GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION




![Original equilibrium Immediately following disturbance [SO 3]2 Q= = Kc 2 [SO 2] [O Original equilibrium Immediately following disturbance [SO 3]2 Q= = Kc 2 [SO 2] [O](https://slidetodoc.com/presentation_image_h2/75f29782718defa244def84e657e447f/image-30.jpg)



- Slides: 38

GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION PETRUCCI HERRING MADURA BISSONNETTE Principles of Chemical Equilibrium 15 PHILIP DUTTON UNIVERSITY OF WINDSOR DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY Slide 15 - 1 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

Solutions and their Physical Properties Slide 15 - 2 CONTENTS 15 -1 The Nature of the Equilibrium State 15 -2 The Equilibrium Constant Expression 15 -3 Relationships Involving Equilibrium Constants 15 -4 The Magnitude of an Equilibrium Constant 15 -5 Predicting the Direction of Net Chemical Change 15 -6 Altering Equilibrium Conditions: Le Châtelier’s Principle 15 -7 Equilibrium Calculations: Some Illustrative Examples General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

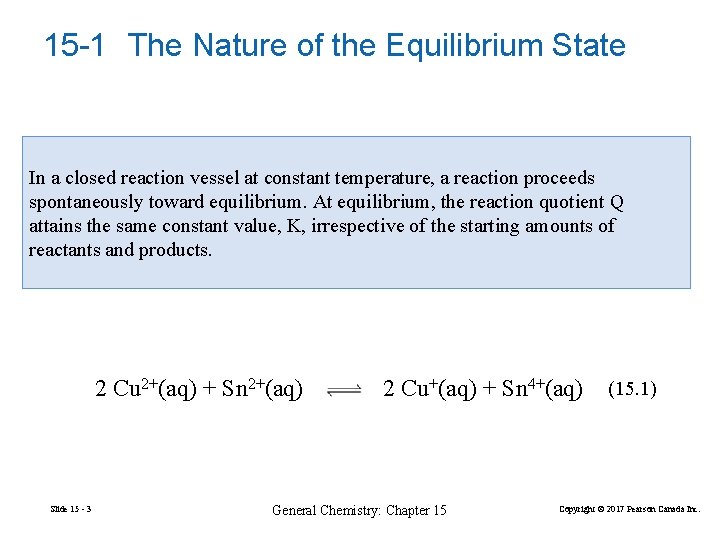
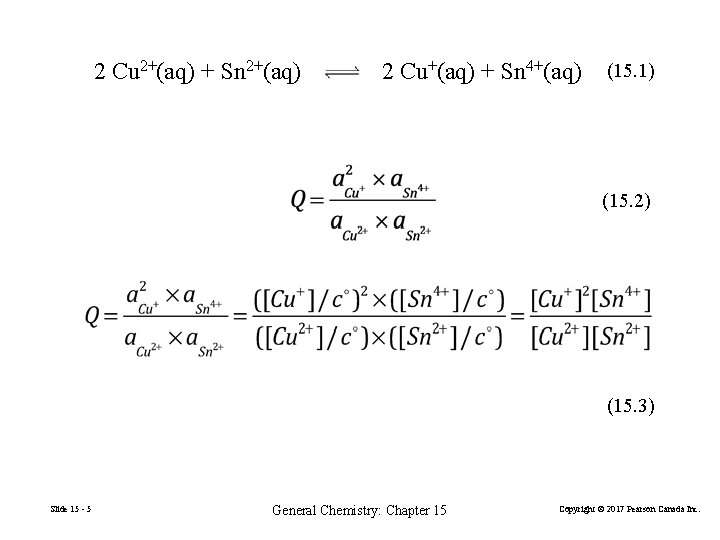
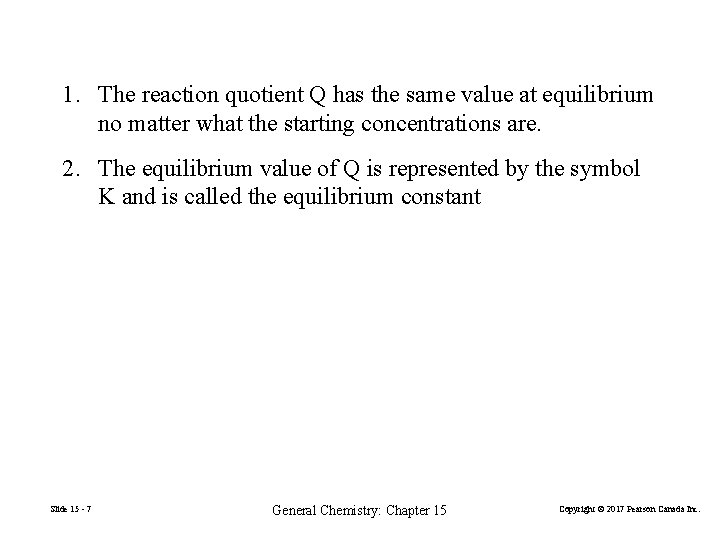
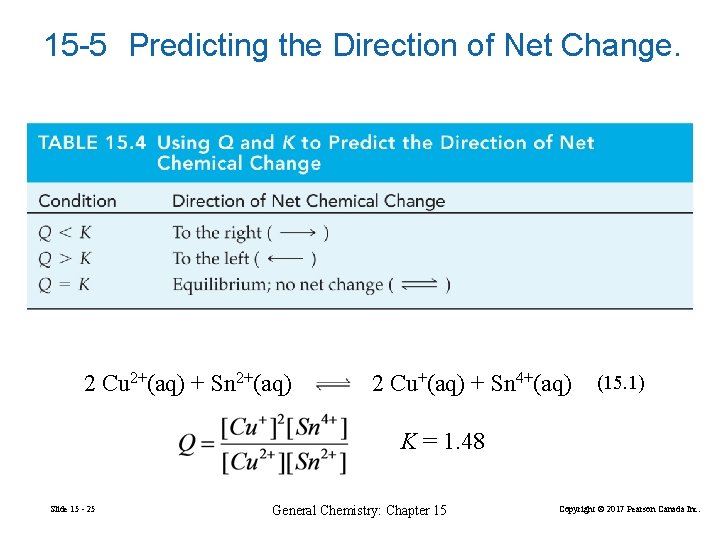
15 -1 The Nature of the Equilibrium State In a closed reaction vessel at constant temperature, a reaction proceeds spontaneously toward equilibrium. At equilibrium, the reaction quotient Q attains the same constant value, K, irrespective of the starting amounts of reactants and products. 2 Cu 2+(aq) + Sn 2+(aq) Slide 15 - 3 2 Cu+(aq) + Sn 4+(aq) General Chemistry: Chapter 15 (15. 1) Copyright © 2017 Pearson Canada Inc.

