 - 7 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

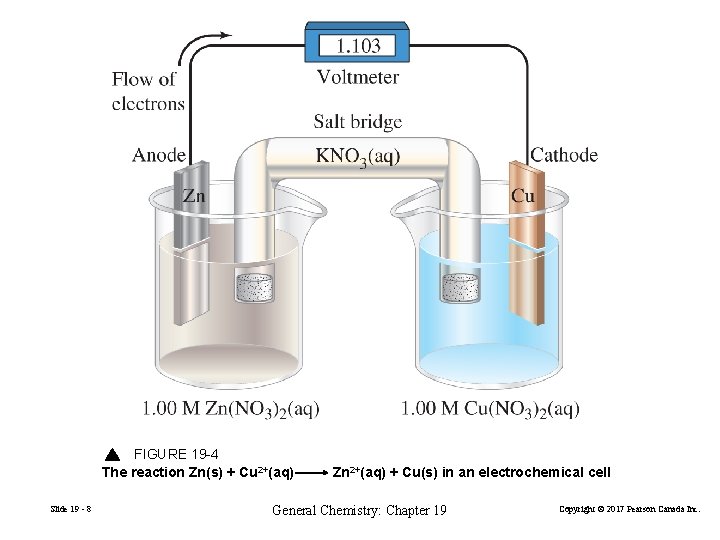
FIGURE 19 -4 The reaction Zn(s) + Cu 2+(aq) Slide 19 - 8 Zn 2+(aq) + Cu(s) in an electrochemical cell General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

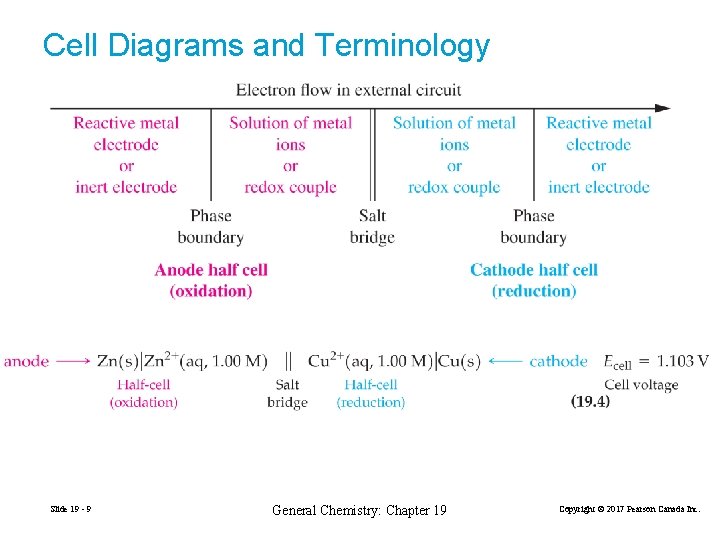
Cell Diagrams and Terminology Slide 19 - 9 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

Galvanic (or voltaic) cells Produce electricity as a result of spontaneous reactions. Electrolytic cells Non-spontaneous chemical change driven by electricity. Slide 19 - 10 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

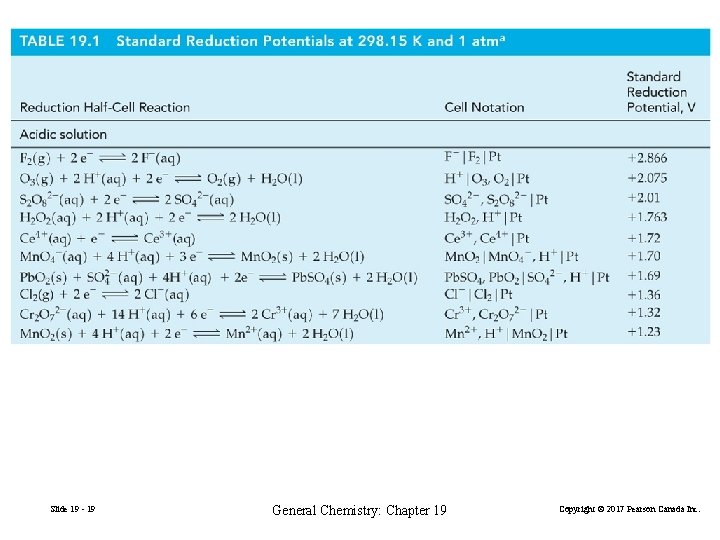
19 -2 Standard Electrode Potentials Cell voltages – potential differences between electrodes – are among the most precise scientific measurements possible. The potential of an individual electrode is difficult to establish. Arbitrary zero is chosen. The standard hydrogen electrode (SHE) Slide 19 - 11 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

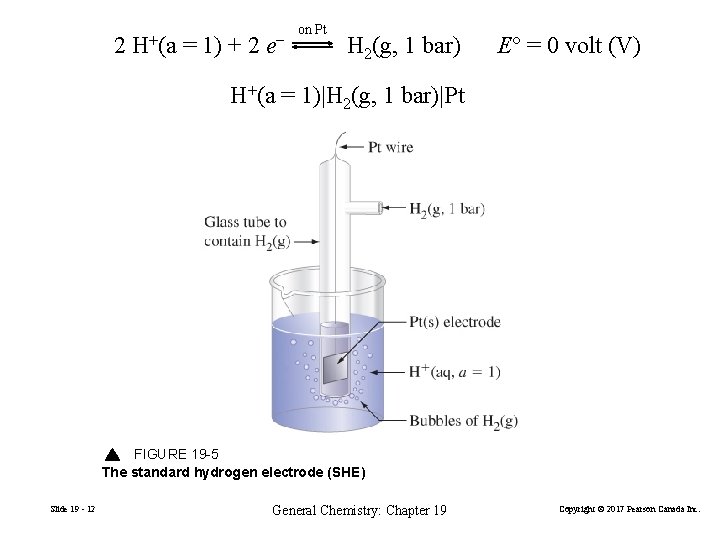
2 H+(a = 1) + 2 e− on Pt H 2(g, 1 bar) E° = 0 volt (V) H+(a = 1)|H 2(g, 1 bar)|Pt FIGURE 19 -5 The standard hydrogen electrode (SHE) Slide 19 - 12 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

The standard electrode potential, Eº The potential difference, or voltage, of a cell formed from two standard electrodes. Difference is always taken as Eºcell = Eº (right) − Eº (left) (cathode) (anode) Measures the tendency for a reduction process to occur at an electrode. All ionic species present at a = 1 (approximately 1 M). All gases are at 1 bar (approximately 1 atm). Where no metallic substance is indicated, the potential is established on an inert metallic electrode (ex. Pt). Slide 19 - 13 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

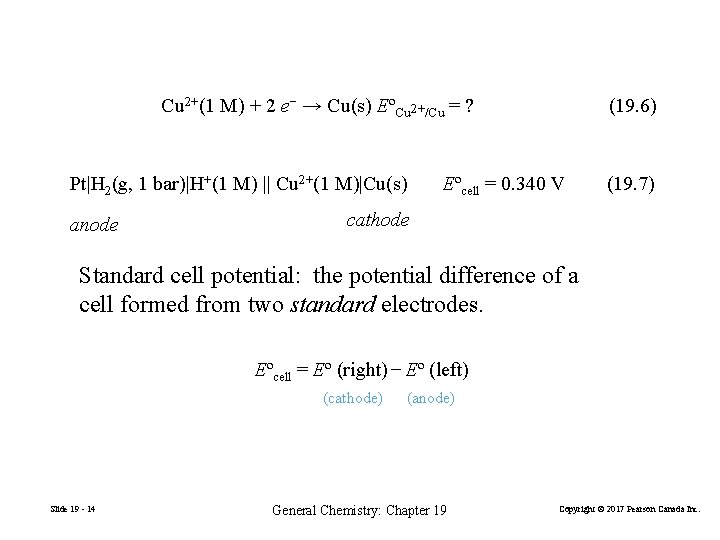
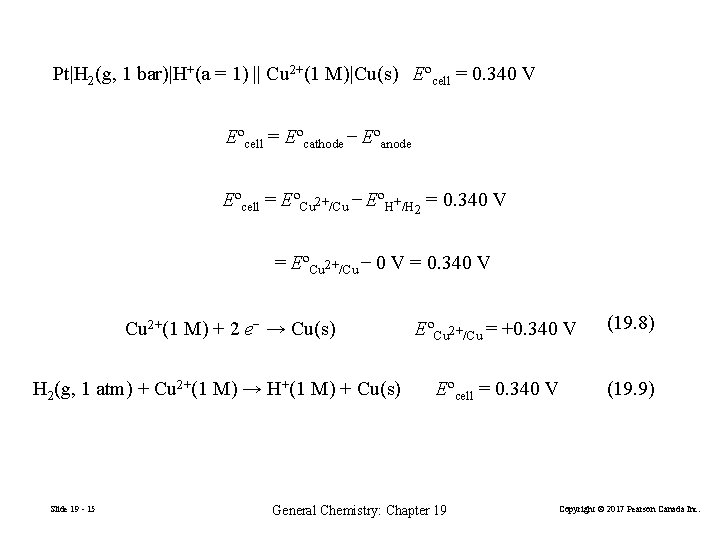
Cu 2+(1 M) + 2 e− → Cu(s) EºCu 2+/Cu = ? Pt|H 2(g, 1 bar)|H+(1 M) || Cu 2+(1 M)|Cu(s) anode (19. 6) Eºcell = 0. 340 V (19. 7) cathode Standard cell potential: the potential difference of a cell formed from two standard electrodes. Eºcell = Eº (right) − Eº (left) (cathode) Slide 19 - 14 (anode) General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

Pt|H 2(g, 1 bar)|H+(a = 1) || Cu 2+(1 M)|Cu(s) Eºcell = 0. 340 V Eºcell = Eºcathode − Eºanode Eºcell = EºCu 2+/Cu − EºH+/H 2 = 0. 340 V = EºCu 2+/Cu − 0 V = 0. 340 V Cu 2+(1 M) + 2 e− → Cu(s) H 2(g, 1 atm) + Cu 2+(1 M) → H+(1 M) + Cu(s) Slide 19 - 15 EºCu 2+/Cu = +0. 340 V (19. 8) Eºcell = 0. 340 V (19. 9) General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

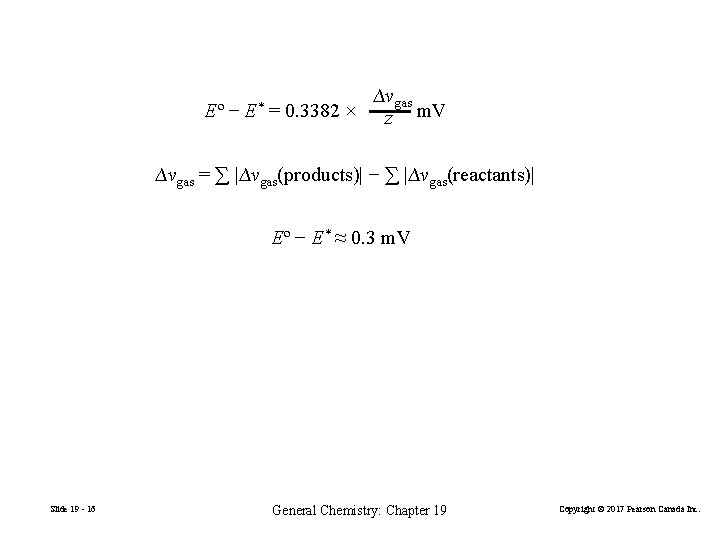
Eº − E* ∆vgas = 0. 3382 × z m. V ∆vgas = ∑ |∆vgas(products)| − ∑ |∆vgas(reactants)| Eº − E* ≈ 0. 3 m. V Slide 19 - 16 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

