GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION





![Formation constant, Kf Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl−(aq) Formation constant, Kf Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl−(aq)](https://slidetodoc.com/presentation_image_h/3254c6a184ba7c00b57b77abb4866f2d/image-24.jpg)



- Slides: 34

GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION PETRUCCI HERRING MADURA BISSONNETTE Solubility and Complex-Ion Equilibria 18 PHILIP DUTTON UNIVERSITY OF WINDSOR DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY Slide 18 - 1 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

Additional Aspects of Acid-Base Equilibria Slide 18 - 2 CONTENTS 18 -1 Solubility Product Constant, Ksp 18 -2 Relationship Between Solubility and Ksp 18 -3 Common-Ion Effect in Solubility Equilibria 18 -4 Limitations of the Ksp Concept 18 -5 Criteria for Precipitation and Its Completeness 18 -6 Fractional Precipitation 18 -7 Solubility and p. H 18 -8 Equilibria Involving Complex Ions 18 -9 Qualitative Cation Analysis General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

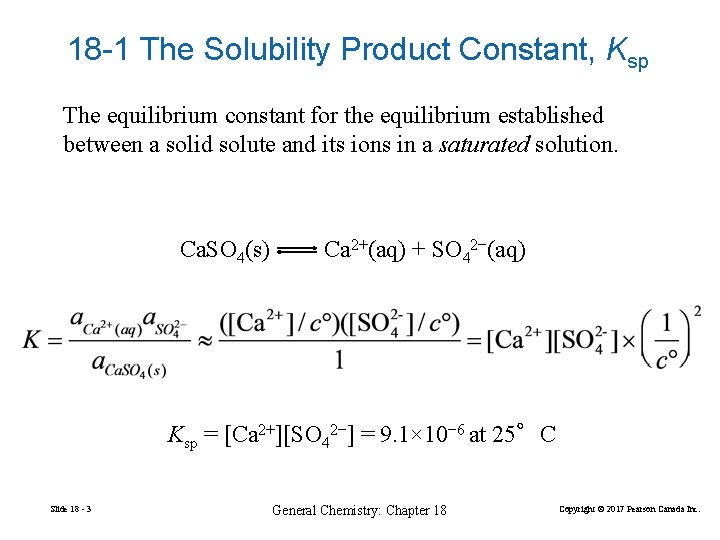
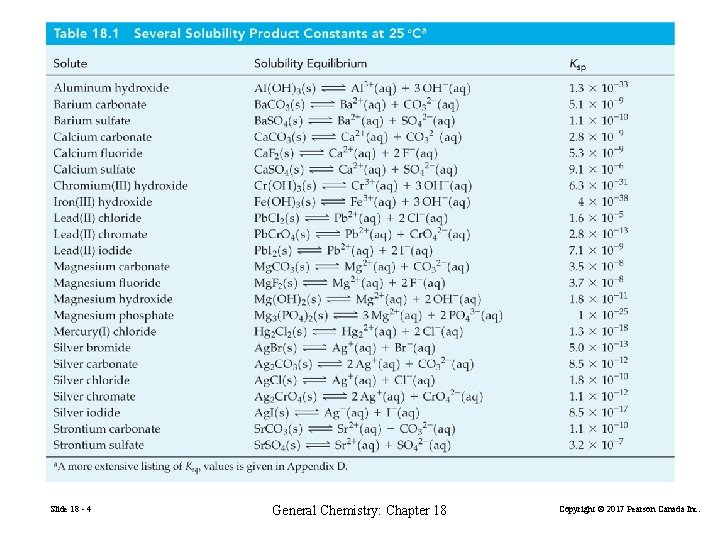
18 -1 The Solubility Product Constant, Ksp The equilibrium constant for the equilibrium established between a solid solute and its ions in a saturated solution. Ca. SO 4(s) Ca 2+(aq) + SO 42−(aq) Ksp = [Ca 2+][SO 42−] = 9. 1× 10− 6 at 25°C Slide 18 - 3 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

Slide 18 - 4 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

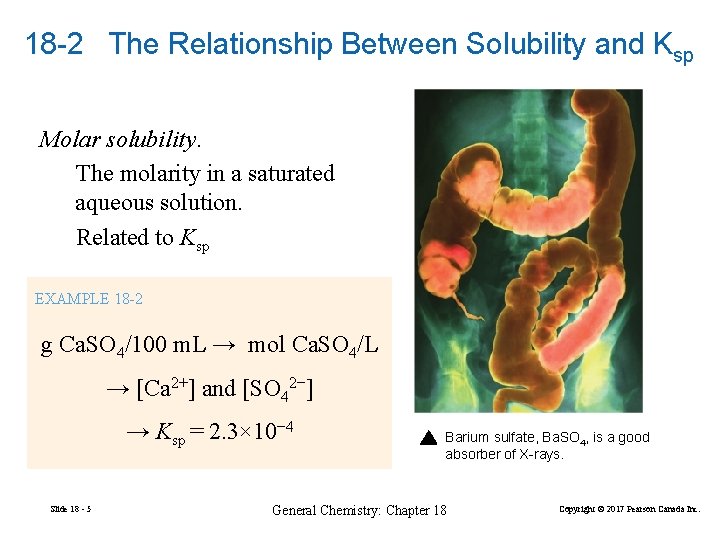
18 -2 The Relationship Between Solubility and Ksp Molar solubility. The molarity in a saturated aqueous solution. Related to Ksp EXAMPLE 18 -2 g Ca. SO 4/100 m. L → mol Ca. SO 4/L → [Ca 2+] and [SO 42−] → Ksp = 2. 3× 10− 4 Slide 18 - 5 Barium sulfate, Ba. SO 4, is a good absorber of X-rays. General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

