GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION








- Slides: 56

GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION PETRUCCI HERRING MADURA BISSONNETTE Chemical Bonding II: Valence Bond and Molecular Orbital Theories 11 PHILIP DUTTON UNIVERSITY OF WINDSOR DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY Slide 11 - 1 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

Chemical Bonding II: Valence Bond and Molecular Orbital Theories Slide 11 - 2 CONTENTS 11 -1 What a Bonding Theory Should Do 11 -2 Introduction to the Valence-Bond Method 11 -3 Hybridization of Atomic Orbitals 11 -4 Multiple Covalent Bonds 11 -5 Molecular Orbital Theory 11 -6 Delocalized Electrons: An Explanation Based on Molecular Orbital Theory 11 -7 Some Unresolved Issues: Can Electron Charge-Density Plots Help? General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

11 -1 What a Bonding Theory Should Do FIGURE 11 -1 Type of interactions between two hydrogen atoms Slide 11 - 3 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

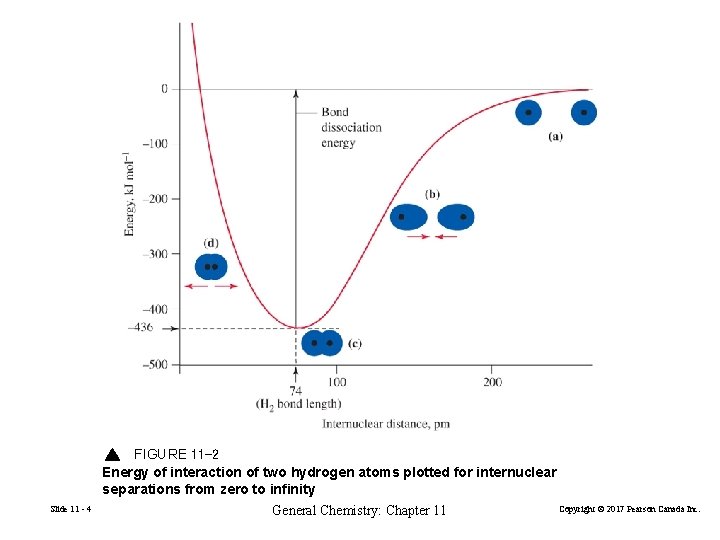
FIGURE 11 -2 Energy of interaction of two hydrogen atoms plotted for internuclear separations from zero to infinity Slide 11 - 4 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

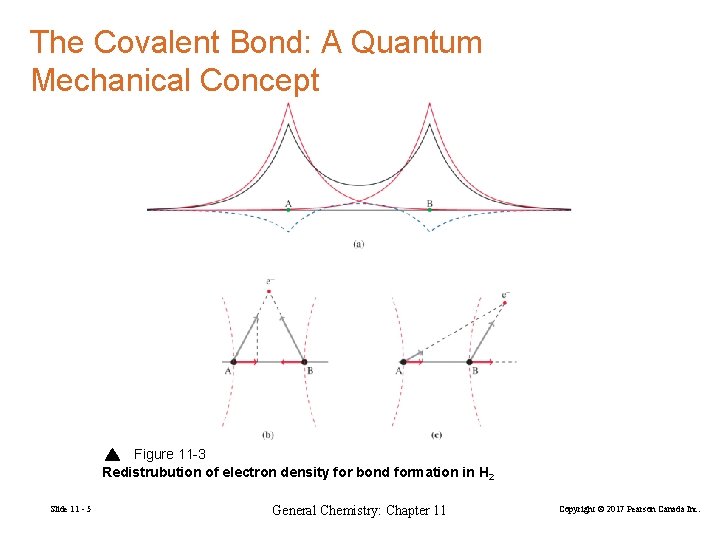
The Covalent Bond: A Quantum Mechanical Concept Figure 11 -3 Redistrubution of electron density for bond formation in H 2 Slide 11 - 5 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

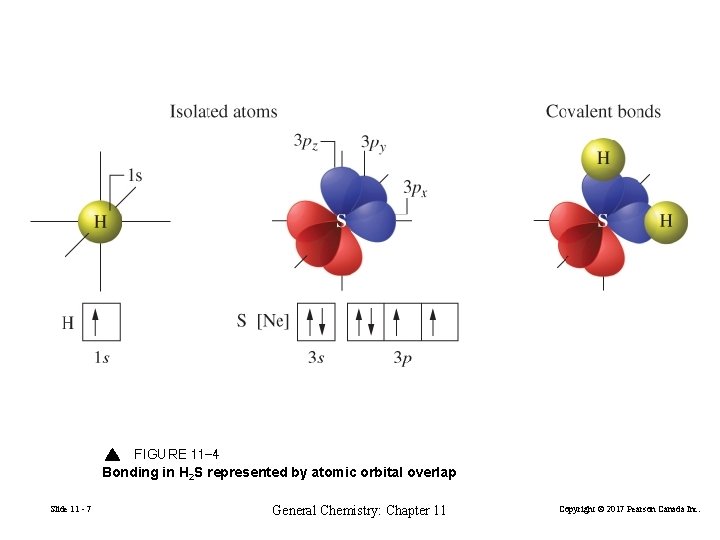
11 -2 Introduction to the Valence-Bond Method Overlap of half-filled atomic orbital overlap normally describes covalent bonding. Sometimes involves the overlap of a filled orbital on one atom with an empty orbital on another. A localized electron model of bonding. Slide 11 - 6 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

FIGURE 11 -4 Bonding in H 2 S represented by atomic orbital overlap Slide 11 - 7 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

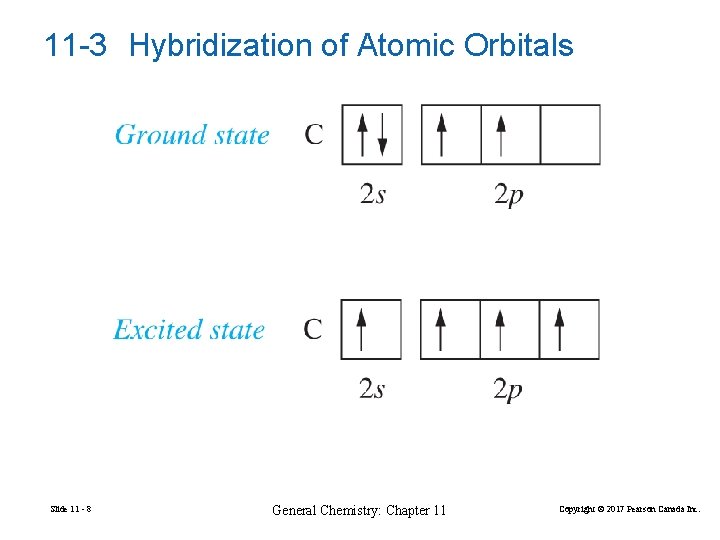
11 -3 Hybridization of Atomic Orbitals Slide 11 - 8 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

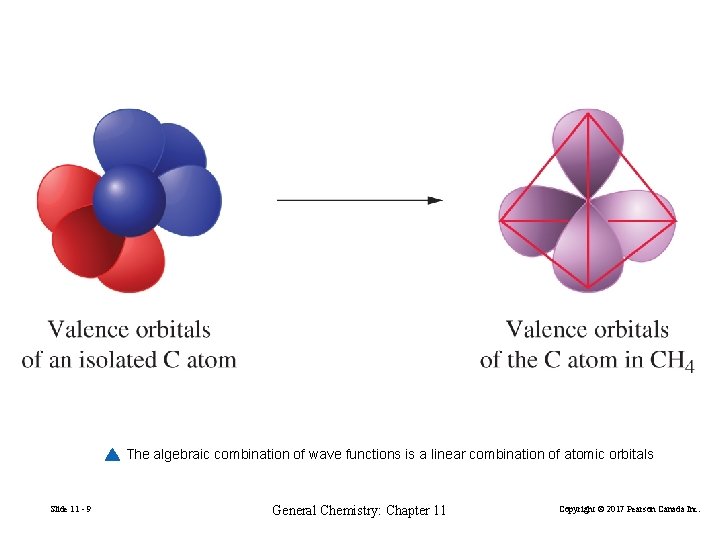
The algebraic combination of wave functions is a linear combination of atomic orbitals Slide 11 - 9 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