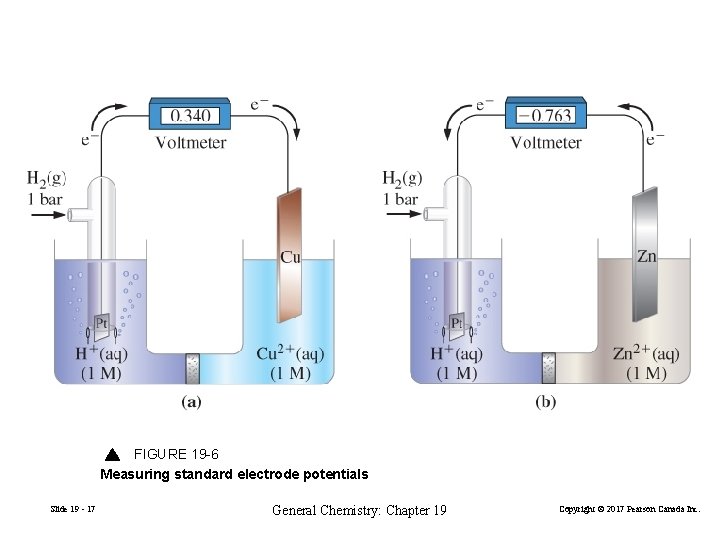
FIGURE 19 -6 Measuring standard electrode potentials Slide 19 - 17 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

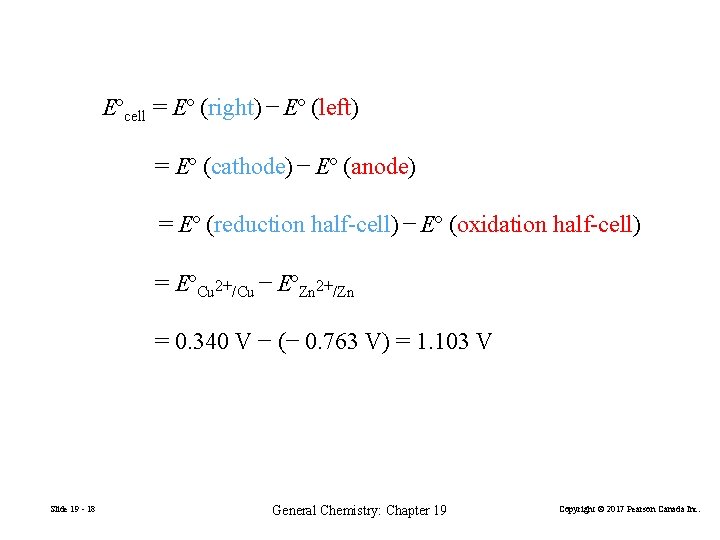
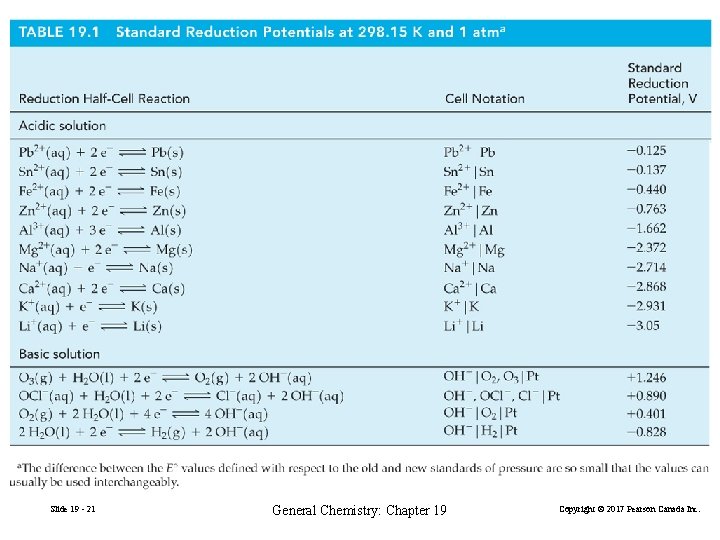
Eºcell = Eº (right) − Eº (left) = Eº (cathode) − Eº (anode) = Eº (reduction half-cell) − Eº (oxidation half-cell) = EºCu 2+/Cu − EºZn 2+/Zn = 0. 340 V − (− 0. 763 V) = 1. 103 V Slide 19 - 18 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

Slide 19 - 19 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

Slide 19 - 20 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

Slide 19 - 21 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

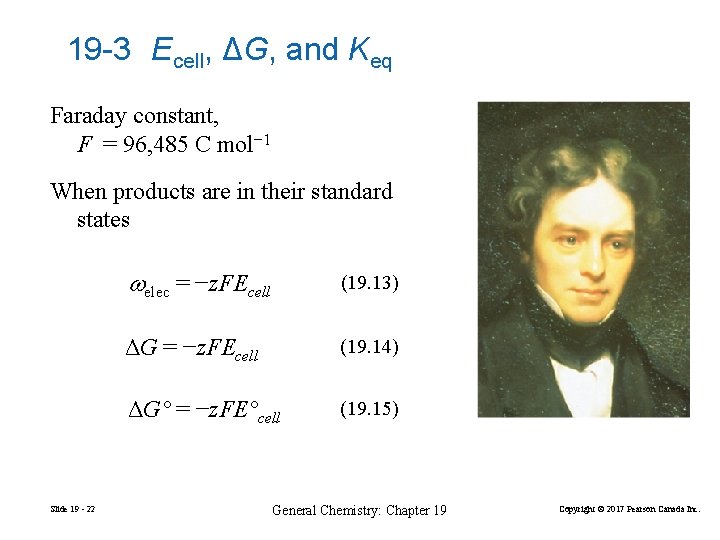
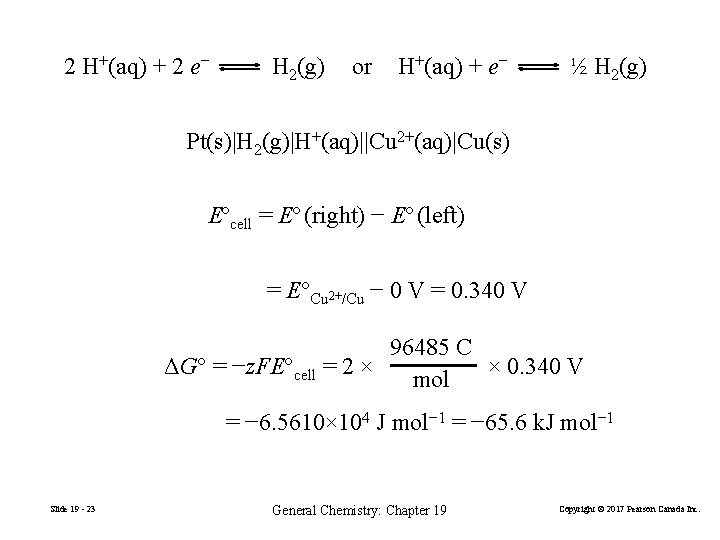
19 -3 Ecell, ΔG, and Keq Faraday constant, F = 96, 485 C mol− 1 When products are in their standard states Slide 19 - 22 elec = −z. FEcell (19. 13) ΔG = −z. FEcell (19. 14) ΔG° = −z. FE°cell (19. 15) General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

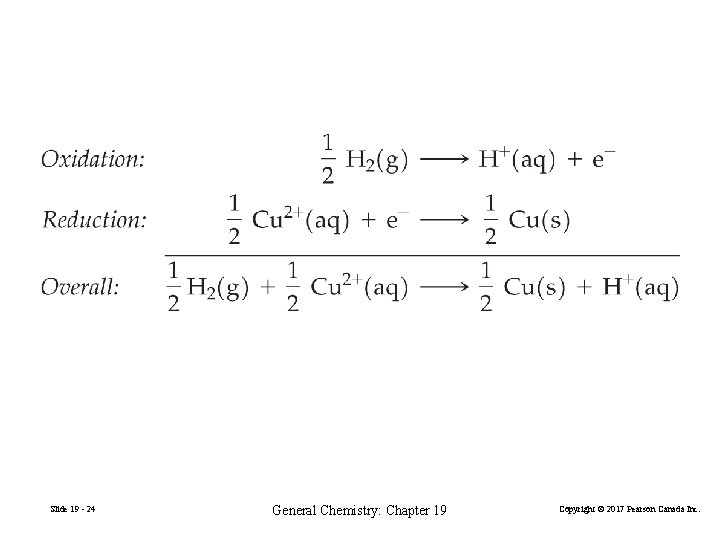
2 H+(aq) + 2 e− H 2(g) or H+(aq) + e− ½ H 2(g) Pt(s)|H 2(g)|H+(aq)||Cu 2+(aq)|Cu(s) Eºcell = Eº (right) − Eº (left) = E°Cu 2+/Cu − 0 V = 0. 340 V 96485 C ΔG° = −z. FE°cell = 2 × × 0. 340 V mol = − 6. 5610× 104 J mol− 1 = − 65. 6 k. J mol− 1 Slide 19 - 23 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

Slide 19 - 24 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

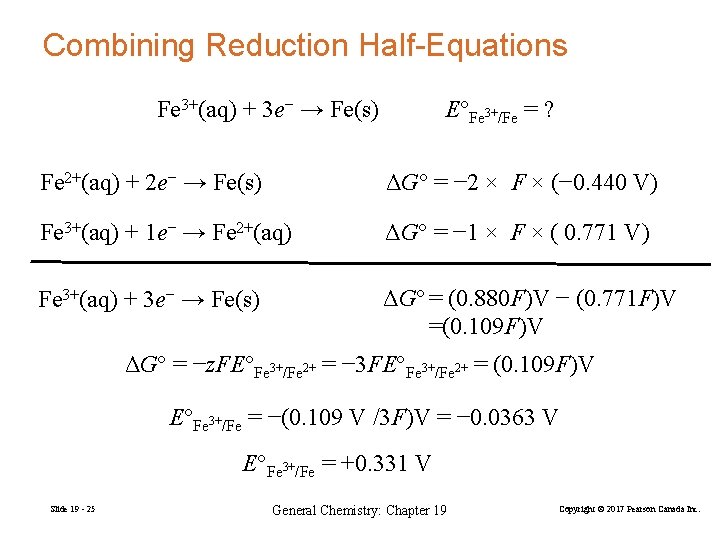
Combining Reduction Half-Equations Fe 3+(aq) + 3 e− → Fe(s) E°Fe 3+/Fe = ? Fe 2+(aq) + 2 e− → Fe(s) ΔG° = − 2 × F × (− 0. 440 V) Fe 3+(aq) + 1 e− → Fe 2+(aq) ΔG° = − 1 × F × ( 0. 771 V) Fe 3+(aq) + 3 e− → Fe(s) ΔG° = (0. 880 F)V − (0. 771 F)V =(0. 109 F)V ΔG° = −z. FE°Fe 3+/Fe 2+ = − 3 FE°Fe 3+/Fe 2+ = (0. 109 F)V E°Fe 3+/Fe = −(0. 109 V /3 F)V = − 0. 0363 V E°Fe 3+/Fe = +0. 331 V Slide 19 - 25 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