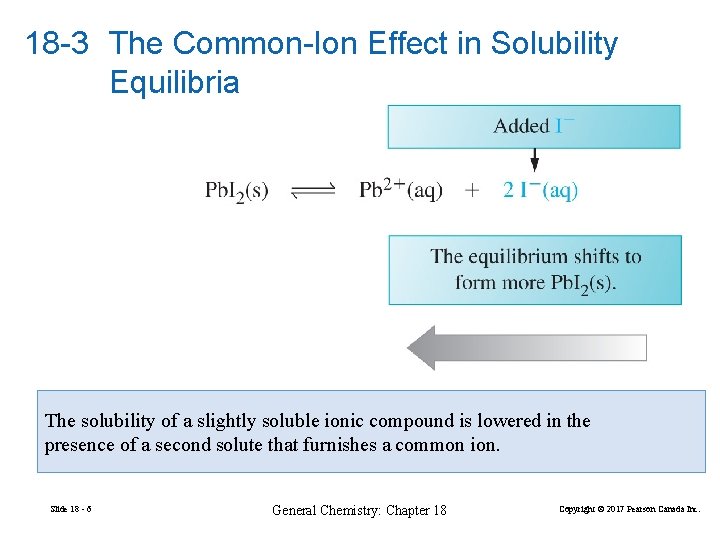
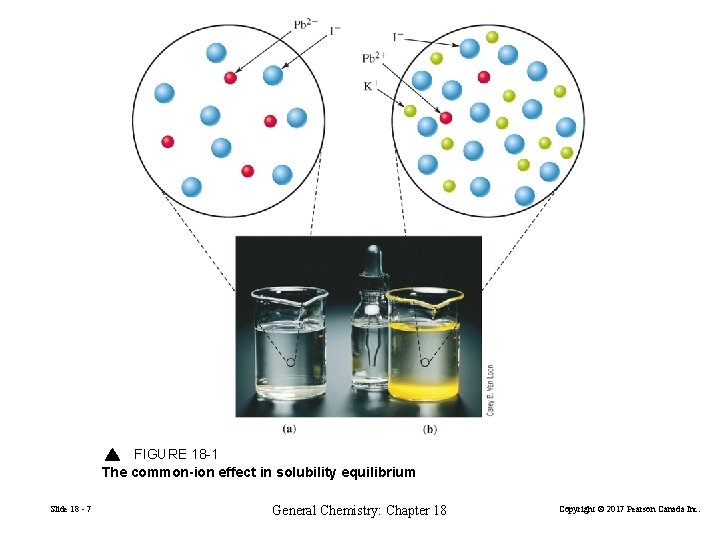
18 -3 The Common-Ion Effect in Solubility Equilibria The solubility of a slightly soluble ionic compound is lowered in the presence of a second solute that furnishes a common ion. Slide 18 - 6 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

FIGURE 18 -1 The common-ion effect in solubility equilibrium Slide 18 - 7 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

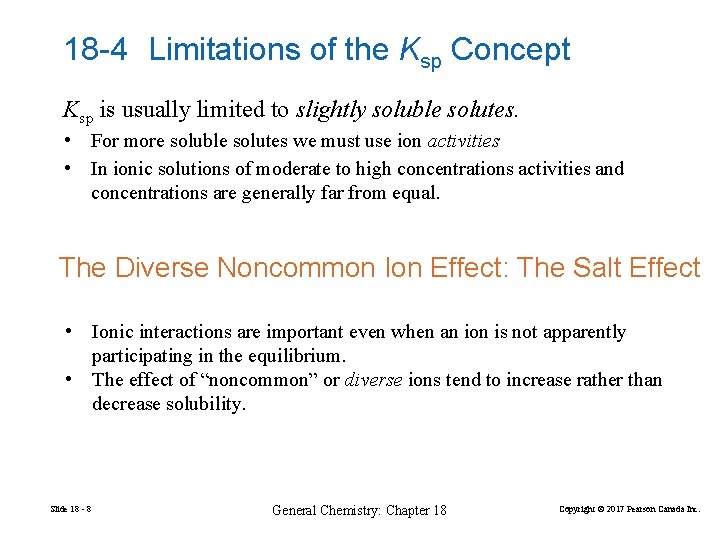
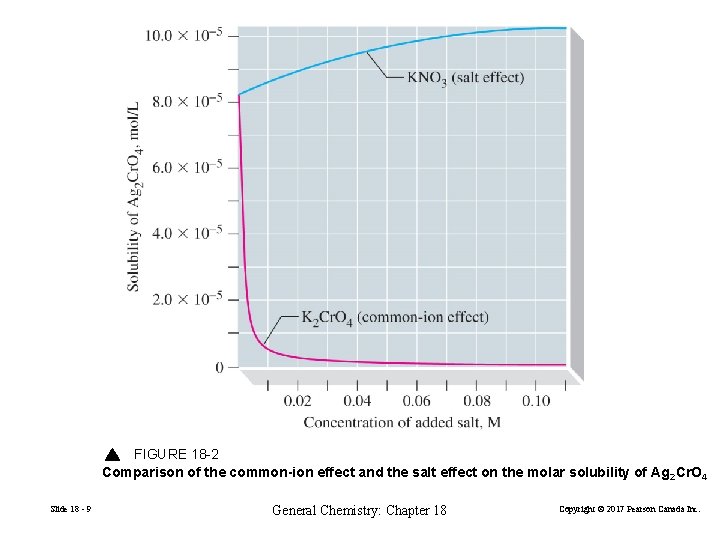
18 -4 Limitations of the Ksp Concept Ksp is usually limited to slightly soluble solutes. • For more soluble solutes we must use ion activities • In ionic solutions of moderate to high concentrations activities and concentrations are generally far from equal. The Diverse Noncommon Ion Effect: The Salt Effect • Ionic interactions are important even when an ion is not apparently participating in the equilibrium. • The effect of “noncommon” or diverse ions tend to increase rather than decrease solubility. Slide 18 - 8 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