Slide 15 - 4 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

2 Cu 2+(aq) + Sn 2+(aq) 2 Cu+(aq) + Sn 4+(aq) (15. 1) (15. 2) (15. 3) Slide 15 - 5 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

Slide 15 - 6 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

1. The reaction quotient Q has the same value at equilibrium no matter what the starting concentrations are. 2. The equilibrium value of Q is represented by the symbol K and is called the equilibrium constant Slide 15 - 7 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

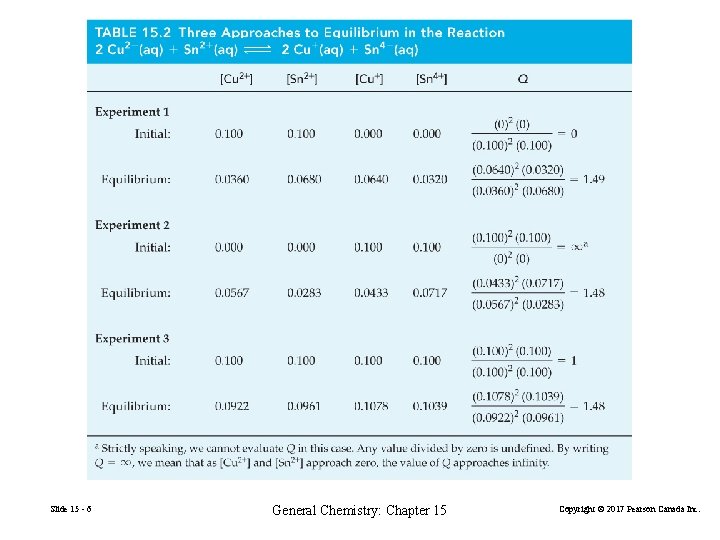
FIGURE 15 -1 Three approaches to equilibrium in the reaction 2 Cu 2+(aq) + Sn 2+(aq) 2 Cu+(aq) + Sn 4+(aq) Slide 15 - 8 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

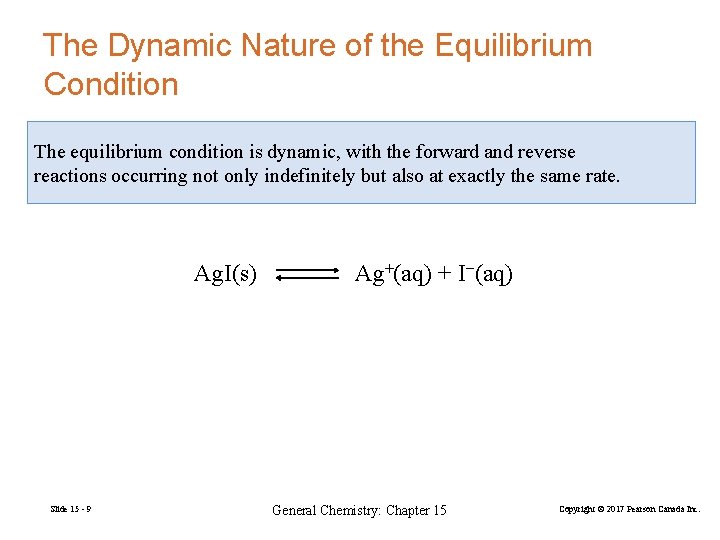
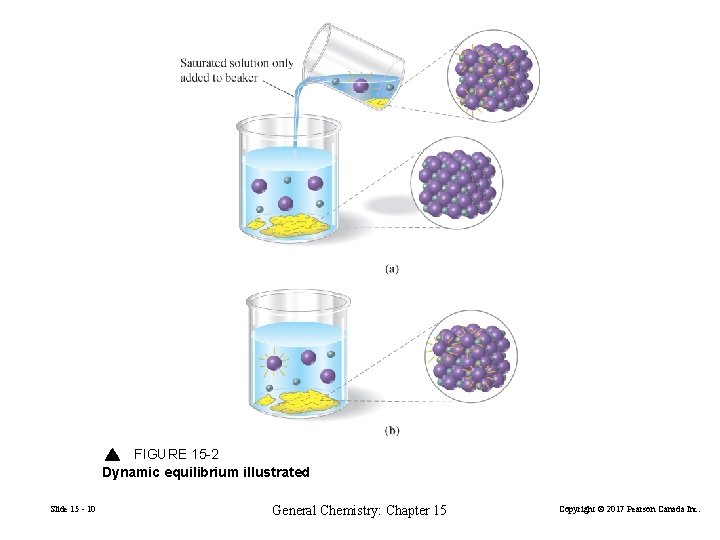
The Dynamic Nature of the Equilibrium Condition The equilibrium condition is dynamic, with the forward and reverse reactions occurring not only indefinitely but also at exactly the same rate. Ag. I(s) Slide 15 - 9 Ag+(aq) + I−(aq) General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

FIGURE 15 -2 Dynamic equilibrium illustrated Slide 15 - 10 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