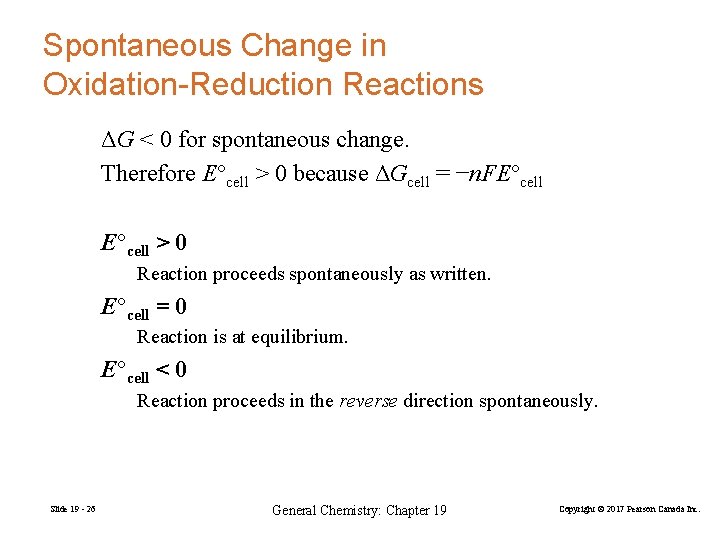
Spontaneous Change in Oxidation-Reduction Reactions ΔG < 0 for spontaneous change. Therefore E°cell > 0 because ΔGcell = −n. FE°cell > 0 Reaction proceeds spontaneously as written. E°cell = 0 Reaction is at equilibrium. E°cell < 0 Reaction proceeds in the reverse direction spontaneously. Slide 19 - 26 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

FIGURE 19 -7 Reaction of Al(s) and Cu 2+(aq) Slide 19 - 27 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

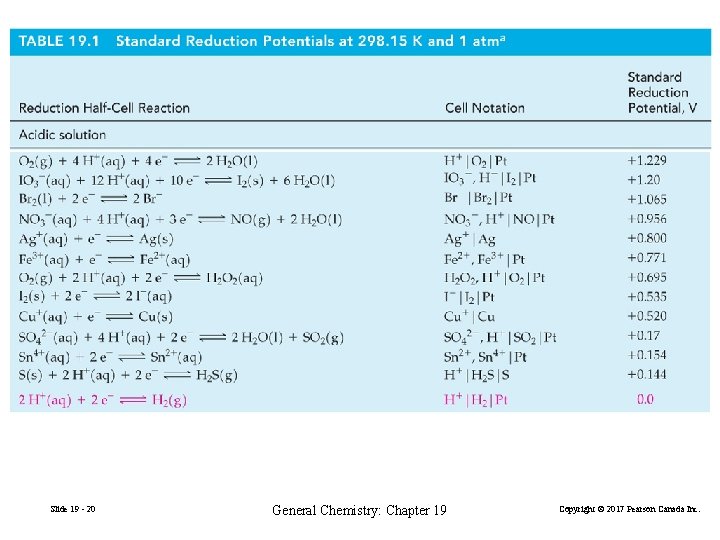
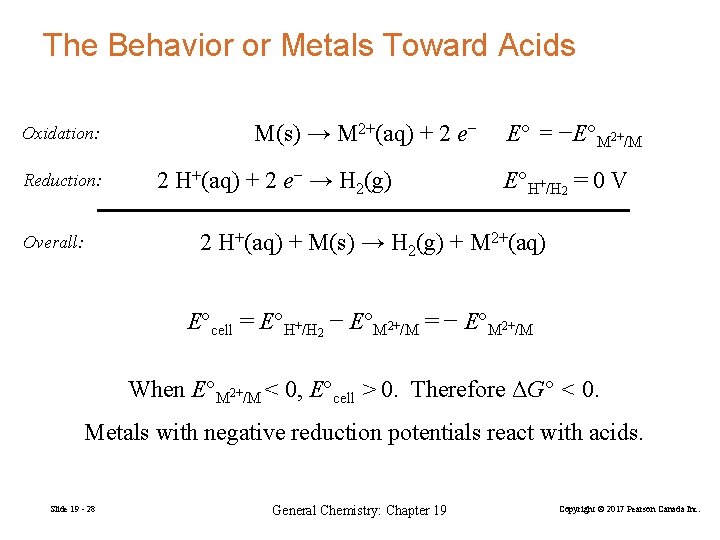
The Behavior or Metals Toward Acids Oxidation: Reduction: M(s) → M 2+(aq) + 2 e− 2 H+(aq) + 2 e− → H 2(g) E° = −E°M 2+/M E°H+/H 2 = 0 V 2 H+(aq) + M(s) → H 2(g) + M 2+(aq) Overall: E°cell = E°H+/H 2 − E°M 2+/M = − E°M 2+/M When E°M 2+/M < 0, E°cell > 0. Therefore ΔG° < 0. Metals with negative reduction potentials react with acids. Slide 19 - 28 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

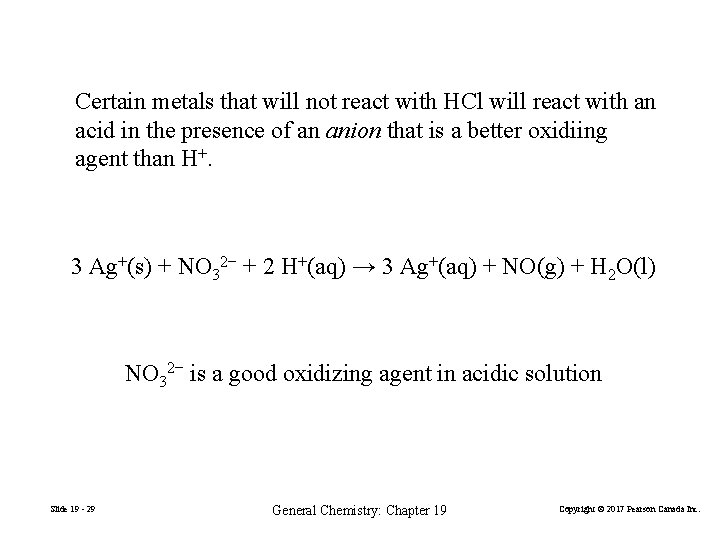
Certain metals that will not react with HCl will react with an acid in the presence of an anion that is a better oxidiing agent than H+. 3 Ag+(s) + NO 32− + 2 H+(aq) → 3 Ag+(aq) + NO(g) + H 2 O(l) NO 32− is a good oxidizing agent in acidic solution Slide 19 - 29 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

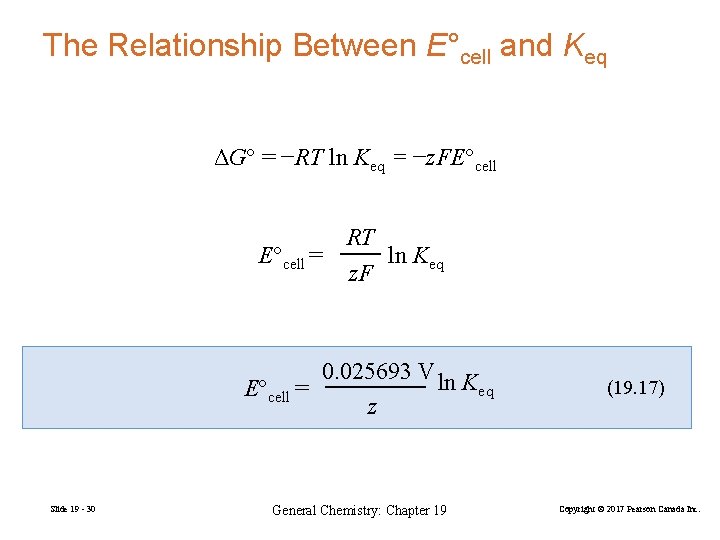
The Relationship Between E°cell and Keq ΔG° = −RT ln Keq = −z. FE°cell RT E°cell = ln Keq z. F 0. 025693 V ln K E°cell = eq z Slide 19 - 30 General Chemistry: Chapter 19 (19. 17) Copyright © 2017 Pearson Canada Inc.

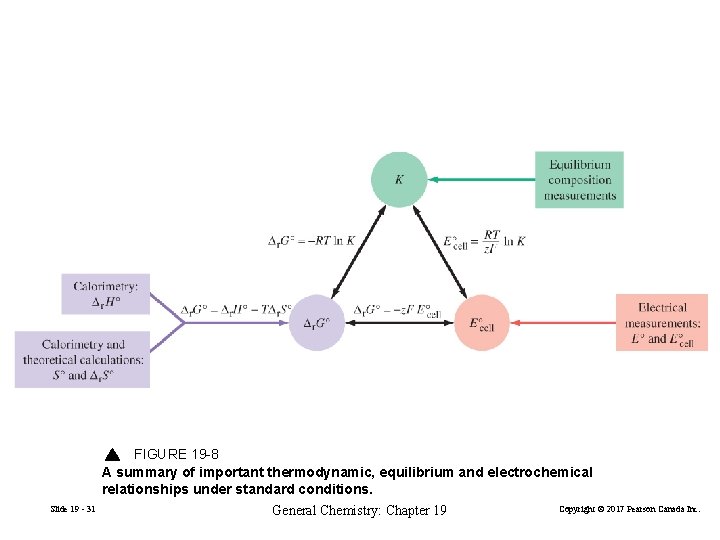
FIGURE 19 -8 A summary of important thermodynamic, equilibrium and electrochemical relationships under standard conditions. Slide 19 - 31 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

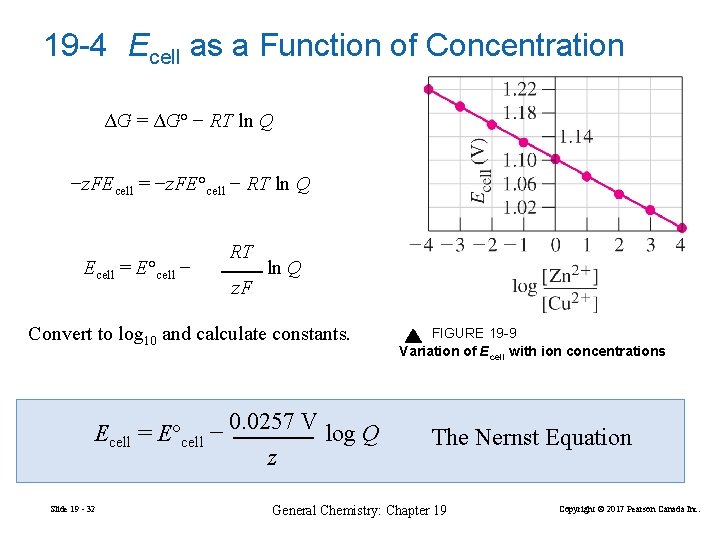
19 -4 Ecell as a Function of Concentration ΔG = ΔG° − RT ln Q −z. FEcell = −z. FE°cell − RT ln Q Ecell = E°cell − RT z. F ln Q Convert to log 10 and calculate constants. Ecell = E°cell − Slide 19 - 32 0. 0257 V z log Q FIGURE 19 -9 Variation of Ecell with ion concentrations The Nernst Equation General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

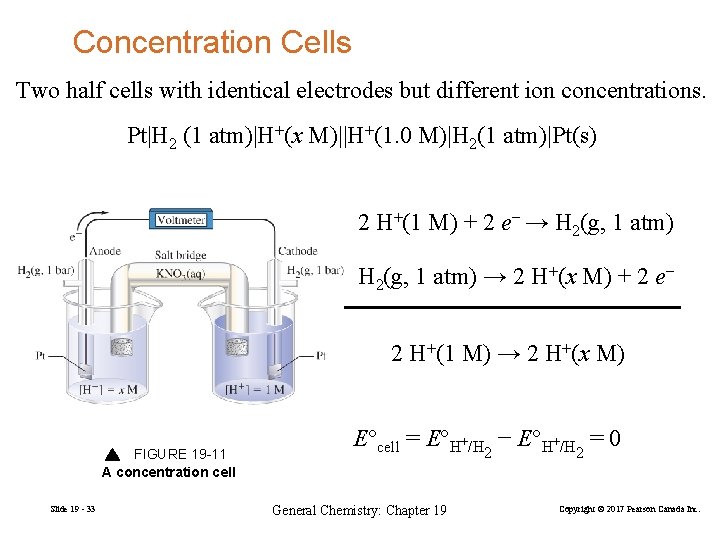
Concentration Cells Two half cells with identical electrodes but different ion concentrations. Pt|H 2 (1 atm)|H+(x M)||H+(1. 0 M)|H 2(1 atm)|Pt(s) 2 H+(1 M) + 2 e− → H 2(g, 1 atm) → 2 H+(x M) + 2 e− 2 H+(1 M) → 2 H+(x M) FIGURE 19 -11 A concentration cell Slide 19 - 33 E°cell = E°H+/H − E°H+/H = 0 2 General Chemistry: Chapter 19 2 Copyright © 2017 Pearson Canada Inc.

