GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION




![[Co(NH 3)6]3+ [Co. Cl 4(NH 3)2]− Complex cation Complex anion [Co. Cl 3(NH 3)3] [Co(NH 3)6]3+ [Co. Cl 4(NH 3)2]− Complex cation Complex anion [Co. Cl 3(NH 3)3]](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-5.jpg)

![FIGURE 24 -3 Three representations of the chelate [Pt(en)2]2+ Slide 24 - 9 General FIGURE 24 -3 Three representations of the chelate [Pt(en)2]2+ Slide 24 - 9 General](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-9.jpg)


![24 -4 Isomerism Ionization Isomerism [Cr. Cl(NH 3)5]SO 4 [Cr(NH 3)5 SO 4]Cl Pentamminesulfatochromium 24 -4 Isomerism Ionization Isomerism [Cr. Cl(NH 3)5]SO 4 [Cr(NH 3)5 SO 4]Cl Pentamminesulfatochromium](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-12.jpg)
![Linkage Isomerism [Co(NH 3)5(ONO)]2+ [Co(NO 2)(NH 3)5]2+ Pentaamminenitrito-N-cobalt(III) ion Pentaamminenitrito-O-cobalt(III) ion Geometric Isomerism [Pt. Linkage Isomerism [Co(NH 3)5(ONO)]2+ [Co(NO 2)(NH 3)5]2+ Pentaamminenitrito-N-cobalt(III) ion Pentaamminenitrito-O-cobalt(III) ion Geometric Isomerism [Pt.](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-13.jpg)
![The geometric isomers of [Pt. Cl 2(NH 3)2] Slide 24 - 15 General Chemistry: The geometric isomers of [Pt. Cl 2(NH 3)2] Slide 24 - 15 General Chemistry:](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-15.jpg)


![The geometric isomers of [Co. Cl 3(NH 3)3] Slide 24 - 18 General Chemistry: The geometric isomers of [Co. Cl 3(NH 3)3] Slide 24 - 18 General Chemistry:](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-18.jpg)
![Isomerism and Werner’s Theory FIGURE 24 -10 Hypothetical strucutres for [Co. Cl 2(NH 3)4]+ Isomerism and Werner’s Theory FIGURE 24 -10 Hypothetical strucutres for [Co. Cl 2(NH 3)4]+](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-22.jpg)



![24 -11 Applications of Coordination Chemistry Cisplatin: A Cancer-Fighting Drug trans-[Pt. Cl 2(NH 3)2] 24 -11 Applications of Coordination Chemistry Cisplatin: A Cancer-Fighting Drug trans-[Pt. Cl 2(NH 3)2]](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-40.jpg)
 2 Co(Cl. O 4)2 • 6 H 2 Hydrates [Co(H 2 O)6](Cl. O 4) 2 Co(Cl. O 4)2 • 6 H 2](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-42.jpg)
![Qualitative Analysis [Co(SCN)4]2− complex ion [Fe(H 2 O)4 (SCN)]2+ complex ion mixture of [Fe. Qualitative Analysis [Co(SCN)4]2− complex ion [Fe(H 2 O)4 (SCN)]2+ complex ion mixture of [Fe.](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-45.jpg)



- Slides: 50

GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION PETRUCCI HERRING MADURA BISSONNETTE Complex Ions and Coordination Compounds 24 PHILIP DUTTON UNIVERSITY OF WINDSOR DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY Slide 24 - 1 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

Complex Ions and Coordination Compounds Slide 24 - 2 CONTENTS 24 -1 Werner’s Theory of Coordination Compounds: An Overview 24 -2 Ligands 24 -3 Nomenclature 24 -4 Isomerism 24 -5 Bonding in Complex Ions: Crystal Field Theory 24 -6 Magnetic Properties of Coordination Compounds and Crystal Field Theory 24 -7 Color and the colors of Complexes 24 -8 Aspects of Complex-Ion Equilibria General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

Complex Ions and Coordination Compounds Slide 24 - 3 CONTENTS 24 -9 Acid-Base Reactions of Complex Ions 24 -10 Some Kinetic Considerations 24 -11 Applications of Coordination Chemistry General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

24 -1 Werner’s Theory of Coordination Compounds: An Overview FIGURE 24 -1 Two coordination compounds Slide 24 - 4 General Chemistry: Chapter 24 Alfred Werner (1866 -1919) Copyright © 2017 Pearson Canada Inc.
![CoNH 363 Co Cl 4NH 32 Complex cation Complex anion Co Cl 3NH 33 [Co(NH 3)6]3+ [Co. Cl 4(NH 3)2]− Complex cation Complex anion [Co. Cl 3(NH 3)3]](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-5.jpg)
[Co(NH 3)6]3+ [Co. Cl 4(NH 3)2]− Complex cation Complex anion [Co. Cl 3(NH 3)3] K 4[Fe(CN)6] Neutral complex Coordination compound Slide 24 - 5 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

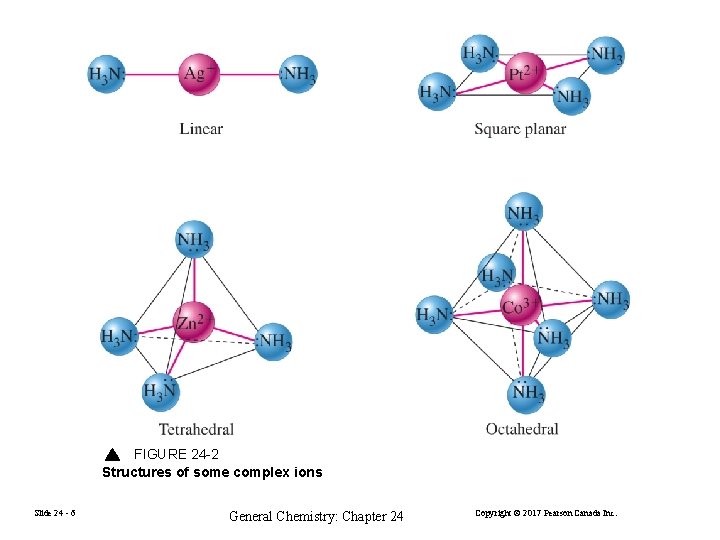
FIGURE 24 -2 Structures of some complex ions Slide 24 - 6 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