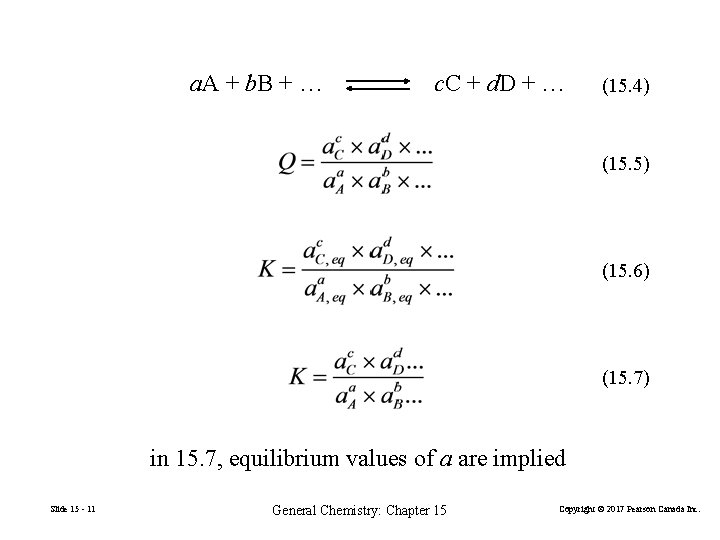
a. A + b. B + … c. C + d. D + … (15. 4) (15. 5) (15. 6) (15. 7) in 15. 7, equilibrium values of a are implied Slide 15 - 11 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

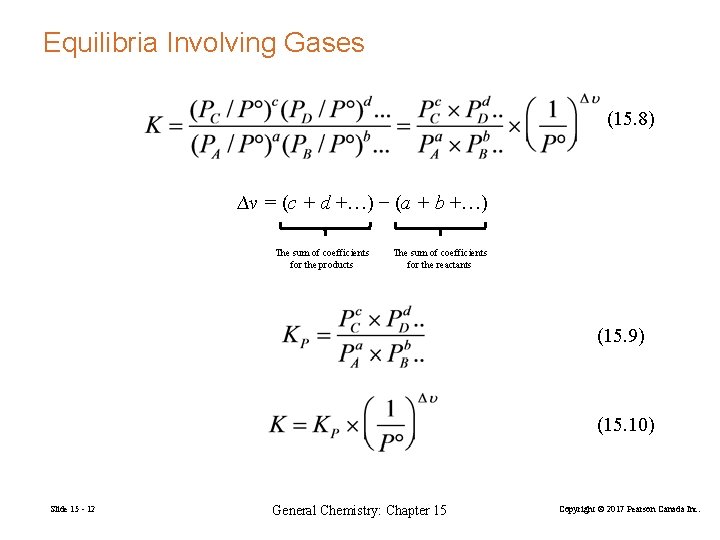
Equilibria Involving Gases (15. 8) ∆ν = (c + d +…) − (a + b +…) The sum of coefficients for the products The sum of coefficients for the reactants (15. 9) (15. 10) Slide 15 - 12 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

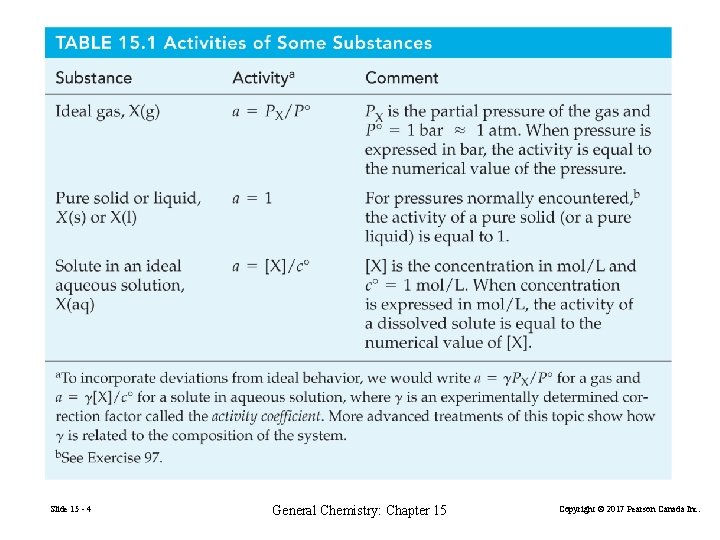
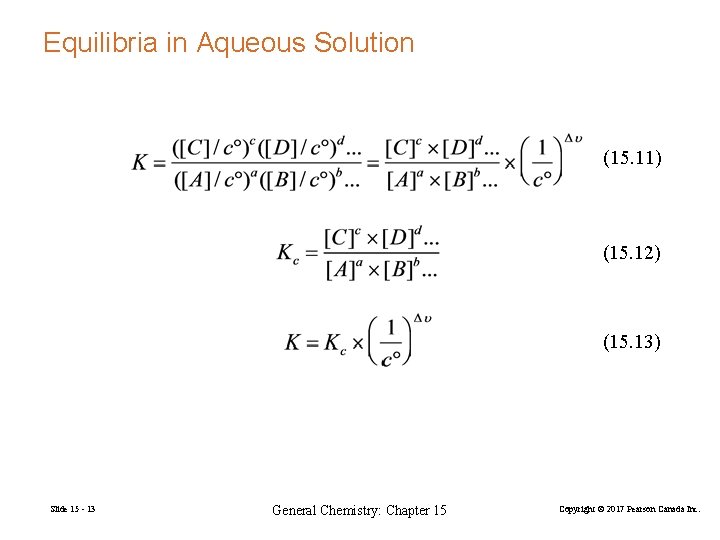
Equilibria in Aqueous Solution (15. 11) (15. 12) (15. 13) Slide 15 - 13 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

