GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION

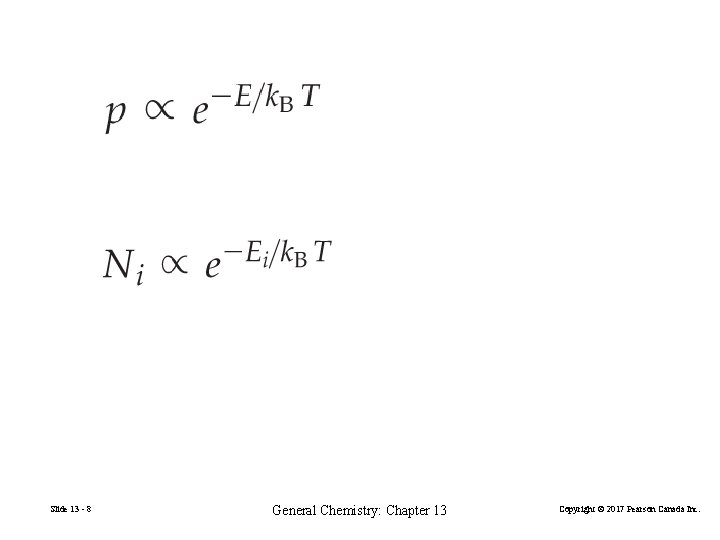


- Slides: 71

GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION PETRUCCI HERRING MADURA BISSONNETTE Spontaneous Change: Entropy and Gibbs Energy 13 PHILIP DUTTON UNIVERSITY OF WINDSOR DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY Slide 13 - 1 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

Spontaneous Change: Entropy and Gibbs Energy Slide 13 - 2 CONTENTS 13 -1 Spontaneity: The Meaning of Spontaneous Change 13 -2 The Concept of Entropy 13 -3 Evaluating Entropy and entropy Changes 13 -4 Criteria for Spontaneous Change: The Second Law of Thermodynamics 13 -5 Standard Gibbs Energy Change, ΔG 13 -6 Gibbs Energy Change and Equilibrium 13 -7 ΔG° and K as Functions of Temperature 13 -8 Coupled Reactions General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

The melting of an ice cube occurs spontaneously at temperatures above 0°C Slide 13 - 3 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

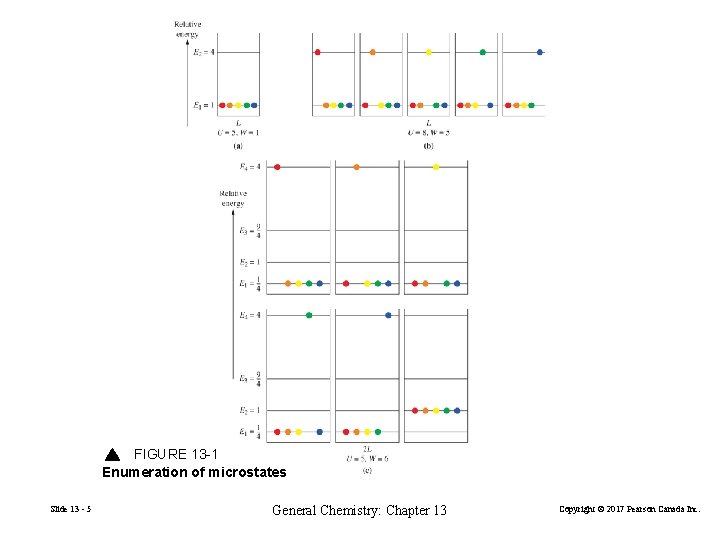
13 -1 Entropy: Boltzmann’s View Microstates Macroscopic systems are made up of many particles. System is easily characterized (n, T, V, P) Microscopic systems are not easily characterized. positions, velocity and energy of individual molecules change from one instant to the next. According to quantum mechanics a microstate is a specific microscopic configuration describing how the particles of a system are distributed among the available energy levels. Slide 13 - 4 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

FIGURE 13 -1 Enumeration of microstates Slide 13 - 5 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

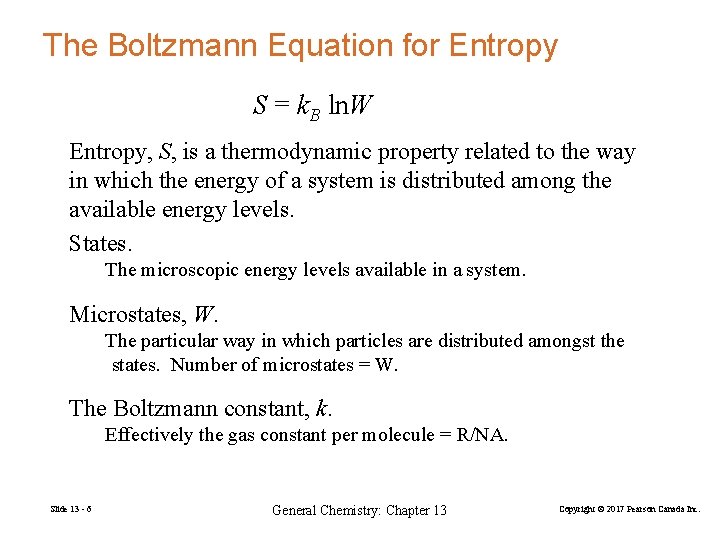
The Boltzmann Equation for Entropy S = k. B ln. W Entropy, S, is a thermodynamic property related to the way in which the energy of a system is distributed among the available energy levels. States. The microscopic energy levels available in a system. Microstates, W. The particular way in which particles are distributed amongst the states. Number of microstates = W. The Boltzmann constant, k. Effectively the gas constant per molecule = R/NA. Slide 13 - 6 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

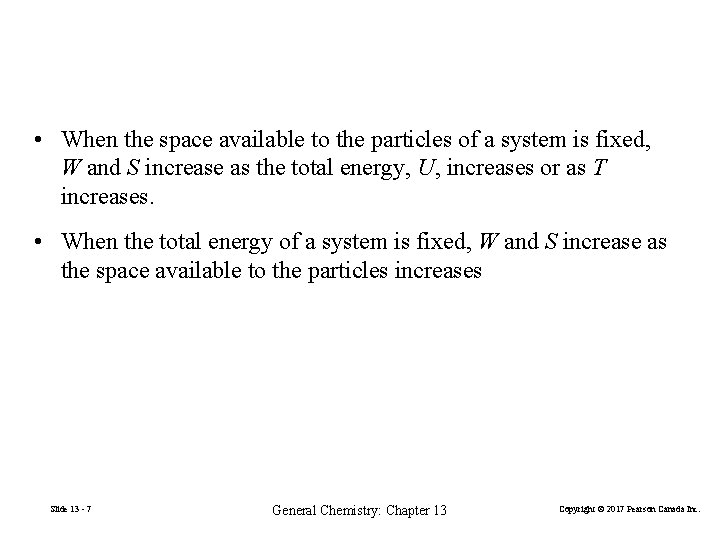
• When the space available to the particles of a system is fixed, W and S increase as the total energy, U, increases or as T increases. • When the total energy of a system is fixed, W and S increase as the space available to the particles increases Slide 13 - 7 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.
Slide 13 - 8 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

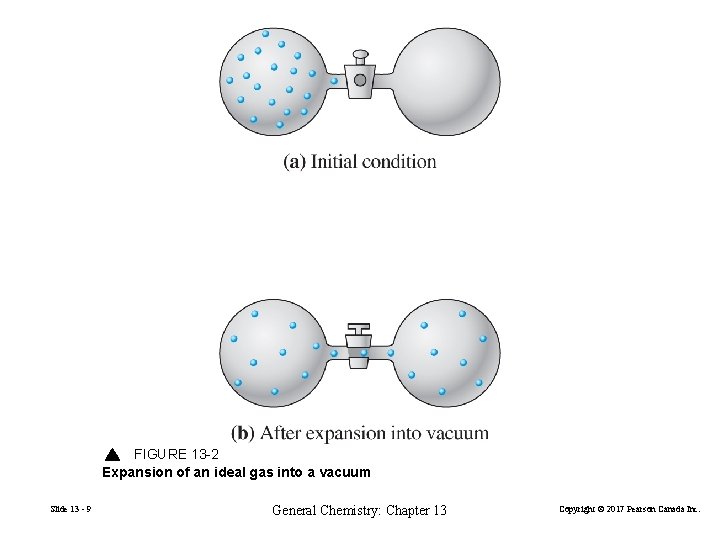
FIGURE 13 -2 Expansion of an ideal gas into a vacuum Slide 13 - 9 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

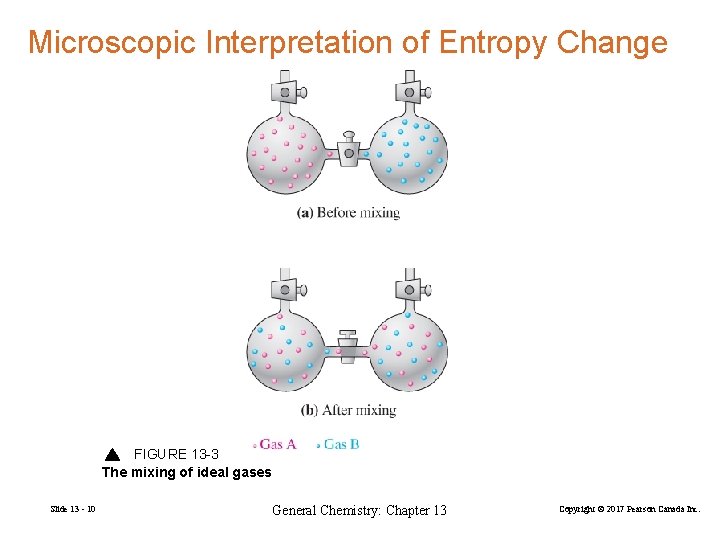
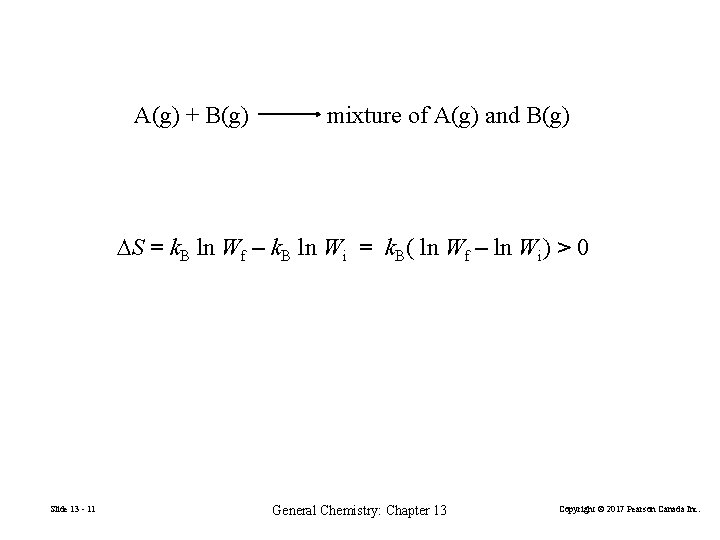
Microscopic Interpretation of Entropy Change FIGURE 13 -3 The mixing of ideal gases Slide 13 - 10 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

A(g) + B(g) mixture of A(g) and B(g) ∆S = k. B ln Wf – k. B ln Wi = k. B( ln Wf – ln Wi) > 0 Slide 13 - 11 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

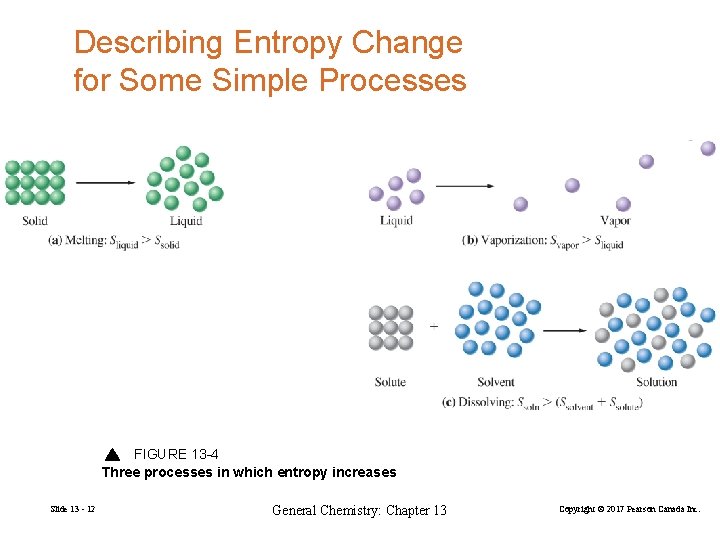
Describing Entropy Change for Some Simple Processes FIGURE 13 -4 Three processes in which entropy increases Slide 13 - 12 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