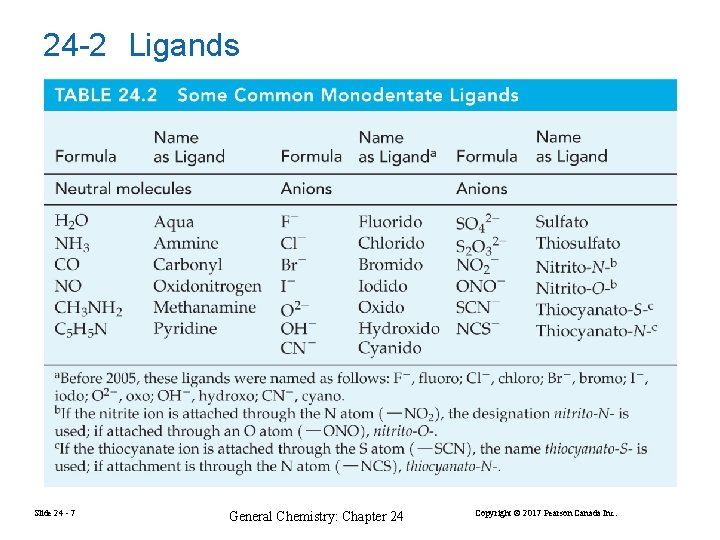
24 -2 Ligands Slide 24 - 7 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

Slide 24 - 8 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.
![FIGURE 24 3 Three representations of the chelate Pten22 Slide 24 9 General FIGURE 24 -3 Three representations of the chelate [Pt(en)2]2+ Slide 24 - 9 General](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-9.jpg)
FIGURE 24 -3 Three representations of the chelate [Pt(en)2]2+ Slide 24 - 9 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

24 -3 Nomenclature 1) Anions as ligands are named using the ending –o –ide changes to–ido, –ite to –ito, and –ate to –ato. 2) Neutral molecules generally carry the unmodified name 3) The number of ligands is denoted by a prefix mono, di, tri, tetra, penta, hexa 4) Ligands are named first in alphabetical order followed by the name of the metal center. Oxidation state is denoted by a Roman numeral. Anions end the metal name in -ate 5) Formula is written with metal first, followed by the ligand symbols in alphabetical order 6) Cations come first, followed by anions Slide 24 - 10 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

Slide 24 - 11 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.
![24 4 Isomerism Ionization Isomerism Cr ClNH 35SO 4 CrNH 35 SO 4Cl Pentamminesulfatochromium 24 -4 Isomerism Ionization Isomerism [Cr. Cl(NH 3)5]SO 4 [Cr(NH 3)5 SO 4]Cl Pentamminesulfatochromium](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-12.jpg)
24 -4 Isomerism Ionization Isomerism [Cr. Cl(NH 3)5]SO 4 [Cr(NH 3)5 SO 4]Cl Pentamminesulfatochromium (III) chloride Pentamminechloridochromiium(III) sulfate Coordination Isomerism [Cr(NH 3)6][Co(CN)6] [Co(NH 3)6][Cr(CN)6] Hexaamminecobalt(III) hexacyanidochromate(III) Slide 24 - 12 Hexaamminechromium(III) hexacyanidocobaltate(III) General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.
![Linkage Isomerism CoNH 35ONO2 CoNO 2NH 352 PentaamminenitritoNcobaltIII ion PentaamminenitritoOcobaltIII ion Geometric Isomerism Pt Linkage Isomerism [Co(NH 3)5(ONO)]2+ [Co(NO 2)(NH 3)5]2+ Pentaamminenitrito-N-cobalt(III) ion Pentaamminenitrito-O-cobalt(III) ion Geometric Isomerism [Pt.](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-13.jpg)
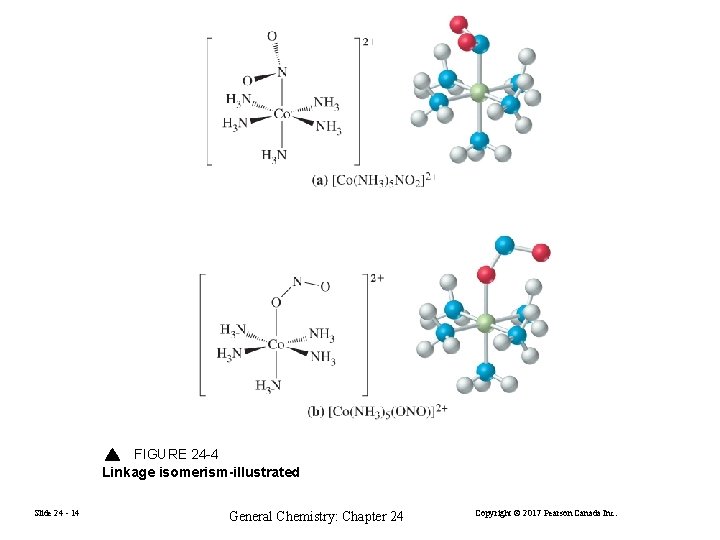
Linkage Isomerism [Co(NH 3)5(ONO)]2+ [Co(NO 2)(NH 3)5]2+ Pentaamminenitrito-N-cobalt(III) ion Pentaamminenitrito-O-cobalt(III) ion Geometric Isomerism [Pt. Cl 2(NH 3)2] cis-diamminedichloridoplatinum(II) or trans-diamminedichloridoplatinum(II) Slide 24 - 13 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

FIGURE 24 -4 Linkage isomerism-illustrated Slide 24 - 14 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.
![The geometric isomers of Pt Cl 2NH 32 Slide 24 15 General Chemistry The geometric isomers of [Pt. Cl 2(NH 3)2] Slide 24 - 15 General Chemistry:](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-15.jpg)
The geometric isomers of [Pt. Cl 2(NH 3)2] Slide 24 - 15 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

FIGURE 24 -5 Geometric isomerism-illustrated Slide 24 - 16 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

FIGURE 24 -6 Cis and trans isomers of an octahedral complex Slide 24 - 17 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.
![The geometric isomers of Co Cl 3NH 33 Slide 24 18 General Chemistry The geometric isomers of [Co. Cl 3(NH 3)3] Slide 24 - 18 General Chemistry:](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-18.jpg)
The geometric isomers of [Co. Cl 3(NH 3)3] Slide 24 - 18 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