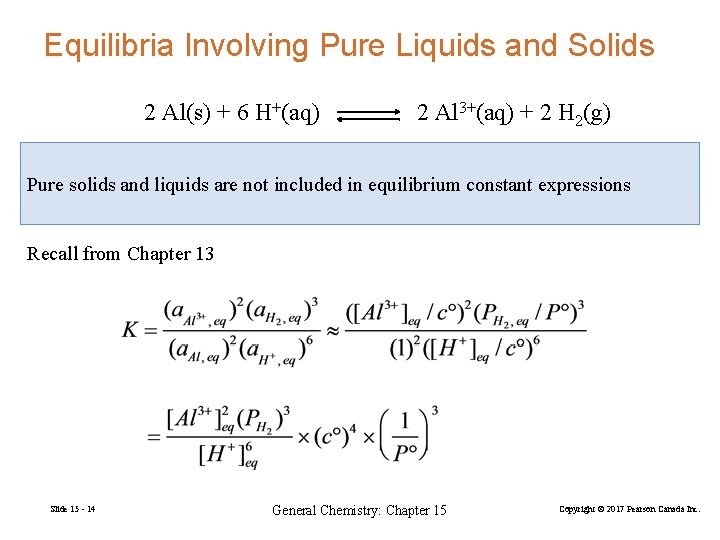
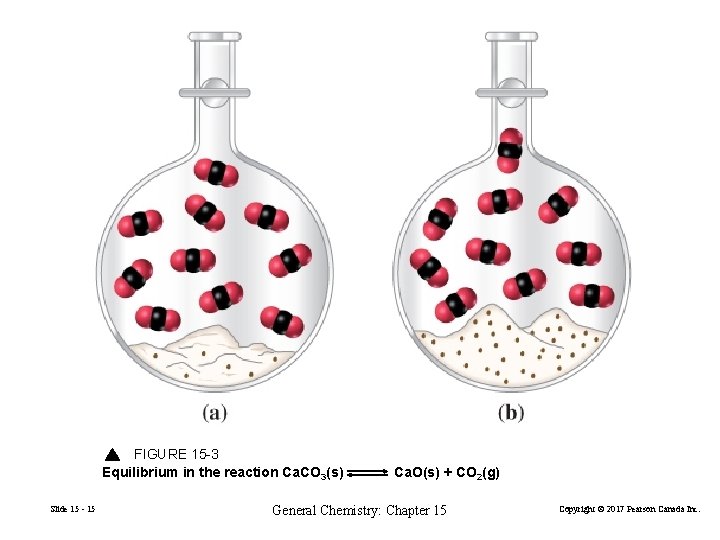
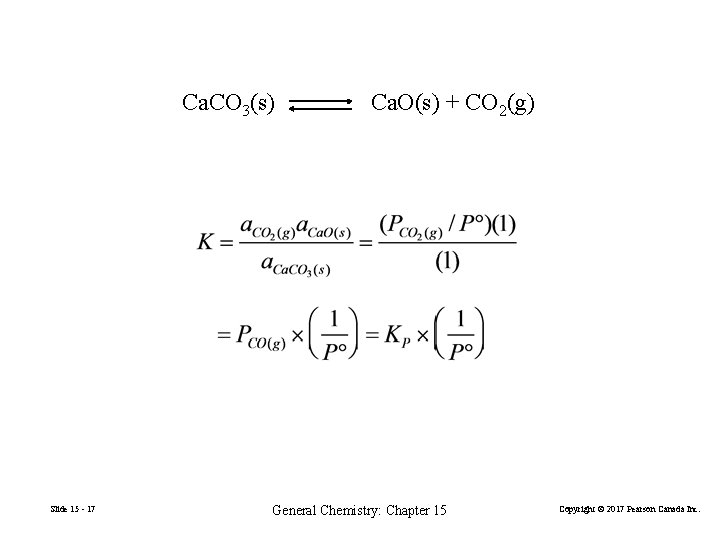
Equilibria Involving Pure Liquids and Solids 2 Al(s) + 6 H+(aq) 2 Al 3+(aq) + 2 H 2(g) Pure solids and liquids are not included in equilibrium constant expressions Recall from Chapter 13 Slide 15 - 14 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

FIGURE 15 -3 Equilibrium in the reaction Ca. CO 3(s) Slide 15 - 15 Ca. O(s) + CO 2(g) General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

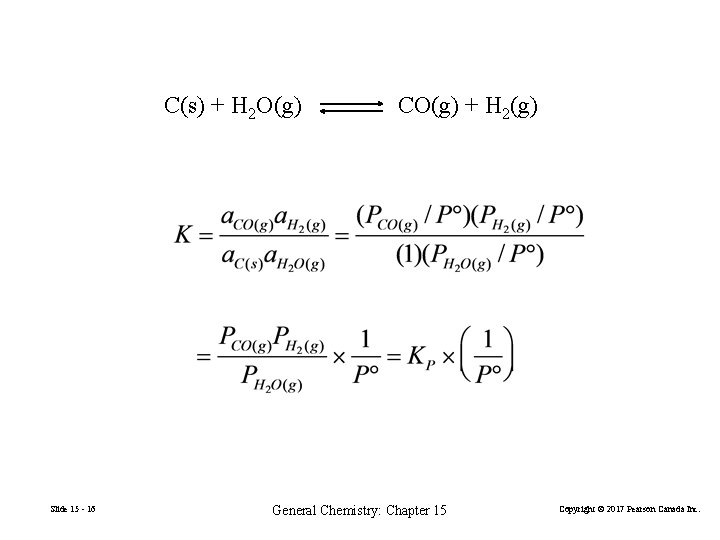
C(s) + H 2 O(g) Slide 15 - 16 CO(g) + H 2(g) General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

Ca. CO 3(s) Slide 15 - 17 Ca. O(s) + CO 2(g) General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