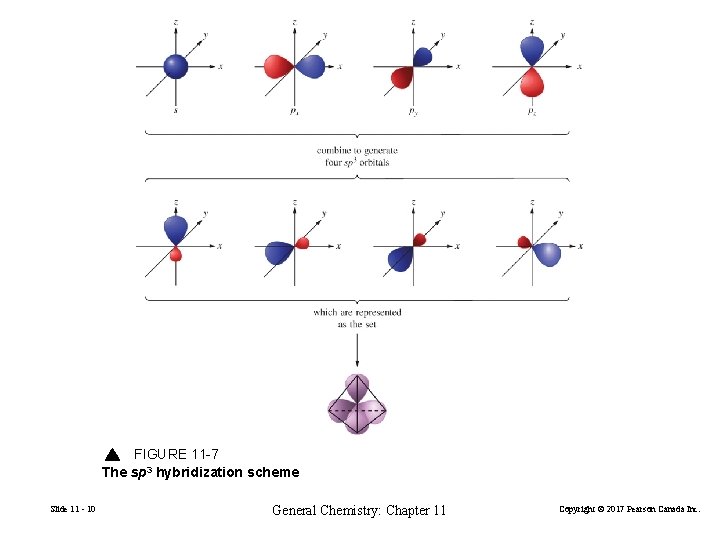
FIGURE 11 -7 The sp 3 hybridization scheme Slide 11 - 10 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

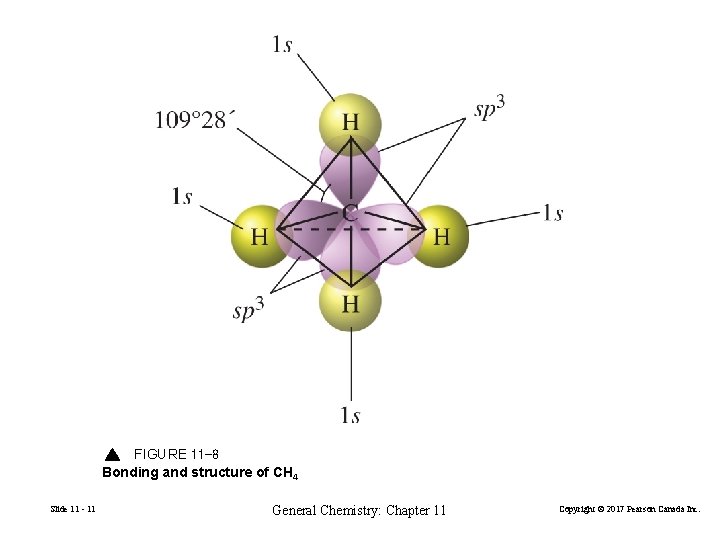
FIGURE 11 -8 Bonding and structure of CH 4 Slide 11 - 11 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

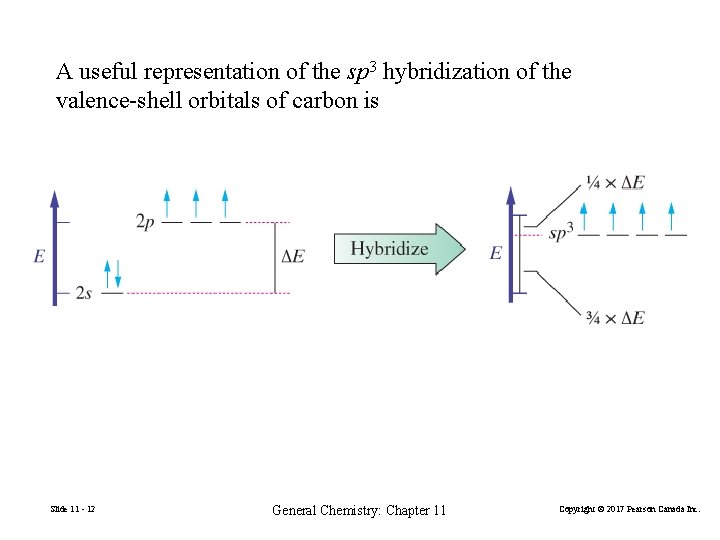
A useful representation of the sp 3 hybridization of the valence-shell orbitals of carbon is Slide 11 - 12 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

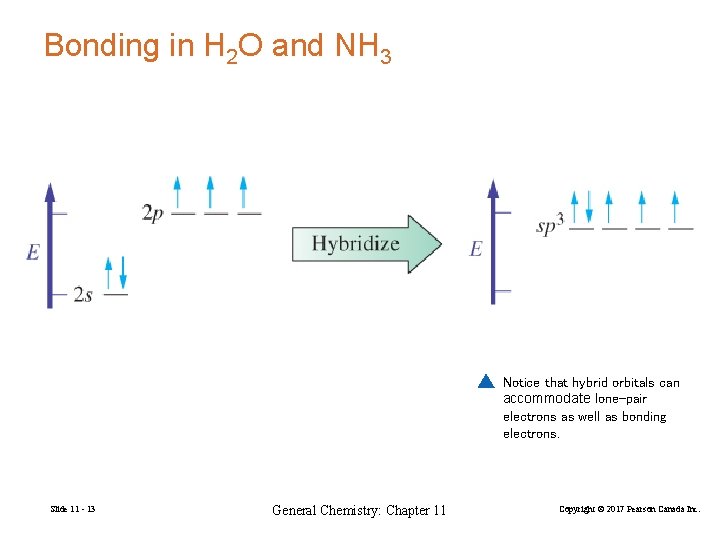
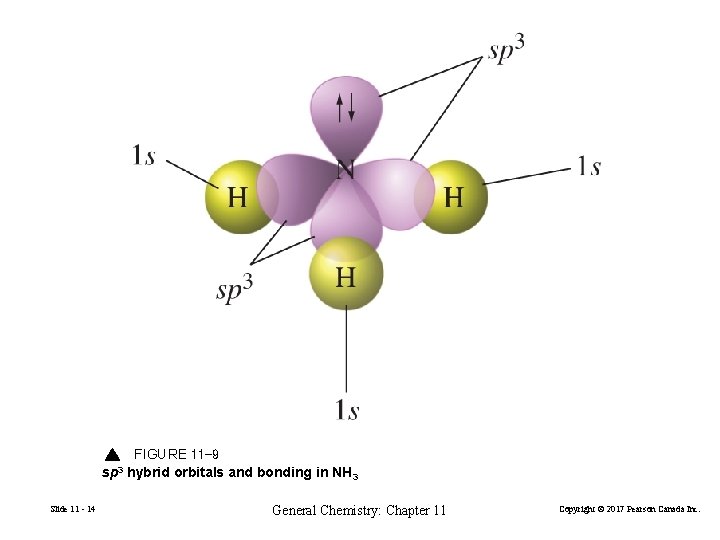
Bonding in H 2 O and NH 3 Notice that hybrid orbitals can accommodate lone-pair electrons as well as bonding electrons. Slide 11 - 13 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

FIGURE 11 -9 sp 3 hybrid orbitals and bonding in NH 3 Slide 11 - 14 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

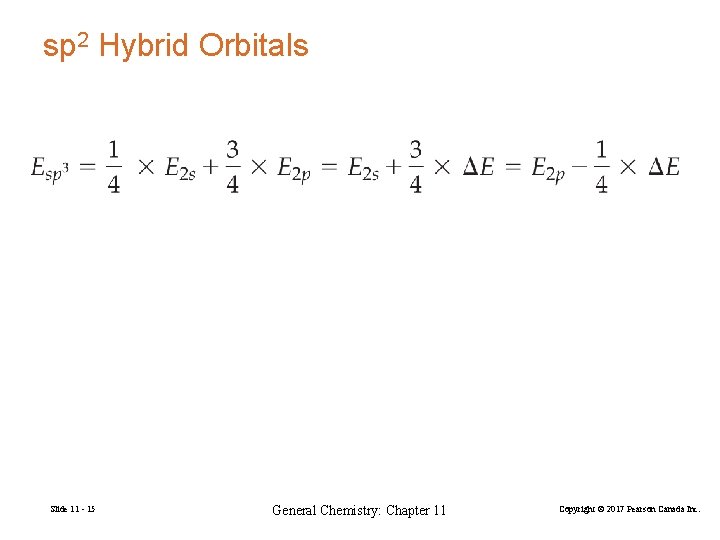
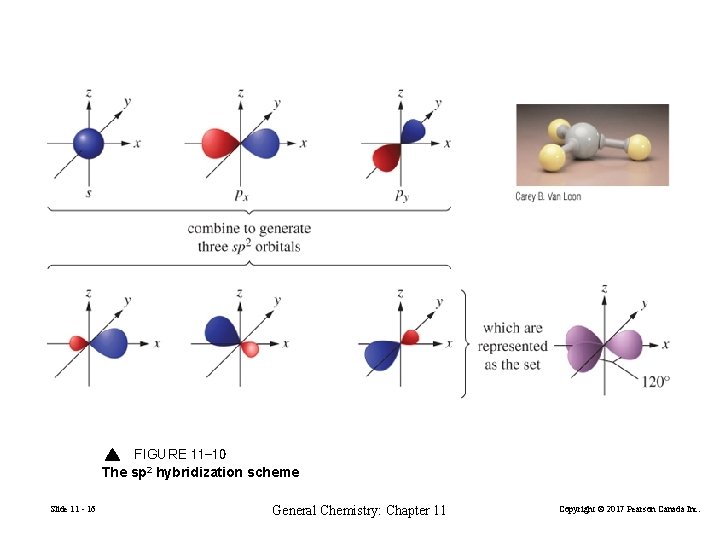
sp 2 Hybrid Orbitals Slide 11 - 15 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