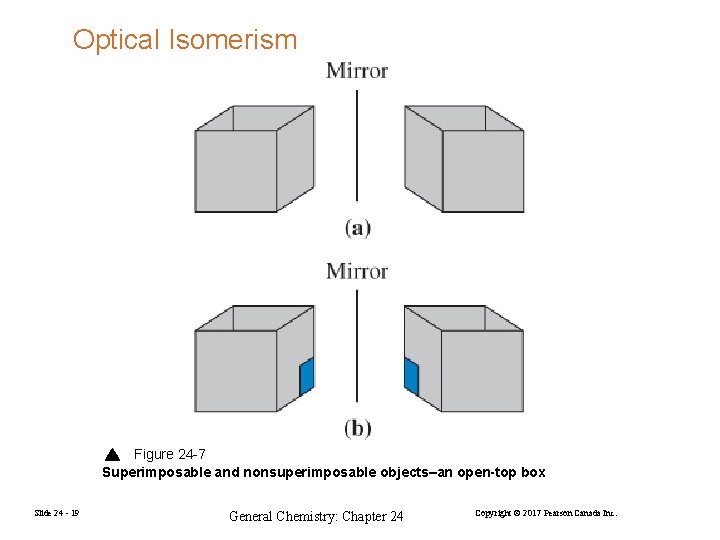
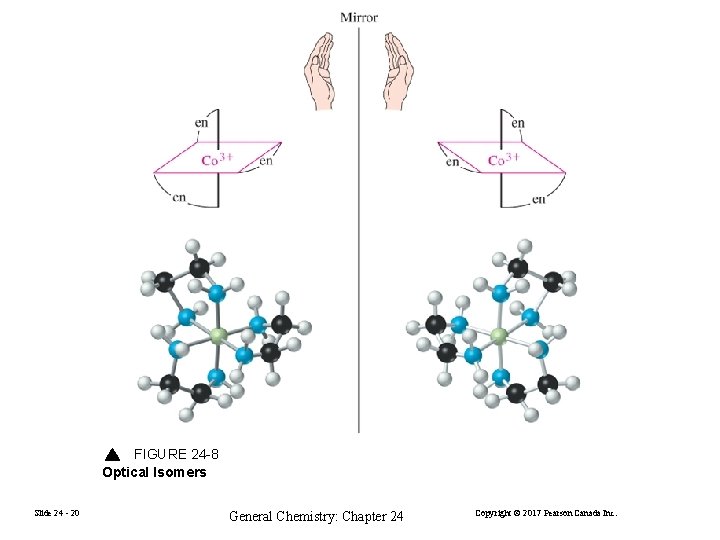
Optical Isomerism Figure 24 -7 Superimposable and nonsuperimposable objects–an open-top box Slide 24 - 19 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

FIGURE 24 -8 Optical Isomers Slide 24 - 20 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

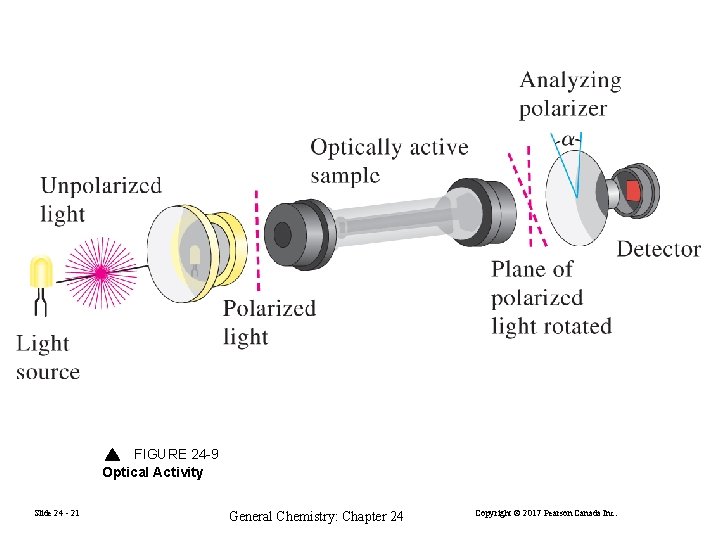
FIGURE 24 -9 Optical Activity Slide 24 - 21 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.
![Isomerism and Werners Theory FIGURE 24 10 Hypothetical strucutres for Co Cl 2NH 34 Isomerism and Werner’s Theory FIGURE 24 -10 Hypothetical strucutres for [Co. Cl 2(NH 3)4]+](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-22.jpg)
Isomerism and Werner’s Theory FIGURE 24 -10 Hypothetical strucutres for [Co. Cl 2(NH 3)4]+ Slide 24 - 22 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

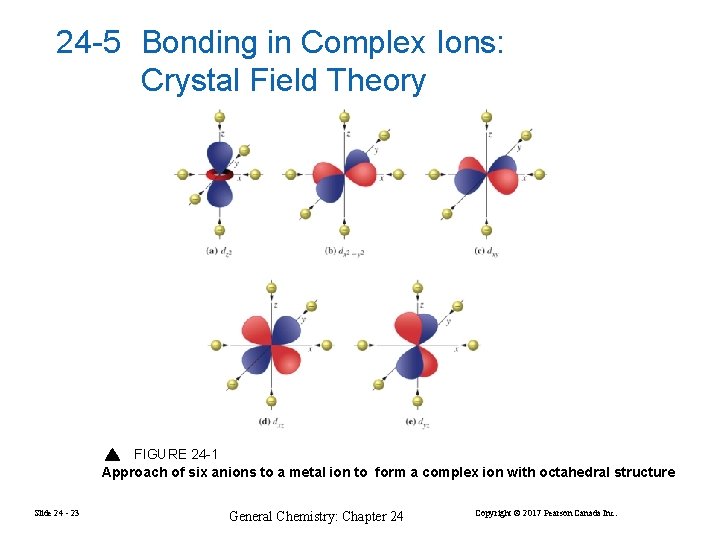
24 -5 Bonding in Complex Ions: Crystal Field Theory FIGURE 24 -1 Approach of six anions to a metal ion to form a complex ion with octahedral structure Slide 24 - 23 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