15 -3 Relationships Involving the Equilibrium Constant Relationship of K to the Balanced Chemical Equation • When we reverse an equation, we invert the value of K. • When we multiply the coefficients in a balanced equation by a common factor we raise the equilibrium constant to the corresponding power. • When we divide the coefficients in a balanced equation by a common factor we take the corresponding root of the equilibrium constant. Slide 15 - 18 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

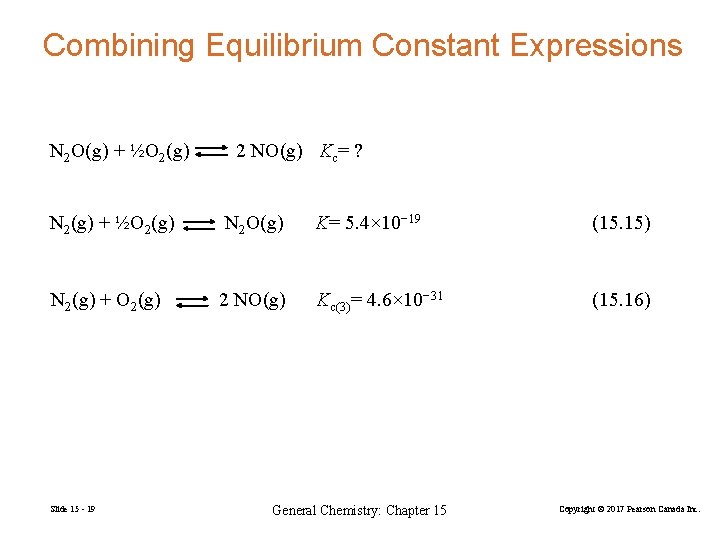
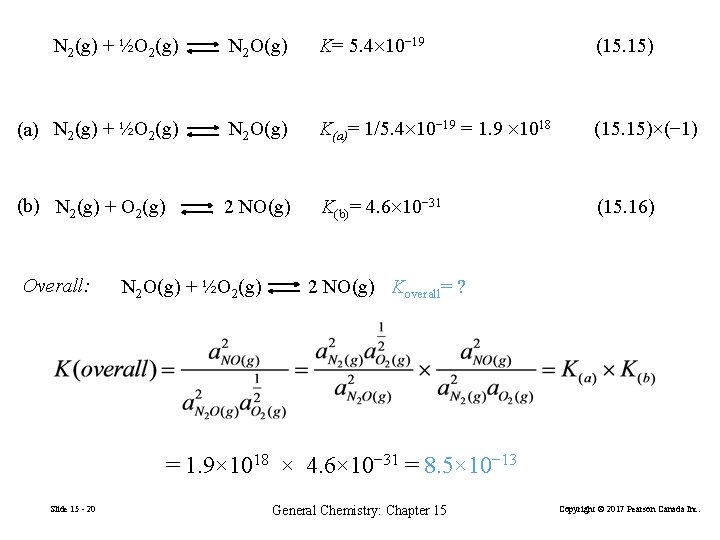
Combining Equilibrium Constant Expressions N 2 O(g) + ½O 2(g) 2 NO(g) Kc= ? N 2(g) + ½O 2(g) N 2 O(g) K= 5. 4× 10− 19 (15. 15) N 2(g) + O 2(g) 2 NO(g) Kc(3)= 4. 6× 10− 31 (15. 16) Slide 15 - 19 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

N 2(g) + ½O 2(g) N 2 O(g) K= 5. 4 10− 19 (15. 15) (a) N 2(g) + ½O 2(g) N 2 O(g) K(a)= 1/5. 4 10− 19 = 1. 9 1018 (15. 15)×(− 1) (b) N 2(g) + O 2(g) 2 NO(g) K(b)= 4. 6 10− 31 (15. 16) Overall: N 2 O(g) + ½O 2(g) 2 NO(g) Koverall= ? = 1. 9× 1018 × 4. 6× 10− 31 = 8. 5× 10− 13 Slide 15 - 20 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

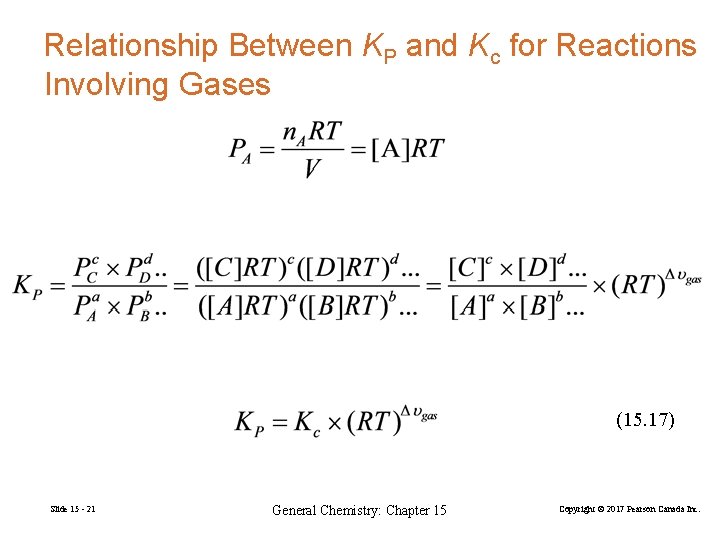
Relationship Between KP and Kc for Reactions Involving Gases (15. 17) Slide 15 - 21 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