FIGURE 18 -2 Comparison of the common-ion effect and the salt effect on the molar solubility of Ag 2 Cr. O 4 Slide 18 - 9 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

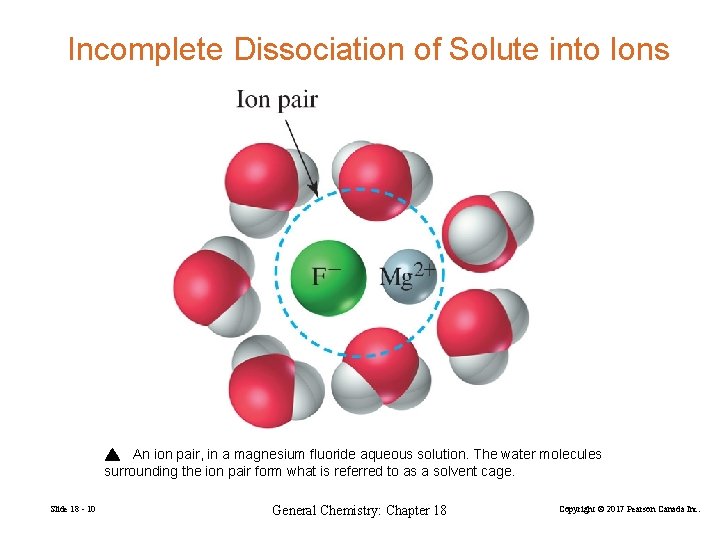
Incomplete Dissociation of Solute into Ions An ion pair, in a magnesium fluoride aqueous solution. The water molecules surrounding the ion pair form what is referred to as a solvent cage. Slide 18 - 10 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

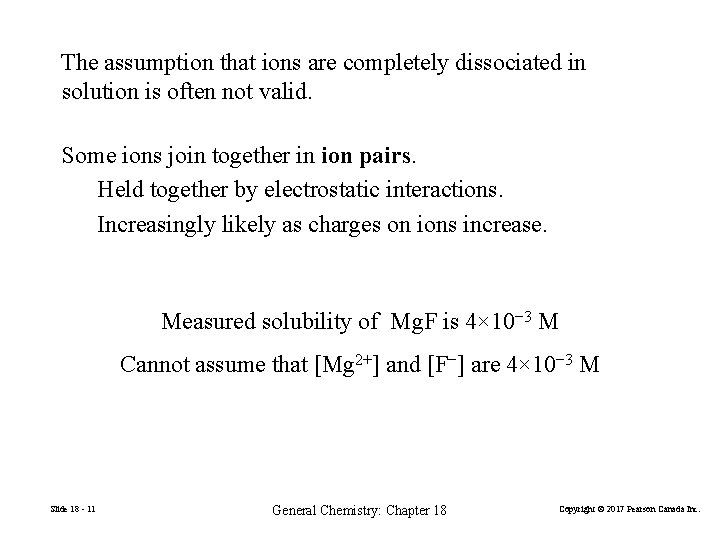
The assumption that ions are completely dissociated in solution is often not valid. Some ions join together in ion pairs. Held together by electrostatic interactions. Increasingly likely as charges on ions increase. Measured solubility of Mg. F is 4× 10− 3 M Cannot assume that [Mg 2+] and [F−] are 4× 10− 3 M Slide 18 - 11 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

Simultaneous Equilibria Other equilibria are usually present in a solution. Kw for example. Reactions between solute ions and other species. Acid base reactions. Complex-ion formation These must be taken into account if they affect the equilibrium in question. Slide 18 - 12 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

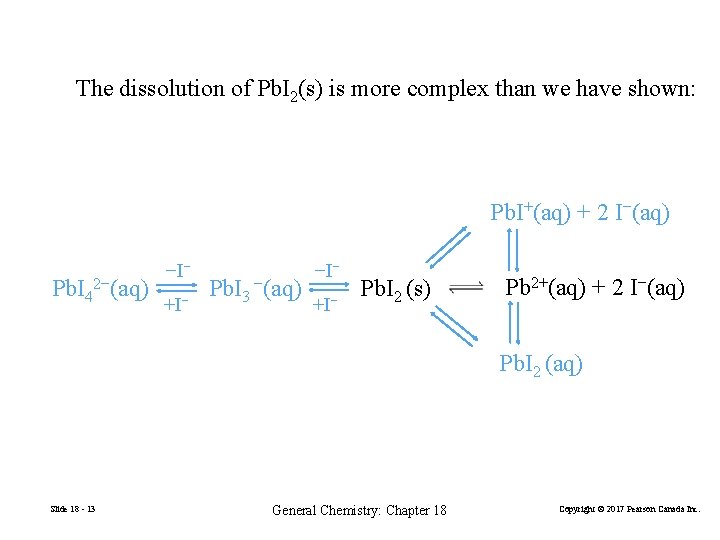
The dissolution of Pb. I 2(s) is more complex than we have shown: Pb. I+(aq) + 2 I−(aq) Pb. I 42−(aq) −I− +I− Pb. I 3 −(aq) −I− +I− Pb. I 2 (s) Pb 2+(aq) + 2 I−(aq) Pb. I 2 (aq) Slide 18 - 13 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