FIGURE 11 -10 The sp 2 hybridization scheme Slide 11 - 16 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

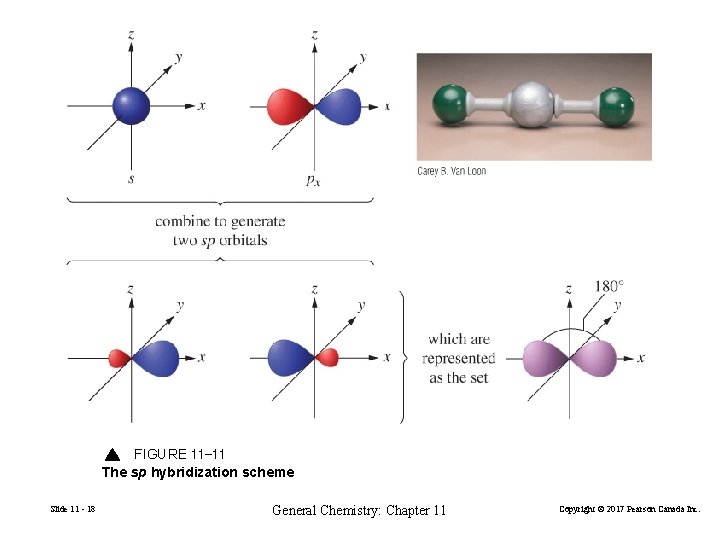
sp Hybridization Slide 11 - 17 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

FIGURE 11 -11 The sp hybridization scheme Slide 11 - 18 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

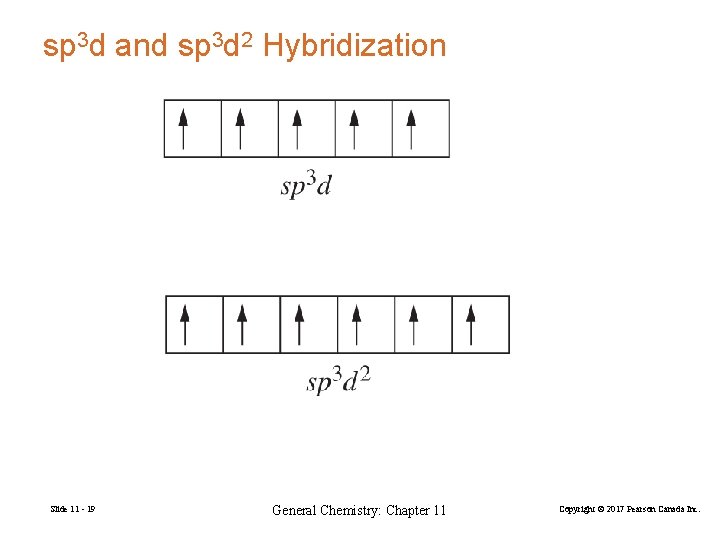
sp 3 d and sp 3 d 2 Hybridization Slide 11 - 19 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

FIGURE 11 -12 The construction of sp hybrid orbitls Slide 11 - 20 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

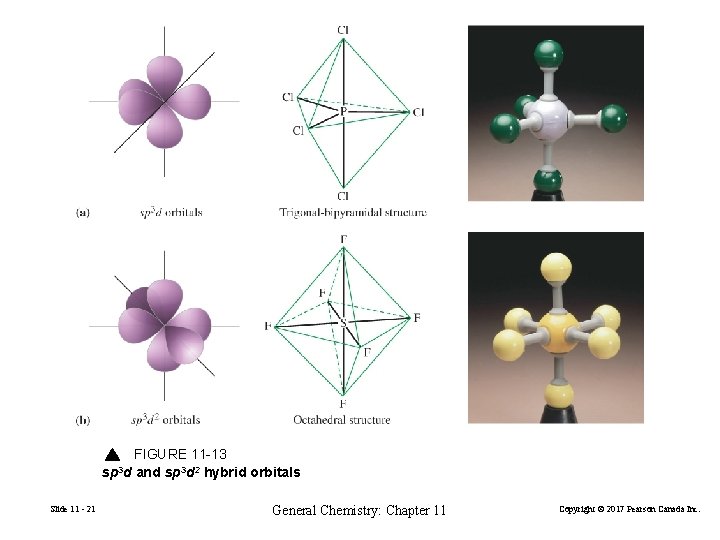
FIGURE 11 -13 sp 3 d and sp 3 d 2 hybrid orbitals Slide 11 - 21 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

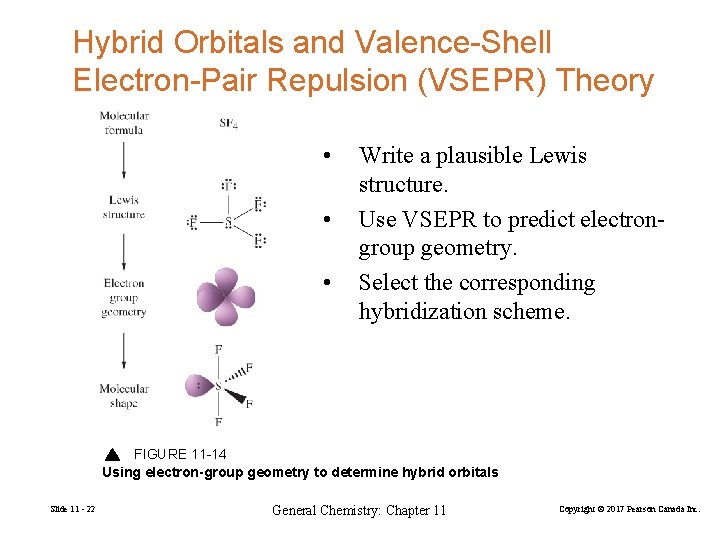
Hybrid Orbitals and Valence-Shell Electron-Pair Repulsion (VSEPR) Theory • • • Write a plausible Lewis structure. Use VSEPR to predict electrongroup geometry. Select the corresponding hybridization scheme. FIGURE 11 -14 Using electron-group geometry to determine hybrid orbitals Slide 11 - 22 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

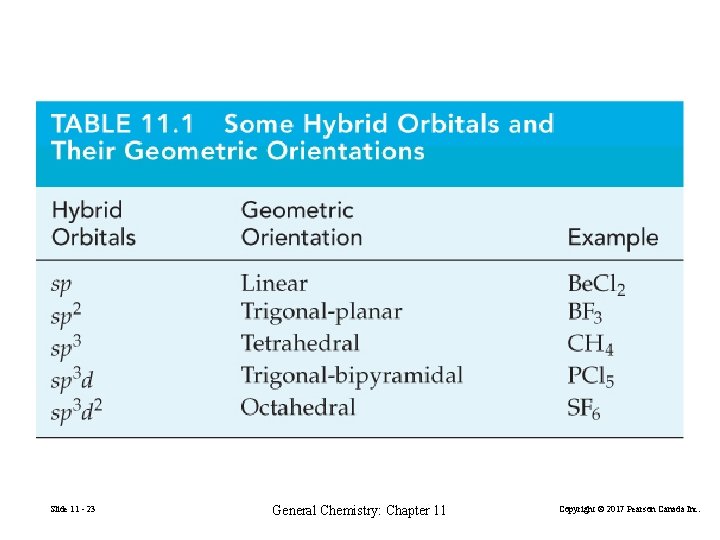
Slide 11 - 23 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