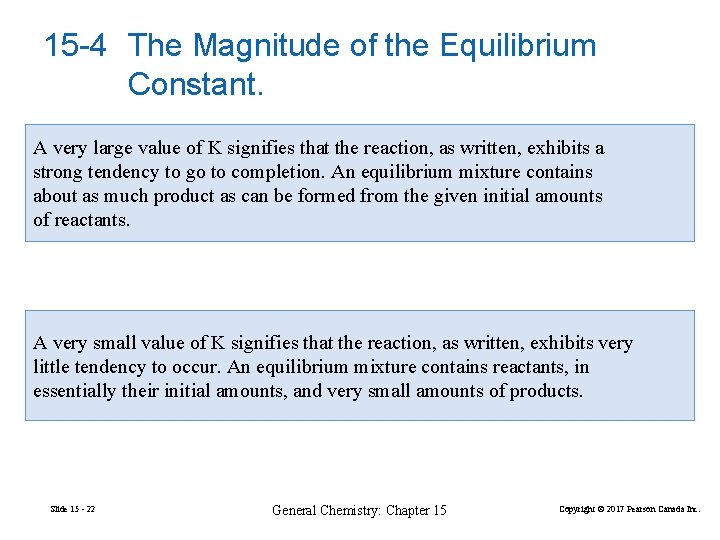
15 -4 The Magnitude of the Equilibrium Constant. A very large value of K signifies that the reaction, as written, exhibits a strong tendency to go to completion. An equilibrium mixture contains about as much product as can be formed from the given initial amounts of reactants. A very small value of K signifies that the reaction, as written, exhibits very little tendency to occur. An equilibrium mixture contains reactants, in essentially their initial amounts, and very small amounts of products. Slide 15 - 22 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

Slide 15 - 23 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

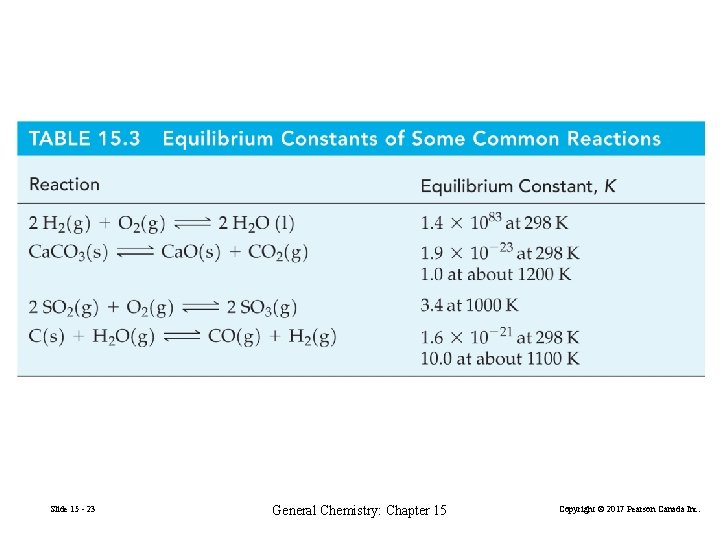
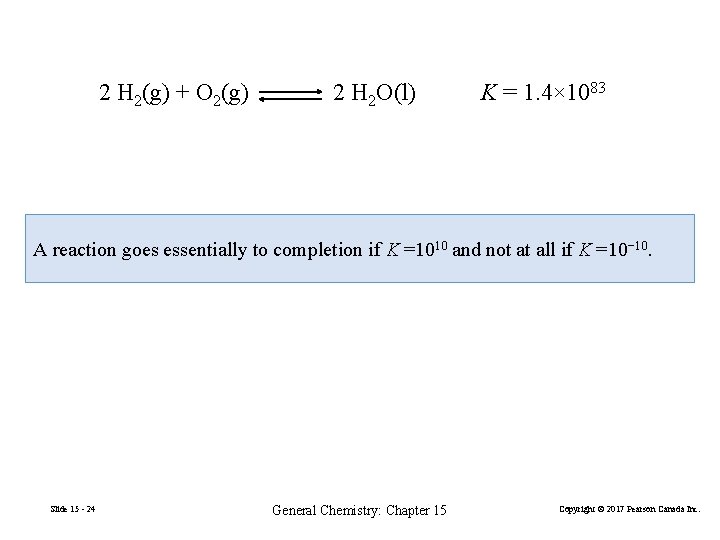
2 H 2(g) + O 2(g) 2 H 2 O(l) K = 1. 4× 1083 A reaction goes essentially to completion if K =1010 and not at all if K =10− 10. Slide 15 - 24 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

15 -5 Predicting the Direction of Net Change. 2 Cu 2+(aq) + Sn 2+(aq) 2 Cu+(aq) + Sn 4+(aq) (15. 1) K = 1. 48 Slide 15 - 25 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

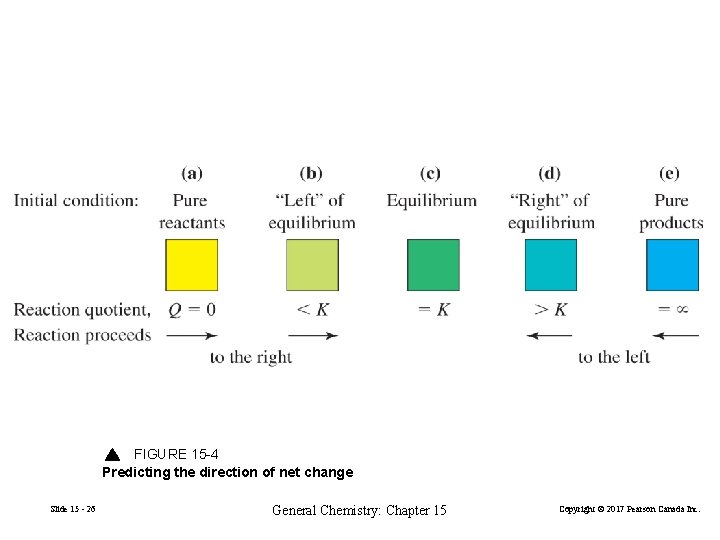
FIGURE 15 -4 Predicting the direction of net change Slide 15 - 26 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

15 -6 Altering Equilibrium Conditions: Le Châtelier’s Principle When an equilibrium system is subjected to a change in temperature, pressure, or concentration of a reacting species, the system responds by attaining a new equilibrium that partially offsets the impact of the change. Slide 15 - 27 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