Assessing the Limitations of Ksp Concentrations are usually substituted for activities. Conflicting results are obtained as a result. Useful predictions can still be made. Slide 18 - 14 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

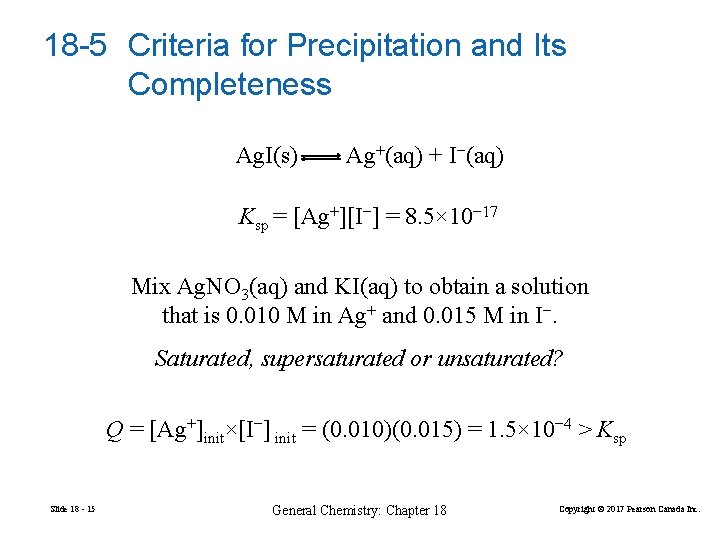
18 -5 Criteria for Precipitation and Its Completeness Ag. I(s) Ag+(aq) + I−(aq) Ksp = [Ag+][I−] = 8. 5× 10− 17 Mix Ag. NO 3(aq) and KI(aq) to obtain a solution that is 0. 010 M in Ag+ and 0. 015 M in I−. Saturated, supersaturated or unsaturated? Q = [Ag+]init×[I−] init = (0. 010)(0. 015) = 1. 5× 10− 4 > Ksp Slide 18 - 15 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

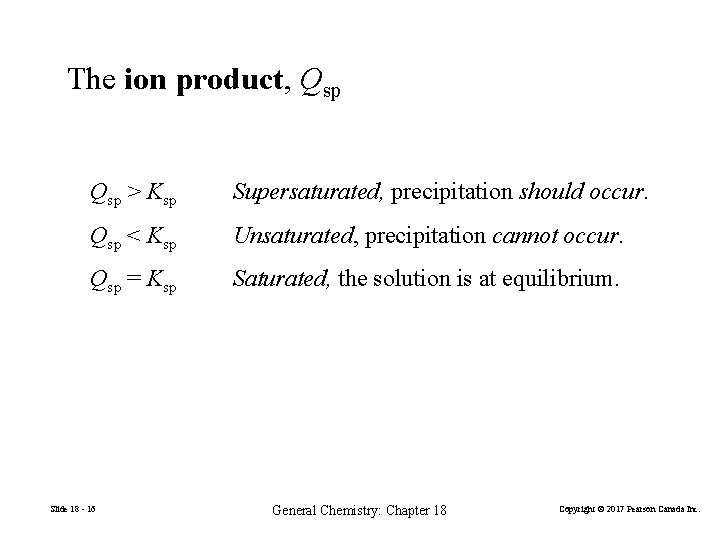
The ion product, Qsp > Ksp Supersaturated, precipitation should occur. Qsp < Ksp Unsaturated, precipitation cannot occur. Qsp = Ksp Saturated, the solution is at equilibrium. Slide 18 - 16 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

FIGURE 18 -3 Applying the criteria for precipitation from solution – Example 18 -5 illustrated Slide 18 - 17 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

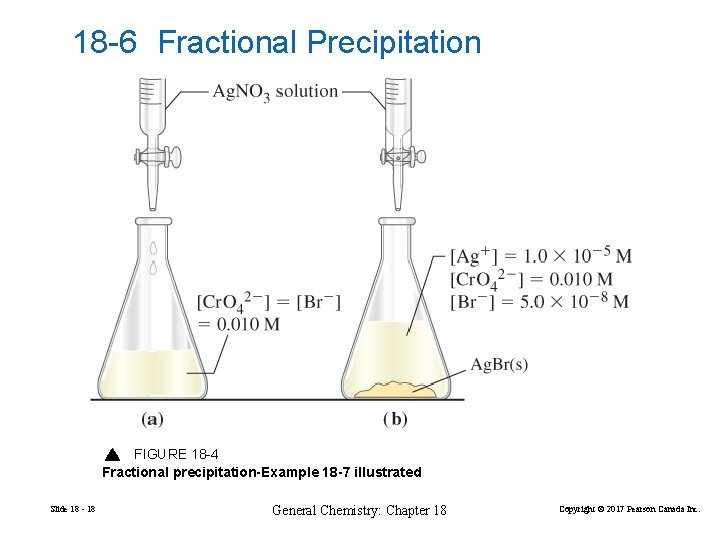
18 -6 Fractional Precipitation FIGURE 18 -4 Fractional precipitation-Example 18 -7 illustrated Slide 18 - 18 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