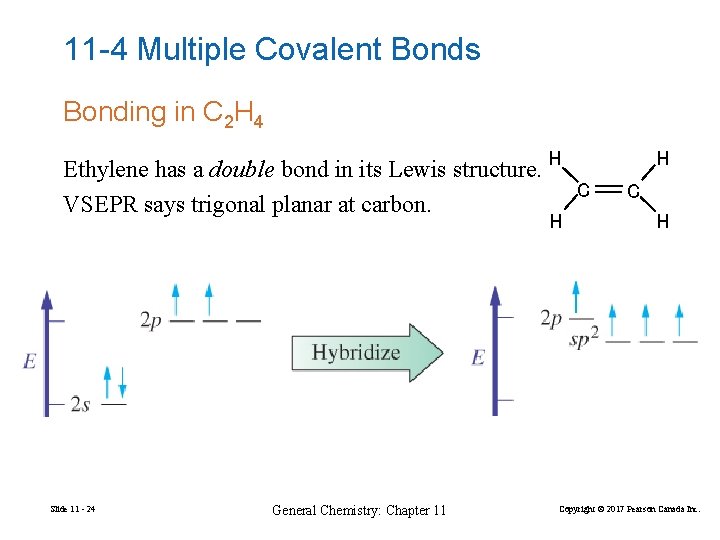
11 -4 Multiple Covalent Bonds Bonding in C 2 H 4 Ethylene has a double bond in its Lewis structure. H C VSEPR says trigonal planar at carbon. H Slide 11 - 24 General Chemistry: Chapter 11 H Copyright © 2017 Pearson Canada Inc.

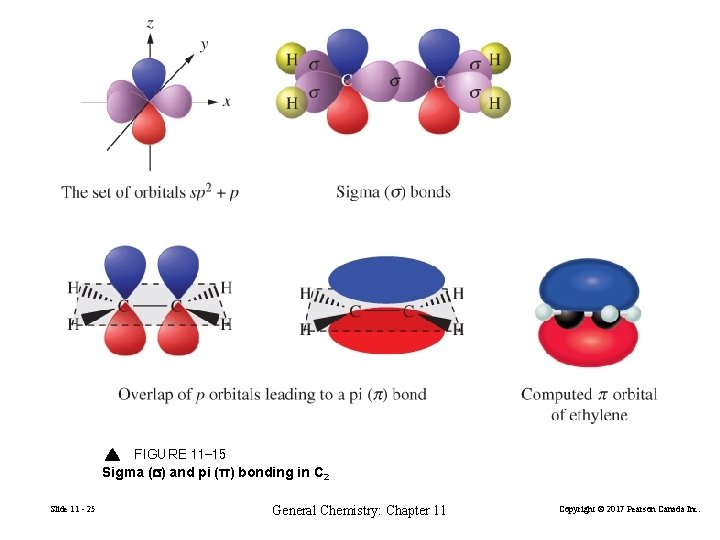
FIGURE 11 -15 Sigma (s) and pi (π) bonding in C 2 Slide 11 - 25 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

Bonding in C 2 H 2 Slide 11 - 26 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

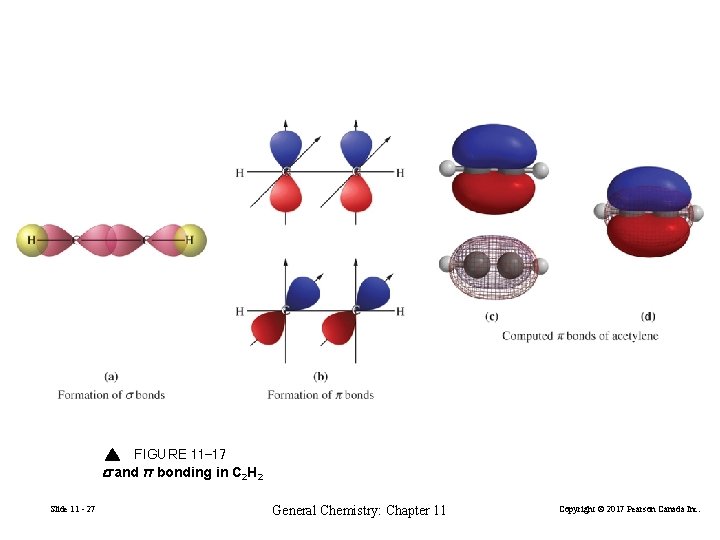
FIGURE 11 -17 s and π bonding in C 2 H 2 Slide 11 - 27 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

FIGURE 11 -19 Bonding in H 2 CO – a schematic representation Slide 11 - 28 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

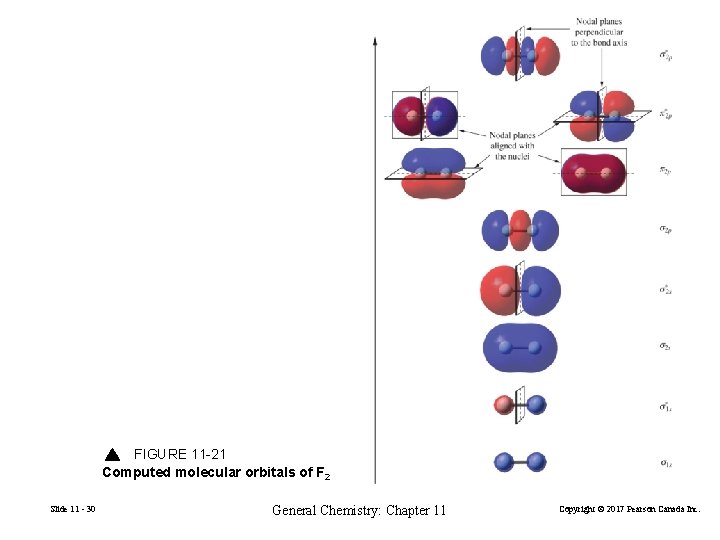
11 -5 Molecular Orbital Theory Atomic orbitals are isolated on atoms. Molecular orbitals span two or more atoms. Calculated orbitals for F 2 Analysis of the total wave function describing F 2 in its lowest energy state MO’s are distributed around both nuclei Linear Combination of Atomic Orbitals Slide 11 - 29 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

FIGURE 11 -21 Computed molecular orbitals of F 2 Slide 11 - 30 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

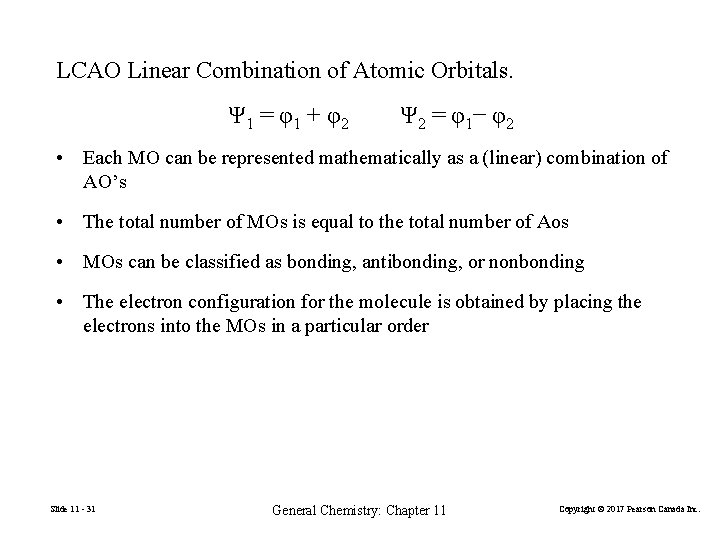
LCAO Linear Combination of Atomic Orbitals. Ψ 1 = φ1 + φ2 Ψ 2 = φ 1− φ 2 • Each MO can be represented mathematically as a (linear) combination of AO’s • The total number of MOs is equal to the total number of Aos • MOs can be classified as bonding, antibonding, or nonbonding • The electron configuration for the molecule is obtained by placing the electrons into the MOs in a particular order Slide 11 - 31 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