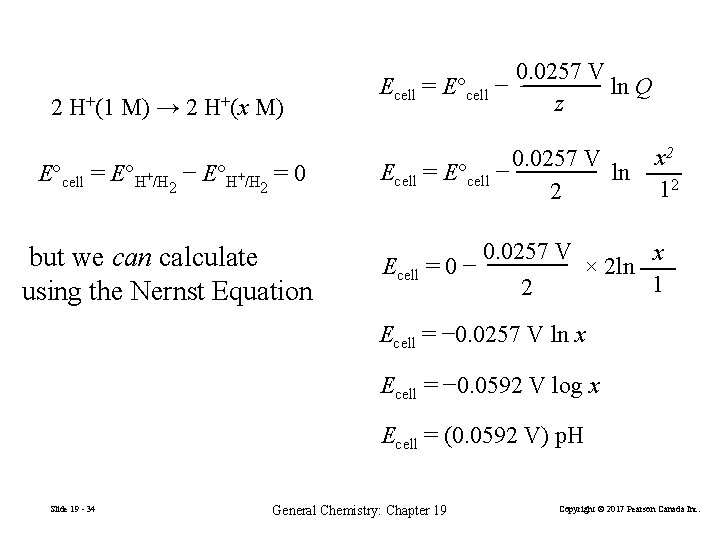
2 H+(1 M) → 2 H+(x M) E°cell = E°H+/H − E°H+/H = 0 2 2 but we can calculate using the Nernst Equation 0. 0257 V Ecell = E°cell − ln Q z 0. 0257 V Ecell = E°cell − ln 2 x 2 12 0. 0257 V x Ecell = 0 − × 2 ln 1 2 Ecell = − 0. 0257 V ln x Ecell = − 0. 0592 V log x Ecell = (0. 0592 V) p. H Slide 19 - 34 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

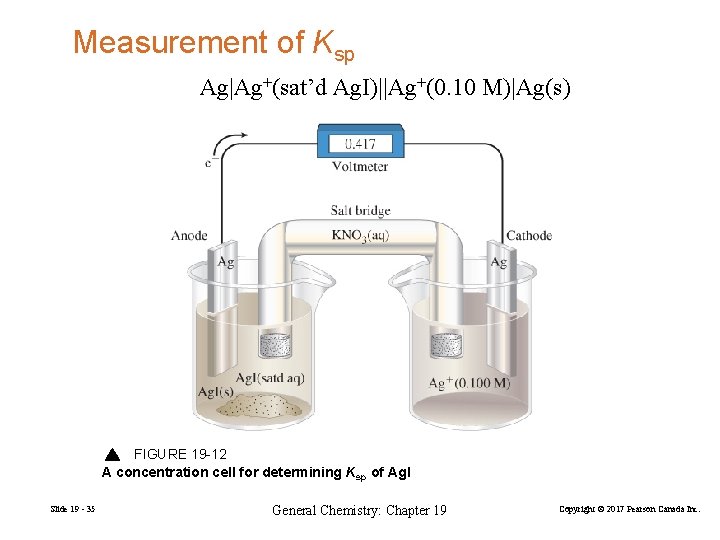
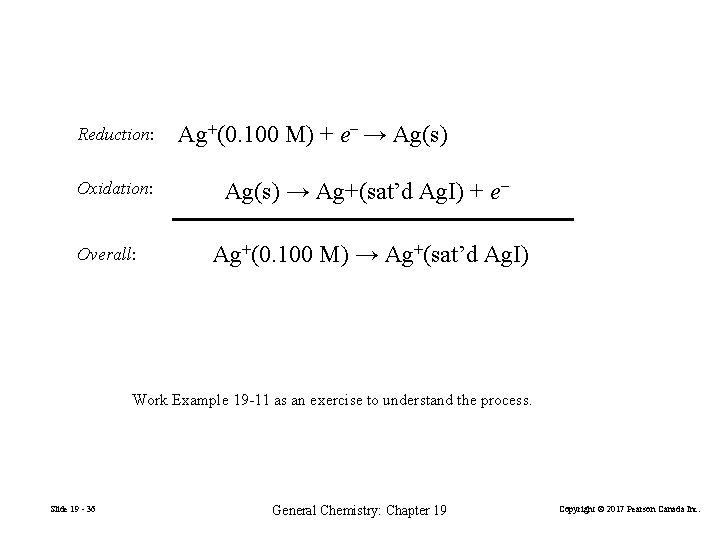
Measurement of Ksp Ag|Ag+(sat’d Ag. I)||Ag+(0. 10 M)|Ag(s) FIGURE 19 -12 A concentration cell for determining Ksp of Ag. I Slide 19 - 35 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

Reduction: Oxidation: Overall: Ag+(0. 100 M) + e− → Ag(s) → Ag+(sat’d Ag. I) + e− Ag+(0. 100 M) → Ag+(sat’d Ag. I) Work Example 19 -11 as an exercise to understand the process. Slide 19 - 36 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

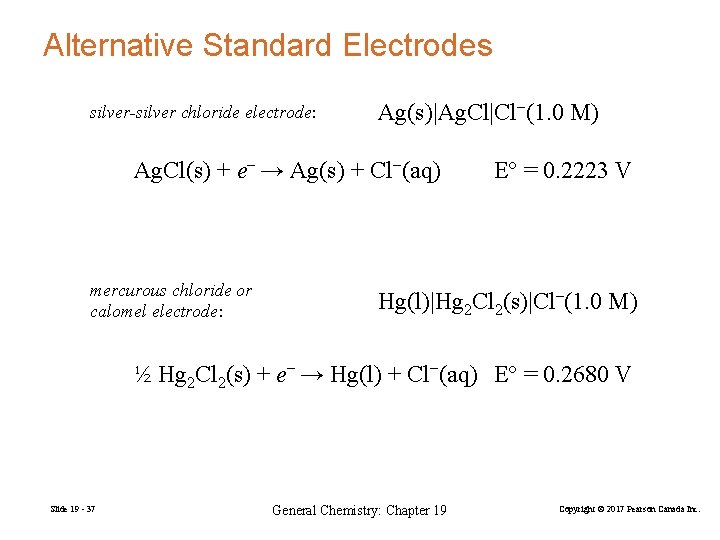
Alternative Standard Electrodes silver-silver chloride electrode: Ag(s)|Ag. Cl|Cl−(1. 0 M) Ag. Cl(s) + e− → Ag(s) + Cl−(aq) mercurous chloride or calomel electrode: E° = 0. 2223 V Hg(l)|Hg 2 Cl 2(s)|Cl−(1. 0 M) ½ Hg 2 Cl 2(s) + e− → Hg(l) + Cl−(aq) E° = 0. 2680 V Slide 19 - 37 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

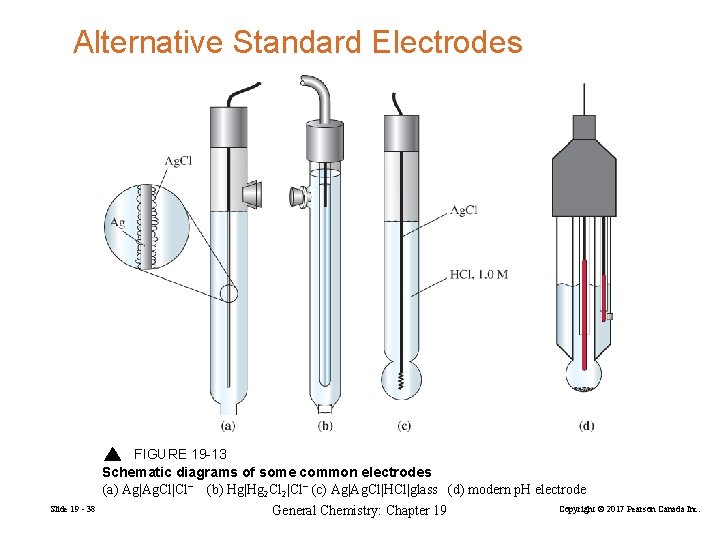
Alternative Standard Electrodes FIGURE 19 -13 Schematic diagrams of some common electrodes (a) Ag|Ag. Cl|Cl− (b) Hg|Hg 2 Cl 2|Cl− (c) Ag|Ag. Cl|HCl|glass (d) modern p. H electrode Slide 19 - 38 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

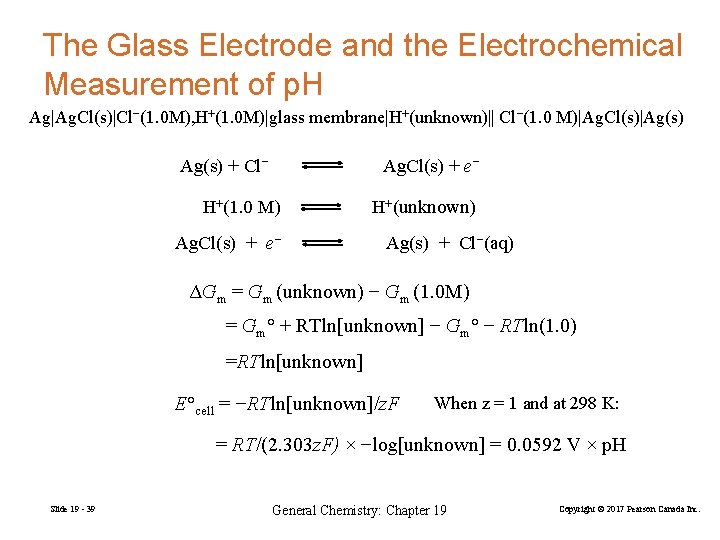
The Glass Electrode and the Electrochemical Measurement of p. H Ag|Ag. Cl(s)|Cl−(1. 0 M), H+(1. 0 M)|glass membrane|H+(unknown)|| Cl−(1. 0 M)|Ag. Cl(s)|Ag(s) + Cl− Ag. Cl(s) + e− H+(1. 0 M) Ag. Cl(s) + e− H+(unknown) Ag(s) + Cl−(aq) ΔGm = Gm (unknown) − Gm (1. 0 M) = Gm° + RTln[unknown] − Gm° − RTln(1. 0) =RTln[unknown] E°cell = −RTln[unknown]/z. F When z = 1 and at 298 K: = RT/(2. 303 z. F) × −log[unknown] = 0. 0592 V × p. H Slide 19 - 39 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

19 -5 Batteries: Producing Electricity Through Chemical Reactions • Primary cells (or batteries). Cell reaction is not reversible. • Secondary cells. Cell reaction can be reversed by passing electricity through the cell (charging). Several hundred or more cycles are possible. • Reserve batteries. Electrolyte is isolated from the rest of the battery to reduce selfdischarge or chemical degradation. Designed for long term storage and to deliver high power over a relatively short time. • Flow batteries and fuel cells. Materials pass through the battery which converts chemical energy to electric energy. Can be run indefinitely when supplied with electrolytes. Slide 19 - 40 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

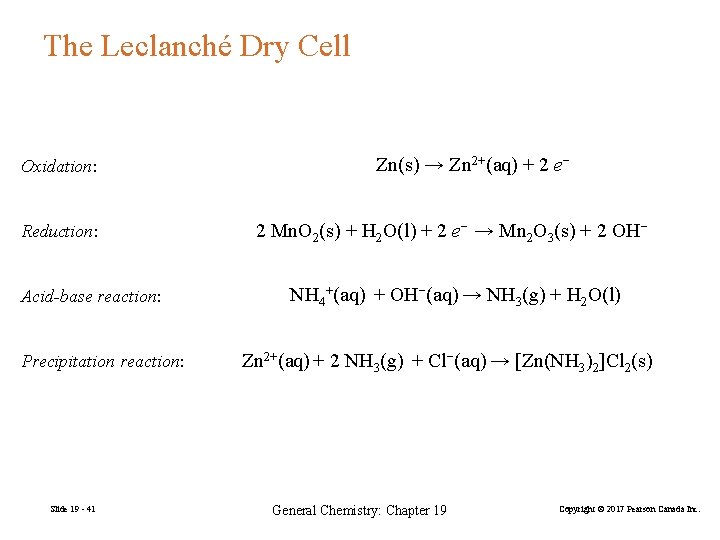
The Leclanché Dry Cell Oxidation: Reduction: Acid-base reaction: Precipitation reaction: Slide 19 - 41 Zn(s) → Zn 2+(aq) + 2 e− 2 Mn. O 2(s) + H 2 O(l) + 2 e− → Mn 2 O 3(s) + 2 OH− NH 4+(aq) + OH−(aq) → NH 3(g) + H 2 O(l) Zn 2+(aq) + 2 NH 3(g) + Cl−(aq) → [Zn(NH 3)2]Cl 2(s) General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

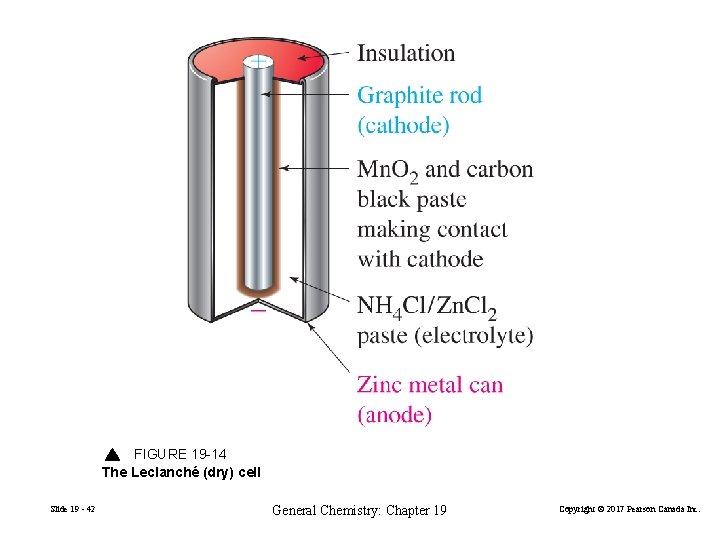
FIGURE 19 -14 The Leclanché (dry) cell Slide 19 - 42 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

The alkaline cell Reduction: 2 Mn. O 2(s) + H 2 O(l) + 2 e− → Mn 2 O 3(s) + 2 OH− Oxidation: can be thought of as occurring in two steps Zn(s) → Zn 2+(aq) + 2 e− Zn 2+(aq) + 2 OH−→ Zn (OH)2(s) Zn (s) + 2 OH− → Zn (OH)2(s) + 2 e− E°cell is an intensive property Slide 19 - 43 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

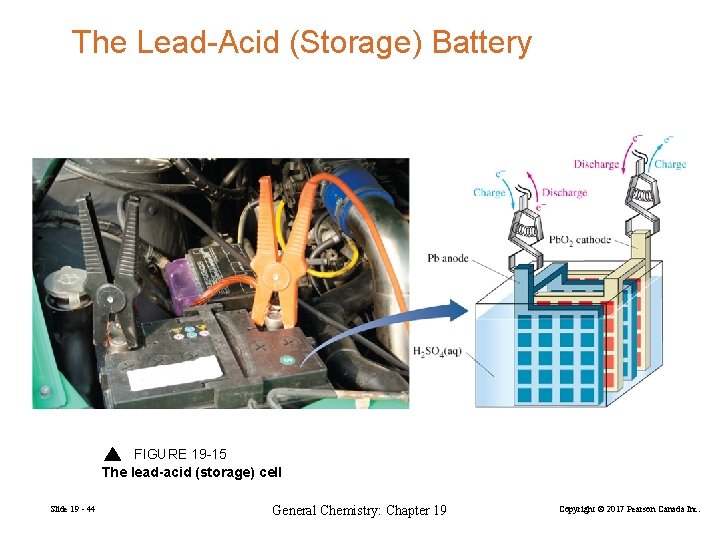
The Lead-Acid (Storage) Battery FIGURE 19 -15 The lead-acid (storage) cell Slide 19 - 44 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

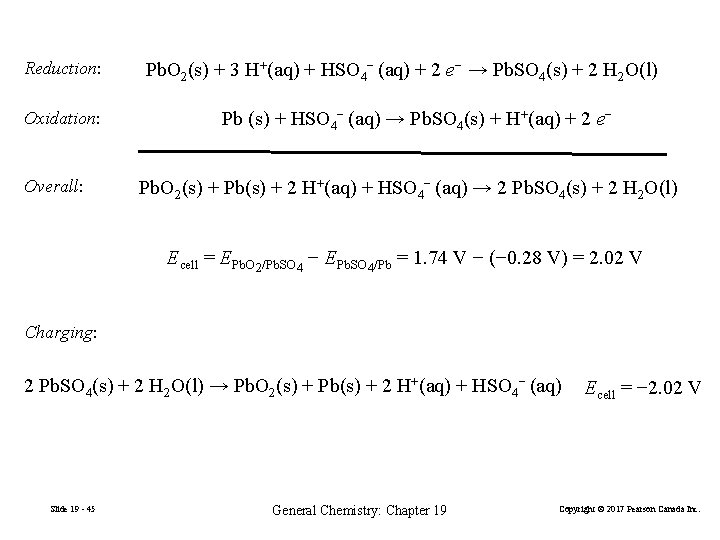
Reduction: Oxidation: Overall: Pb. O 2(s) + 3 H+(aq) + HSO 4− (aq) + 2 e− → Pb. SO 4(s) + 2 H 2 O(l) Pb (s) + HSO 4− (aq) → Pb. SO 4(s) + H+(aq) + 2 e− Pb. O 2(s) + Pb(s) + 2 H+(aq) + HSO 4− (aq) → 2 Pb. SO 4(s) + 2 H 2 O(l) Ecell = EPb. O 2/Pb. SO 4 − EPb. SO 4/Pb = 1. 74 V − (− 0. 28 V) = 2. 02 V Charging: 2 Pb. SO 4(s) + 2 H 2 O(l) → Pb. O 2(s) + Pb(s) + 2 H+(aq) + HSO 4− (aq) Slide 19 - 45 General Chemistry: Chapter 19 Ecell = − 2. 02 V Copyright © 2017 Pearson Canada Inc.

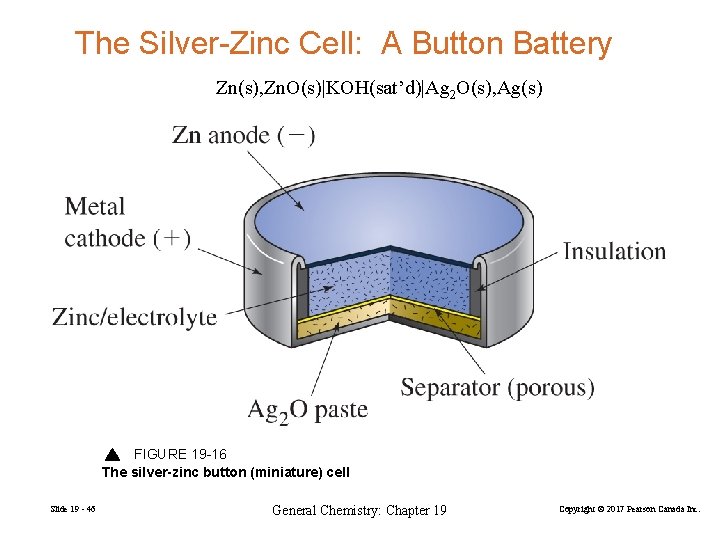
The Silver-Zinc Cell: A Button Battery Zn(s), Zn. O(s)|KOH(sat’d)|Ag 2 O(s), Ag(s) FIGURE 19 -16 The silver-zinc button (miniature) cell Slide 19 - 46 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

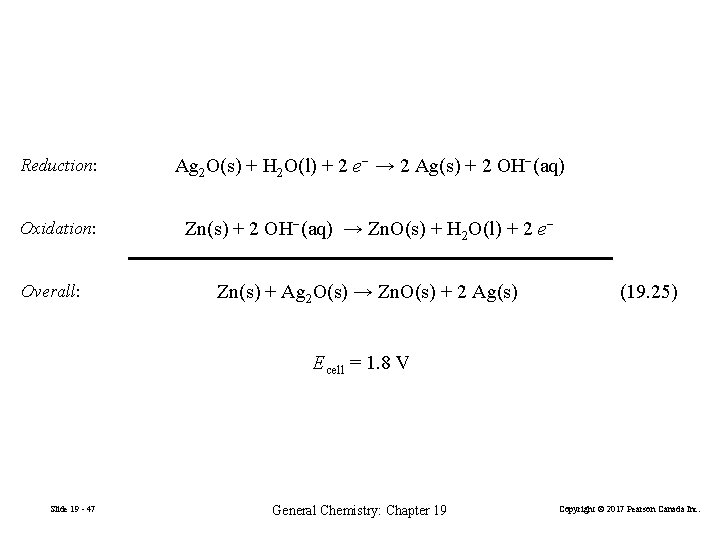
Reduction: Ag 2 O(s) + H 2 O(l) + 2 e− → 2 Ag(s) + 2 OH−(aq) Oxidation: Zn(s) + 2 OH−(aq) → Zn. O(s) + H 2 O(l) + 2 e− Overall: Zn(s) + Ag 2 O(s) → Zn. O(s) + 2 Ag(s) (19. 25) Ecell = 1. 8 V Slide 19 - 47 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

The Nickel-Cadmium Cell: A Rechargeable Battery A rechargeable nickel-cadmium cell, or nicad battery Cd(s) + 2 Ni. O(OH)(s) + 2 H 2 O(l) → 2 Ni(OH)2(s) + Cd(OH)2(s) Slide 19 - 48 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

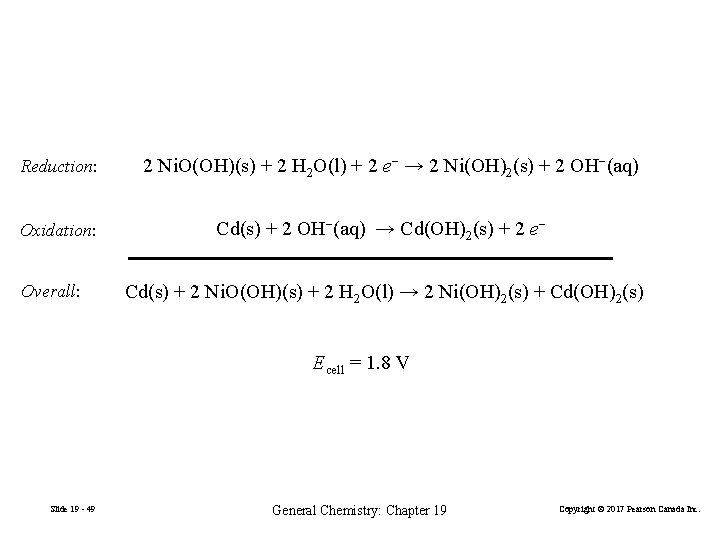
Reduction: Oxidation: Overall: 2 Ni. O(OH)(s) + 2 H 2 O(l) + 2 e− → 2 Ni(OH)2(s) + 2 OH−(aq) Cd(s) + 2 OH−(aq) → Cd(OH)2(s) + 2 e− Cd(s) + 2 Ni. O(OH)(s) + 2 H 2 O(l) → 2 Ni(OH)2(s) + Cd(OH)2(s) Ecell = 1. 8 V Slide 19 - 49 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

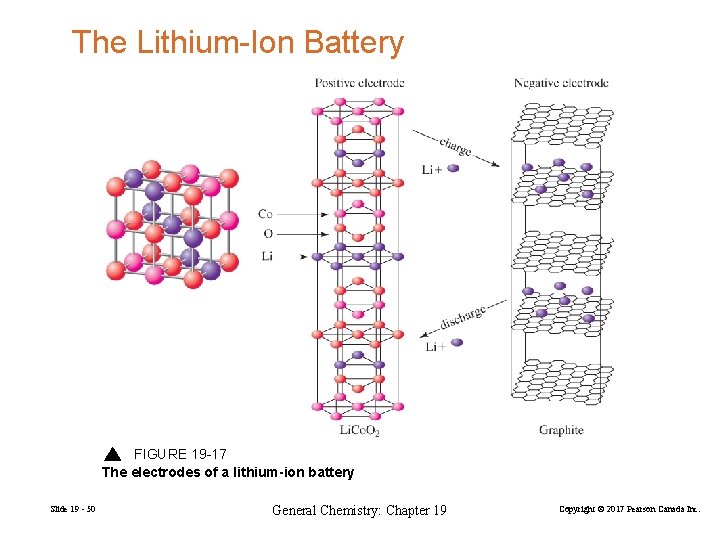
The Lithium-Ion Battery FIGURE 19 -17 The electrodes of a lithium-ion battery Slide 19 - 50 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

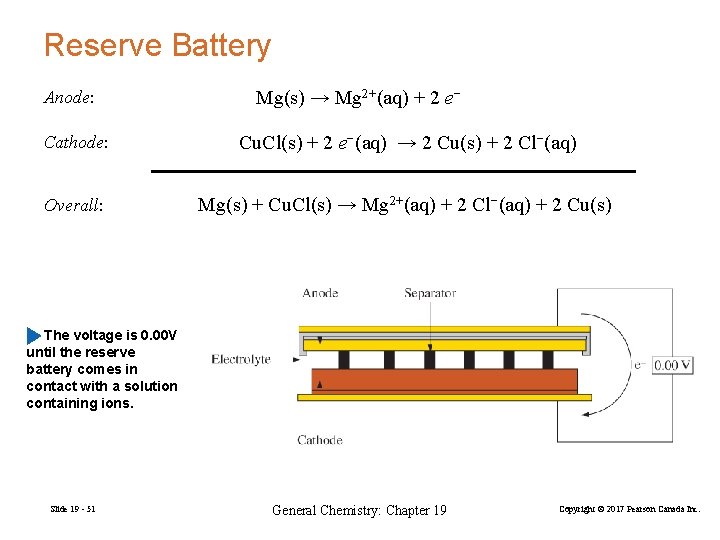
Reserve Battery Anode: Mg(s) → Mg 2+(aq) + 2 e− Cathode: Cu. Cl(s) + 2 e−(aq) → 2 Cu(s) + 2 Cl−(aq) Overall: Mg(s) + Cu. Cl(s) → Mg 2+(aq) + 2 Cl−(aq) + 2 Cu(s) The voltage is 0. 00 V until the reserve battery comes in contact with a solution containing ions. Slide 19 - 51 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

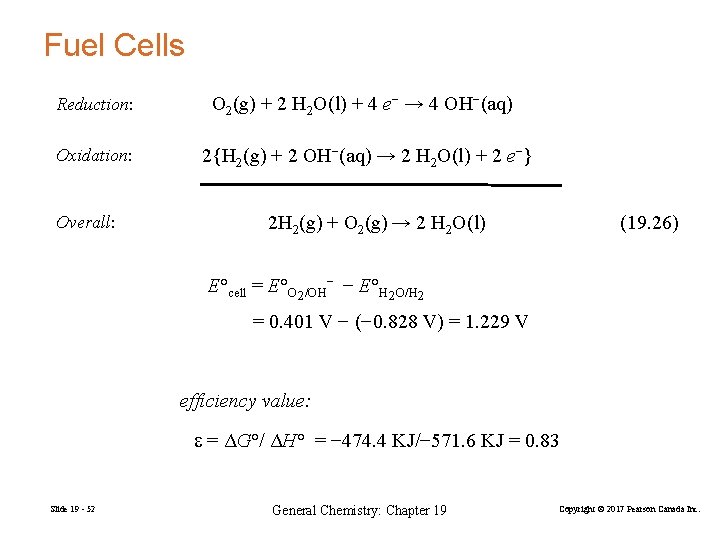
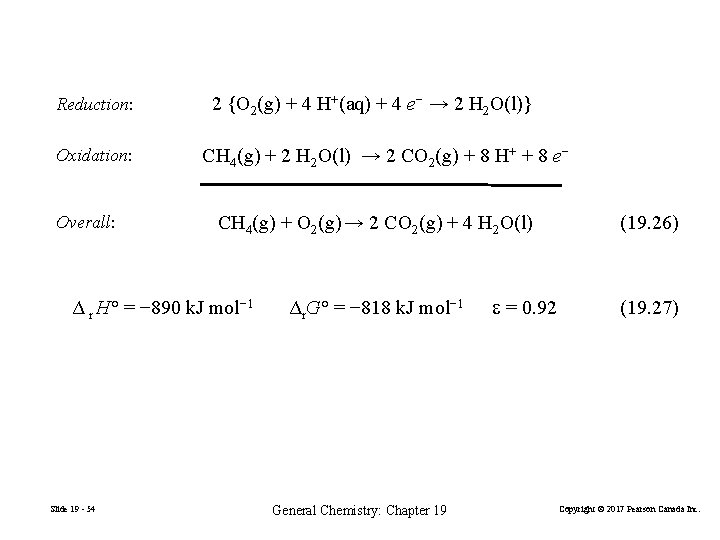
Fuel Cells Reduction: O 2(g) + 2 H 2 O(l) + 4 e− → 4 OH−(aq) Oxidation: 2{H 2(g) + 2 OH−(aq) → 2 H 2 O(l) + 2 e−} Overall: 2 H 2(g) + O 2(g) → 2 H 2 O(l) (19. 26) E°cell = E°O 2/OH− − E°H 2 O/H 2 = 0. 401 V − (− 0. 828 V) = 1. 229 V efficiency value: = ΔG°/ ΔH° = − 474. 4 KJ/− 571. 6 KJ = 0. 83 Slide 19 - 52 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

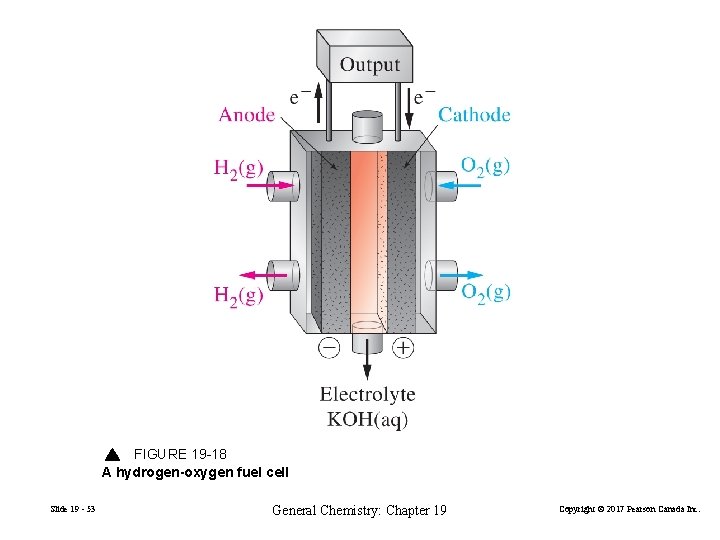
FIGURE 19 -18 A hydrogen-oxygen fuel cell Slide 19 - 53 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

Reduction: Oxidation: Overall: 2 {O 2(g) + 4 H+(aq) + 4 e− → 2 H 2 O(l)} CH 4(g) + 2 H 2 O(l) → 2 CO 2(g) + 8 H+ + 8 e− CH 4(g) + O 2(g) → 2 CO 2(g) + 4 H 2 O(l) Δ r H° = − 890 k. J mol− 1 Slide 19 - 54 Δr. G° = − 818 k. J mol− 1 General Chemistry: Chapter 19 = 0. 92 (19. 26) (19. 27) Copyright © 2017 Pearson Canada Inc.

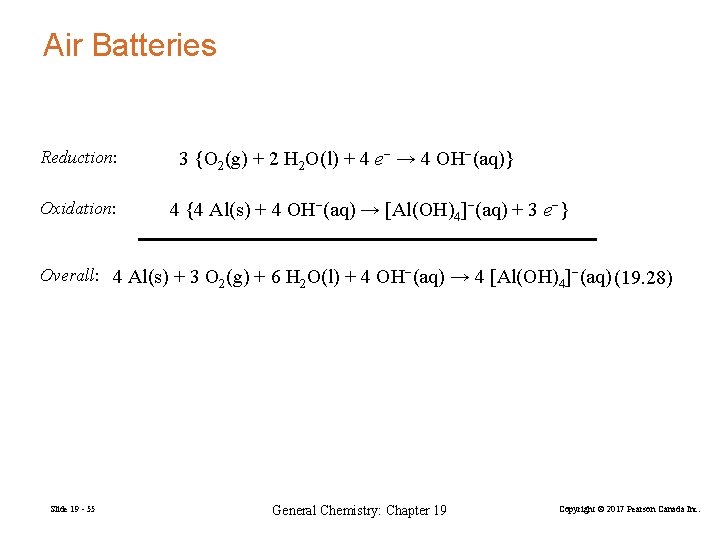
Air Batteries Reduction: Oxidation: 3 {O 2(g) + 2 H 2 O(l) + 4 e− → 4 OH−(aq)} 4 {4 Al(s) + 4 OH−(aq) → [Al(OH)4]−(aq) + 3 e−} Overall: 4 Al(s) + 3 O 2(g) + 6 H 2 O(l) + 4 OH−(aq) → 4 [Al(OH)4]−(aq) (19. 28) Slide 19 - 55 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

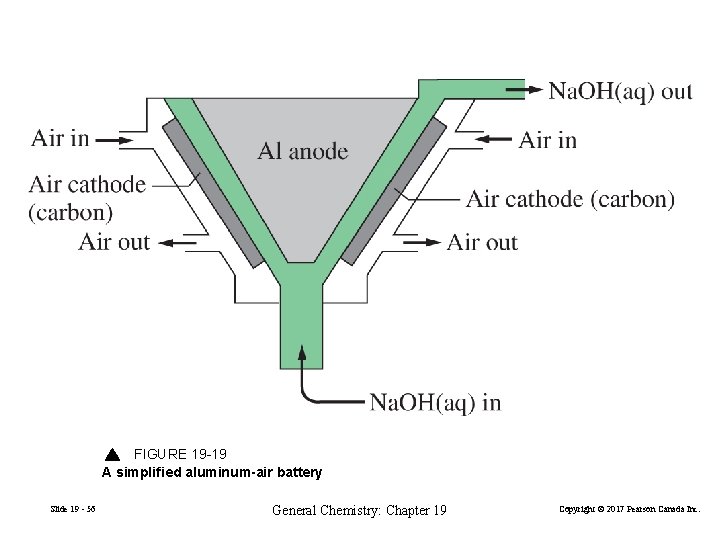
FIGURE 19 -19 A simplified aluminum-air battery Slide 19 - 56 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

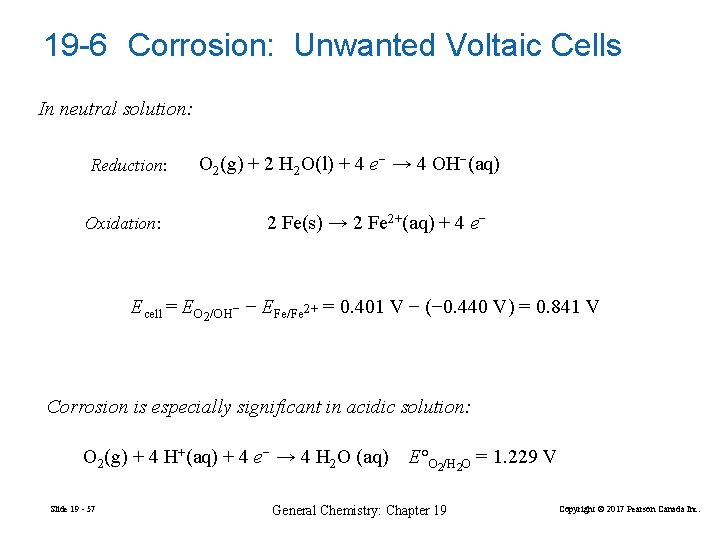
19 -6 Corrosion: Unwanted Voltaic Cells In neutral solution: Reduction: Oxidation: O 2(g) + 2 H 2 O(l) + 4 e− → 4 OH−(aq) 2 Fe(s) → 2 Fe 2+(aq) + 4 e− Ecell = EO 2/OH− − EFe/Fe 2+ = 0. 401 V − (− 0. 440 V) = 0. 841 V Corrosion is especially significant in acidic solution: O 2(g) + 4 H+(aq) + 4 e− → 4 H 2 O (aq) Slide 19 - 57 E°O 2/H 2 O = 1. 229 V General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

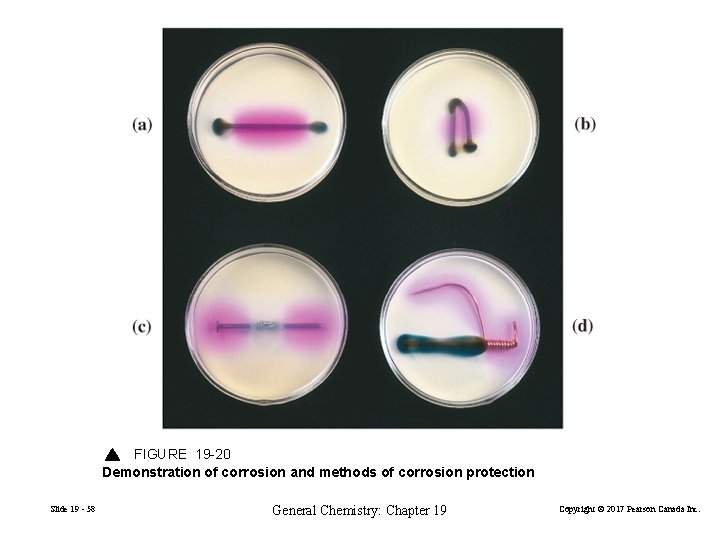
FIGURE 19 -20 Demonstration of corrosion and methods of corrosion protection Slide 19 - 58 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

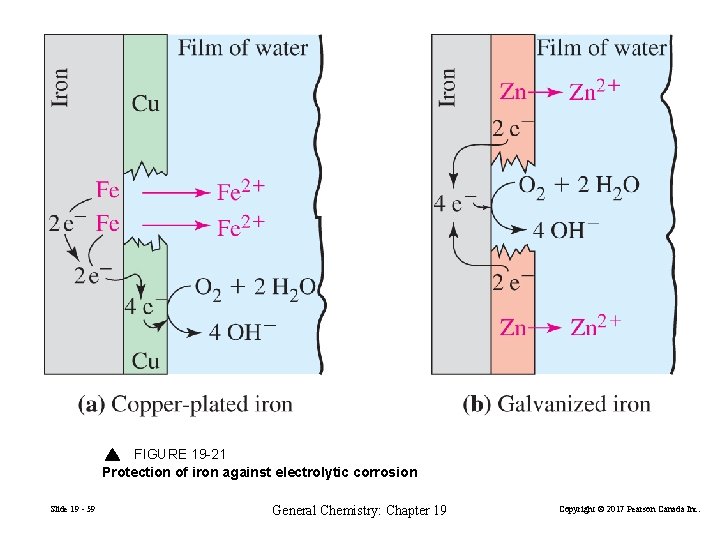
FIGURE 19 -21 Protection of iron against electrolytic corrosion Slide 19 - 59 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

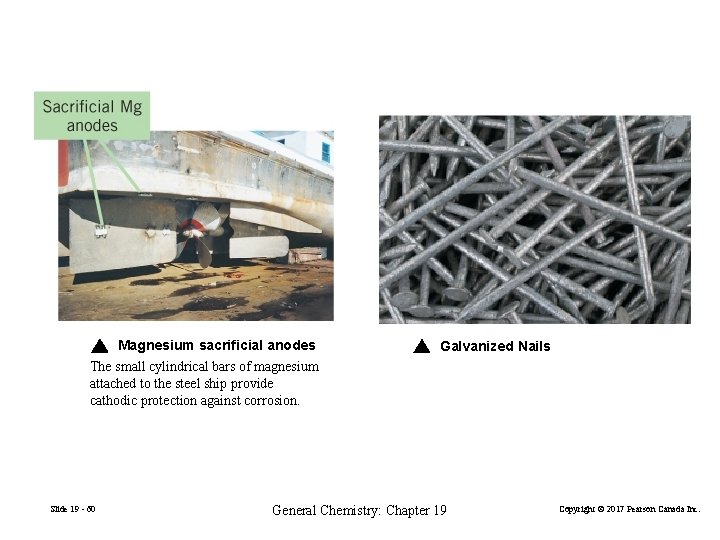
Magnesium sacrificial anodes The small cylindrical bars of magnesium attached to the steel ship provide cathodic protection against corrosion. Slide 19 - 60 Galvanized Nails General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

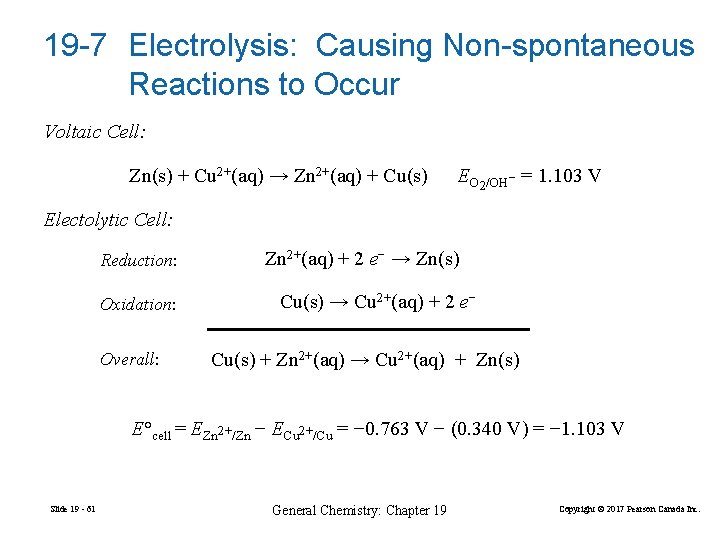
19 -7 Electrolysis: Causing Non-spontaneous Reactions to Occur Voltaic Cell: Zn(s) + Cu 2+(aq) → Zn 2+(aq) + Cu(s) EO 2/OH− = 1. 103 V Electolytic Cell: Reduction: Oxidation: Overall: Zn 2+(aq) + 2 e− → Zn(s) Cu(s) → Cu 2+(aq) + 2 e− Cu(s) + Zn 2+(aq) → Cu 2+(aq) + Zn(s) E°cell = EZn 2+/Zn − ECu 2+/Cu = − 0. 763 V − (0. 340 V) = − 1. 103 V Slide 19 - 61 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

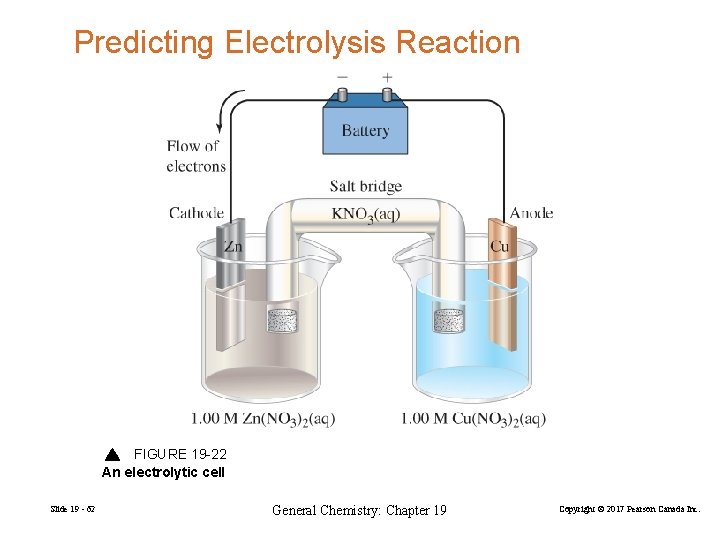
Predicting Electrolysis Reaction FIGURE 19 -22 An electrolytic cell Slide 19 - 62 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

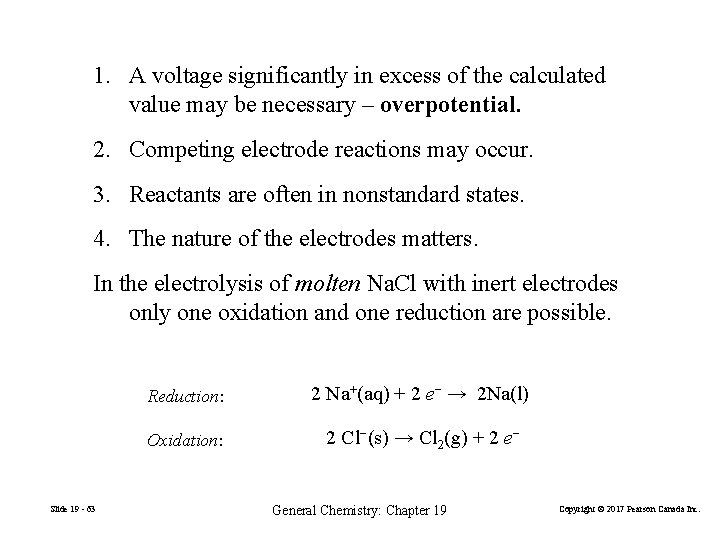
1. A voltage significantly in excess of the calculated value may be necessary – overpotential. 2. Competing electrode reactions may occur. 3. Reactants are often in nonstandard states. 4. The nature of the electrodes matters. In the electrolysis of molten Na. Cl with inert electrodes only one oxidation and one reduction are possible. Slide 19 - 63 Reduction: 2 Na+(aq) + 2 e− → 2 Na(l) Oxidation: 2 Cl−(s) → Cl 2(g) + 2 e− General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

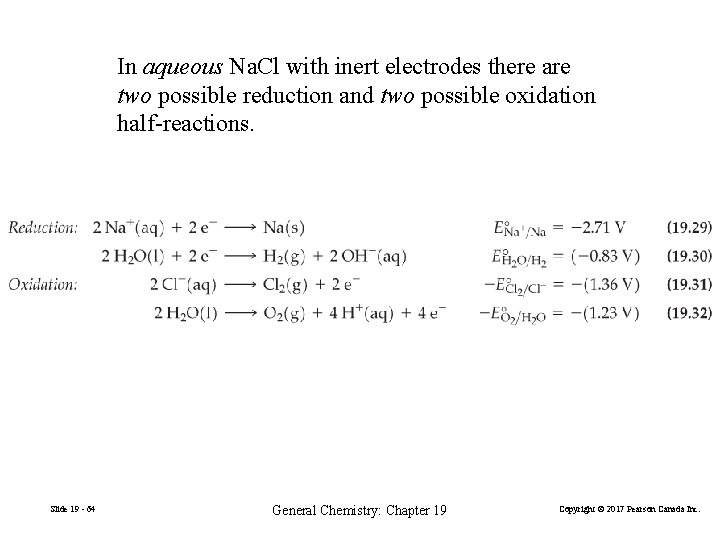
In aqueous Na. Cl with inert electrodes there are two possible reduction and two possible oxidation half-reactions. Slide 19 - 64 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

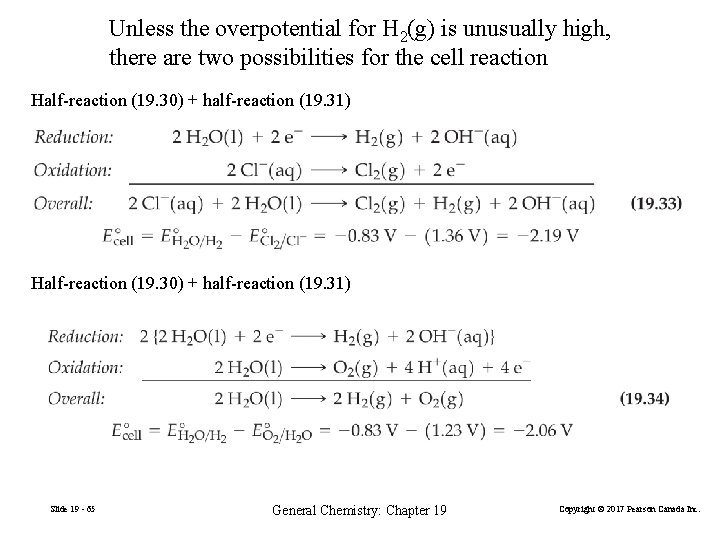
Unless the overpotential for H 2(g) is unusually high, there are two possibilities for the cell reaction Half-reaction (19. 30) + half-reaction (19. 31) Slide 19 - 65 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

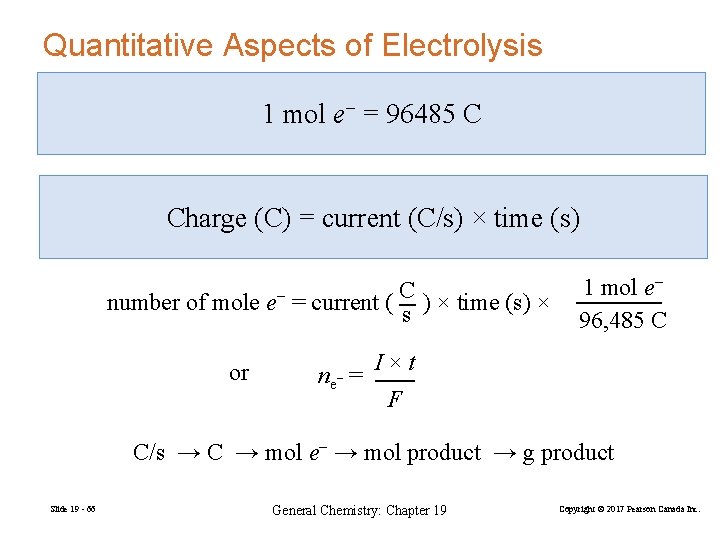
Quantitative Aspects of Electrolysis 1 mol e− = 96485 C Charge (C) = current (C/s) × time (s) number of mole e− = current ( C ) × time (s) × s or ne− = 1 mol e− 96, 485 C I×t F C/s → C → mol e− → mol product → g product Slide 19 - 66 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

19 -8 Industrial Electrolysis Processes The refining of copper by electrolysis. Slide 19 - 67 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

Electrorefining Electroplating Electrosynthesis A rack of metal parts being lifted from the electrolyte solution after electroplating. Slide 19 - 68 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

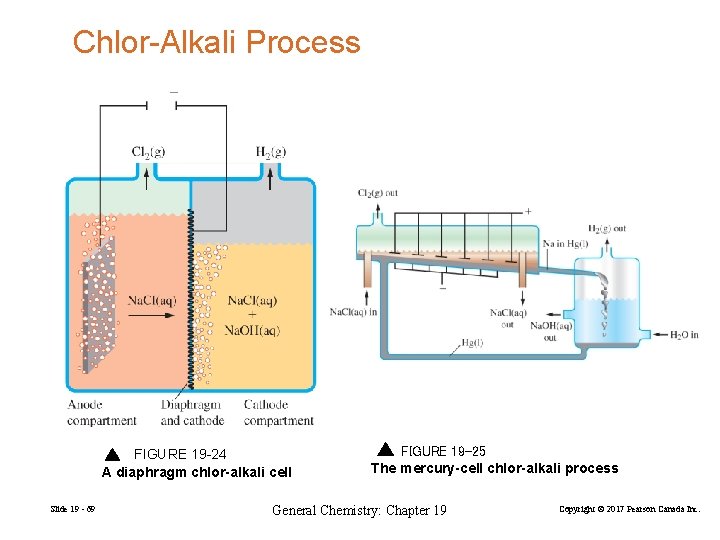
Chlor-Alkali Process FIGURE 19 -24 A diaphragm chlor-alkali cell Slide 19 - 69 FIGURE 19 -25 The mercury-cell chlor-alkali process General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

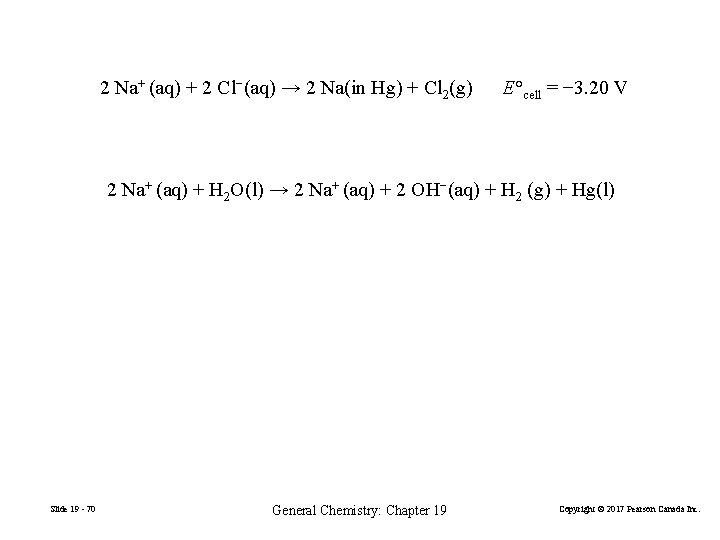
2 Na+ (aq) + 2 Cl−(aq) → 2 Na(in Hg) + Cl 2(g) E°cell = − 3. 20 V 2 Na+ (aq) + H 2 O(l) → 2 Na+ (aq) + 2 OH−(aq) + H 2 (g) + Hg(l) Slide 19 - 70 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.

End of Chapter Slide 19 - 71 General Chemistry: Chapter 19 Copyright © 2017 Pearson Canada Inc.
 Management eleventh edition stephen p robbins
Management eleventh edition stephen p robbins Management eleventh edition
Management eleventh edition Management eleventh edition
Management eleventh edition Management eleventh edition
Management eleventh edition General chemistry 11th edition
General chemistry 11th edition Eleventh 5 year plan
Eleventh 5 year plan Eleventh 5 year plan
Eleventh 5 year plan Eleventh plan
Eleventh plan For his eleventh birthday elvis presley
For his eleventh birthday elvis presley Introduction to genetic analysis tenth edition
Introduction to genetic analysis tenth edition No slip condition
No slip condition Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Modern systems analysis and design 7th edition
Modern systems analysis and design 7th edition Discrete mathematics with applications susanna s. epp
Discrete mathematics with applications susanna s. epp Using mis (10th edition) 10th edition
Using mis (10th edition) 10th edition Using mis (10th edition)
Using mis (10th edition) Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Electrical engineering
Electrical engineering Learning principles and applications
Learning principles and applications Applications of nuclear chemistry
Applications of nuclear chemistry Applications of nuclear chemistry
Applications of nuclear chemistry Modern labor economics 12th edition solution
Modern labor economics 12th edition solution Modern labor economics 12th edition pdf
Modern labor economics 12th edition pdf Modern real estate practice in pennsylvania
Modern real estate practice in pennsylvania Modern database management 12th edition ppt
Modern database management 12th edition ppt Modern database management
Modern database management Modern operating systems 3rd edition
Modern operating systems 3rd edition Tanenbaum structured computer organization
Tanenbaum structured computer organization Modern database management 8th edition
Modern database management 8th edition University physics with modern physics fifteenth edition
University physics with modern physics fifteenth edition Modern database management 11th edition
Modern database management 11th edition Modern labor economics 12th edition
Modern labor economics 12th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Expert systems: principles and programming, fourth edition
Expert systems: principles and programming, fourth edition Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Pericyclic
Pericyclic Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein Introductory chemistry 4th edition
Introductory chemistry 4th edition Prefix multipliers
Prefix multipliers Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro The central science 14th edition
The central science 14th edition Acid chloride + grignard reagent
Acid chloride + grignard reagent Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Ap chemistry notes zumdahl
Ap chemistry notes zumdahl Organic chemistry third edition david klein
Organic chemistry third edition david klein Drop in molecular views answer key
Drop in molecular views answer key Chemistry by raymond chang 10th edition
Chemistry by raymond chang 10th edition Democritus atomic model diagram
Democritus atomic model diagram Halohydrin
Halohydrin Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein Klein
Klein Properties of smart and modern materials
Properties of smart and modern materials Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry What is the chemical formula for tetranitrogen heptoxide?
What is the chemical formula for tetranitrogen heptoxide? Modern chemistry chapter 15
Modern chemistry chapter 15 Chapter 14 review acids and bases section 1
Chapter 14 review acids and bases section 1 Chapter 13 ions in aqueous solutions
Chapter 13 ions in aqueous solutions Chapter 12 review solutions answer key
Chapter 12 review solutions answer key Modern chemistry chapter 4
Modern chemistry chapter 4 Modern chemistry solutions
Modern chemistry solutions Ib organic chemistry
Ib organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Principles of network applications in computer networks
Principles of network applications in computer networks Principles of network applications
Principles of network applications Principles of information security 5th edition pdf
Principles of information security 5th edition pdf Principles of electronic communication systems 3rd edition
Principles of electronic communication systems 3rd edition Principles of economics third edition oxford pdf
Principles of economics third edition oxford pdf Accounting principles second canadian edition
Accounting principles second canadian edition Accounting principles second canadian edition
Accounting principles second canadian edition