Four situations generally produce an increase in entropy: • Pure liquids or liquid solutions are formed from solids. • Gases are formed from either solids or liquids. • The number of molecules of gas increases as a result of a chemical reaction. • The temperature of a substance increases. Slide 13 - 13 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

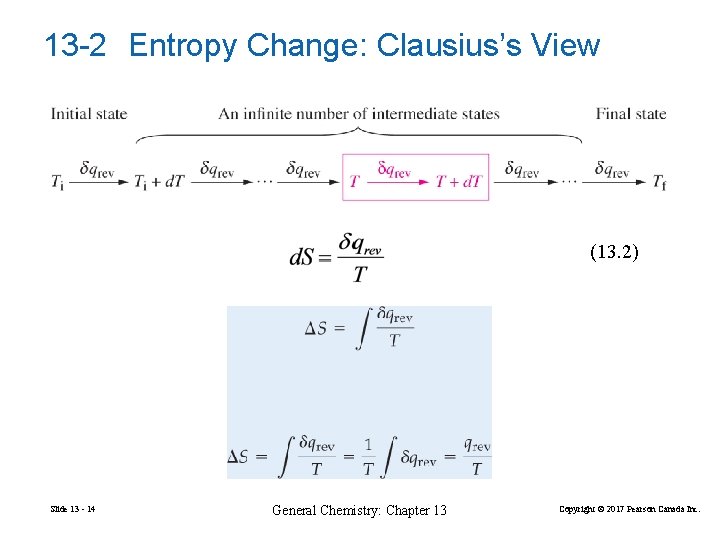
13 -2 Entropy Change: Clausius’s View (13. 2) Slide 13 - 14 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

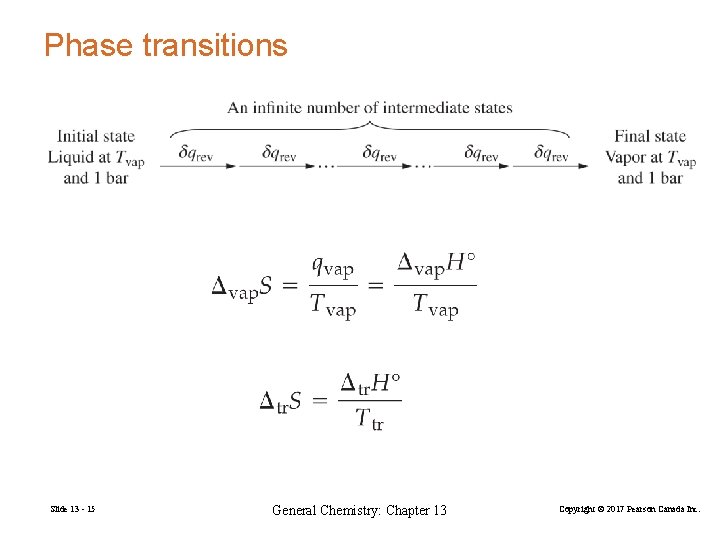
Phase transitions Slide 13 - 15 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

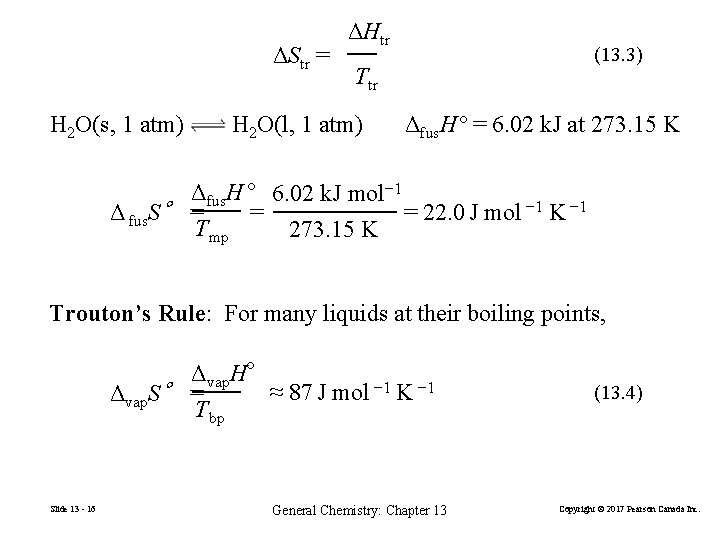
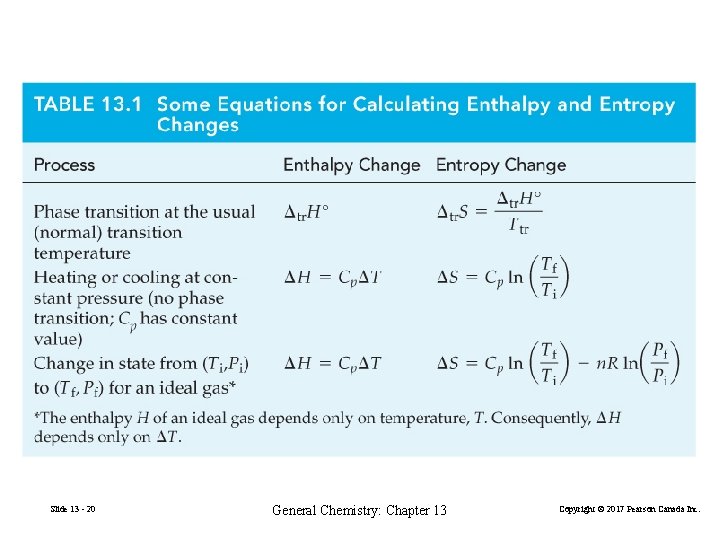
ΔStr = H 2 O(s, 1 atm) ΔHtr (13. 3) Ttr H 2 O(l, 1 atm) Δfus. H° = 6. 02 k. J at 273. 15 K Δfus. H ° 6. 02 k. J mol− 1 = 22. 0 J mol − 1 K − 1 Δ fus. S° = = Tmp 273. 15 K Trouton’s Rule: For many liquids at their boiling points, Δvap. H° ≈ 87 J mol − 1 K − 1 Δvap. S° = Tbp Slide 13 - 16 General Chemistry: Chapter 13 (13. 4) Copyright © 2017 Pearson Canada Inc.

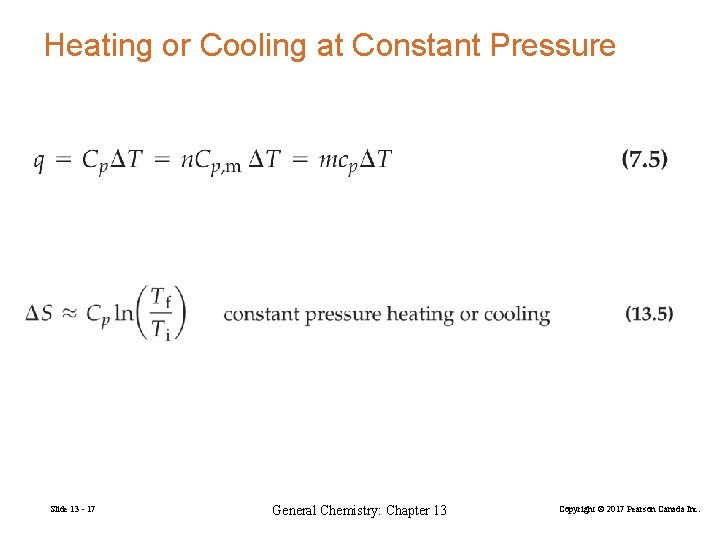
Heating or Cooling at Constant Pressure Slide 13 - 17 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

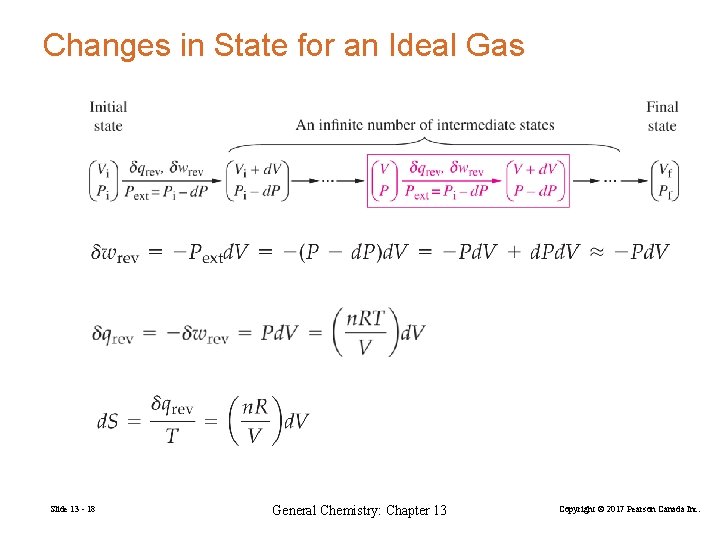
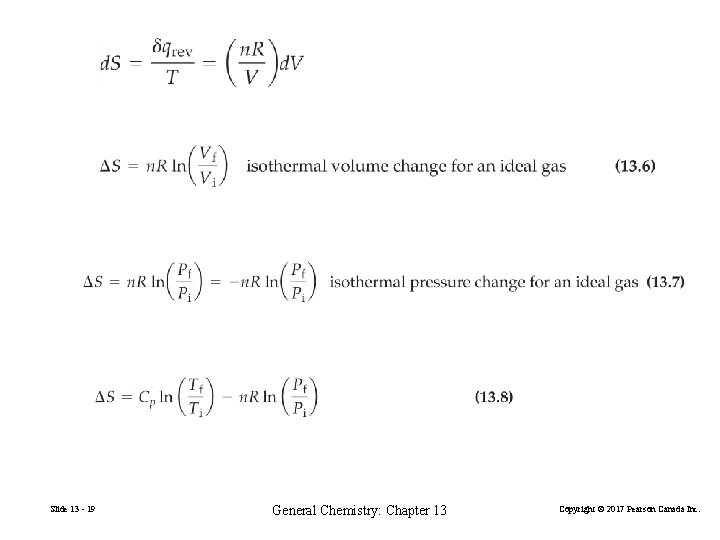
Changes in State for an Ideal Gas Slide 13 - 18 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