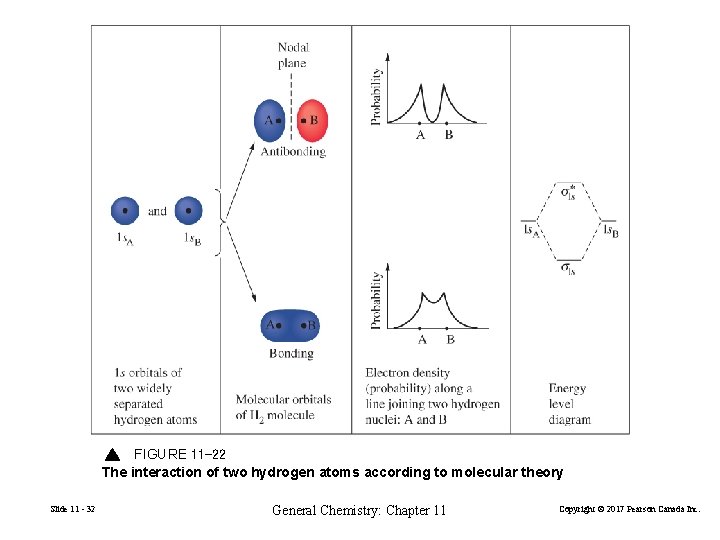
FIGURE 11 -22 The interaction of two hydrogen atoms according to molecular theory Slide 11 - 32 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

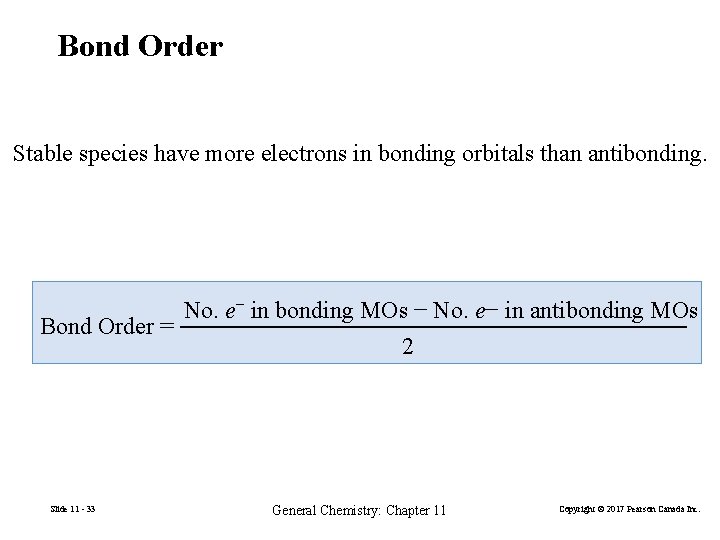
Bond Order Stable species have more electrons in bonding orbitals than antibonding. No. e− in bonding MOs − No. e− in antibonding MOs Bond Order = 2 Slide 11 - 33 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

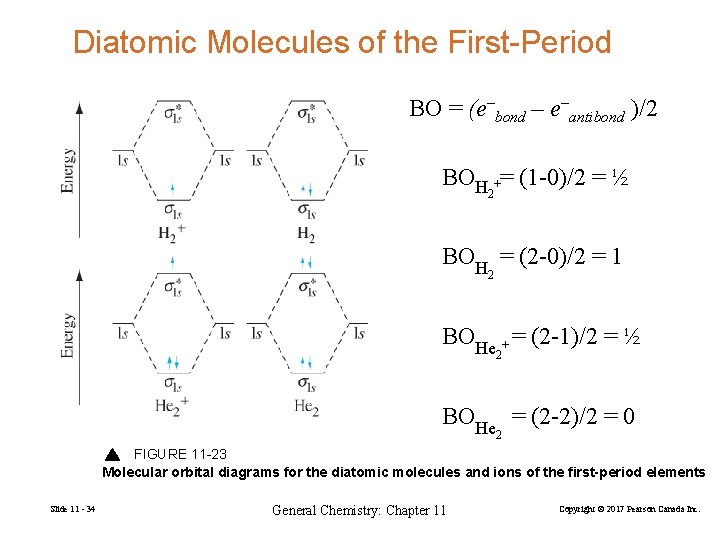
Diatomic Molecules of the First-Period BO = (e–bond – e–antibond )/2 BOH += (1 -0)/2 = ½ 2 BOH = (2 -0)/2 = 1 2 BOHe + = (2 -1)/2 = ½ 2 BOHe = (2 -2)/2 = 0 2 FIGURE 11 -23 Molecular orbital diagrams for the diatomic molecules and ions of the first-period elements Slide 11 - 34 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

Molecular Orbitals of the Second Period Elements First period use only 1 s orbitals. Second period have 2 s and 2 p orbitals available. p orbital overlap: End-to end overlap – sigma bond (σ). Side-to-side overlap– pi bond (π). Slide 11 - 35 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

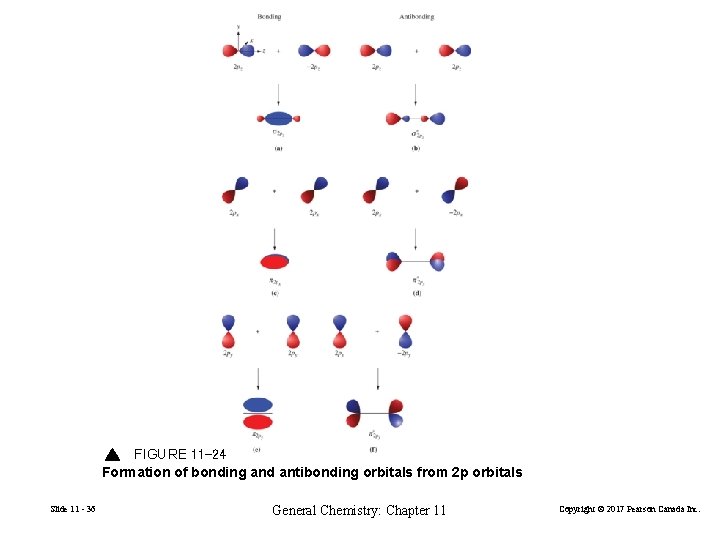
FIGURE 11 -24 Formation of bonding and antibonding orbitals from 2 p orbitals Slide 11 - 36 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

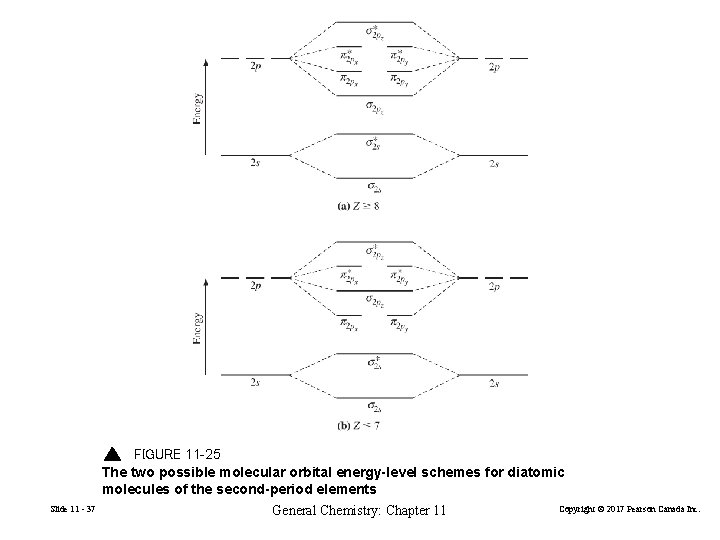
FIGURE 11 -25 The two possible molecular orbital energy-level schemes for diatomic molecules of the second-period elements Slide 11 - 37 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

FIGURE 11 -26 Molecular orbital occupancy diagrams for the homonuclear diatomic molecules of the second-period elements Slide 11 - 38 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

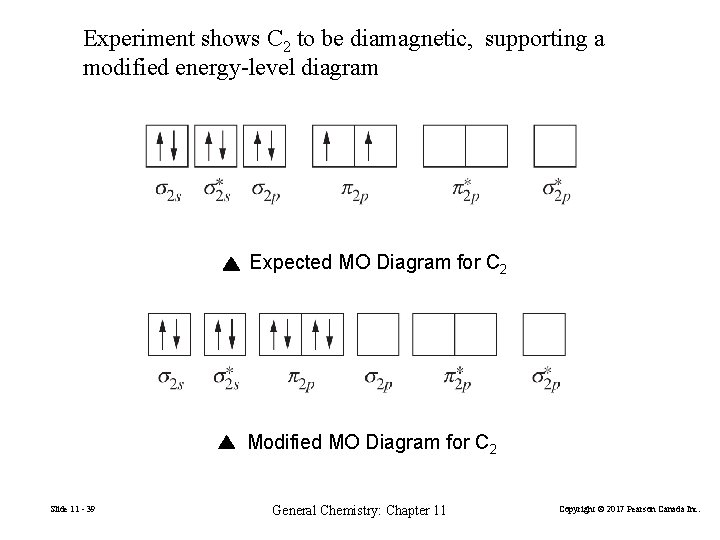
Experiment shows C 2 to be diamagnetic, supporting a modified energy-level diagram Expected MO Diagram for C 2 Modified MO Diagram for C 2 Slide 11 - 39 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