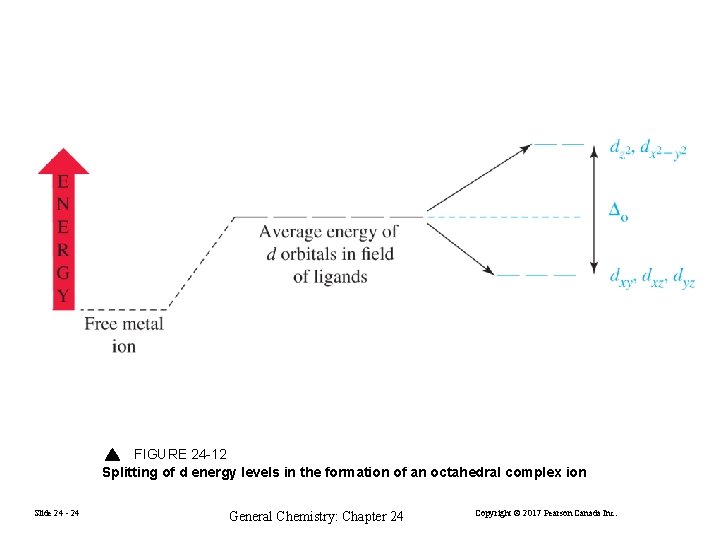
FIGURE 24 -12 Splitting of d energy levels in the formation of an octahedral complex ion Slide 24 - 24 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

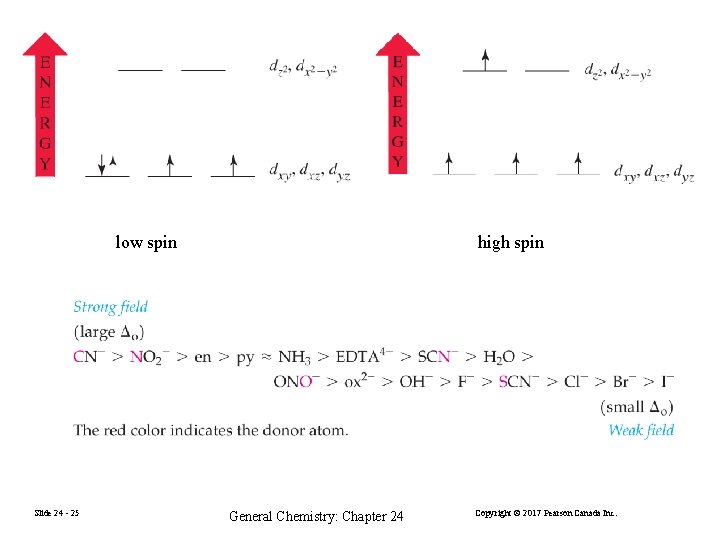
low spin Slide 24 - 25 high spin General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

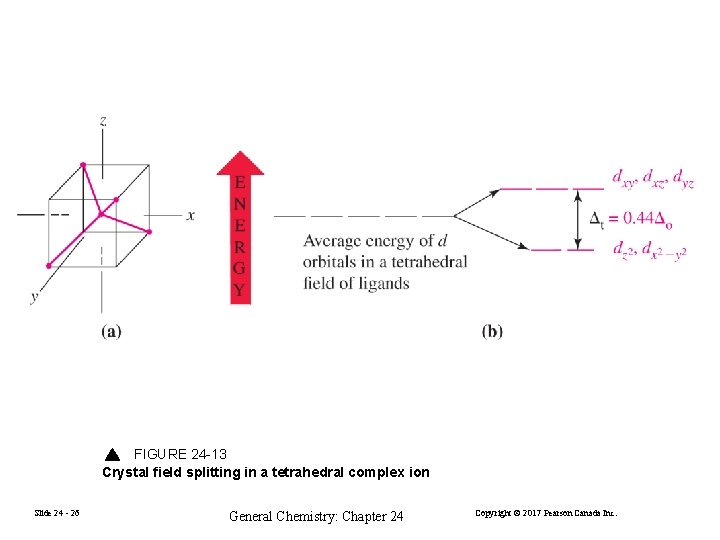
FIGURE 24 -13 Crystal field splitting in a tetrahedral complex ion Slide 24 - 26 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

Slide 24 - 27 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

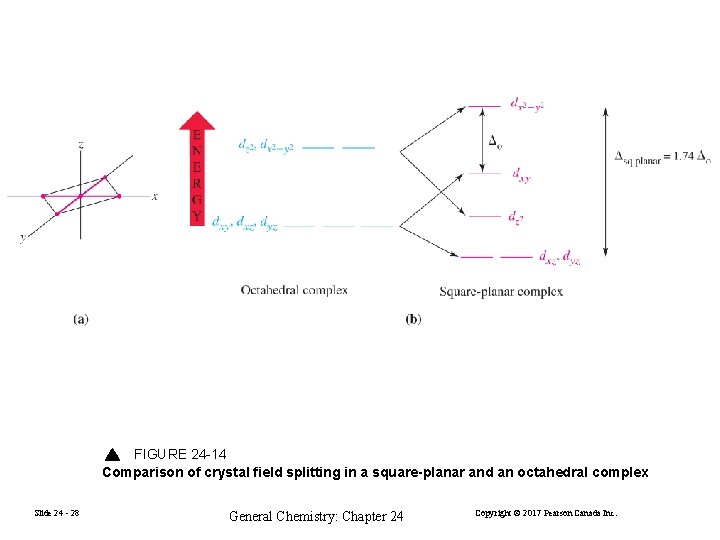
FIGURE 24 -14 Comparison of crystal field splitting in a square-planar and an octahedral complex Slide 24 - 28 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

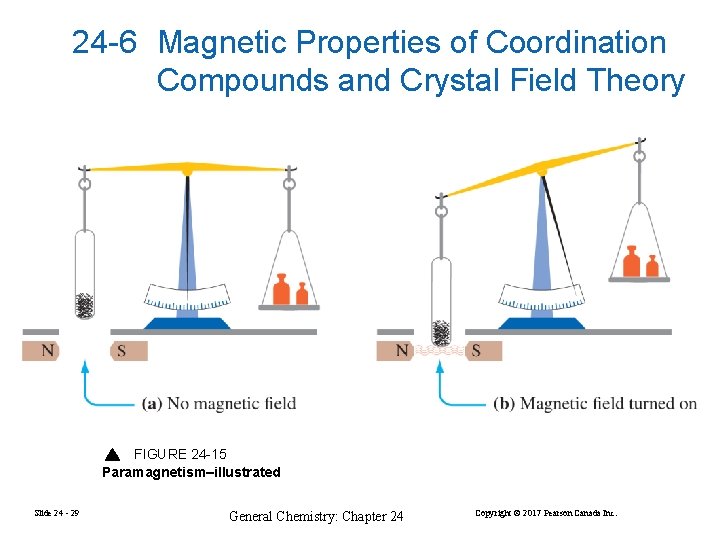
24 -6 Magnetic Properties of Coordination Compounds and Crystal Field Theory FIGURE 24 -15 Paramagnetism–illustrated Slide 24 - 29 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

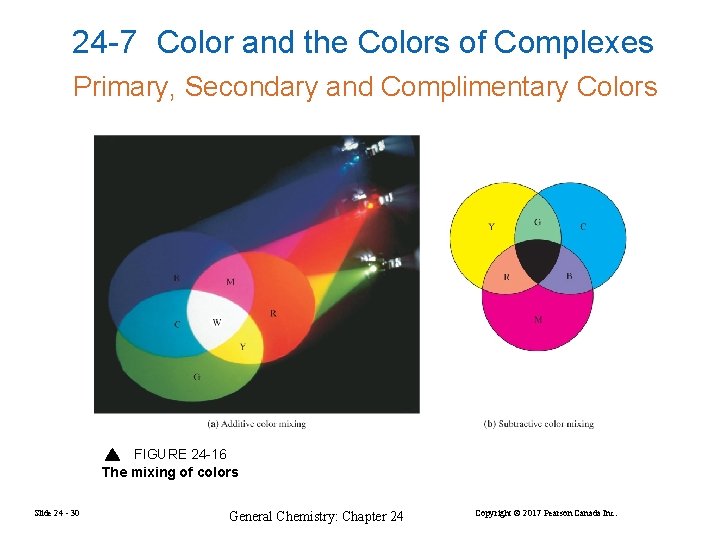
24 -7 Color and the Colors of Complexes Primary, Secondary and Complimentary Colors FIGURE 24 -16 The mixing of colors Slide 24 - 30 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

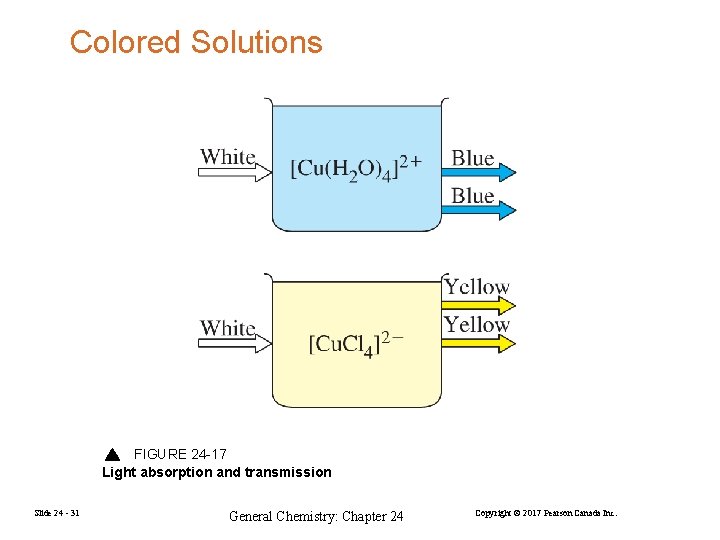
Colored Solutions FIGURE 24 -17 Light absorption and transmission Slide 24 - 31 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

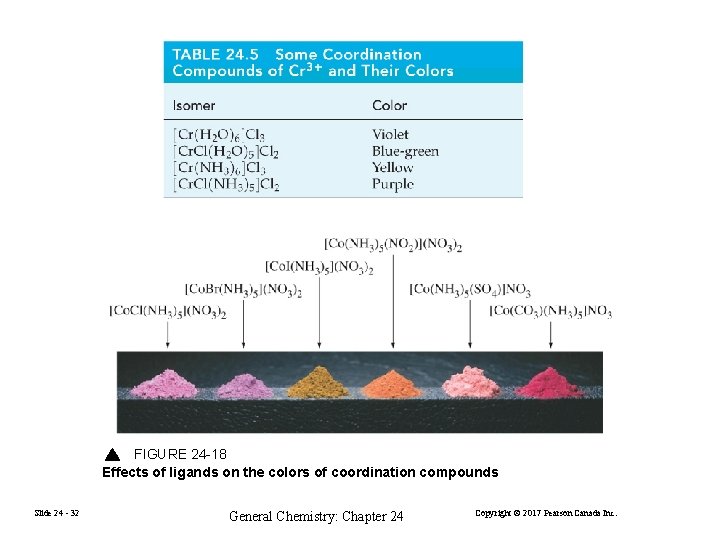
FIGURE 24 -18 Effects of ligands on the colors of coordination compounds Slide 24 - 32 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

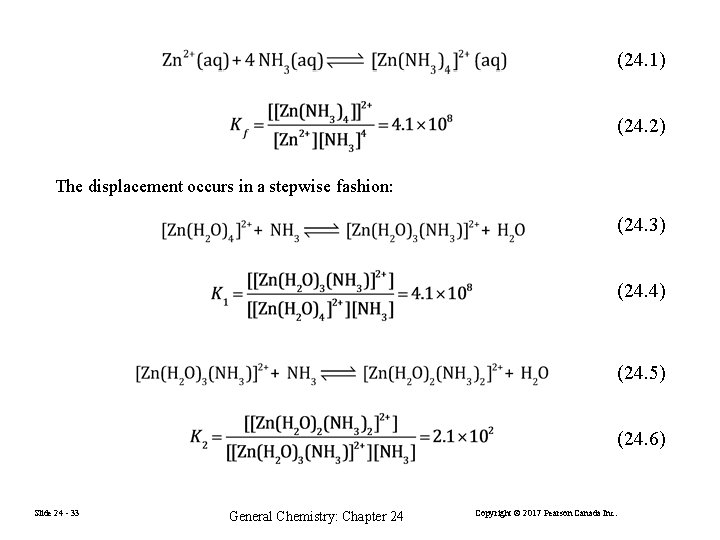
(24. 1) (24. 2) The displacement occurs in a stepwise fashion: (24. 3) (24. 4) (24. 5) (24. 6) Slide 24 - 33 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

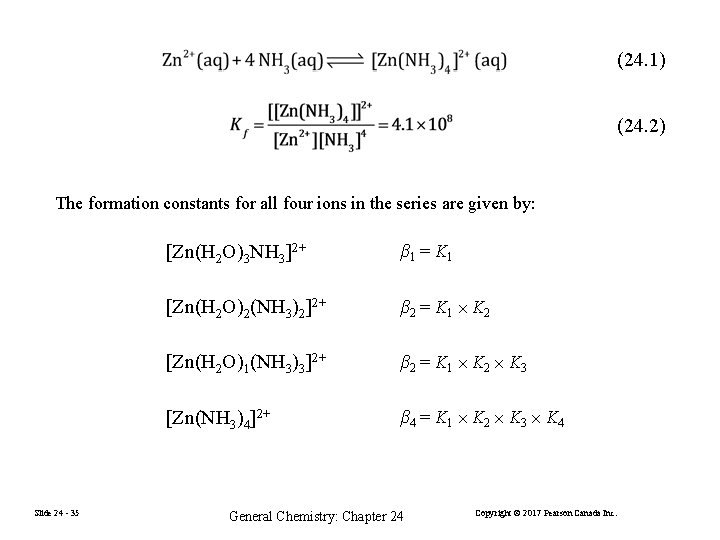
(24. 1) (24. 2) K 1 is often designated β 1 and is called the formation constant for [Zn(H 2 O)3 NH 3]2+ β 2 is the formation constant for [Zn(H 2 O)2(NH 3) 2]2+, the sum of (23. 3) and (23. 4) (24. 7) (24. 8) Slide 24 - 34 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

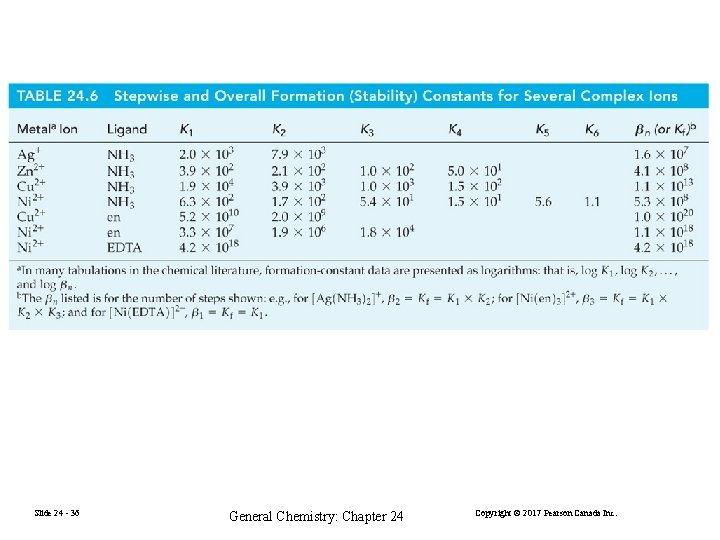
(24. 1) (24. 2) The formation constants for all four ions in the series are given by: Slide 24 - 35 [Zn(H 2 O)3 NH 3]2+ β 1 = K 1 [Zn(H 2 O)2(NH 3)2]2+ β 2 = K 1 K 2 [Zn(H 2 O)1(NH 3)3]2+ β 2 = K 1 K 2 K 3 [Zn(NH 3)4]2+ β 4 = K 1 K 2 K 3 K 4 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

Slide 24 - 36 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

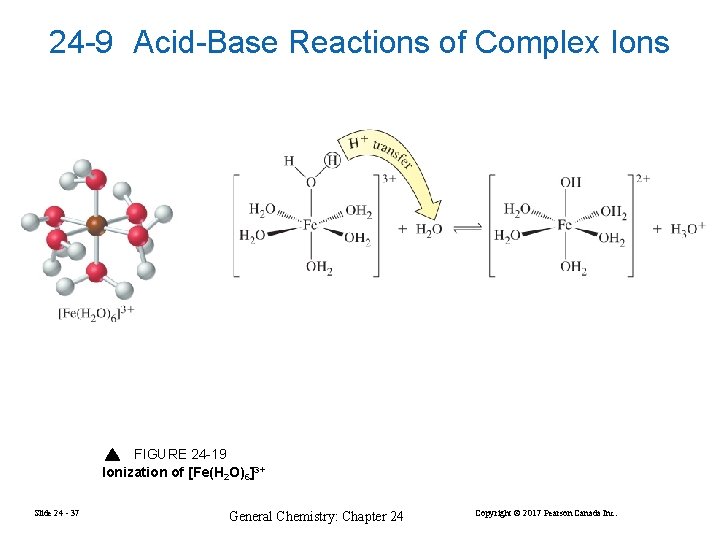
24 -9 Acid-Base Reactions of Complex Ions FIGURE 24 -19 Ionization of [Fe(H 2 O)6]3+ Slide 24 - 37 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

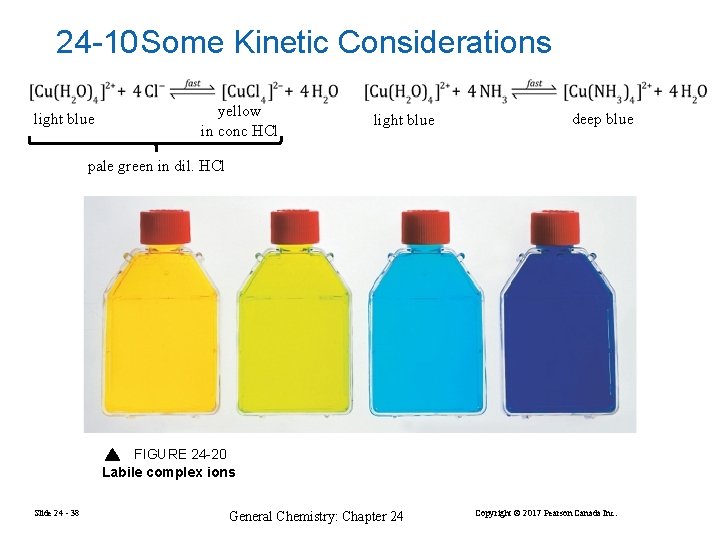
24 -10 Some Kinetic Considerations light blue yellow in conc HCl light blue deep blue pale green in dil. HCl FIGURE 24 -20 Labile complex ions Slide 24 - 38 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

FIGURE 24 -2 Inert complex ions Slide 24 - 39 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.
![24 11 Applications of Coordination Chemistry Cisplatin A CancerFighting Drug transPt Cl 2NH 32 24 -11 Applications of Coordination Chemistry Cisplatin: A Cancer-Fighting Drug trans-[Pt. Cl 2(NH 3)2]](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-40.jpg)
24 -11 Applications of Coordination Chemistry Cisplatin: A Cancer-Fighting Drug trans-[Pt. Cl 2(NH 3)2] (transplatin) cis-[Pt. Cl 2(NH 3)2] (cisplatin) K 2[Pt. Cl 4] + 4 KI K 2[Pt. I 4] + 4 KCl K 2[Pt. I 4] + NH 3 + KI K 2[Pt. I 2(NH 3)] + NH 3 + KI treat with Ag. NO 3 followed by KCl to obtain cisplatin Slide 24 - 40 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

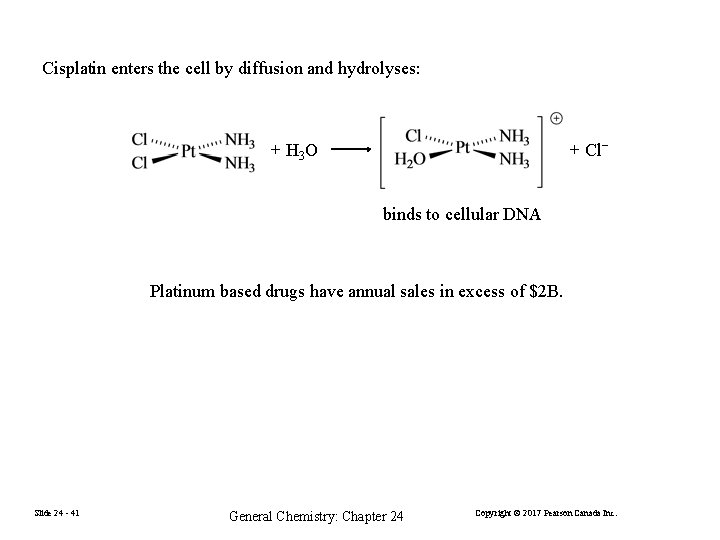
Cisplatin enters the cell by diffusion and hydrolyses: + H 3 O + Cl− binds to cellular DNA Platinum based drugs have annual sales in excess of $2 B. Slide 24 - 41 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.
 2 Co(Cl. O 4)2 • 6 H 2](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-42.jpg)
Hydrates [Co(H 2 O)6](Cl. O 4) 2 Co(Cl. O 4)2 • 6 H 2 O [Cu. SO 4] • 5 H 2 O [Cu(H 2 O)4][SO 4 • H 2 O] Ba. Cl 2 • 2 H 2 O KAl(SO 4)2 • 12 H 2 O Slide 24 - 42 lattice water In alums, some water is coordinated to an ion and some is lattice water General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

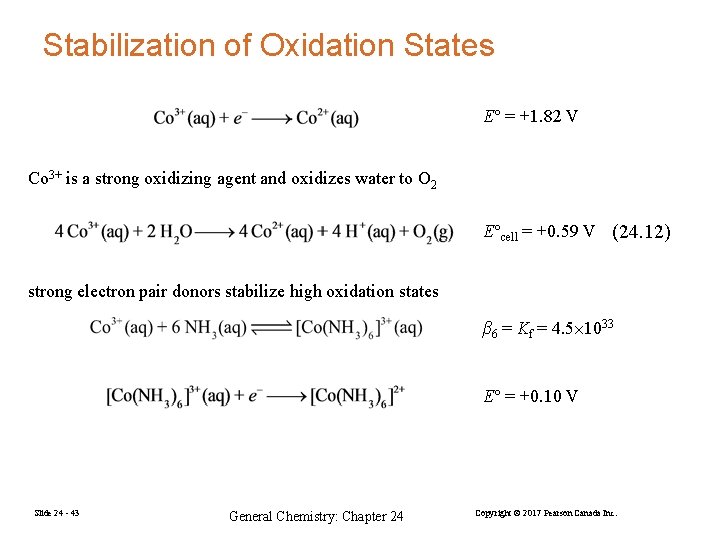
Stabilization of Oxidation States E° = +1. 82 V Co 3+ is a strong oxidizing agent and oxidizes water to O 2 E°cell = +0. 59 V (24. 12) strong electron pair donors stabilize high oxidation states β 6 = Kf = 4. 5 1033 E° = +0. 10 V Slide 24 - 43 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

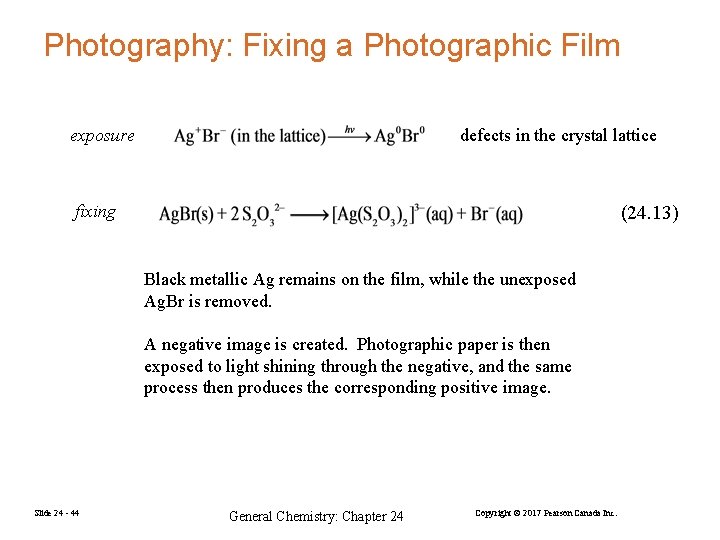
Photography: Fixing a Photographic Film exposure defects in the crystal lattice fixing (24. 13) Black metallic Ag remains on the film, while the unexposed Ag. Br is removed. A negative image is created. Photographic paper is then exposed to light shining through the negative, and the same process then produces the corresponding positive image. Slide 24 - 44 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.
![Qualitative Analysis CoSCN42 complex ion FeH 2 O4 SCN2 complex ion mixture of Fe Qualitative Analysis [Co(SCN)4]2− complex ion [Fe(H 2 O)4 (SCN)]2+ complex ion mixture of [Fe.](https://slidetodoc.com/presentation_image_h/9916051ee8dde0bb7e61d79e1744dfc0/image-45.jpg)
Qualitative Analysis [Co(SCN)4]2− complex ion [Fe(H 2 O)4 (SCN)]2+ complex ion mixture of [Fe. F 6] and [Co(SCN)4]2− complex ion FIGURE 24 -22 Qualitative tests for Co 2+ and Fe 3+ Slide 24 - 45 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

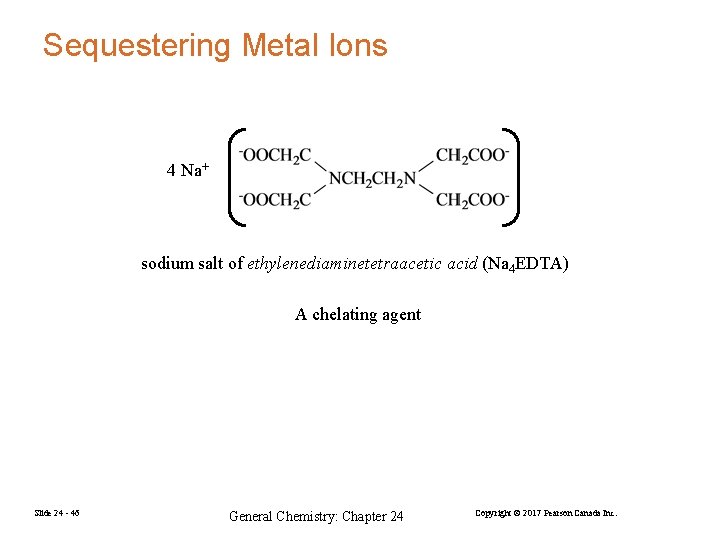
Sequestering Metal Ions 4 Na+ sodium salt of ethylenediaminetetraacetic acid (Na 4 EDTA) A chelating agent Slide 24 - 46 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

FIGURE 23 -23 Structure of a metal-EDTA complex Slide 24 - 47 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

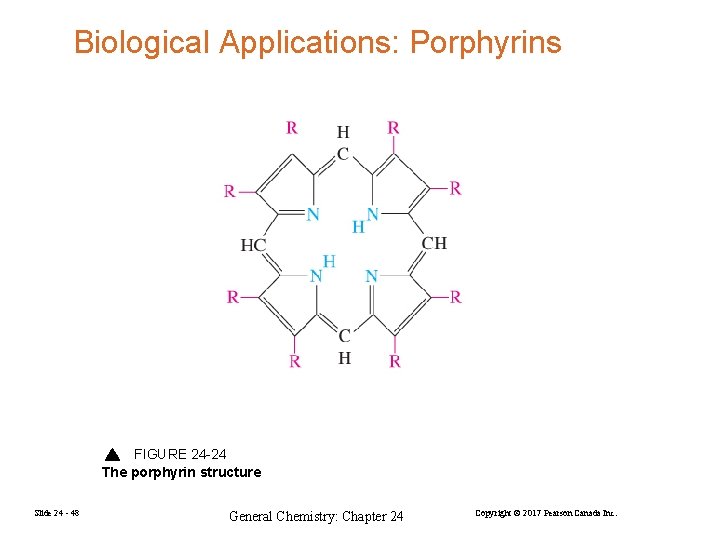
Biological Applications: Porphyrins FIGURE 24 -24 The porphyrin structure Slide 24 - 48 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

FIGURE 24 -25 Structure of chlorophyll a Slide 24 - 49 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.

End of Chapter Slide 24 - 50 General Chemistry: Chapter 24 Copyright © 2017 Pearson Canada Inc.
 Management eleventh edition
Management eleventh edition Management stephen p robbins 11th edition
Management stephen p robbins 11th edition Management eleventh edition
Management eleventh edition Management eleventh edition
Management eleventh edition General chemistry 11th edition
General chemistry 11th edition Chadha committee
Chadha committee Eleventh 5 year plan
Eleventh 5 year plan Eleventh plan
Eleventh plan For his eleventh birthday elvis presley
For his eleventh birthday elvis presley Human genetics concepts and applications 10th edition
Human genetics concepts and applications 10th edition Fluid mechanics fundamentals and applications
Fluid mechanics fundamentals and applications Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Modern systems analysis and design 7th edition
Modern systems analysis and design 7th edition Discrete mathematics with applications fourth edition
Discrete mathematics with applications fourth edition Using mis (10th edition) 10th edition
Using mis (10th edition) 10th edition Zulily case study
Zulily case study Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Principles and applications of electrical engineering
Principles and applications of electrical engineering Learning principles and applications
Learning principles and applications 25 m/s
25 m/s Irradiated food
Irradiated food Modern labor economics 12th edition solution
Modern labor economics 12th edition solution Modern labor economics 12th edition pdf
Modern labor economics 12th edition pdf Modern real estate practice in pennsylvania
Modern real estate practice in pennsylvania Modern database management 12th edition ppt
Modern database management 12th edition ppt Modern database management system
Modern database management system Modern operating systems 3rd edition
Modern operating systems 3rd edition Tanenbaum structured computer organization
Tanenbaum structured computer organization Modern database management 8th edition
Modern database management 8th edition University physics with modern physics fifteenth edition
University physics with modern physics fifteenth edition Transaction cannot be subdivided
Transaction cannot be subdivided Modern labor economics 12th edition
Modern labor economics 12th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Expert systems: principles and programming, fourth edition
Expert systems: principles and programming, fourth edition Rearranged most stable carbocation is
Rearranged most stable carbocation is Pericyclic
Pericyclic Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Introductory chemistry 4th edition
Introductory chemistry 4th edition Prefix multipliers
Prefix multipliers Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Chemistry the central science 14th edition
Chemistry the central science 14th edition Acid chloride + grignard reagent
Acid chloride + grignard reagent David klein organic chemistry
David klein organic chemistry Ap chemistry notes zumdahl
Ap chemistry notes zumdahl Organic chemistry third edition david klein
Organic chemistry third edition david klein Drop in molecular views answer key
Drop in molecular views answer key Chemistry by raymond chang 10th edition
Chemistry by raymond chang 10th edition Democritus atomic model diagram
Democritus atomic model diagram