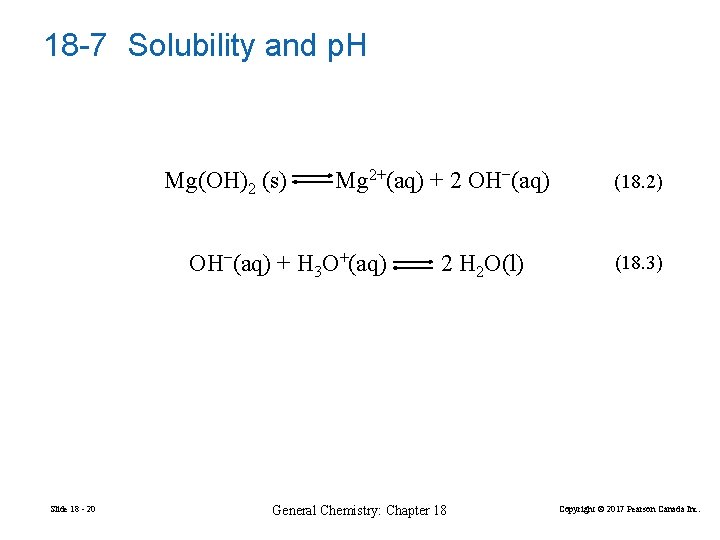
18 -7 Solubility and p. H can affect the solubility of a salt. Especially when the anion of the salt is the conjugate base of a weak acid. Mg(OH)2 Milk of Magnesia. Slide 18 - 19 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

18 -7 Solubility and p. H Mg(OH)2 (s) Mg 2+(aq) + 2 OH−(aq) + H 3 O+(aq) Slide 18 - 20 2 H 2 O(l) General Chemistry: Chapter 18 (18. 2) (18. 3) Copyright © 2017 Pearson Canada Inc.

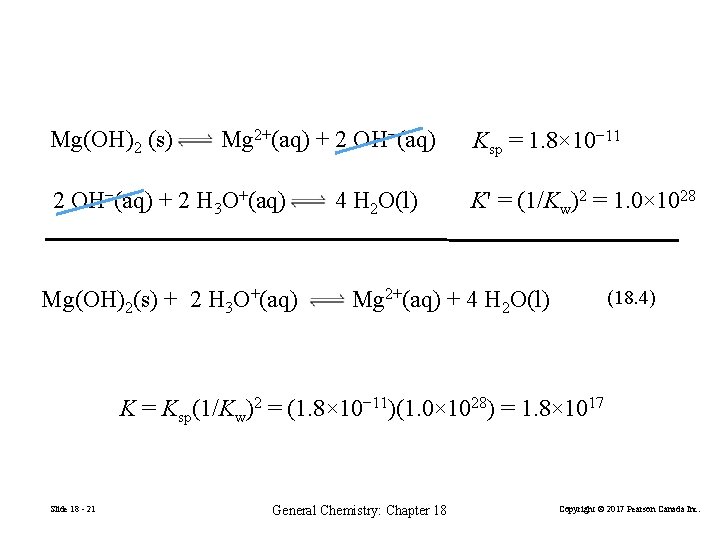
Mg(OH)2 (s) Mg 2+(aq) + 2 OH−(aq) + 2 H 3 O+(aq) Mg(OH)2(s) + 2 H 3 O+(aq) 4 H 2 O(l) Ksp = 1. 8× 10− 11 K' = (1/Kw)2 = 1. 0× 1028 Mg 2+(aq) + 4 H 2 O(l) (18. 4) K = Ksp(1/Kw)2 = (1. 8× 10− 11)(1. 0× 1028) = 1. 8× 1017 Slide 18 - 21 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

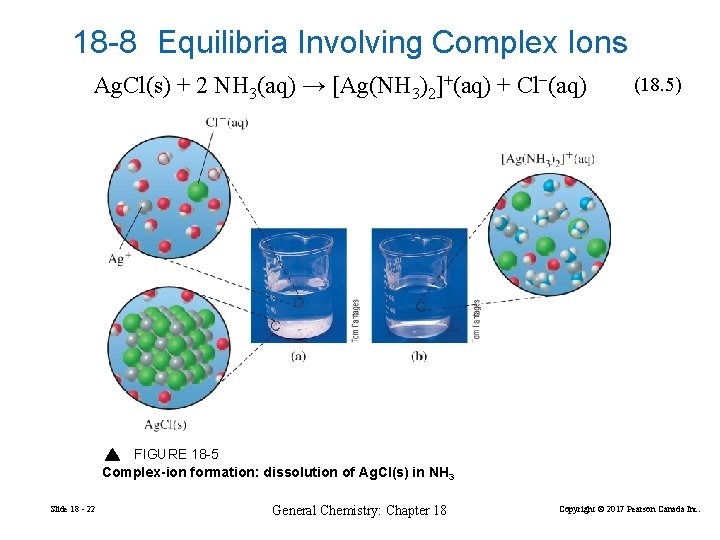
18 -8 Equilibria Involving Complex Ions Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl−(aq) (18. 5) FIGURE 18 -5 Complex-ion formation: dissolution of Ag. Cl(s) in NH 3 Slide 18 - 22 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