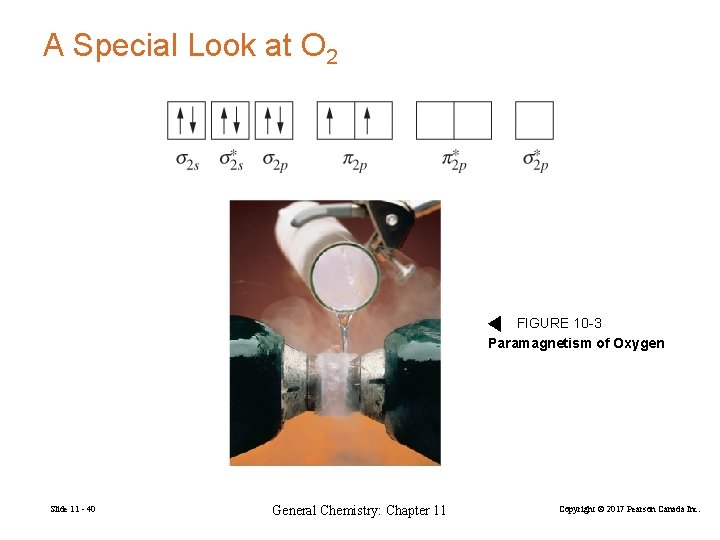
A Special Look at O 2 FIGURE 10 -3 Paramagnetism of Oxygen Slide 11 - 40 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

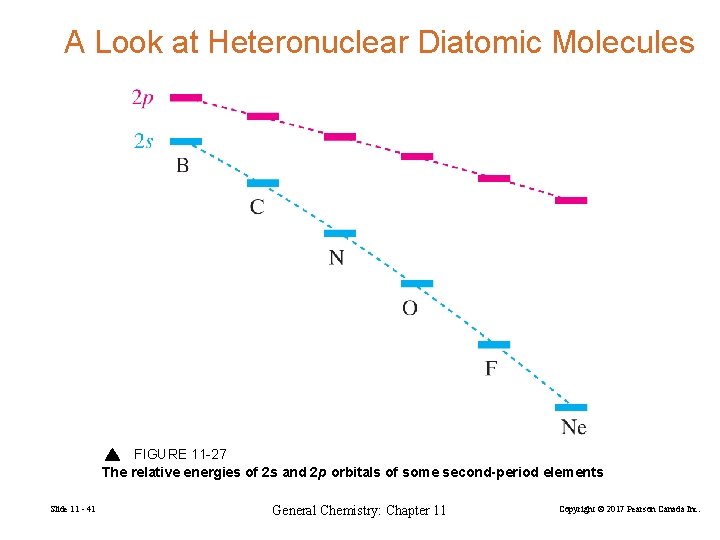
A Look at Heteronuclear Diatomic Molecules FIGURE 11 -27 The relative energies of 2 s and 2 p orbitals of some second-period elements Slide 11 - 41 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

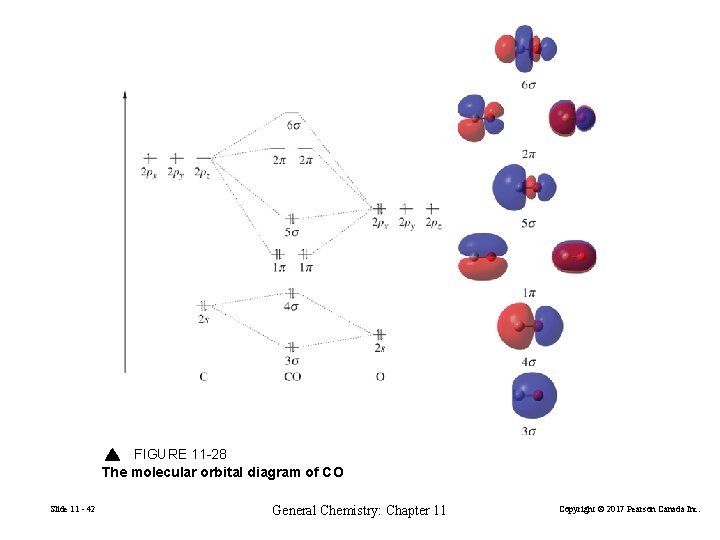
FIGURE 11 -28 The molecular orbital diagram of CO Slide 11 - 42 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

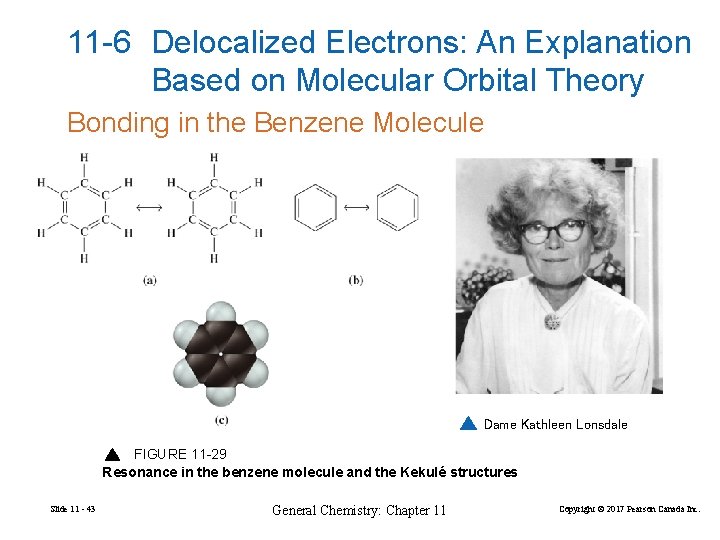
11 -6 Delocalized Electrons: An Explanation Based on Molecular Orbital Theory Bonding in the Benzene Molecule Dame Kathleen Lonsdale FIGURE 11 -29 Resonance in the benzene molecule and the Kekulé structures Slide 11 - 43 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

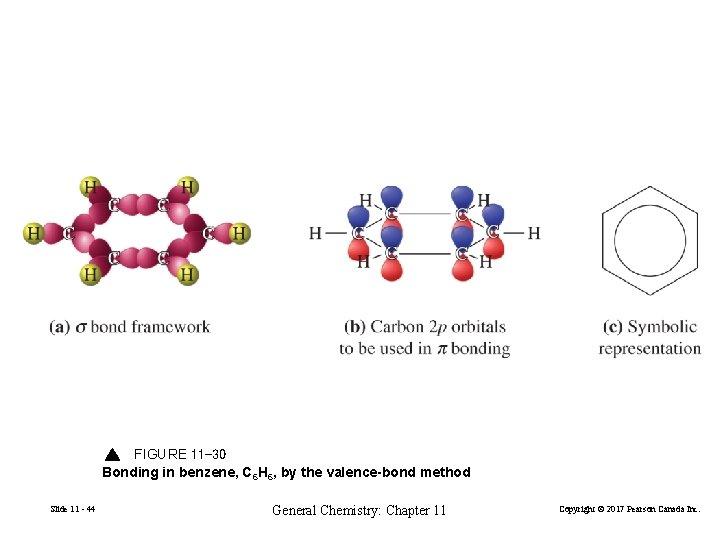
FIGURE 11 -30 Bonding in benzene, C 6 H 6, by the valence-bond method Slide 11 - 44 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

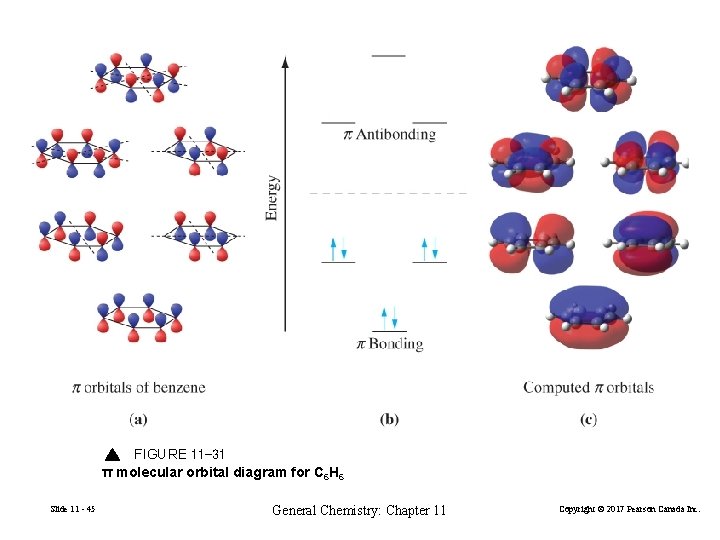
FIGURE 11 -31 π molecular orbital diagram for C 6 H 6 Slide 11 - 45 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

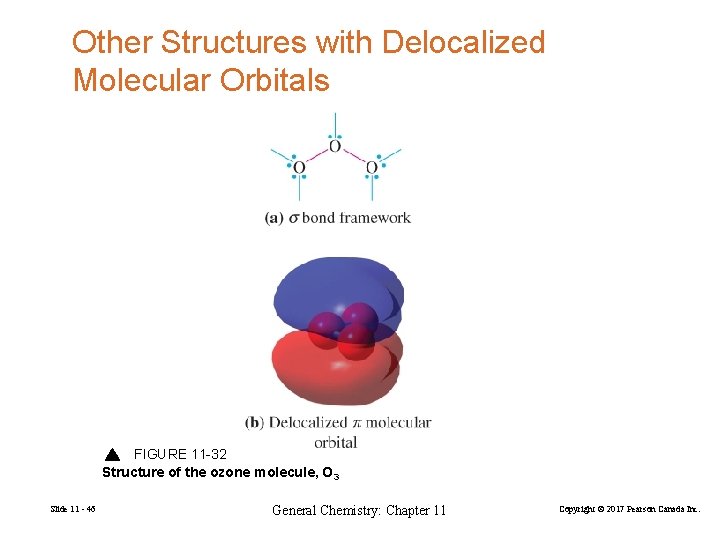
Other Structures with Delocalized Molecular Orbitals FIGURE 11 -32 Structure of the ozone molecule, O 3 Slide 11 - 46 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

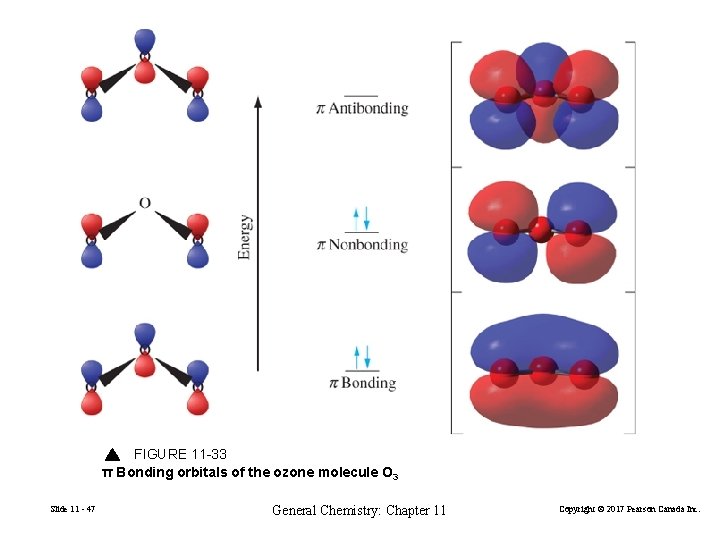
FIGURE 11 -33 π Bonding orbitals of the ozone molecule O 3 Slide 11 - 47 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

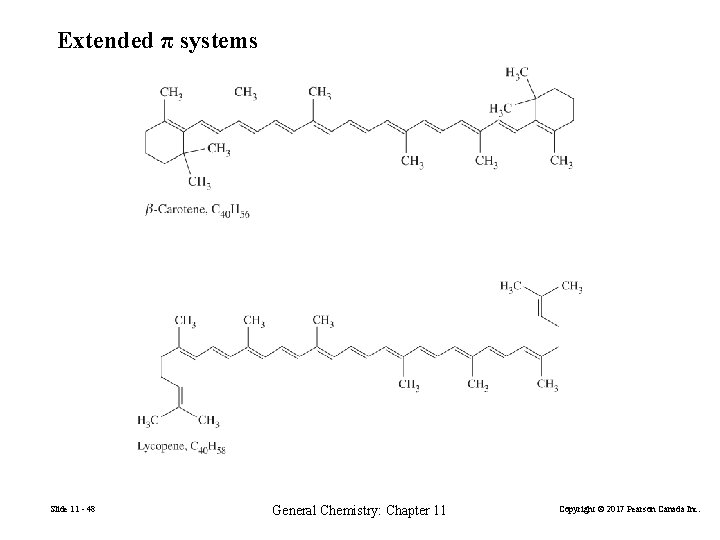
Extended π systems Slide 11 - 48 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

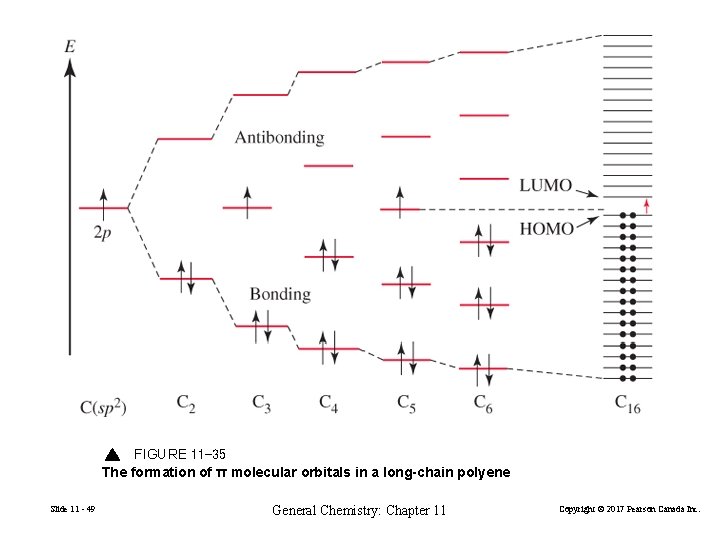
FIGURE 11 -35 The formation of π molecular orbitals in a long-chain polyene Slide 11 - 49 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

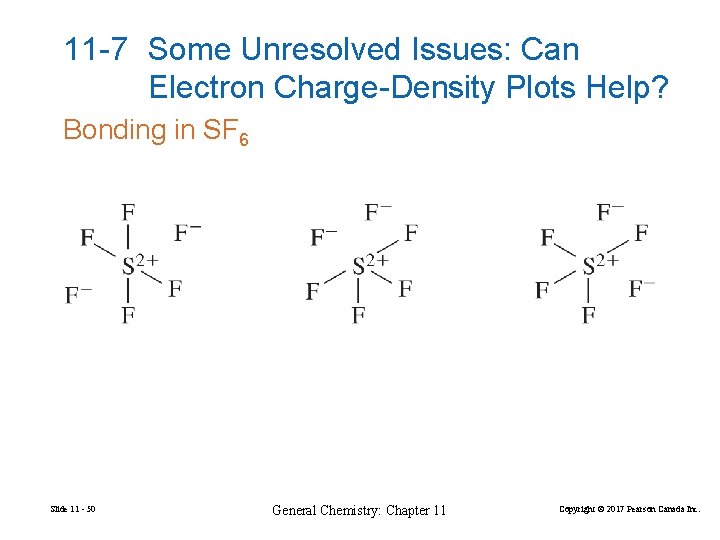
11 -7 Some Unresolved Issues: Can Electron Charge-Density Plots Help? Bonding in SF 6 Slide 11 - 50 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