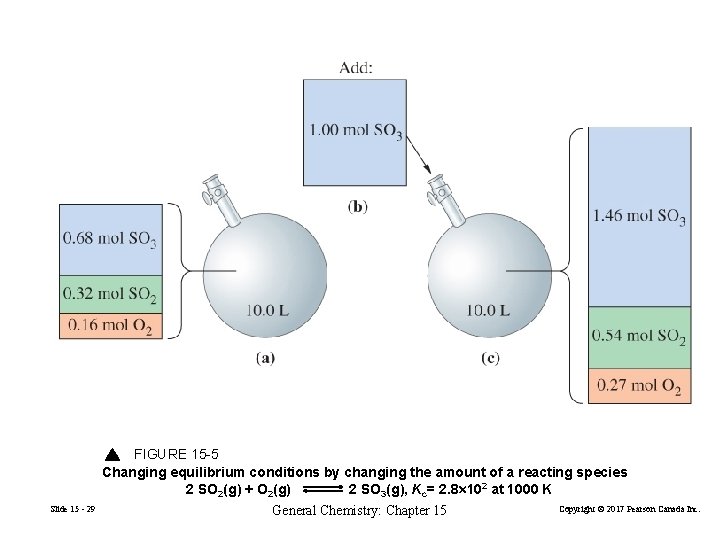
Effect of Changing the Amounts of Reacting Species on Equilibrium 2 SO 2(g) + O 2(g) Slide 15 - 28 2 SO 3 O(g) General Chemistry: Chapter 15 Kc = 2. 8× 102 at 1000 K Copyright © 2017 Pearson Canada Inc.

FIGURE 15 -5 Changing equilibrium conditions by changing the amount of a reacting species 2 SO 2(g) + O 2(g) 2 SO 3(g), Kc= 2. 8 102 at 1000 K Slide 15 - 29 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.
![Original equilibrium Immediately following disturbance SO 32 Q Kc 2 SO 2 O Original equilibrium Immediately following disturbance [SO 3]2 Q= = Kc 2 [SO 2] [O](https://slidetodoc.com/presentation_image_h2/75f29782718defa244def84e657e447f/image-30.jpg)
Original equilibrium Immediately following disturbance [SO 3]2 Q= = Kc 2 [SO 2] [O 2] 2 SO 2(g) + O 2(g) Slide 15 - 30 Q > Kc k 1 k− 1 2 SO 3(g) General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

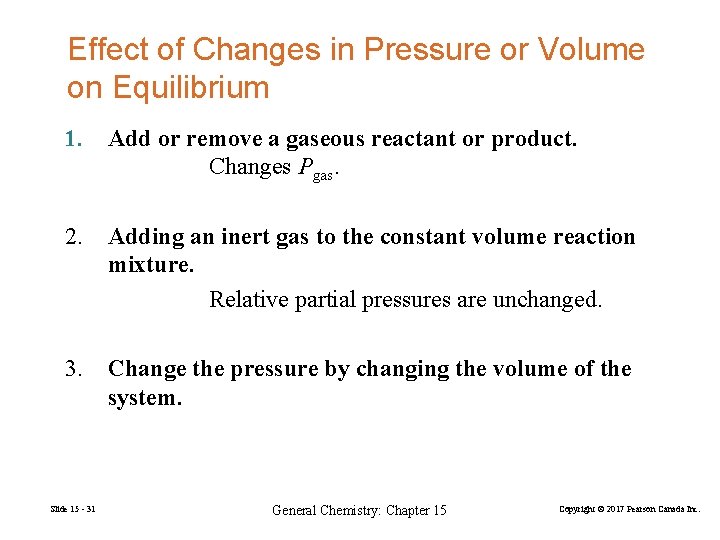
Effect of Changes in Pressure or Volume on Equilibrium 1. Add or remove a gaseous reactant or product. Changes Pgas. 2. Adding an inert gas to the constant volume reaction mixture. Relative partial pressures are unchanged. 3. Change the pressure by changing the volume of the system. Slide 15 - 31 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

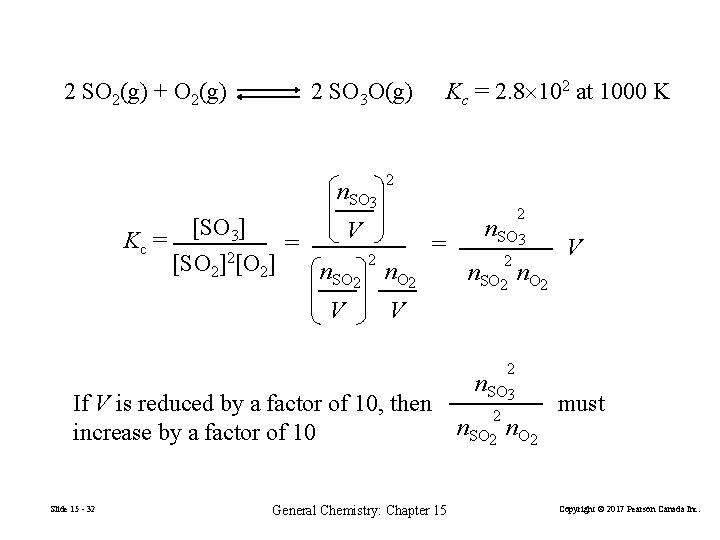
2 SO 2(g) + O 2(g) 2 SO 3 O(g) n. SO 3 Kc = [SO 3] [SO 2]2[O 2] 2 V = n. SO 2 V 2 Kc = 2. 8 102 at 1000 K = n. O 2 2 n. SO 2 n. O 2 V V If V is reduced by a factor of 10, then increase by a factor of 10 Slide 15 - 32 2 n. SO 3 General Chemistry: Chapter 15 2 n. SO 3 2 n. SO 2 n. O 2 must Copyright © 2017 Pearson Canada Inc.