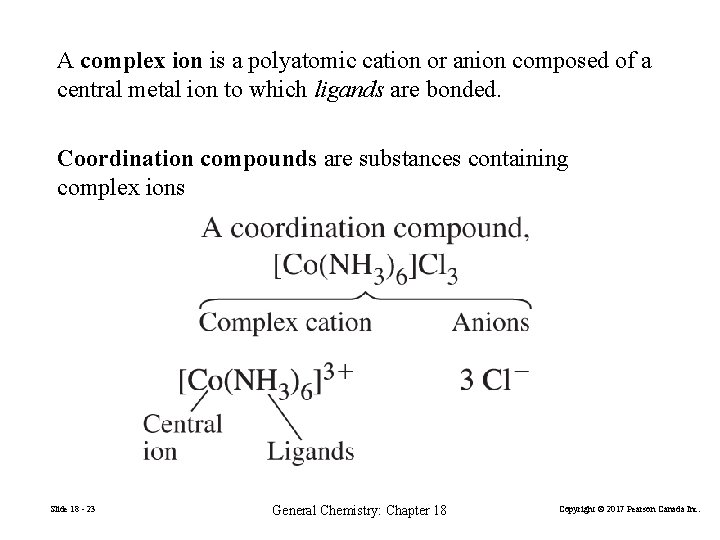
A complex ion is a polyatomic cation or anion composed of a central metal ion to which ligands are bonded. Coordination compounds are substances containing complex ions Slide 18 - 23 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.
![Formation constant Kf Ag Cls 2 NH 3aq AgNH 32aq Claq Formation constant, Kf Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl−(aq)](https://slidetodoc.com/presentation_image_h/3254c6a184ba7c00b57b77abb4866f2d/image-24.jpg)
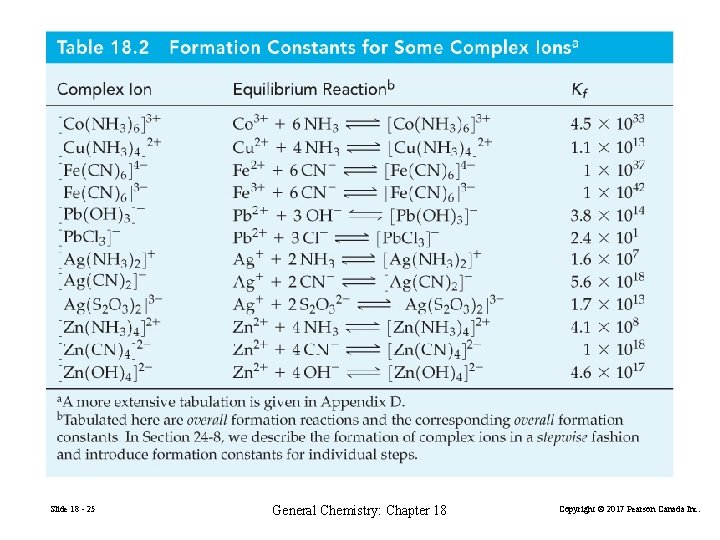
Formation constant, Kf Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl−(aq) (18. 5) It helps to think of this reaction as two simultaneous equilbria. Ag. Cl(s) Ag+(aq) + Cl−(aq) Ag+(aq) + 2 NH 3(aq) [Ag(NH 3)2]+(aq) (18. 6) (18. 7) [[Ag(NH 3)2]+] 7 = 1. 6× 10 Kf = [Ag+][NH 3]2 Slide 18 - 24 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

Slide 18 - 25 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

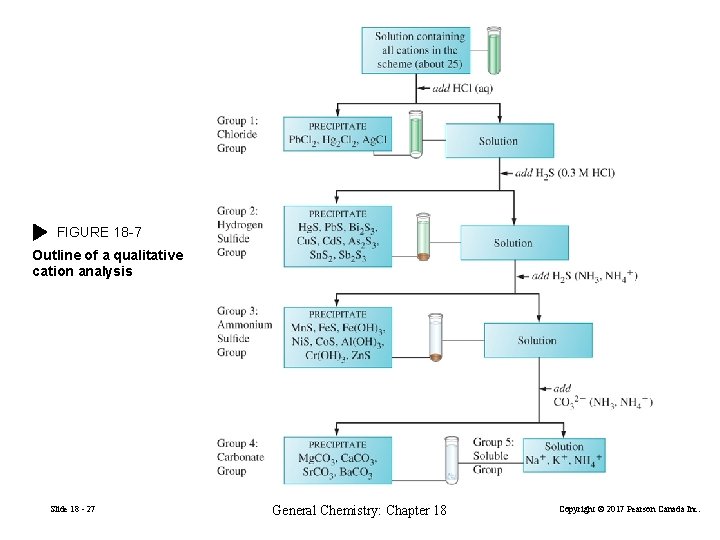
18 -9 Qualitative Cation Analysis An analysis that aims at identifying the cations present in a mixture but not their quantities. We think of cations in solubility groups according to the conditions that causes precipitation. Group 1: chloride group (HCl) Group 2: hydrogen sulfide group (H 2 S) Group 3: ammonium sulfide group (NH 3, NH 4+) Group 4: carbonate group (CO 32−) Group 5: soluble group Selectively precipitate the first group of cations then move on to the next. Slide 18 - 26 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

FIGURE 18 -7 Outline of a qualitative cation analysis Slide 18 - 27 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