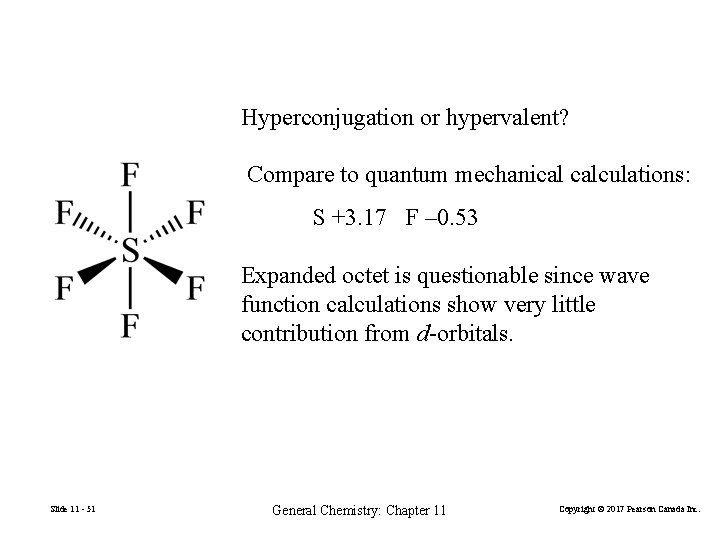
Hyperconjugation or hypervalent? Compare to quantum mechanical calculations: S +3. 17 F – 0. 53 Expanded octet is questionable since wave function calculations show very little contribution from d-orbitals. Slide 11 - 51 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

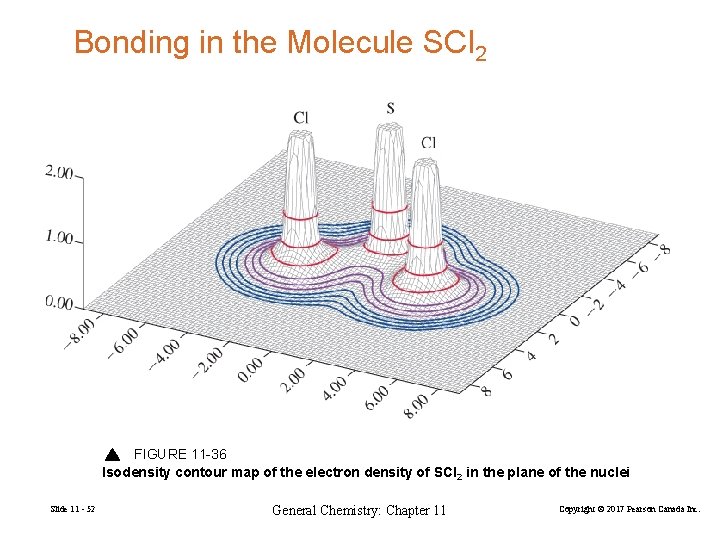
Bonding in the Molecule SCl 2 FIGURE 11 -36 Isodensity contour map of the electron density of SCl 2 in the plane of the nuclei Slide 11 - 52 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

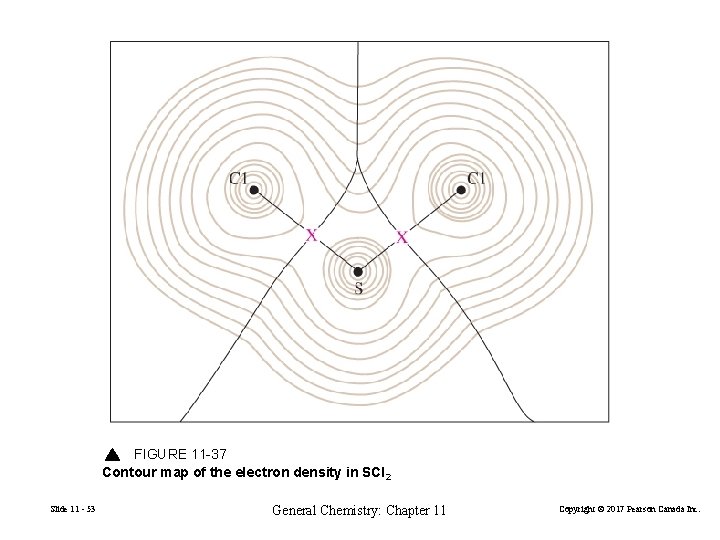
FIGURE 11 -37 Contour map of the electron density in SCl 2 Slide 11 - 53 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

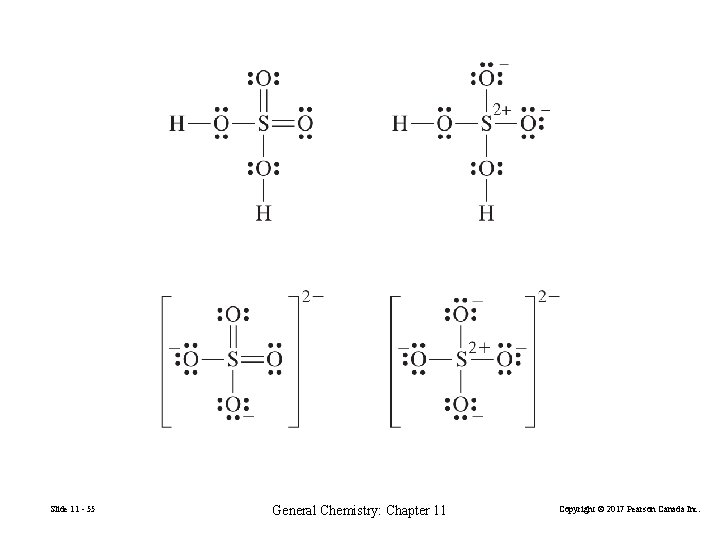
Bonding in the Molecule H 2 SO 4 and the Anion SO 42– FIGURE 11 -38 Three-dimensional plots of the electron density in H 2 SO 4 and SO 42– Slide 11 - 54 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

Slide 11 - 55 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.

End of Chapter Slide 11 - 56 General Chemistry: Chapter 11 Copyright © 2017 Pearson Canada Inc.
 Eleventh edition management
Eleventh edition management Eleventh edition management
Eleventh edition management Management eleventh edition
Management eleventh edition Management eleventh edition
Management eleventh edition General chemistry 11th edition
General chemistry 11th edition Chadha committee
Chadha committee Eleventh 5 year plan
Eleventh 5 year plan Eleventh plan
Eleventh plan For his eleventh birthday elvis presley
For his eleventh birthday elvis presley Human genetics concepts and applications 10th edition
Human genetics concepts and applications 10th edition Fluid mechanics fundamentals and applications
Fluid mechanics fundamentals and applications Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Modern systems analysis and design 7th edition
Modern systems analysis and design 7th edition Susanna s epp
Susanna s epp Using mis (10th edition) 10th edition
Using mis (10th edition) 10th edition Zulily case study
Zulily case study Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Pearson engineering
Pearson engineering Learning principles and applications
Learning principles and applications 25 m/s
25 m/s Irradiated food
Irradiated food Modern labor economics 12th edition solution
Modern labor economics 12th edition solution Vertical economics
Vertical economics Modern real estate practice in pennsylvania
Modern real estate practice in pennsylvania Modern database management 12th edition ppt
Modern database management 12th edition ppt Modern database management 12th edition ppt
Modern database management 12th edition ppt Modern operating systems 3rd edition
Modern operating systems 3rd edition Modern operating systems tanenbaum 5th edition
Modern operating systems tanenbaum 5th edition Modern database management 8th edition
Modern database management 8th edition University physics with modern physics fifteenth edition
University physics with modern physics fifteenth edition Modern database management 11th edition
Modern database management 11th edition Modern labor economics 12th edition
Modern labor economics 12th edition Computer security principles and practice
Computer security principles and practice Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Expert systems: principles and programming, fourth edition
Expert systems: principles and programming, fourth edition Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Is alkane an organic compound
Is alkane an organic compound Introductory chemistry 4th edition
Introductory chemistry 4th edition Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro The central science 14th edition
The central science 14th edition Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Nomenclature of ethers
Nomenclature of ethers Zumdahl chemistry, 9th edition notes
Zumdahl chemistry, 9th edition notes Organic chemistry third edition david klein
Organic chemistry third edition david klein Living by chemistry 2nd edition answers
Living by chemistry 2nd edition answers Chemistry by raymond chang 10th edition
Chemistry by raymond chang 10th edition Fifth edition chemistry a molecular approach
Fifth edition chemistry a molecular approach Organic chemistry (3rd) edition chapter 2 problem 17s
Organic chemistry (3rd) edition chapter 2 problem 17s Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein Organic chemistry second edition david klein
Organic chemistry second edition david klein General properties of smart and modern materials
General properties of smart and modern materials Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Modern chemistry chapter 7 test
Modern chemistry chapter 7 test