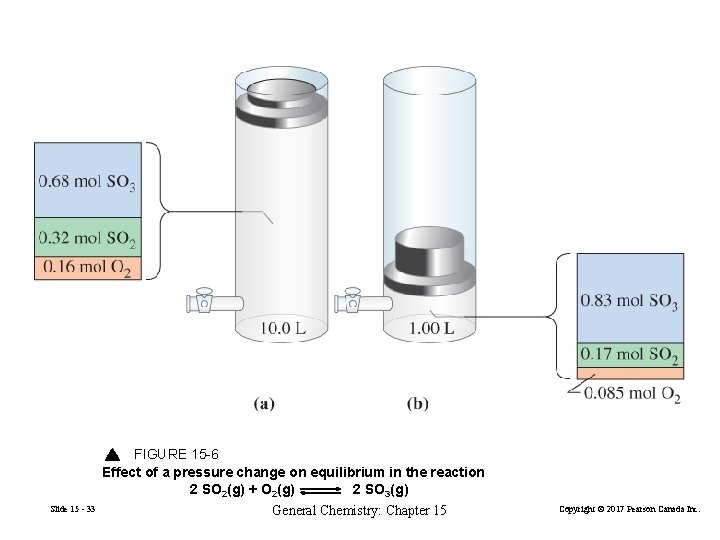
FIGURE 15 -6 Effect of a pressure change on equilibrium in the reaction 2 SO 2(g) + O 2(g) 2 SO 3(g) Slide 15 - 33 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

When the volume of an equilibrium mixture of gases is reduced, a net change occurs in the direction that produces fewer moles of gas. When the volume is increased, a net change occurs in the direction that produces more moles of gas. Slide 15 - 34 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

Effect of Temperature on Equilibrium Raising the temperature of an equilibrium mixture shifts the equilibrium condition in the direction of the endothermic reaction. Lowering the temperature causes a shift in the direction of the exothermic reaction. For endothermic reactions, K increases as temperature increases. For exothermic reactions, K decreases as temperature increases. Slide 15 - 35 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

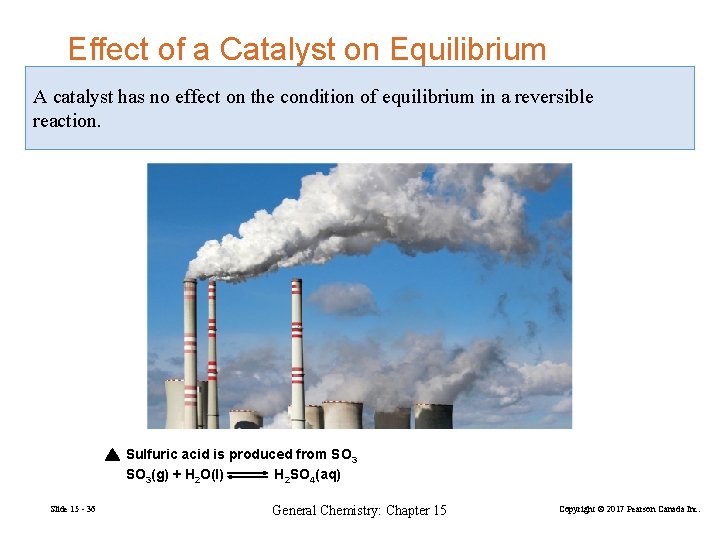
Effect of a Catalyst on Equilibrium A catalyst has no effect on the condition of equilibrium in a reversible reaction. Sulfuric acid is produced from SO 3(g) + H 2 O(l) H 2 SO 4(aq) Slide 15 - 36 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

15 -7 Equilibrium Calculations: Some Illustrative Examples. Five numerical examples are given in the text that illustrate ideas that have been presented in this chapter. Refer to the “comments” which describe the methodology. These will help in subsequent chapters. Exercise your understanding by working through the examples with a pencil and paper. Slide 15 - 37 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.

End of Chapter Slide 15 - 38 General Chemistry: Chapter 15 Copyright © 2017 Pearson Canada Inc.
 Management eleventh edition stephen p robbins
Management eleventh edition stephen p robbins Management stephen p robbins 11th edition
Management stephen p robbins 11th edition Management eleventh edition
Management eleventh edition Management eleventh edition
Management eleventh edition General chemistry 11th edition
General chemistry 11th edition Chadha committee
Chadha committee Eleventh 5 year plan
Eleventh 5 year plan Eleventh plan
Eleventh plan For his eleventh birthday elvis presley
For his eleventh birthday elvis presley Human genetics concepts and applications 10th edition
Human genetics concepts and applications 10th edition No slip condition
No slip condition Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Modern systems analysis and design 7th edition
Modern systems analysis and design 7th edition Discrete math susanna epp
Discrete math susanna epp Using mis (10th edition) 10th edition
Using mis (10th edition) 10th edition Report
Report Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Allan
Allan Learning principles and applications
Learning principles and applications Applications of nuclear chemistry
Applications of nuclear chemistry Applications of nuclear chemistry
Applications of nuclear chemistry Modern labor economics 12th edition solution
Modern labor economics 12th edition solution Modern labor economics 12th edition
Modern labor economics 12th edition Modern real estate practice in pennsylvania
Modern real estate practice in pennsylvania Modern database management 12th edition ppt
Modern database management 12th edition ppt Modern database management 10th edition
Modern database management 10th edition Modern operating systems 3rd edition
Modern operating systems 3rd edition Structured computer organization
Structured computer organization Modern database management 8th edition
Modern database management 8th edition University physics with modern physics fifteenth edition
University physics with modern physics fifteenth edition Modern database management 11th edition
Modern database management 11th edition Modern labor economics 12th edition
Modern labor economics 12th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Expert systems: principles and programming, fourth edition
Expert systems: principles and programming, fourth edition Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s