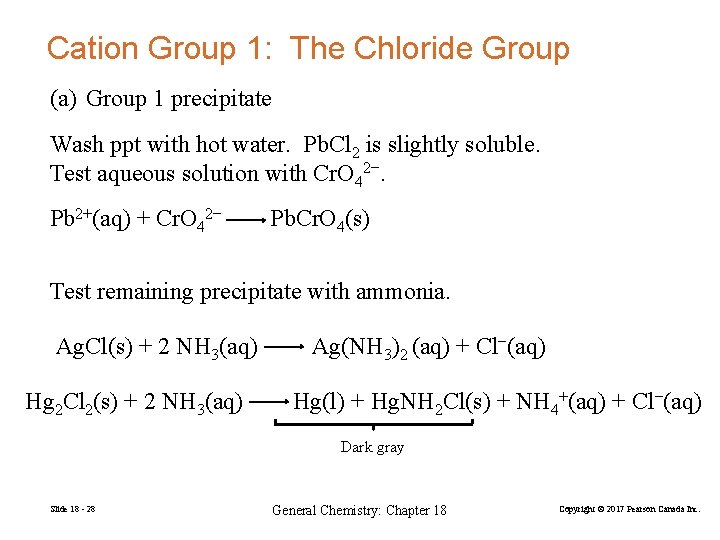
Cation Group 1: The Chloride Group (a) Group 1 precipitate Wash ppt with hot water. Pb. Cl 2 is slightly soluble. Test aqueous solution with Cr. O 42−. Pb 2+(aq) + Cr. O 42− Pb. Cr. O 4(s) Test remaining precipitate with ammonia. Ag. Cl(s) + 2 NH 3(aq) Hg 2 Cl 2(s) + 2 NH 3(aq) Ag(NH 3)2 (aq) + Cl−(aq) Hg(l) + Hg. NH 2 Cl(s) + NH 4+(aq) + Cl−(aq) Dark gray Slide 18 - 28 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

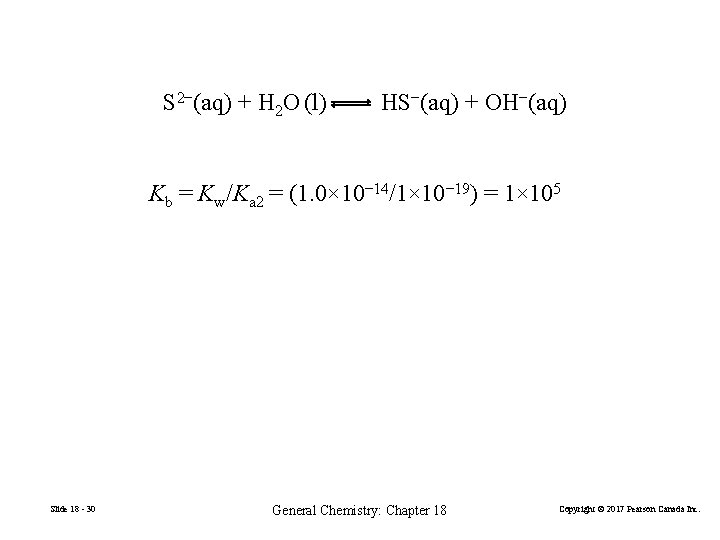
Cation Groups 2 and 3: Equilibria Involving Hydrogen Sulfide. H 2 S, hydrogen sulfide or hydrosulfuric acid, is a weak diprotic acid H 2 S(aq) + H 2 O(l) H 3 O+(aq) + HS−(aq) Ka 1 = 1. 0× 10− 7 HS−(aq) + H 2 O(l) H 3 O+(aq) + S 2−(aq) Ka 2 = 1× 10− 19 S 2− is an extremely strong base and is unlikely to be the precipitating agent for the sulfide groups. Slide 18 - 29 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

S 2−(aq) + H 2 O (l) HS−(aq) + OH−(aq) Kb = Kw/Ka 2 = (1. 0× 10− 14/1× 10− 19) = 1× 105 Slide 18 - 30 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

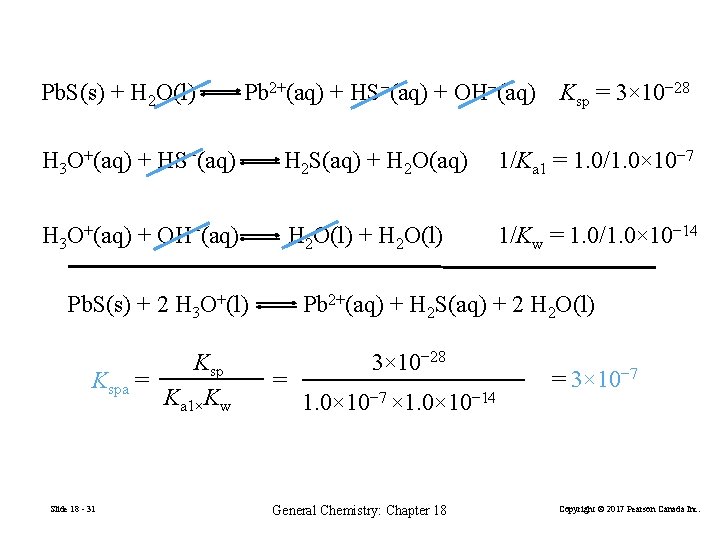
Pb. S(s) + H 2 O(l) Pb 2+(aq) + HS−(aq) + OH−(aq) Ksp = 3× 10− 28 H 3 O+(aq) + HS−(aq) H 2 S(aq) + H 2 O(aq) 1/Ka 1 = 1. 0/1. 0× 10− 7 H 3 O+(aq) + OH−(aq) H 2 O(l) + H 2 O(l) 1/Kw = 1. 0/1. 0× 10− 14 Pb. S(s) + 2 H 3 O+(l) Kspa = Ka 1×Kw Slide 18 - 31 Pb 2+(aq) + H 2 S(aq) + 2 H 2 O(l) = 3× 10− 28 1. 0× 10− 7 × 1. 0× 10− 14 General Chemistry: Chapter 18 = 3× 10− 7 Copyright © 2017 Pearson Canada Inc.