Slide 13 - 19 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

Slide 13 - 20 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

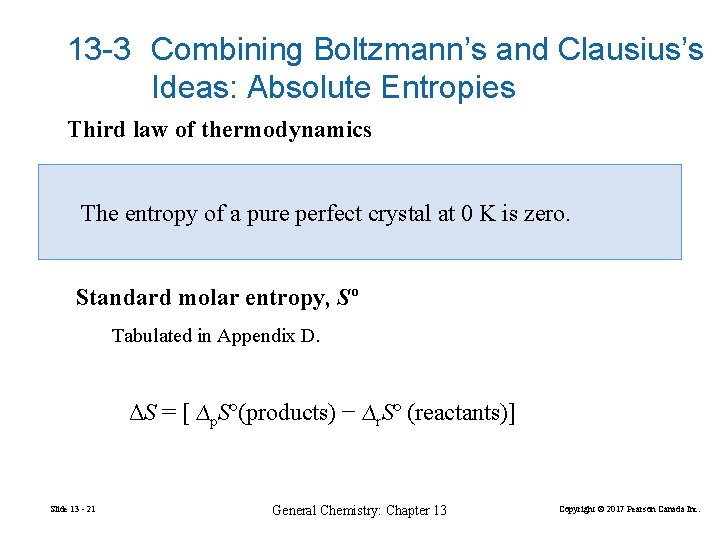
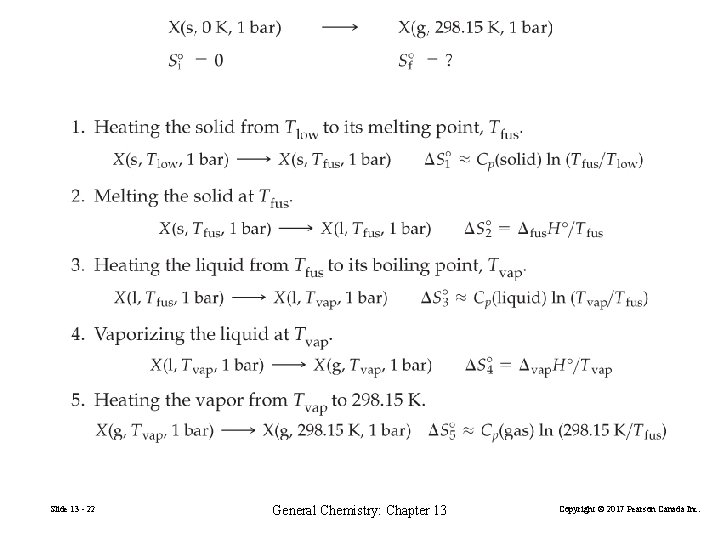
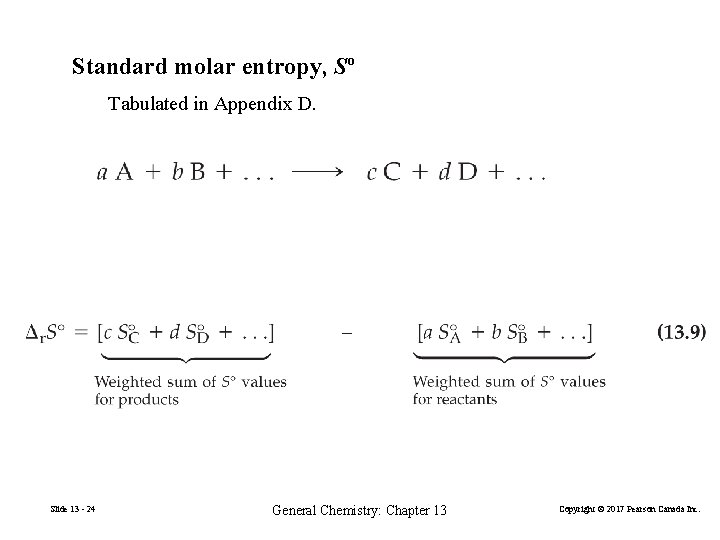
13 -3 Combining Boltzmann’s and Clausius’s Ideas: Absolute Entropies Third law of thermodynamics The entropy of a pure perfect crystal at 0 K is zero. Standard molar entropy, Sº Tabulated in Appendix D. ΔS = [ ∆p. Sº(products) − ∆r. Sº (reactants)] Slide 13 - 21 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

Slide 13 - 22 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

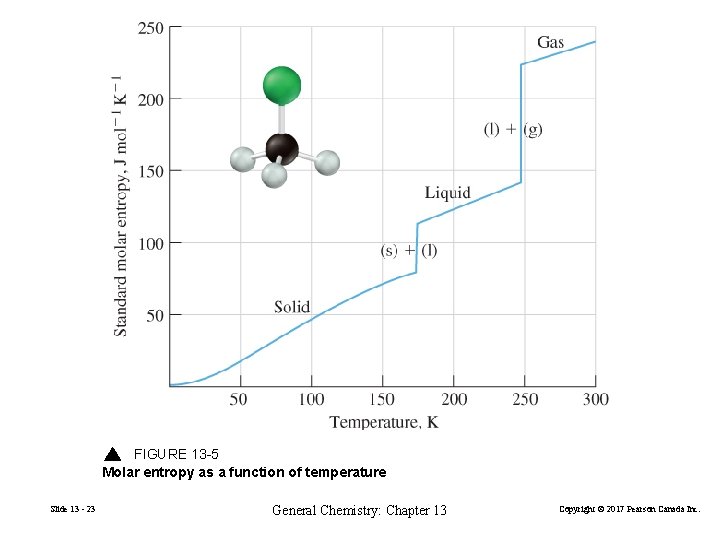
FIGURE 13 -5 Molar entropy as a function of temperature Slide 13 - 23 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

Standard molar entropy, Sº Tabulated in Appendix D. Slide 13 - 24 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

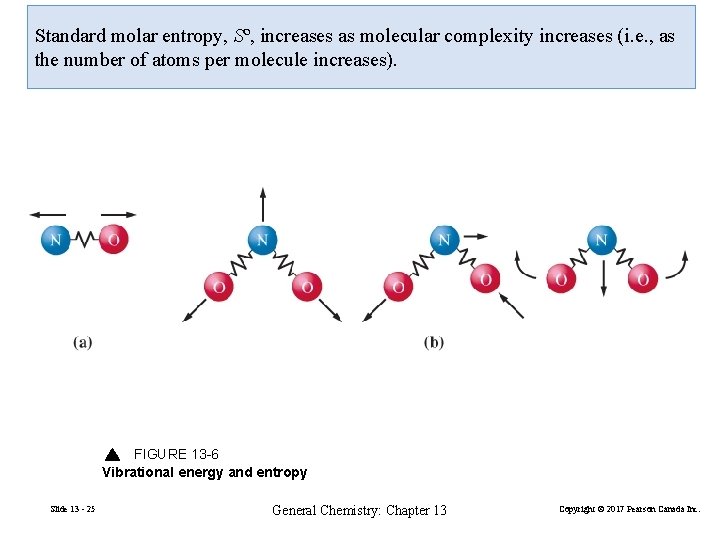
Standard molar entropy, Sº, increases as molecular complexity increases (i. e. , as the number of atoms per molecule increases). FIGURE 13 -6 Vibrational energy and entropy Slide 13 - 25 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

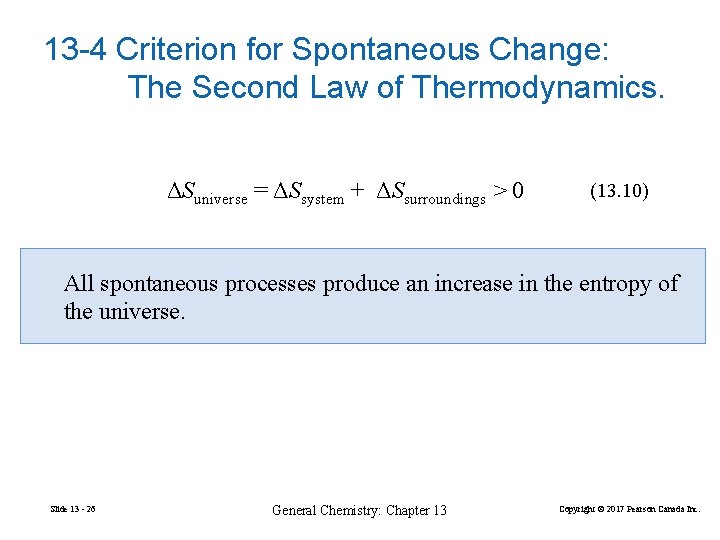
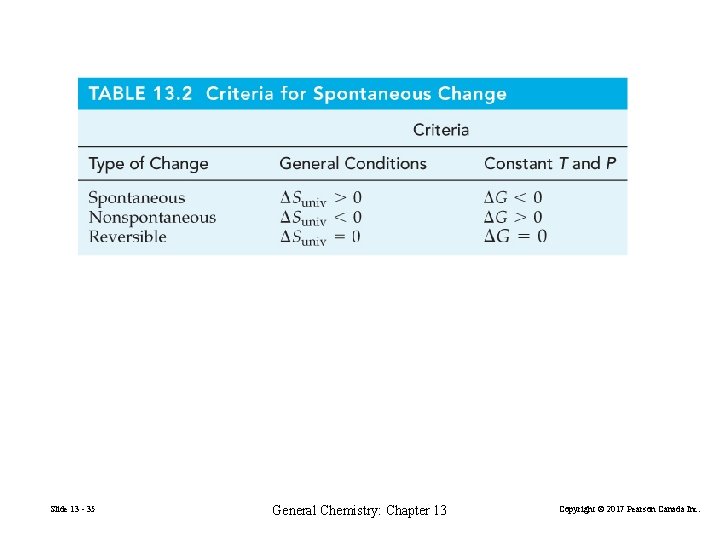
13 -4 Criterion for Spontaneous Change: The Second Law of Thermodynamics. ΔSuniverse = ΔSsystem + ΔSsurroundings > 0 (13. 10) All spontaneous processes produce an increase in the entropy of the universe. Slide 13 - 26 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

Slide 13 - 27 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

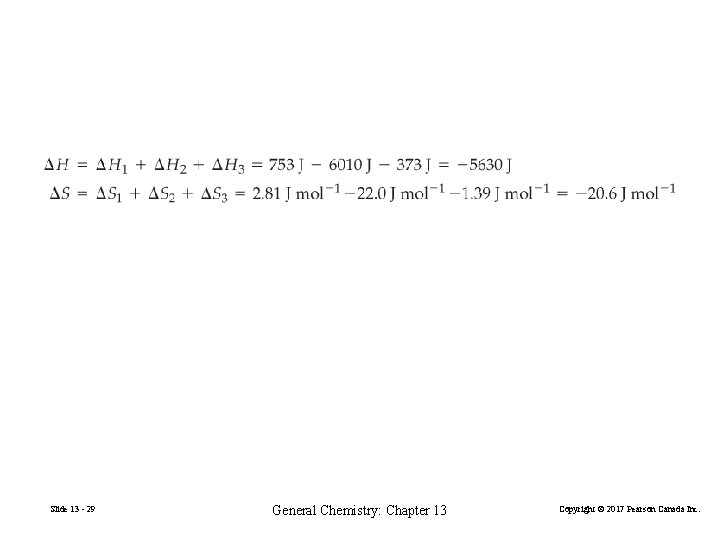
Slide 13 - 28 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

Slide 13 - 29 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

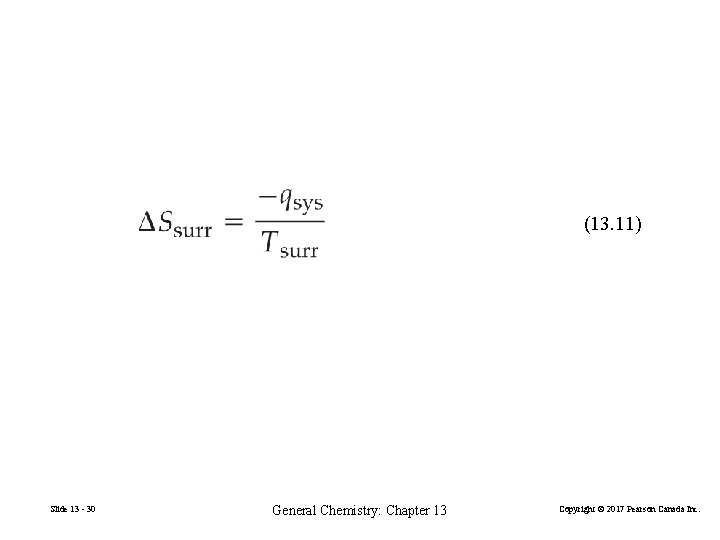
(13. 11) Slide 13 - 30 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

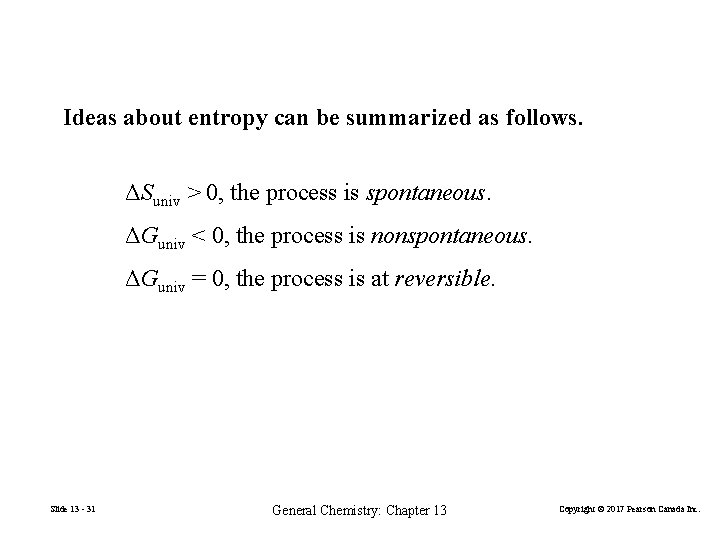
Ideas about entropy can be summarized as follows. ΔSuniv > 0, the process is spontaneous. ΔGuniv < 0, the process is nonspontaneous. ΔGuniv = 0, the process is at reversible. Slide 13 - 31 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

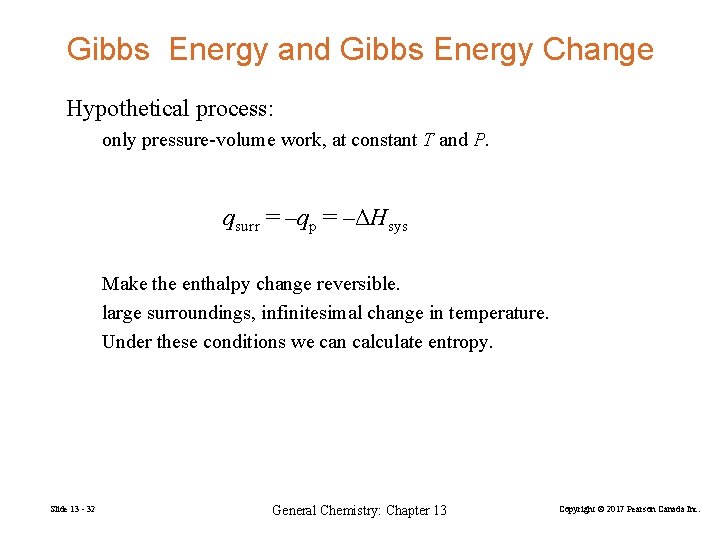
Gibbs Energy and Gibbs Energy Change Hypothetical process: only pressure-volume work, at constant T and P. qsurr = –qp = –ΔHsys Make the enthalpy change reversible. large surroundings, infinitesimal change in temperature. Under these conditions we can calculate entropy. Slide 13 - 32 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

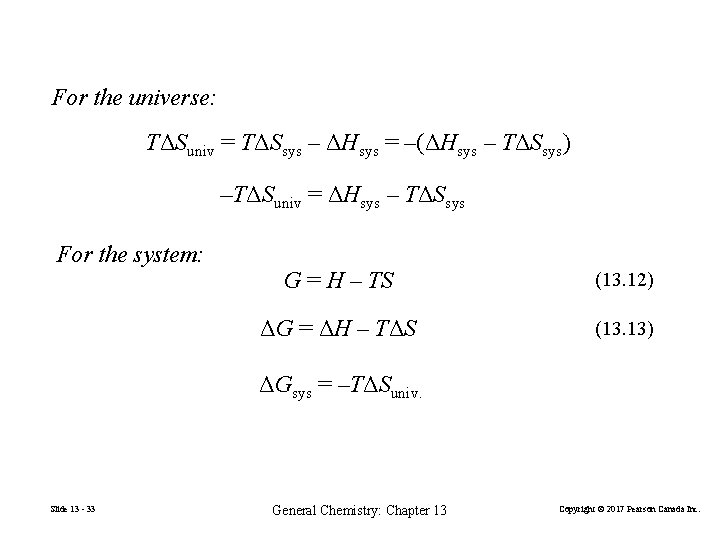
For the universe: TΔSuniv = TΔSsys – ΔHsys = –(ΔHsys – TΔSsys) –TΔSuniv = ΔHsys – TΔSsys For the system: G = H – TS (13. 12) ΔG = ΔH – TΔS (13. 13) ΔGsys = –TΔSuniv. Slide 13 - 33 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

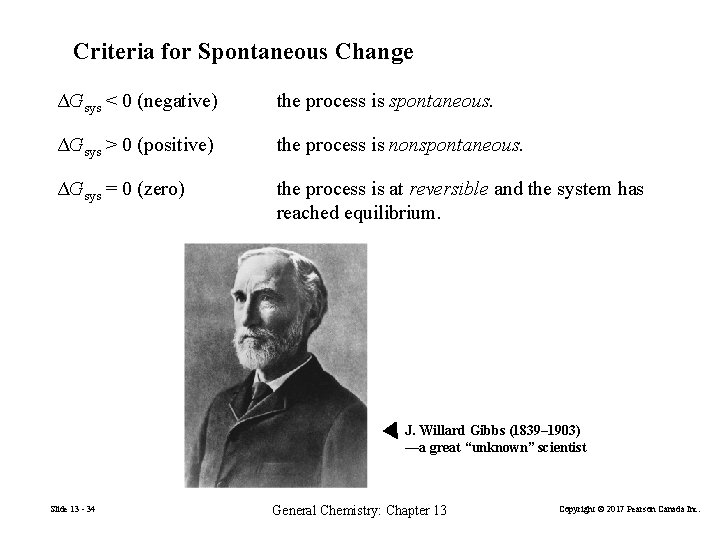
Criteria for Spontaneous Change ΔGsys < 0 (negative) the process is spontaneous. ΔGsys > 0 (positive) the process is nonspontaneous. ΔGsys = 0 (zero) the process is at reversible and the system has reached equilibrium. J. Willard Gibbs (1839– 1903) —a great “unknown” scientist Slide 13 - 34 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

Slide 13 - 35 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

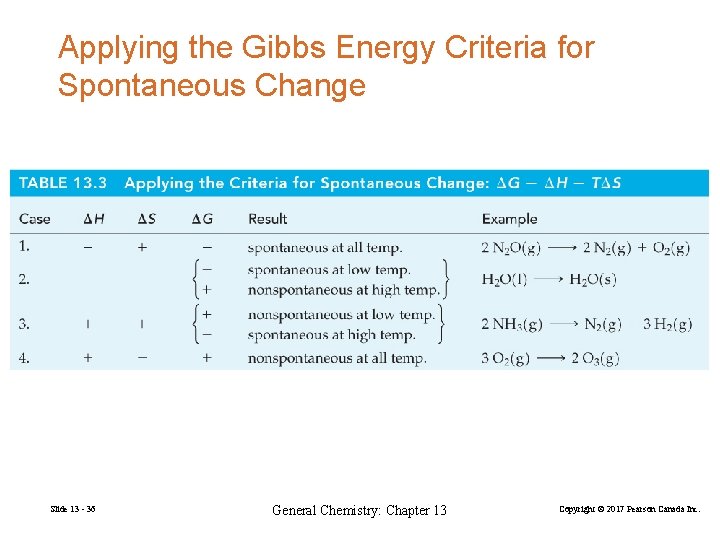
Applying the Gibbs Energy Criteria for Spontaneous Change Slide 13 - 36 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

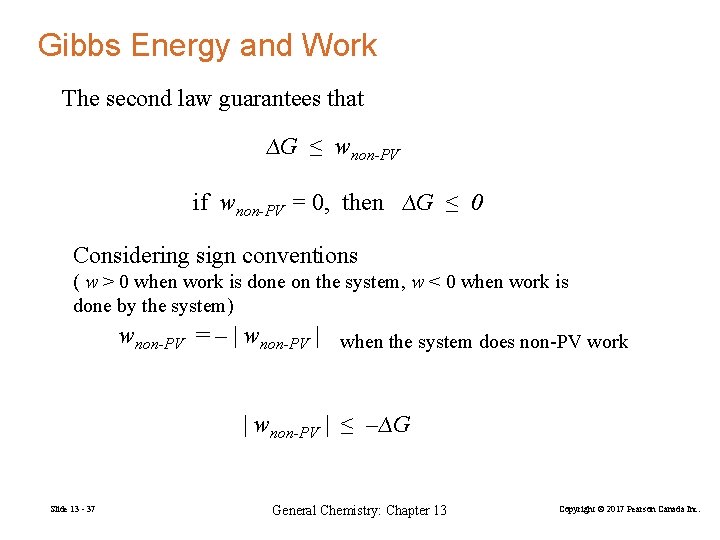
Gibbs Energy and Work The second law guarantees that ∆G ≤ wnon-PV if wnon-PV = 0, then ∆G ≤ 0 Considering sign conventions ( w > 0 when work is done on the system, w < 0 when work is done by the system) wnon-PV = – | wnon-PV | when the system does non-PV work | wnon-PV | ≤ –∆G Slide 13 - 37 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

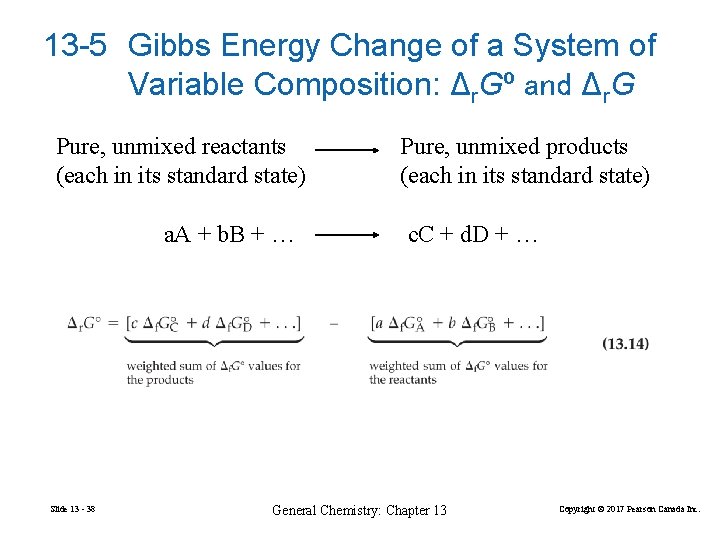
13 -5 Gibbs Energy Change of a System of Variable Composition: Δr. Gº and Δr. G Pure, unmixed reactants (each in its standard state) a. A + b. B + … Slide 13 - 38 Pure, unmixed products (each in its standard state) c. C + d. D + … General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

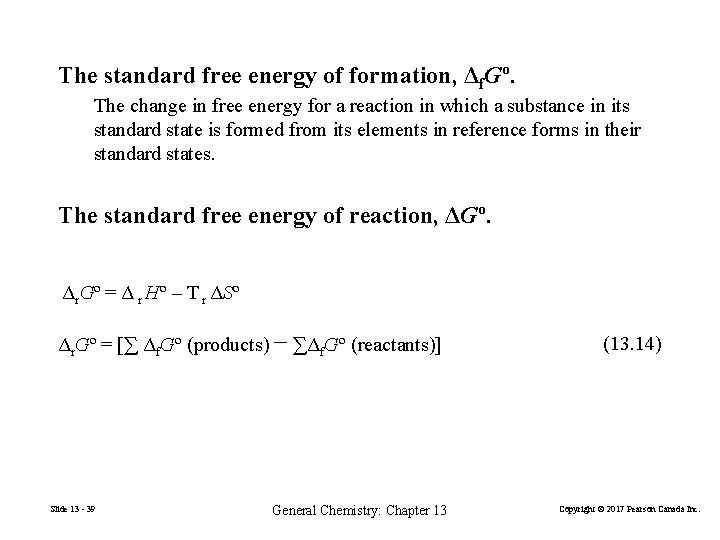
The standard free energy of formation, Δf. Gº. The change in free energy for a reaction in which a substance in its standard state is formed from its elements in reference forms in their standard states. The standard free energy of reaction, ΔGº. Δr. Gº = Δ r Hº – T r ΔSº Δr. Gº = [∑ Δf. Gº (products) − ∑Δf. Gº (reactants)] Slide 13 - 39 General Chemistry: Chapter 13 (13. 14) Copyright © 2017 Pearson Canada Inc.

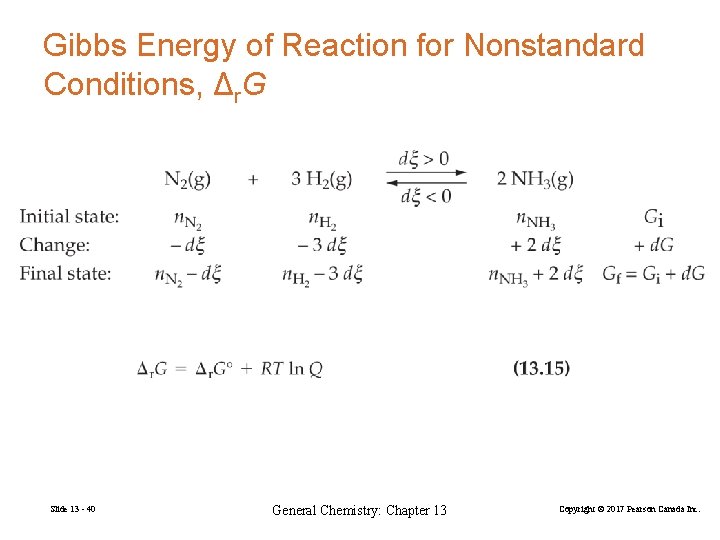
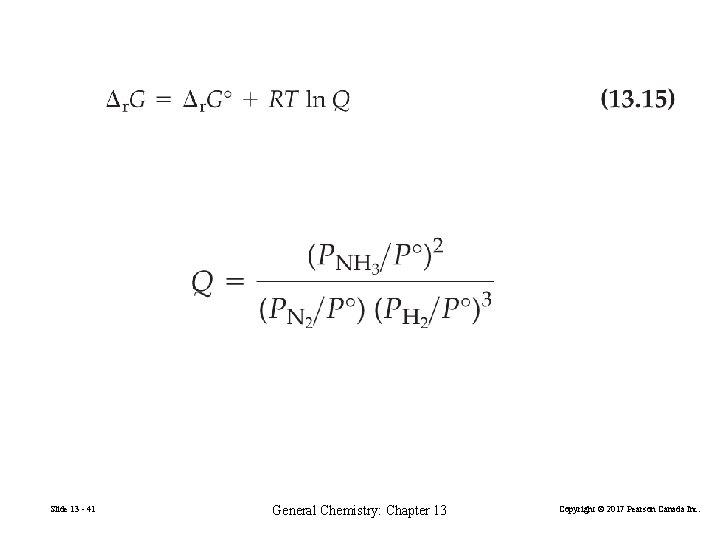
Gibbs Energy of Reaction for Nonstandard Conditions, Δr. G Slide 13 - 40 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

Slide 13 - 41 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

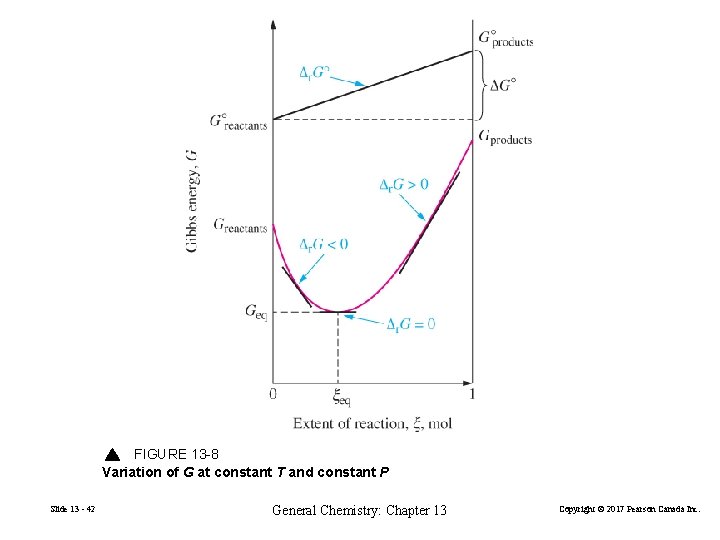
FIGURE 13 -8 Variation of G at constant T and constant P Slide 13 - 42 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

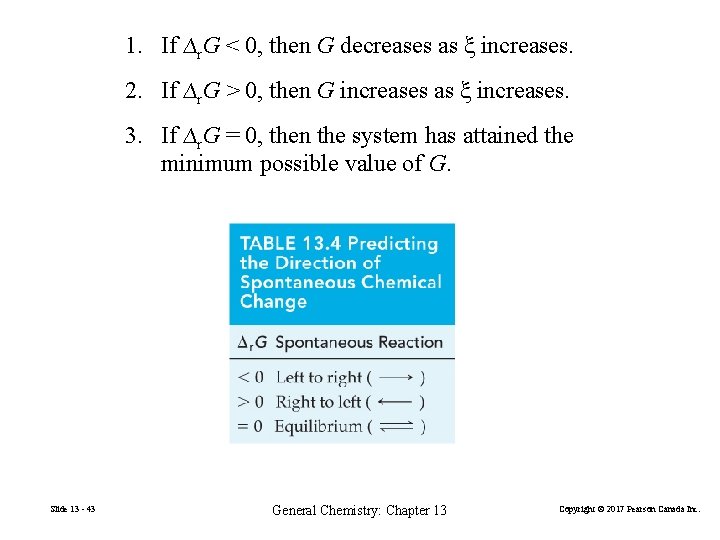
1. If ∆r. G < 0, then G decreases as ξ increases. 2. If ∆r. G > 0, then G increases as ξ increases. 3. If ∆r. G = 0, then the system has attained the minimum possible value of G. Slide 13 - 43 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

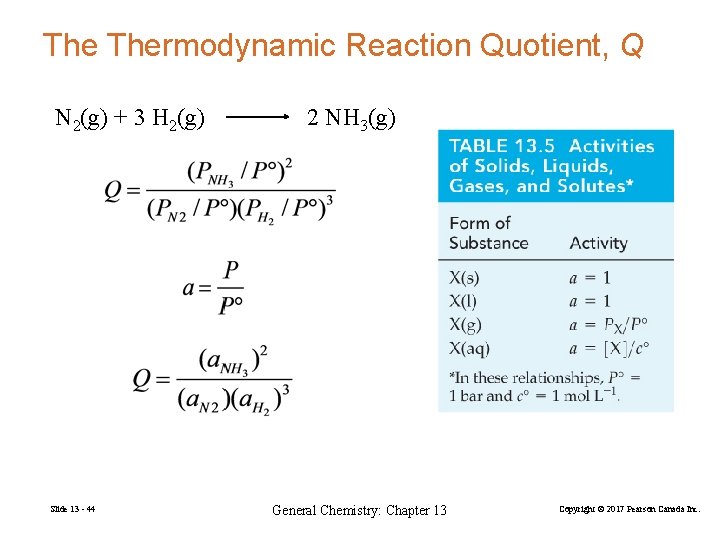
The Thermodynamic Reaction Quotient, Q N 2(g) + 3 H 2(g) Slide 13 - 44 2 NH 3(g) General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

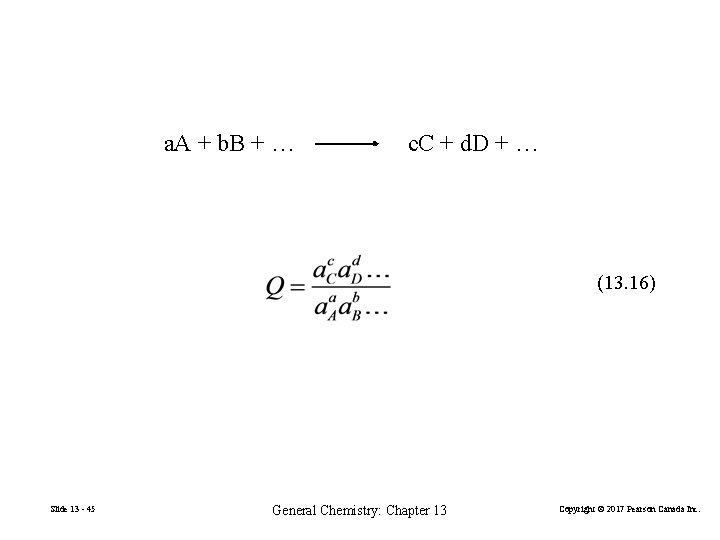
a. A + b. B + … c. C + d. D + … (13. 16) Slide 13 - 45 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

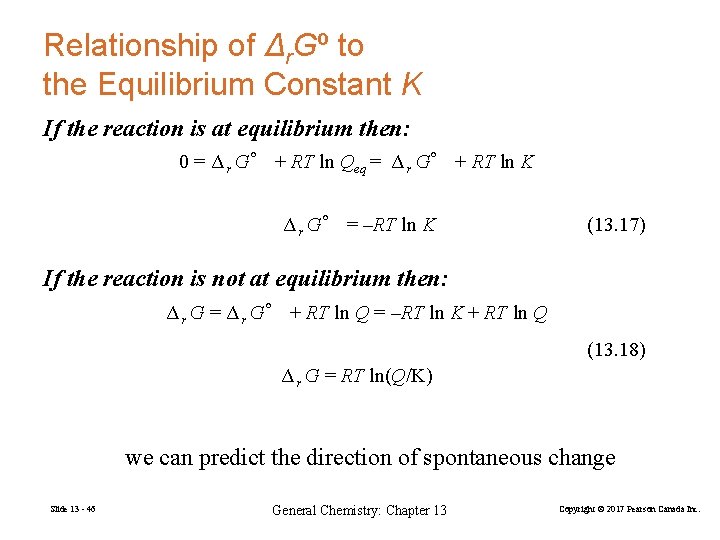
Relationship of Δr. Gº to the Equilibrium Constant K If the reaction is at equilibrium then: 0 = Δ r G° + RT ln Qeq = Δ r G° + RT ln K Δ r G° = –RT ln K (13. 17) If the reaction is not at equilibrium then: Δ r G = Δ r G° + RT ln Q = –RT ln K + RT ln Q (13. 18) Δ r G = RT ln(Q/K) we can predict the direction of spontaneous change Slide 13 - 46 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

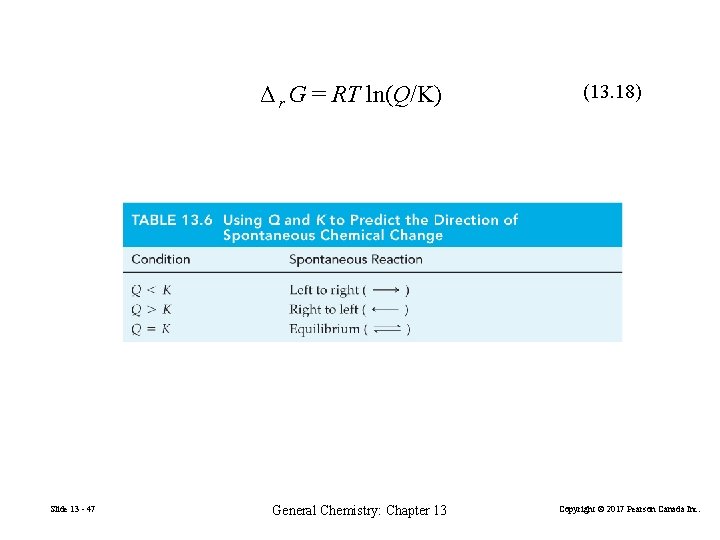
Δ r G = RT ln(Q/K) Slide 13 - 47 General Chemistry: Chapter 13 (13. 18) Copyright © 2017 Pearson Canada Inc.

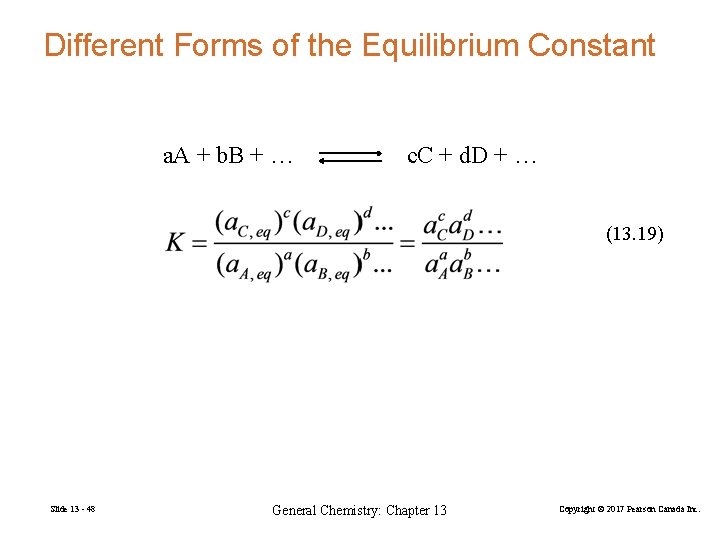
Different Forms of the Equilibrium Constant a. A + b. B + … c. C + d. D + … (13. 19) Slide 13 - 48 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

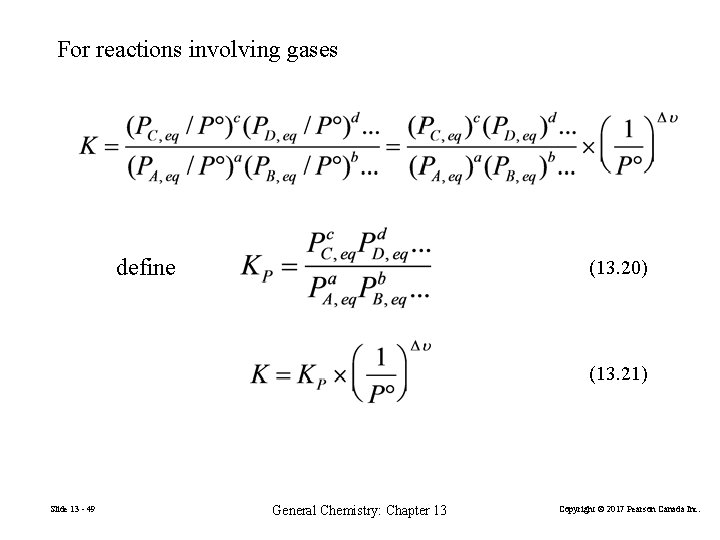
For reactions involving gases define (13. 20) (13. 21) Slide 13 - 49 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

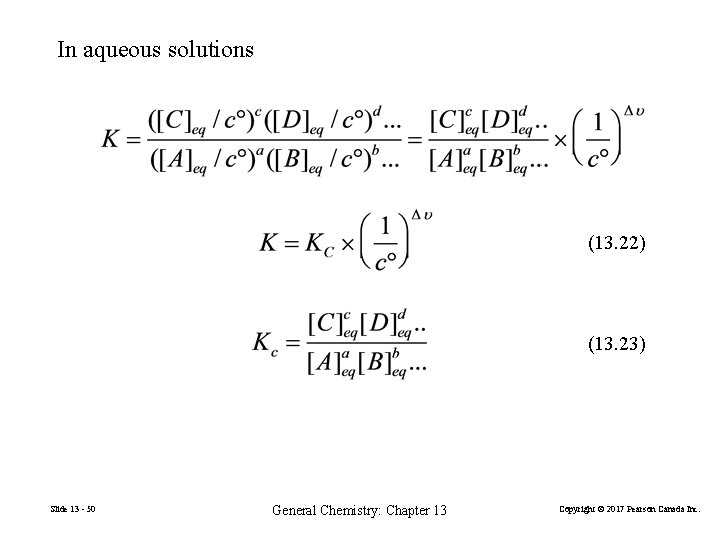
In aqueous solutions (13. 22) (13. 23) Slide 13 - 50 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

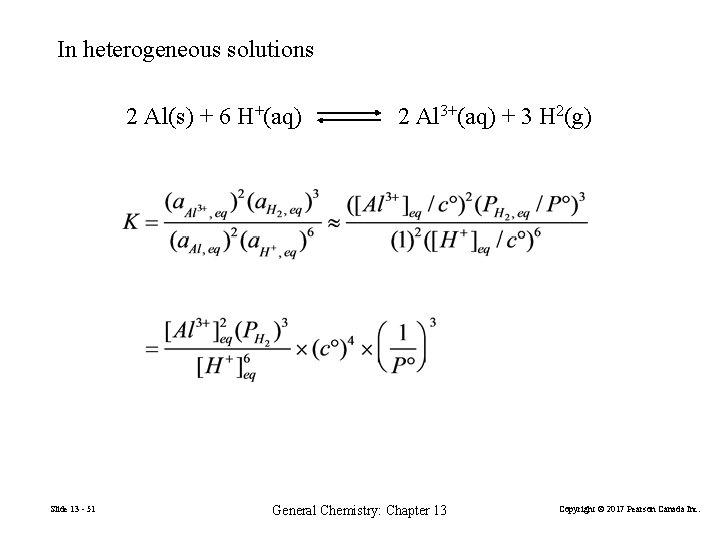
In heterogeneous solutions 2 Al(s) + 6 H+(aq) Slide 13 - 51 2 Al 3+(aq) + 3 H 2(g) General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

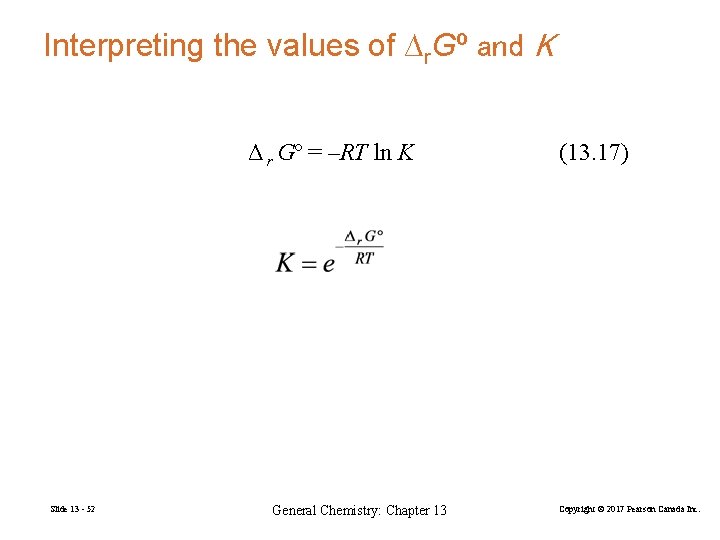
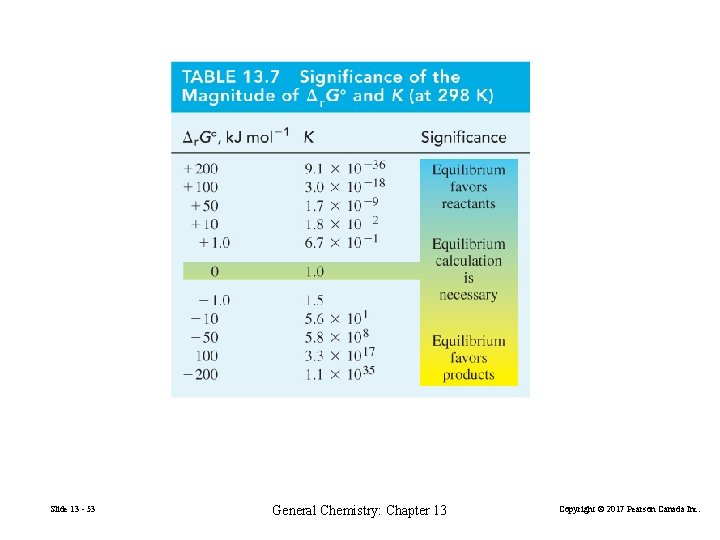
Interpreting the values of ∆r. Gº and K Δ r Gº = –RT ln K Slide 13 - 52 General Chemistry: Chapter 13 (13. 17) Copyright © 2017 Pearson Canada Inc.

Slide 13 - 53 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

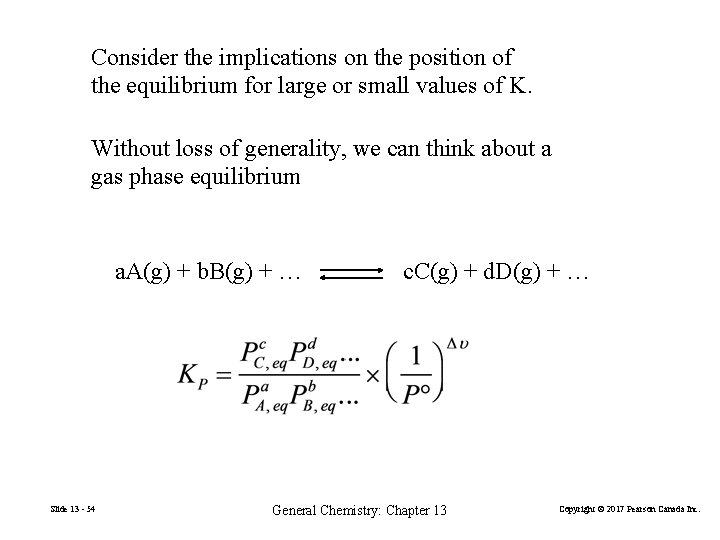
Consider the implications on the position of the equilibrium for large or small values of K. Without loss of generality, we can think about a gas phase equilibrium a. A(g) + b. B(g) + … Slide 13 - 54 c. C(g) + d. D(g) + … General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

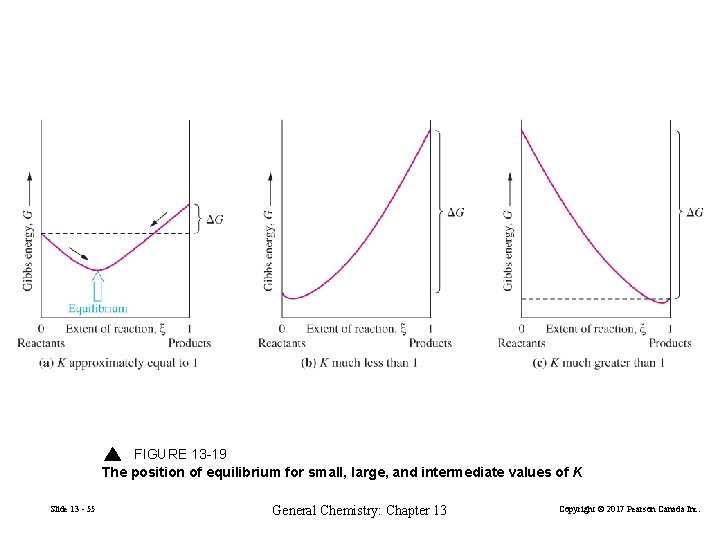
FIGURE 13 -19 The position of equilibrium for small, large, and intermediate values of K Slide 13 - 55 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

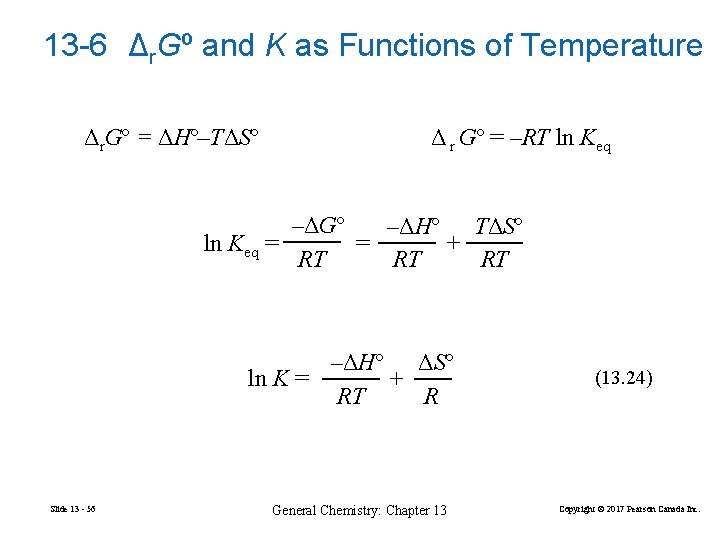
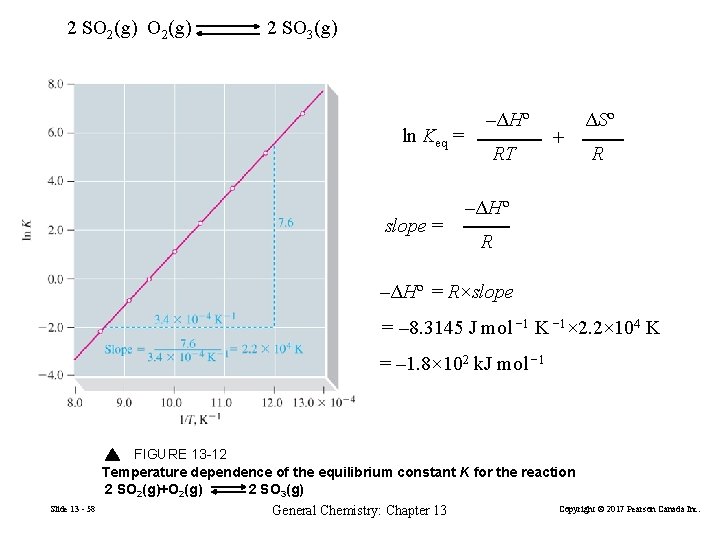
13 -6 Δr. Gº and K as Functions of Temperature Δr. Gº = ΔHº–TΔSº Δ r Gº = –RT ln Keq –ΔGº –ΔHº TΔSº ln Keq = = + RT RT RT –ΔHº ΔSº ln K = + RT R Slide 13 - 56 General Chemistry: Chapter 13 (13. 24) Copyright © 2017 Pearson Canada Inc.

Slide 13 - 57 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

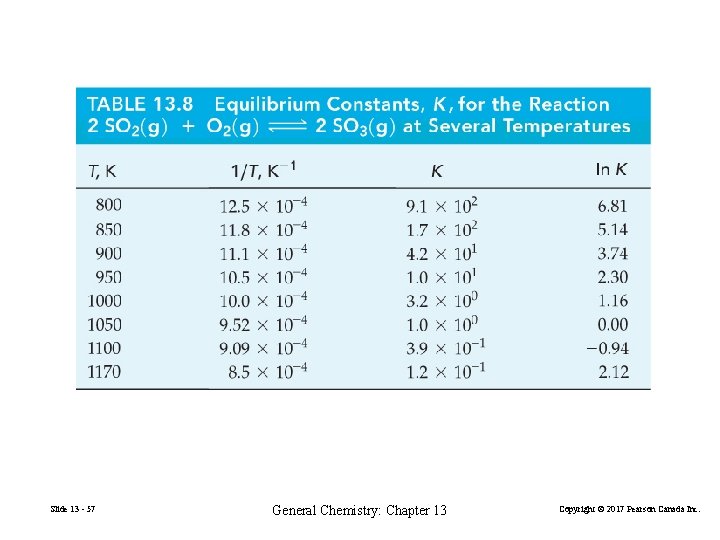
2 SO 2(g) 2 SO 3(g) ln Keq = slope = –ΔHº RT + ΔSº R –ΔHº = R×slope = – 8. 3145 J mol − 1 K − 1× 2. 2× 104 K = – 1. 8× 102 k. J mol − 1 FIGURE 13 -12 Temperature dependence of the equilibrium constant K for the reaction 2 SO 2(g)+O 2(g) 2 SO 3(g) Slide 13 - 58 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

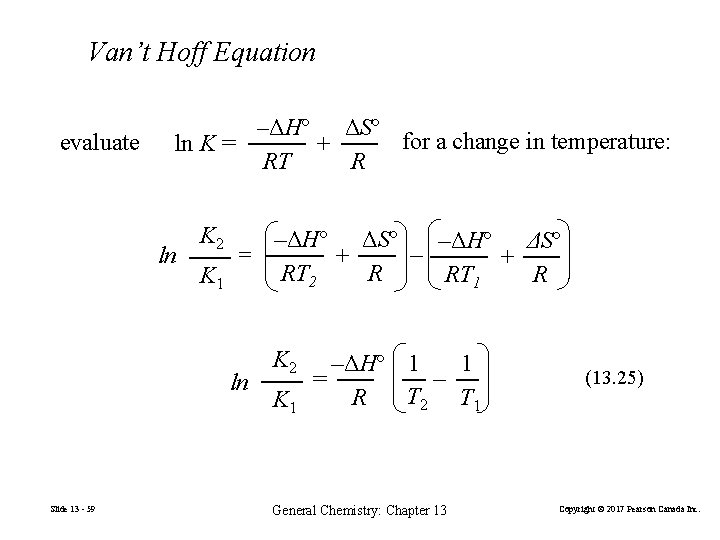
Van’t Hoff Equation evaluate –ΔHº ΔSº for a change in temperature: ln K = + RT R K 2 –ΔHº ΔSº – ln = + + RT 2 R RT 1 R K 1 ln Slide 13 - 59 K 2 K 1 1 –ΔHº 1 – = T 2 T 1 R General Chemistry: Chapter 13 (13. 25) Copyright © 2017 Pearson Canada Inc.

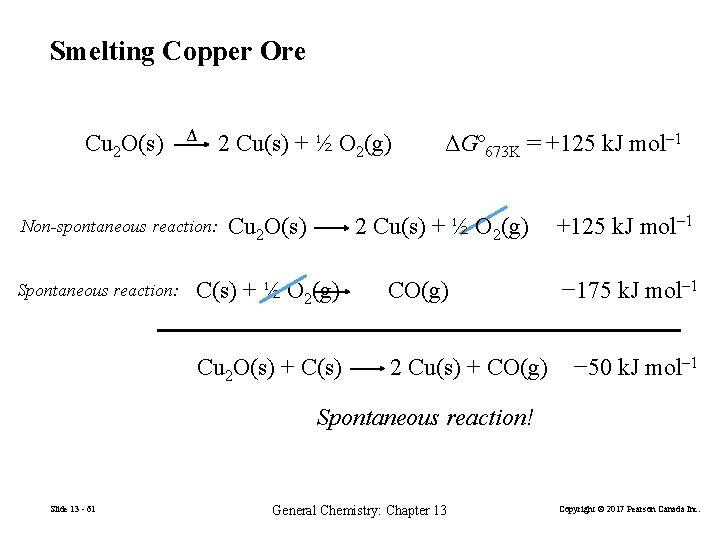
13 -7 Coupled Reactions In order to drive a non-spontaneous reactions we changed the conditions (i. e. temperature or electrolysis). Another method is to couple two reactions. One with a positive ΔG and one with a negative ΔG. Overall spontaneous process. Slide 13 - 60 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

Smelting Copper Ore Cu 2 O(s) Δ Non-spontaneous reaction: Spontaneous reaction: 2 Cu(s) + ½ O 2(g) Cu 2 O(s) ΔGº 673 K = +125 k. J mol– 1 2 Cu(s) + ½ O 2(g) CO(g) Cu 2 O(s) + C(s) 2 Cu(s) + CO(g) +125 k. J mol– 1 − 175 k. J mol– 1 − 50 k. J mol– 1 Spontaneous reaction! Slide 13 - 61 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

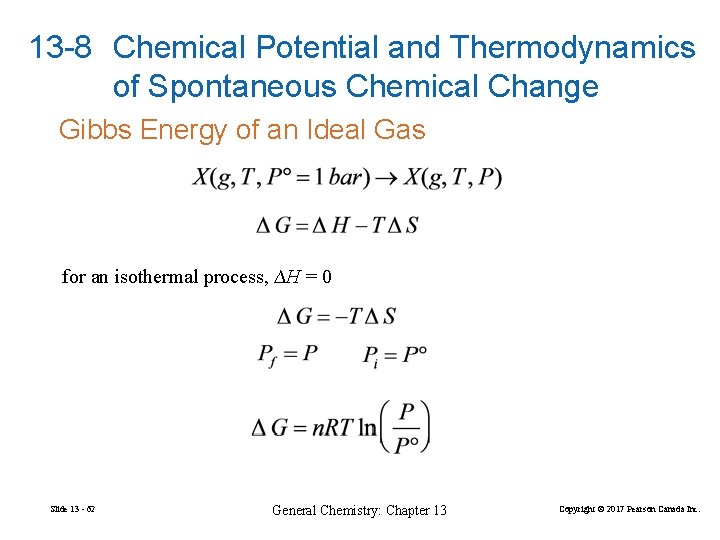
13 -8 Chemical Potential and Thermodynamics of Spontaneous Chemical Change Gibbs Energy of an Ideal Gas for an isothermal process, ∆H = 0 Slide 13 - 62 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

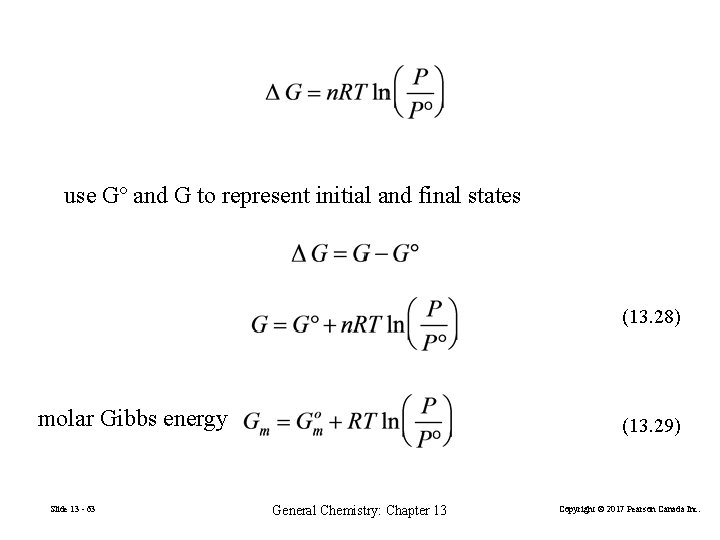
use Gº and G to represent initial and final states (13. 28) molar Gibbs energy Slide 13 - 63 (13. 29) General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

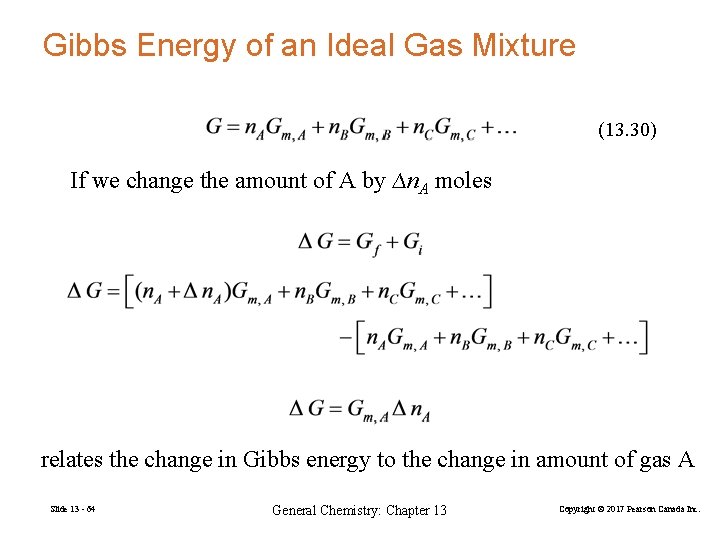
Gibbs Energy of an Ideal Gas Mixture (13. 30) If we change the amount of A by ∆n. A moles relates the change in Gibbs energy to the change in amount of gas A Slide 13 - 64 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

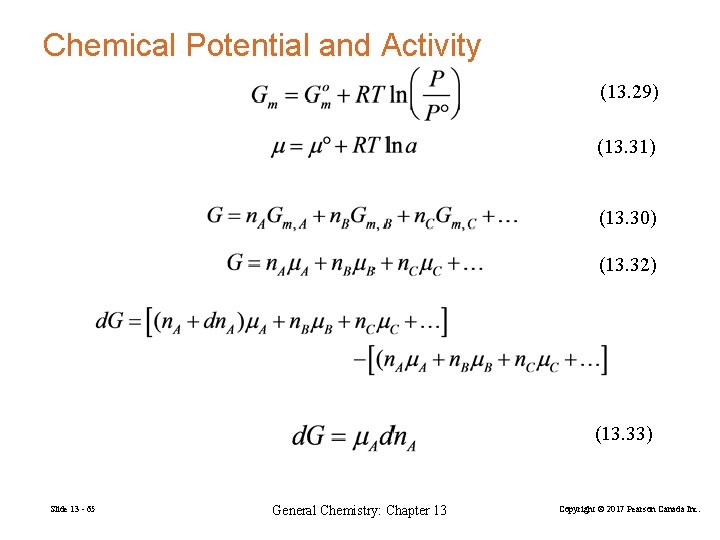
Chemical Potential and Activity (13. 29) (13. 31) (13. 30) (13. 32) (13. 33) Slide 13 - 65 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

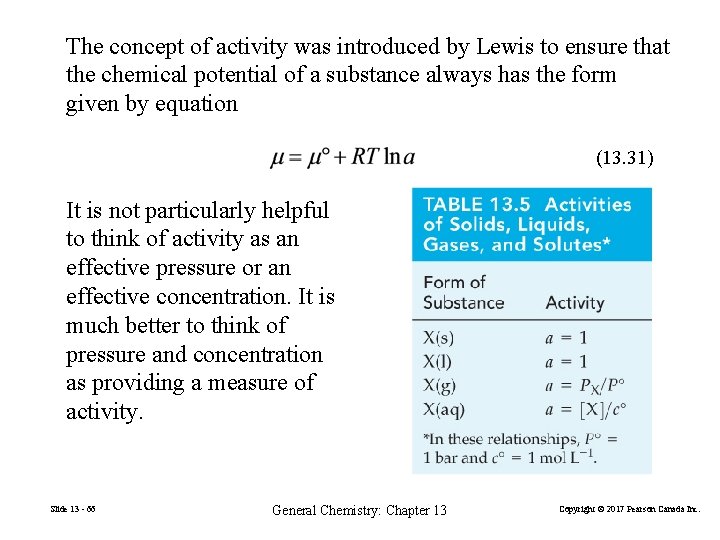
The concept of activity was introduced by Lewis to ensure that the chemical potential of a substance always has the form given by equation (13. 31) It is not particularly helpful to think of activity as an effective pressure or an effective concentration. It is much better to think of pressure and concentration as providing a measure of activity. Slide 13 - 66 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

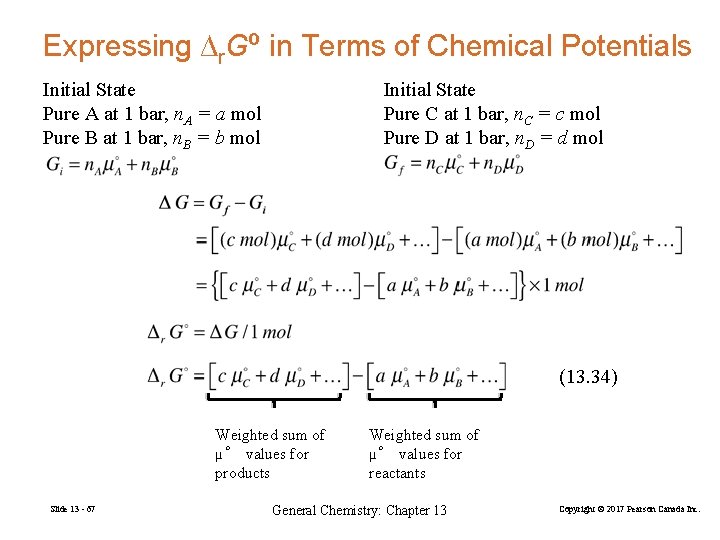
Expressing ∆r. Gº in Terms of Chemical Potentials Initial State Pure A at 1 bar, n. A = a mol Pure B at 1 bar, n. B = b mol Initial State Pure C at 1 bar, n. C = c mol Pure D at 1 bar, n. D = d mol (13. 34) Weighted sum of µ° values for products Slide 13 - 67 Weighted sum of µ° values for reactants General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

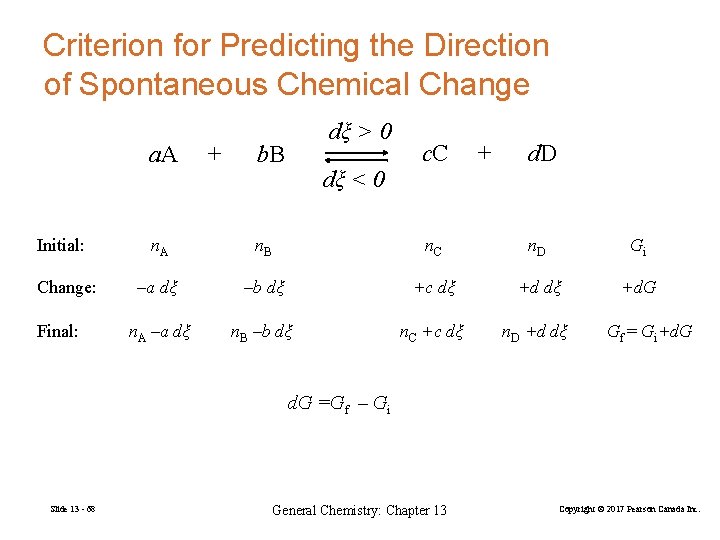
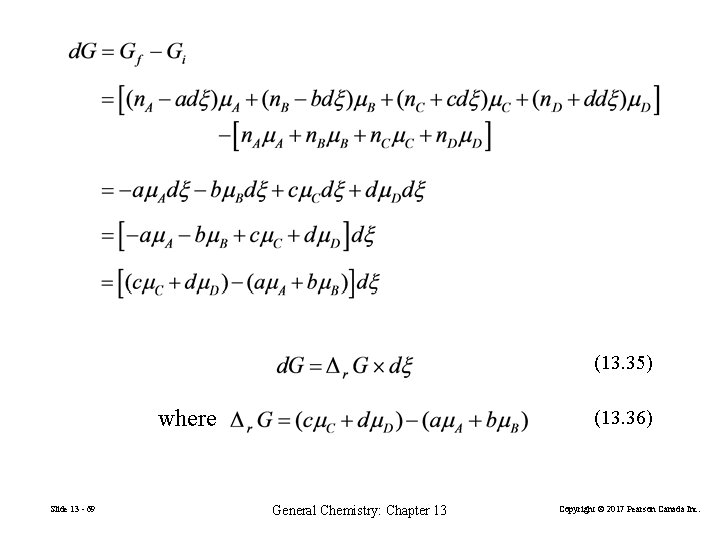
Criterion for Predicting the Direction of Spontaneous Chemical Change a. A Initial: Change: Final: + dξ > 0 b. B dξ < 0 c. C + d. D n. A n. B n. C n. D Gi –a dξ –b dξ +c dξ +d. G n. A –a dξ n. B –b dξ n. C +c dξ n. D +d dξ Gf = Gi+d. G =Gf – Gi Slide 13 - 68 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

(13. 35) where Slide 13 - 69 (13. 36) General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

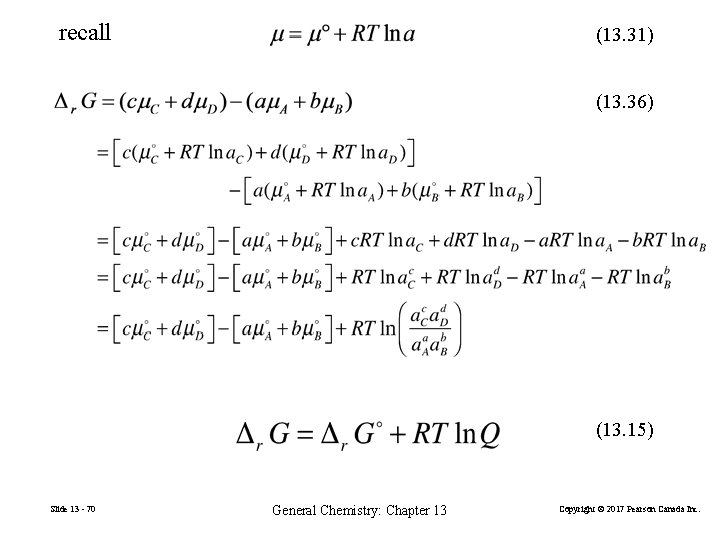
recall (13. 31) (13. 36) (13. 15) Slide 13 - 70 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.

End of Chapter Slide 13 - 71 General Chemistry: Chapter 13 Copyright © 2017 Pearson Canada Inc.
 Management eleventh edition
Management eleventh edition Eleventh edition management
Eleventh edition management Management eleventh edition
Management eleventh edition Management eleventh edition
Management eleventh edition General chemistry 11th edition
General chemistry 11th edition Eleventh 5 year plan
Eleventh 5 year plan Eleventh 5 year plan
Eleventh 5 year plan 11th five year plan
11th five year plan For his eleventh birthday elvis presley
For his eleventh birthday elvis presley Human genetics concepts and applications 10th edition
Human genetics concepts and applications 10th edition No slip condition
No slip condition Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Modern systems analysis and design 7th edition
Modern systems analysis and design 7th edition Discrete mathematics with applications fourth edition
Discrete mathematics with applications fourth edition Mis chapter 6
Mis chapter 6 Chapter 1
Chapter 1 Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Electrical engineering
Electrical engineering Learning principles and applications
Learning principles and applications Applications of nuclear chemistry
Applications of nuclear chemistry Applications of nuclear chemistry
Applications of nuclear chemistry Modern labor economics 12th edition solution
Modern labor economics 12th edition solution Modern labor economics 12th edition pdf
Modern labor economics 12th edition pdf Modern real estate practice in pennsylvania
Modern real estate practice in pennsylvania Modern database management 12th edition ppt
Modern database management 12th edition ppt Modern database management 12th edition
Modern database management 12th edition Modern operating systems 3rd edition
Modern operating systems 3rd edition Tanenbaum structured computer organization
Tanenbaum structured computer organization Modern database management 8th edition
Modern database management 8th edition University physics with modern physics fifteenth edition
University physics with modern physics fifteenth edition Transaction cannot be subdivided
Transaction cannot be subdivided Modern labor economics 12th edition
Modern labor economics 12th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Expert systems: principles and programming, fourth edition
Expert systems: principles and programming, fourth edition Transition state energy diagram
Transition state energy diagram Thermodynamic vs kinetic control
Thermodynamic vs kinetic control Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein Introductory chemistry 4th edition
Introductory chemistry 4th edition Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Introductory chemistry 5th edition answers
Introductory chemistry 5th edition answers The central science 14th edition
The central science 14th edition Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Ap chemistry notes zumdahl
Ap chemistry notes zumdahl Organic chemistry third edition david klein
Organic chemistry third edition david klein Living by chemistry 2nd edition answers
Living by chemistry 2nd edition answers Chemistry by raymond chang 10th edition
Chemistry by raymond chang 10th edition Fifth edition chemistry a molecular approach
Fifth edition chemistry a molecular approach Organic chemistry (3rd) edition chapter 2 problem 17s
Organic chemistry (3rd) edition chapter 2 problem 17s Thermodynamic control
Thermodynamic control Klein
Klein Properties of smart and modern materials
Properties of smart and modern materials Chapter 9 section 3 stoichiometry
Chapter 9 section 3 stoichiometry Modern chemistry chapter 7 review answers
Modern chemistry chapter 7 review answers Modern chemistry chapter 15 review answers
Modern chemistry chapter 15 review answers Modern chemistry chapter 14 review answers
Modern chemistry chapter 14 review answers Modern chemistry chapter 13
Modern chemistry chapter 13 Chapter 12 solutions chemistry
Chapter 12 solutions chemistry Modern chemistry chapter 4
Modern chemistry chapter 4 Modern chemistry solutions
Modern chemistry solutions Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Principles of network applications
Principles of network applications Principles of network applications
Principles of network applications Failure of supporting utilities and structural collapse
Failure of supporting utilities and structural collapse Principles of electronic communication systems 3rd edition
Principles of electronic communication systems 3rd edition Principles of economics third edition oxford pdf
Principles of economics third edition oxford pdf Accounting principles second canadian edition
Accounting principles second canadian edition Accounting principles second canadian edition
Accounting principles second canadian edition