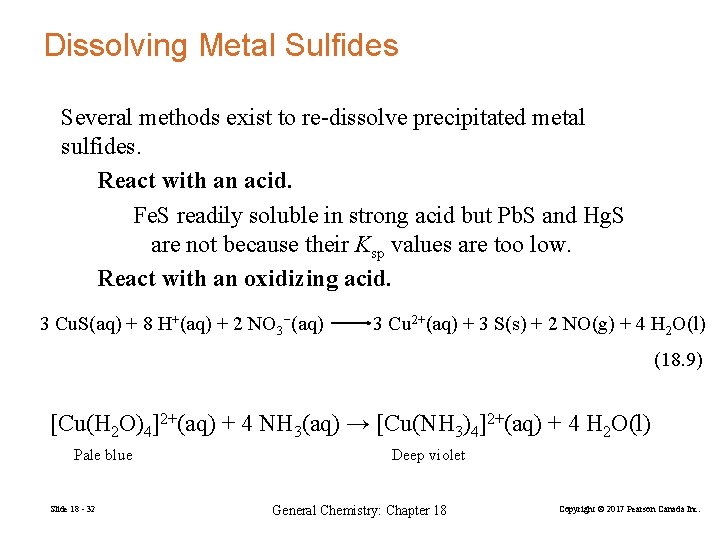
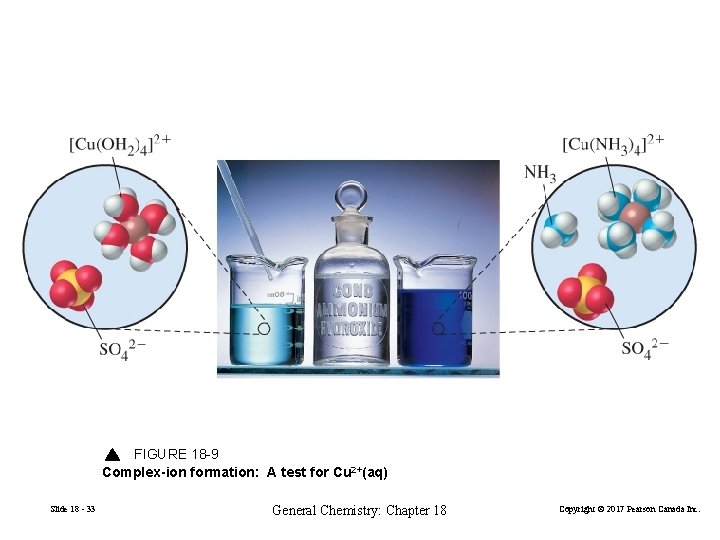
Dissolving Metal Sulfides Several methods exist to re-dissolve precipitated metal sulfides. React with an acid. Fe. S readily soluble in strong acid but Pb. S and Hg. S are not because their Ksp values are too low. React with an oxidizing acid. 3 Cu. S(aq) + 8 H+(aq) + 2 NO 3−(aq) 3 Cu 2+(aq) + 3 S(s) + 2 NO(g) + 4 H 2 O(l) (18. 9) [Cu(H 2 O)4]2+(aq) + 4 NH 3(aq) → [Cu(NH 3)4]2+(aq) + 4 H 2 O(l) Pale blue Slide 18 - 32 Deep violet General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

FIGURE 18 -9 Complex-ion formation: A test for Cu 2+(aq) Slide 18 - 33 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.

End of Chapter Slide 18 - 34 General Chemistry: Chapter 18 Copyright © 2017 Pearson Canada Inc.
 Management eleventh edition stephen p robbins
Management eleventh edition stephen p robbins Management eleventh edition stephen p robbins
Management eleventh edition stephen p robbins Management eleventh edition
Management eleventh edition Management eleventh edition stephen p robbins
Management eleventh edition stephen p robbins General chemistry 11th edition
General chemistry 11th edition Chadha committee
Chadha committee Eleventh 5 year plan
Eleventh 5 year plan Eleventh plan
Eleventh plan For his eleventh birthday elvis presley
For his eleventh birthday elvis presley Introduction to genetic analysis tenth edition
Introduction to genetic analysis tenth edition Fluid mechanics fundamentals and applications 3rd edition
Fluid mechanics fundamentals and applications 3rd edition Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Modern systems analysis and design 7th edition
Modern systems analysis and design 7th edition Discrete mathematics with applications fourth edition
Discrete mathematics with applications fourth edition Using mis (10th edition) 10th edition
Using mis (10th edition) 10th edition Mis
Mis Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Electrical engineering
Electrical engineering Learning principles and applications
Learning principles and applications Applications of nuclear chemistry
Applications of nuclear chemistry Applications of nuclear chemistry
Applications of nuclear chemistry Modern labor economics 12th edition solution
Modern labor economics 12th edition solution Modern labor economics 12th edition
Modern labor economics 12th edition Modern real estate practice in pennsylvania 14th edition
Modern real estate practice in pennsylvania 14th edition Modern database management 12th edition ppt
Modern database management 12th edition ppt Modern database management 12th edition ppt
Modern database management 12th edition ppt Modern operating systems 3rd edition
Modern operating systems 3rd edition Structured computer organization
Structured computer organization Modern database management 8th edition
Modern database management 8th edition University physics with modern physics fifteenth edition
University physics with modern physics fifteenth edition Transaction cannot be subdivided
Transaction cannot be subdivided Modern labor economics 12th edition
Modern labor economics 12th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition