GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION
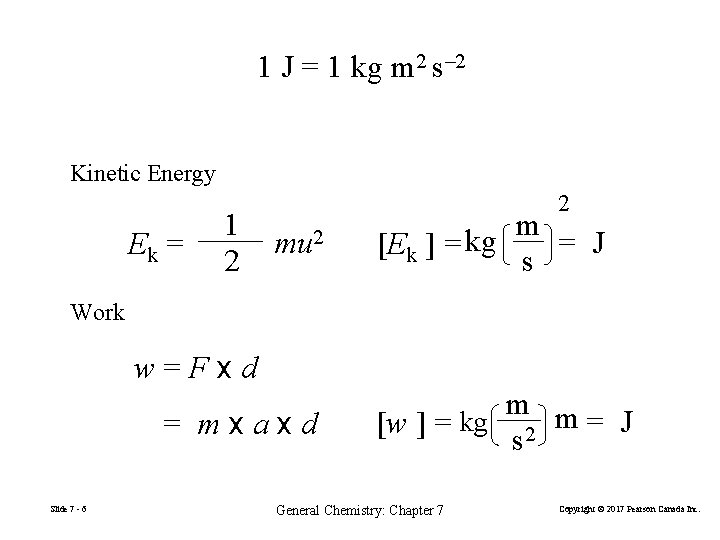




- Slides: 62

GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION PETRUCCI HERRING MADURA Thermochemistry BISSONNETTE 7 PHILIP DUTTON UNIVERSITY OF WINDSOR DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY Slide 7 - 1 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

Thermochemistry CONTENTS 7 -1 Getting Started: Some Terminology 7 -2 Heat 7 -3 Heats of Reaction and Calorimetry 7 -4 Work 7 -5 The First Law of Thermodynamics 7 -6 Application of the First Law to Chemical and Physical Changes 7 -7 Indirect Determination of Dr. H: Hess’s Law 7 -8 Standard Enthalpies of Formation 7 -9 Fuels as Sources of Energy 7 -10 Spontaneous and Nonspontaneous Processes: An Introduction Slide 7 - 2 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

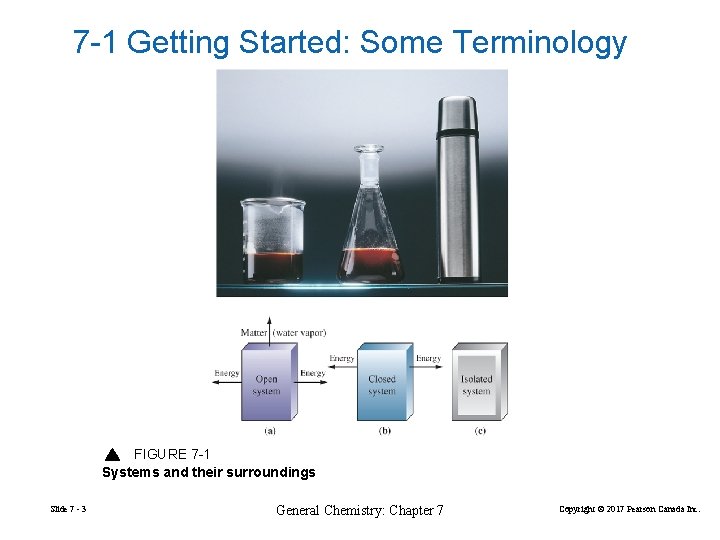
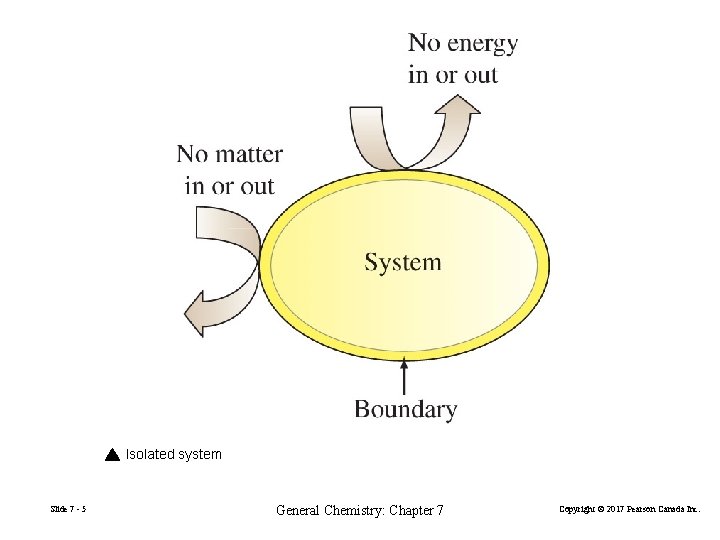
7 -1 Getting Started: Some Terminology FIGURE 7 -1 Systems and their surroundings Slide 7 - 3 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

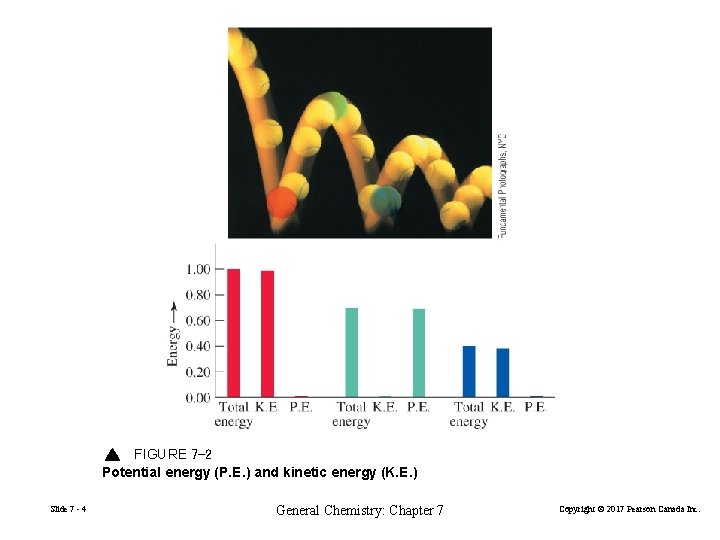
FIGURE 7 -2 Potential energy (P. E. ) and kinetic energy (K. E. ) Slide 7 - 4 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

Isolated system Slide 7 - 5 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.
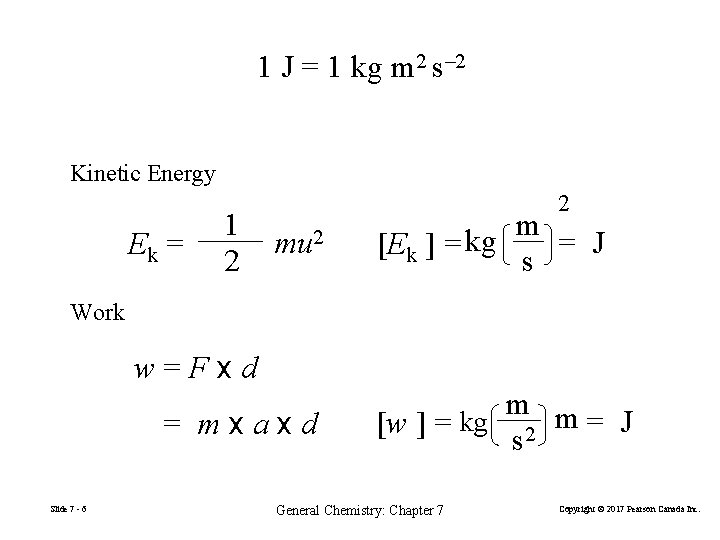
1 J = 1 kg m 2 s– 2 Kinetic Energy Ek = 1 2 2 mu 2 m = J [Ek ] = kg s Work w=Fxd = mxaxd Slide 7 - 6 m m = J [w ] = kg 2 s General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

7 -2 Heat Thermal Energy Kinetic energy associated with random molecular motion. In general proportional to temperature. An intensive property. Heat and Work q and w. Energy changes. Slide 7 - 7 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

Internal energy is transferred between a system and its surroundings as a result of a temperature difference. Heat “flows” from hotter to colder. Temperature may change. Phase may change (an isothermal process). Slide 7 - 8 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

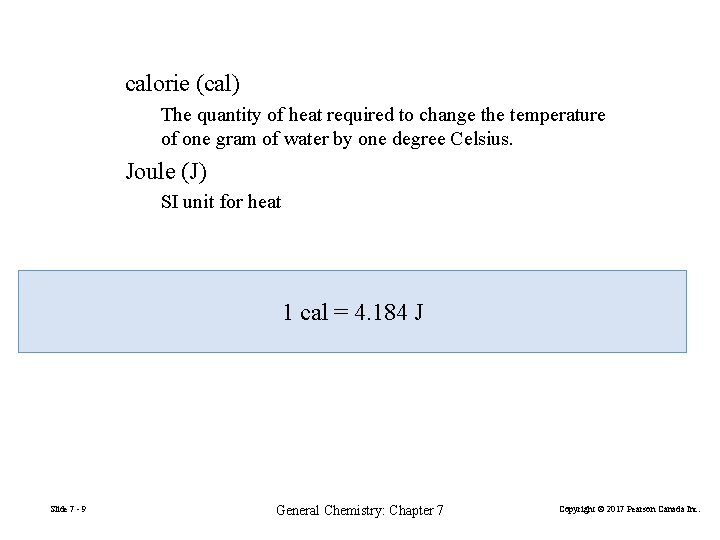
calorie (cal) The quantity of heat required to change the temperature of one gram of water by one degree Celsius. Joule (J) SI unit for heat 1 cal = 4. 184 J Slide 7 - 9 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

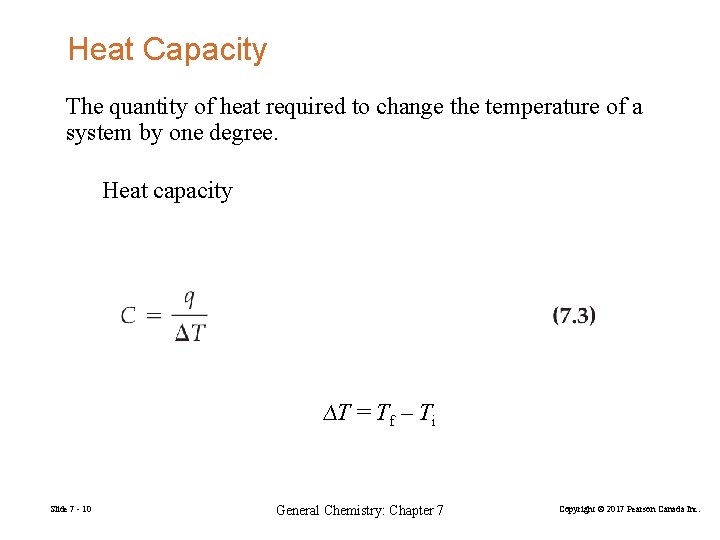
Heat Capacity The quantity of heat required to change the temperature of a system by one degree. Heat capacity ∆T = Tf – Ti Slide 7 - 10 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

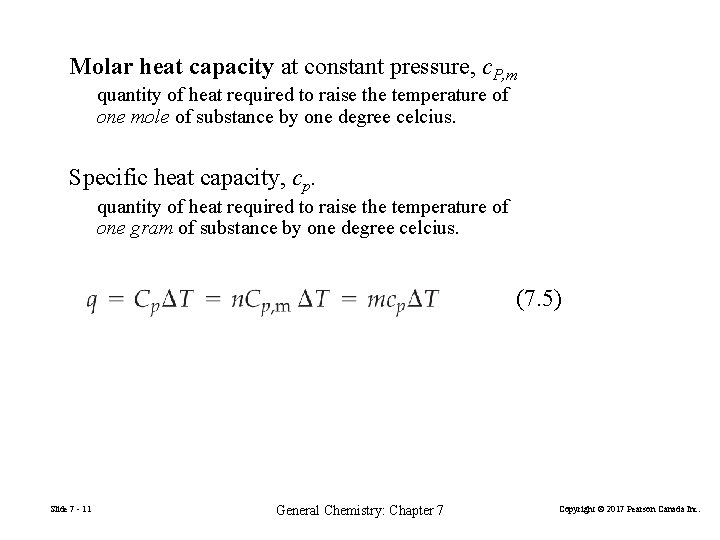
Molar heat capacity at constant pressure, c. P, m quantity of heat required to raise the temperature of one mole of substance by one degree celcius. Specific heat capacity, cp. quantity of heat required to raise the temperature of one gram of substance by one degree celcius. (7. 5) Slide 7 - 11 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

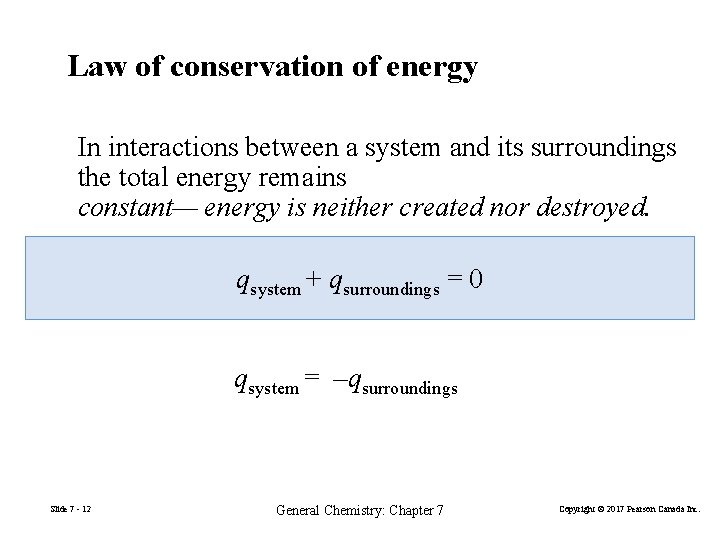
Law of conservation of energy In interactions between a system and its surroundings the total energy remains constant— energy is neither created nor destroyed. qsystem + qsurroundings = 0 qsystem = –qsurroundings Slide 7 - 12 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

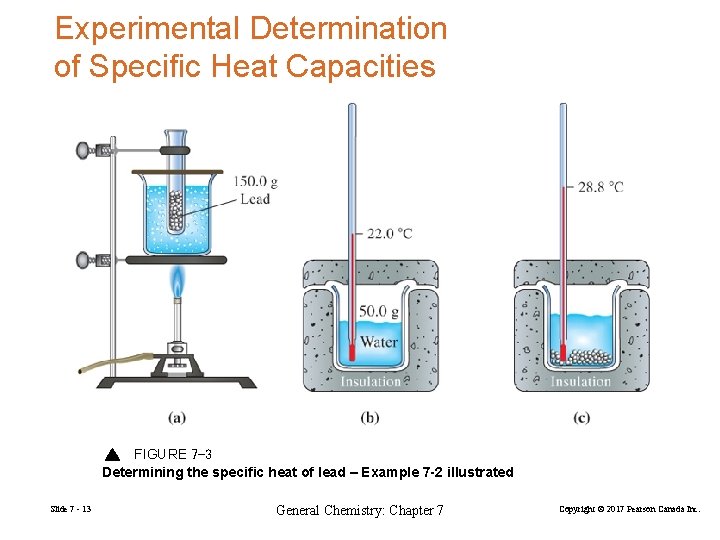
Experimental Determination of Specific Heat Capacities FIGURE 7 -3 Determining the specific heat of lead – Example 7 -2 illustrated Slide 7 - 13 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

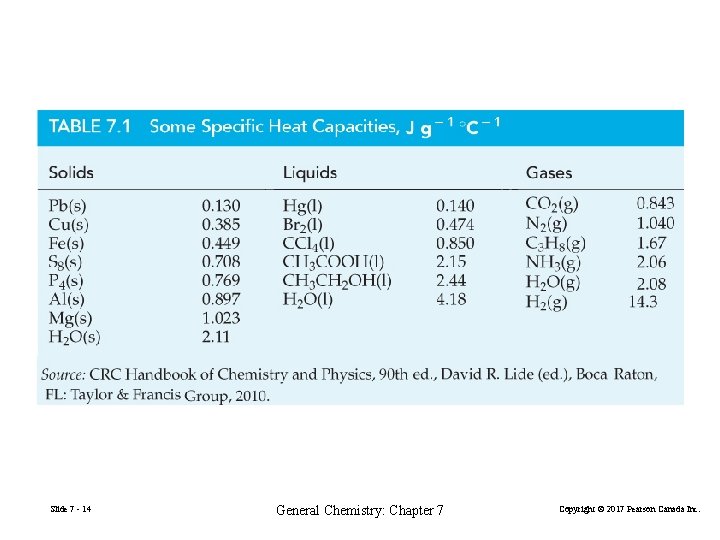
Slide 7 - 14 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

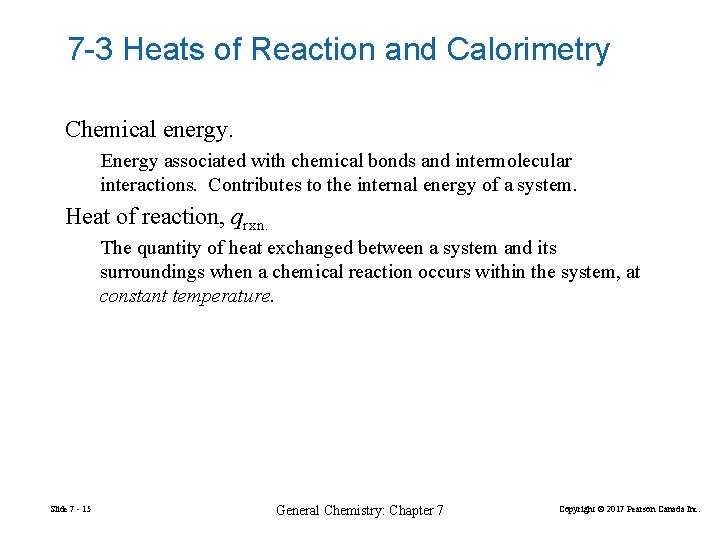
7 -3 Heats of Reaction and Calorimetry Chemical energy. Energy associated with chemical bonds and intermolecular interactions. Contributes to the internal energy of a system. Heat of reaction, qrxn. The quantity of heat exchanged between a system and its surroundings when a chemical reaction occurs within the system, at constant temperature. Slide 7 - 15 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

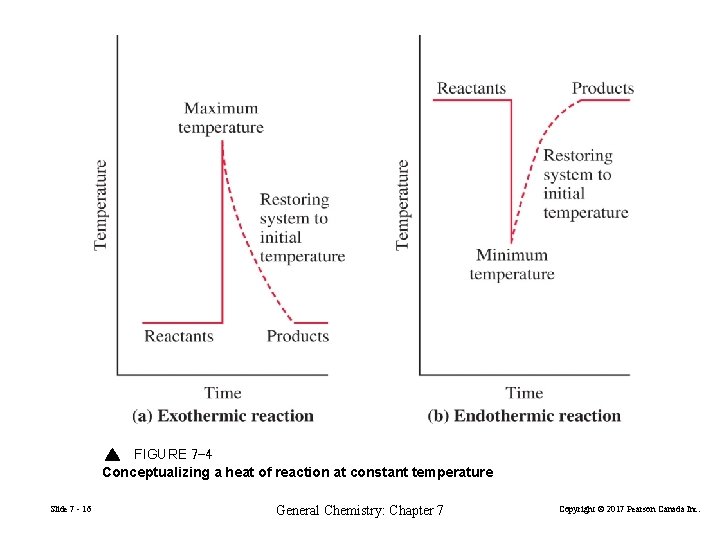
FIGURE 7 -4 Conceptualizing a heat of reaction at constant temperature Slide 7 - 16 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

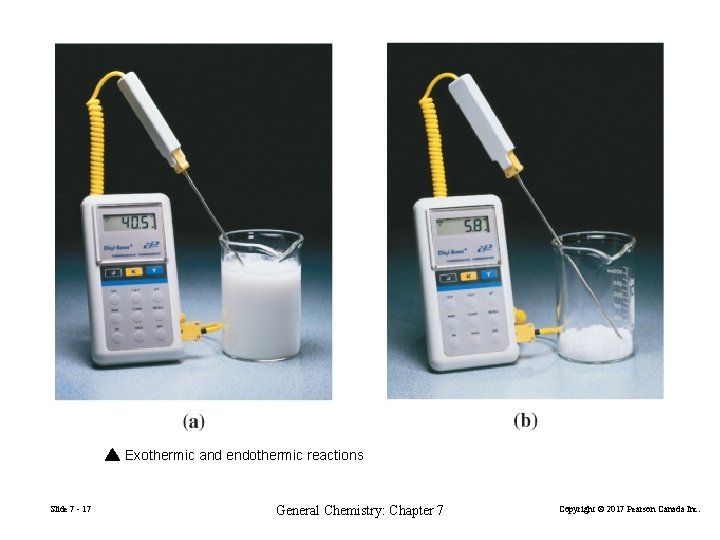
Exothermic and endothermic reactions Slide 7 - 17 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

KEEP IN MIND that the temperature of a reaction mixture usually changes during a reaction, so the mixture must be returned to the initial temperature (actually or hypothetically) before we assess how much heat is exchanged with the surroundings. Slide 7 - 18 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

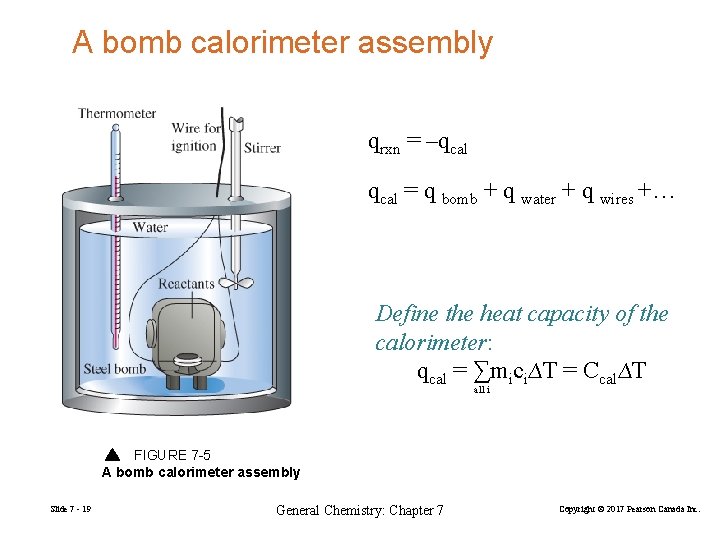
A bomb calorimeter assembly qrxn = –qcal = q bomb + q water + q wires +… Define the heat capacity of the calorimeter: qcal = ∑mici∆T = Ccal∆T all i FIGURE 7 -5 A bomb calorimeter assembly Slide 7 - 19 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

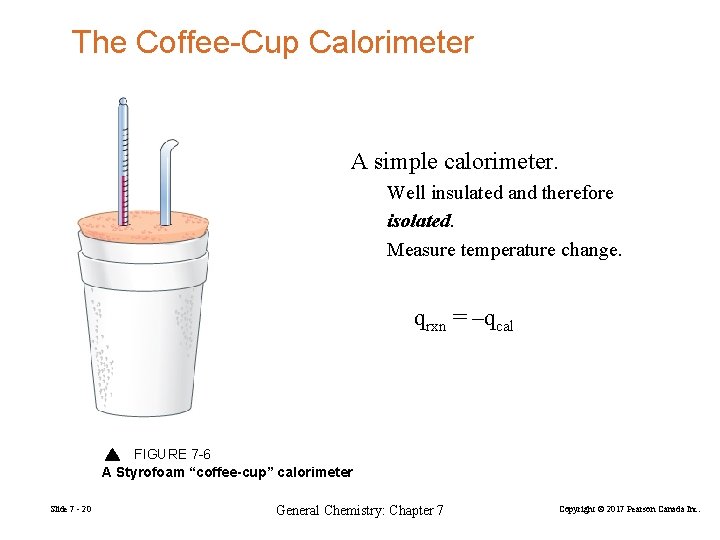
The Coffee-Cup Calorimeter A simple calorimeter. Well insulated and therefore isolated. Measure temperature change. qrxn = –qcal FIGURE 7 -6 A Styrofoam “coffee-cup” calorimeter Slide 7 - 20 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

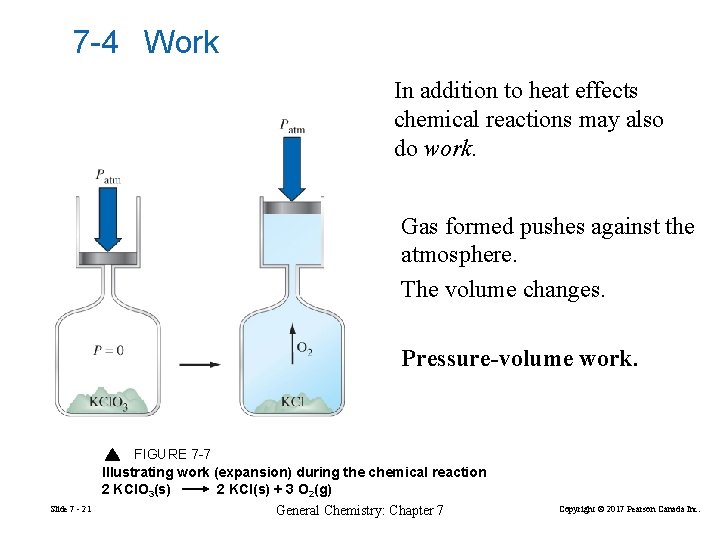
7 -4 Work In addition to heat effects chemical reactions may also do work. Gas formed pushes against the atmosphere. The volume changes. Pressure-volume work. FIGURE 7 -7 Illustrating work (expansion) during the chemical reaction 2 KCl. O 3(s) 2 KCl(s) + 3 O 2(g) Slide 7 - 21 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

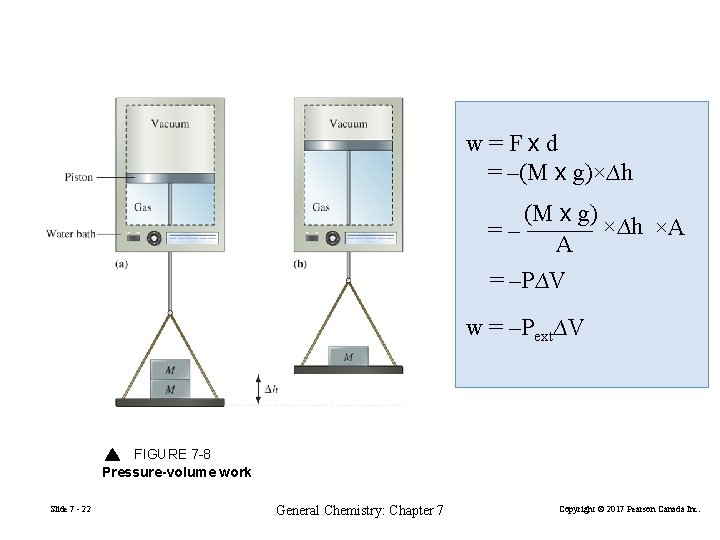
w=Fxd = –(M x g)×∆h (M x g) ×∆h ×A =– A = –P∆V w = –Pext∆V FIGURE 7 -8 Pressure-volume work Slide 7 - 22 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

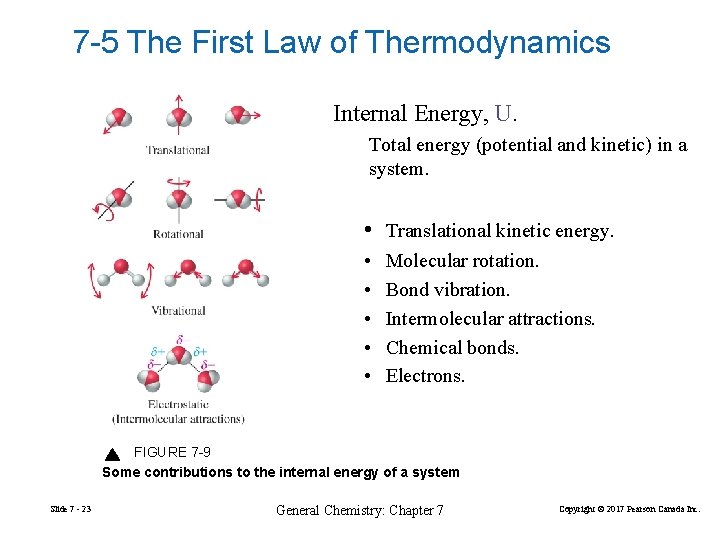
7 -5 The First Law of Thermodynamics Internal Energy, U. Total energy (potential and kinetic) in a system. • Translational kinetic energy. • • • Molecular rotation. Bond vibration. Intermolecular attractions. Chemical bonds. Electrons. FIGURE 7 -9 Some contributions to the internal energy of a system Slide 7 - 23 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

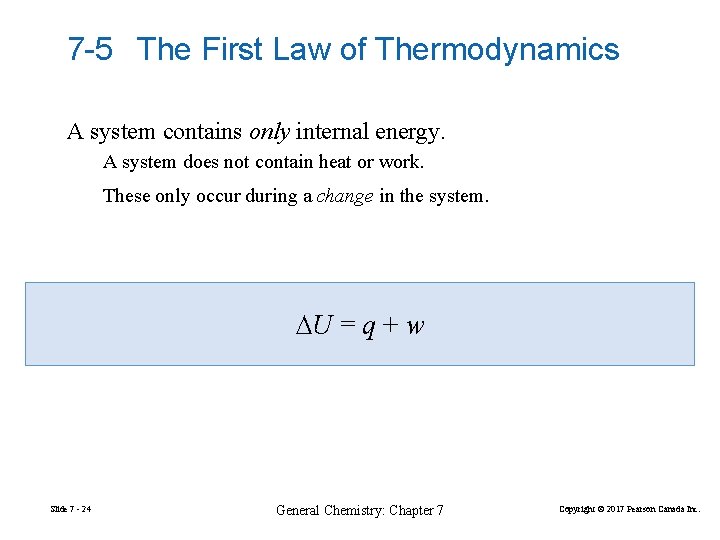
7 -5 The First Law of Thermodynamics A system contains only internal energy. A system does not contain heat or work. These only occur during a change in the system. ∆U = q + w Slide 7 - 24 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

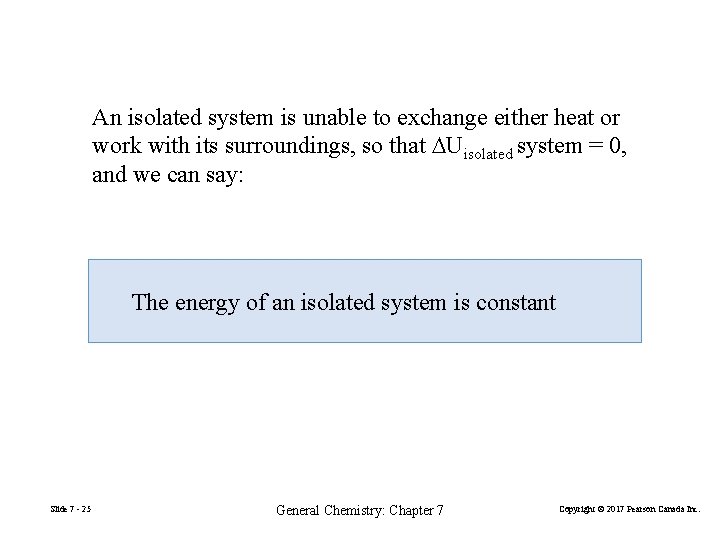
An isolated system is unable to exchange either heat or work with its surroundings, so that DUisolated system = 0, and we can say: The energy of an isolated system is constant Slide 7 - 25 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

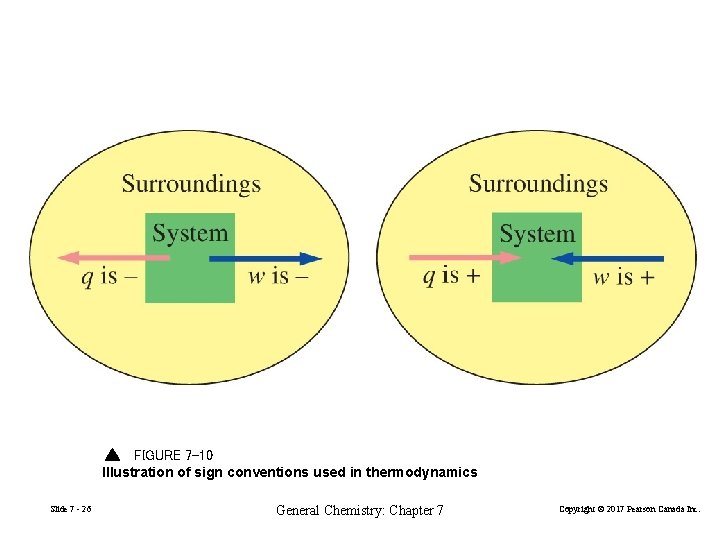
FIGURE 7 -10 Illustration of sign conventions used in thermodynamics Slide 7 - 26 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

Functions of State Any property that has a unique value for a specified state of a system is said to be a function of state or a state function. Water at 293. 15 K and 100 k. Pa is in a specified state. d = 0. 99820 g/m. L This density is a unique function of the state. It does not matter how the state was established. Slide 7 - 27 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

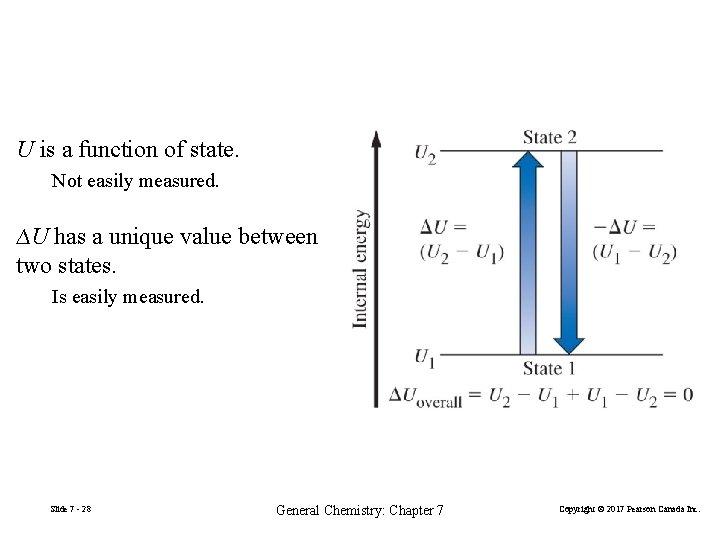
U is a function of state. Not easily measured. ∆U has a unique value between two states. Is easily measured. Slide 7 - 28 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

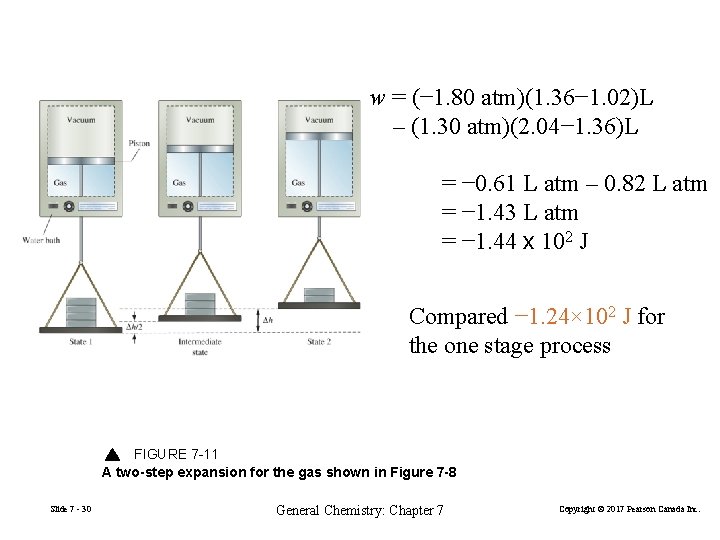
Path Dependent Functions Changes in heat and work are not functions of state. Consider process in Example 7. 5 and Figure 7 -8 w = − 1. 24 x 102 J in a one step expansion of gas: let us consider a two step process from 2. 40 atm to 1. 80 atm, and finally to 1. 20 atm. Slide 7 - 29 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

w = (− 1. 80 atm)(1. 36− 1. 02)L – (1. 30 atm)(2. 04− 1. 36)L = − 0. 61 L atm – 0. 82 L atm = − 1. 43 L atm = − 1. 44 x 102 J Compared − 1. 24× 102 J for the one stage process FIGURE 7 -11 A two-step expansion for the gas shown in Figure 7 -8 Slide 7 - 30 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

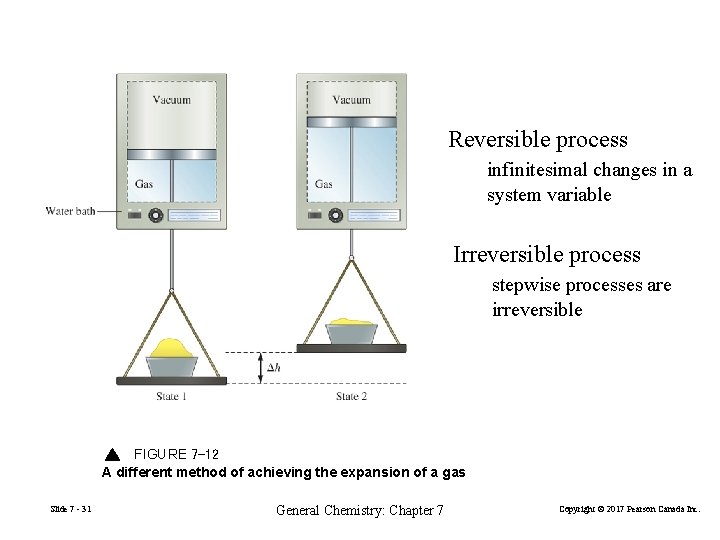
Reversible process infinitesimal changes in a system variable Irreversible process stepwise processes are irreversible FIGURE 7 -12 A different method of achieving the expansion of a gas Slide 7 - 31 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

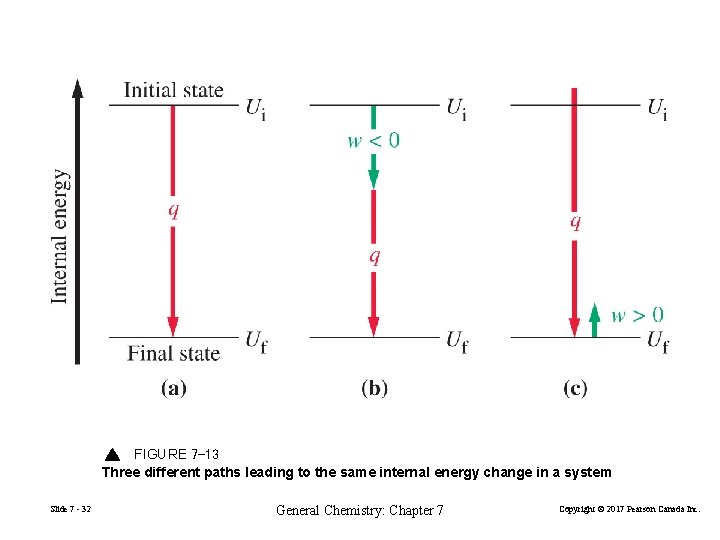
FIGURE 7 -13 Three different paths leading to the same internal energy change in a system Slide 7 - 32 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

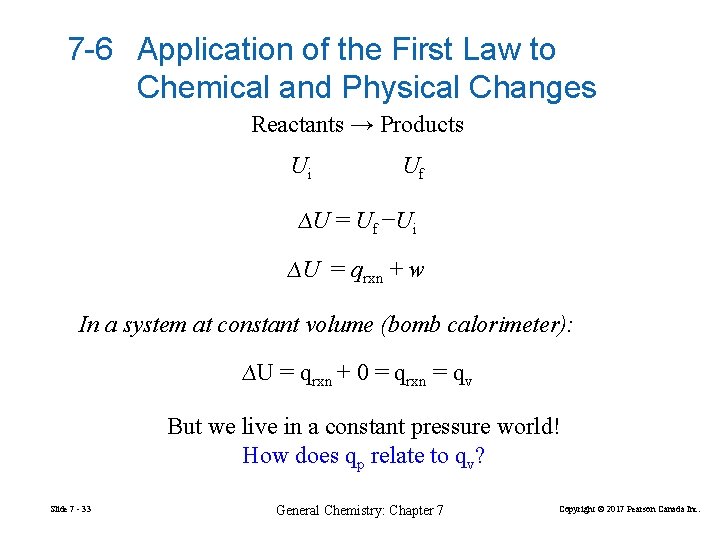
7 -6 Application of the First Law to Chemical and Physical Changes Reactants → Products Ui Uf ∆U = Uf −Ui ∆U = qrxn + w In a system at constant volume (bomb calorimeter): ∆U = qrxn + 0 = qrxn = qv But we live in a constant pressure world! How does qp relate to qv? Slide 7 - 33 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

q. V = q. P + w We know that w = –P∆V and ∆U = qv, therefore: ∆U = q. P – Pext∆V Uf–Ui = q. P – Pext. Vf+Pext. Vi (Uf + Pext. Vf)–(Ui+Pext. Vi)= q. P H = U + PV ∆H = Hf – Hi = q. P = ∆U + P∆V Slide 7 - 34 ∆U = ∆H– P∆V General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

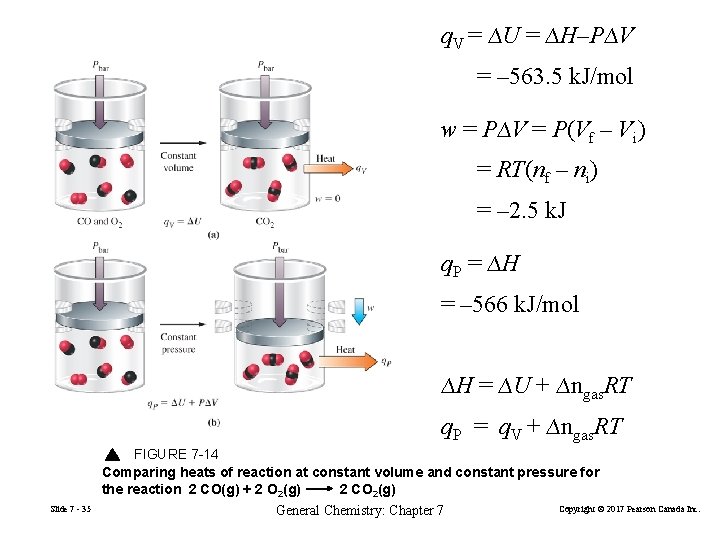
q. V = ∆U = ∆H–P∆V = – 563. 5 k. J/mol w = P∆V = P(Vf – Vi) = RT(nf – ni) = – 2. 5 k. J q. P = ∆H = – 566 k. J/mol ∆H = ∆U + ∆ngas. RT q. P = q. V + ∆ngas. RT FIGURE 7 -14 Comparing heats of reaction at constant volume and constant pressure for the reaction 2 CO(g) + 2 O 2(g) 2 CO 2(g) Slide 7 - 35 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

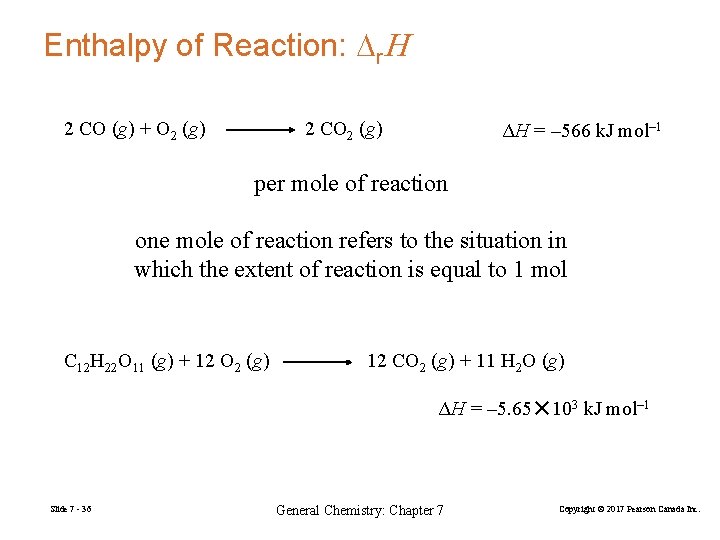
Enthalpy of Reaction: Dr. H 2 CO (g) + O 2 (g) 2 CO 2 (g) ΔH = – 566 k. J mol– 1 per mole of reaction one mole of reaction refers to the situation in which the extent of reaction is equal to 1 mol C 12 H 22 O 11 (g) + 12 O 2 (g) 12 CO 2 (g) + 11 H 2 O (g) ΔH = – 5. 65✕ 103 k. J mol– 1 Slide 7 - 36 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

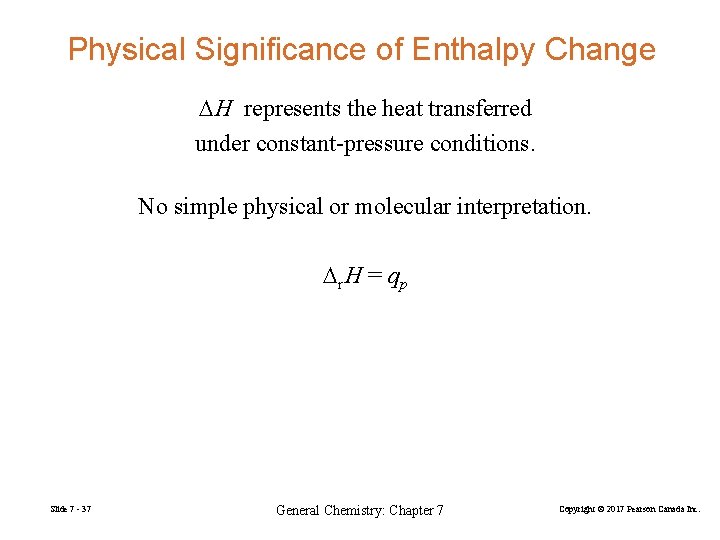
Physical Significance of Enthalpy Change DH represents the heat transferred under constant-pressure conditions. No simple physical or molecular interpretation. Dr. H = qp Slide 7 - 37 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

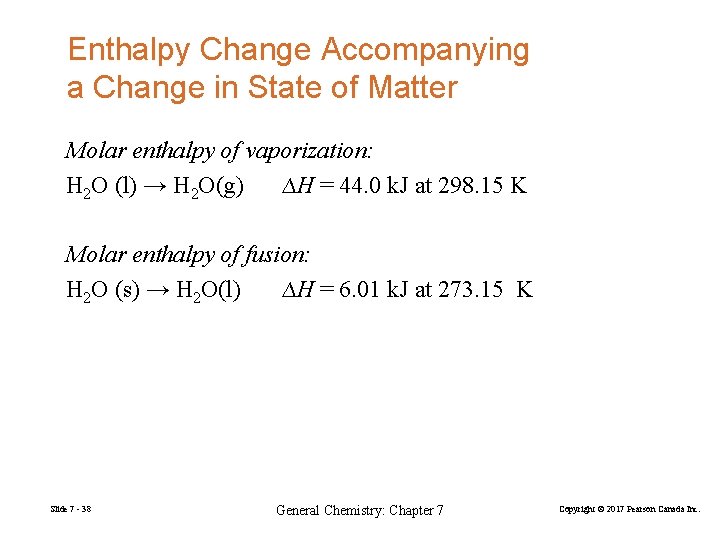
Enthalpy Change Accompanying a Change in State of Matter Molar enthalpy of vaporization: H 2 O (l) → H 2 O(g) ∆H = 44. 0 k. J at 298. 15 K Molar enthalpy of fusion: H 2 O (s) → H 2 O(l) ∆H = 6. 01 k. J at 273. 15 K Slide 7 - 38 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

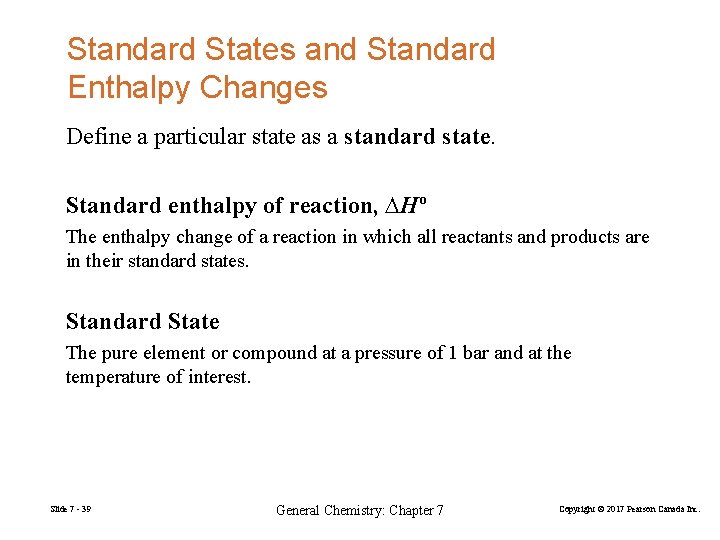
Standard States and Standard Enthalpy Changes Define a particular state as a standard state. Standard enthalpy of reaction, ∆Hº The enthalpy change of a reaction in which all reactants and products are in their standard states. Standard State The pure element or compound at a pressure of 1 bar and at the temperature of interest. Slide 7 - 39 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

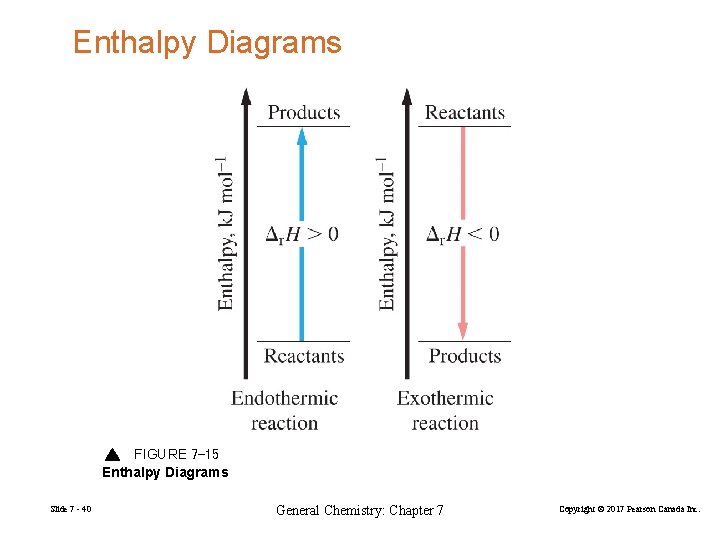
Enthalpy Diagrams FIGURE 7 -15 Enthalpy Diagrams Slide 7 - 40 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

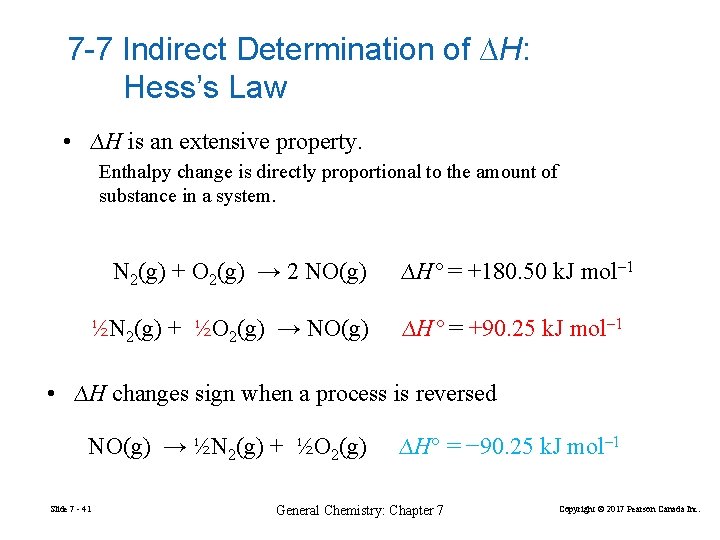
7 -7 Indirect Determination of ∆H: Hess’s Law • ∆H is an extensive property. Enthalpy change is directly proportional to the amount of substance in a system. N 2(g) + O 2(g) → 2 NO(g) ½N 2(g) + ½O 2(g) → NO(g) ∆H° = +180. 50 k. J mol– 1 ∆H° = +90. 25 k. J mol– 1 • ∆H changes sign when a process is reversed NO(g) → ½N 2(g) + ½O 2(g) Slide 7 - 41 ∆H° = − 90. 25 k. J mol– 1 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

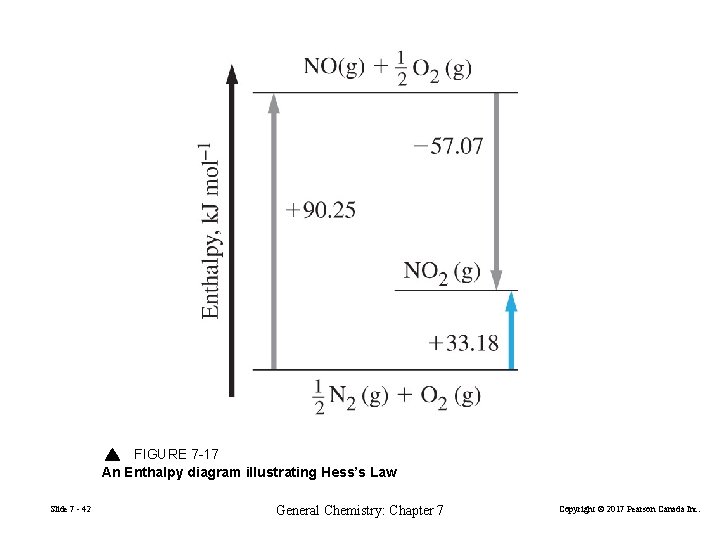
FIGURE 7 -17 An Enthalpy diagram illustrating Hess’s Law Slide 7 - 42 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

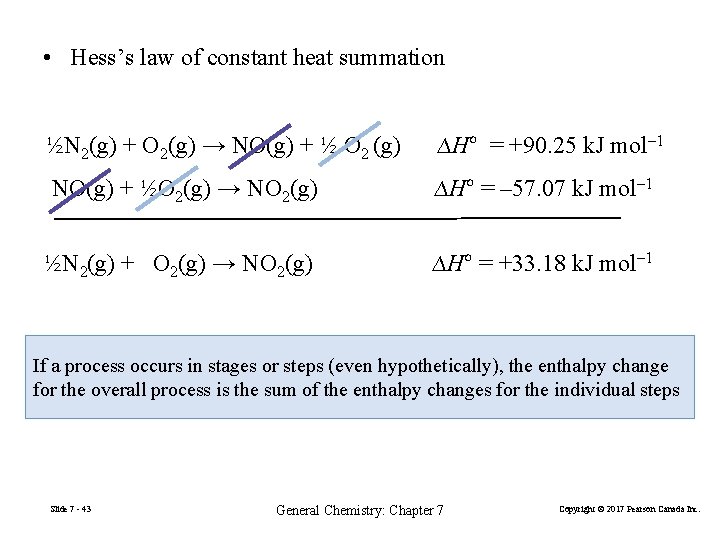
• Hess’s law of constant heat summation ½N 2(g) + O 2(g) → NO(g) + ½ O 2 (g) ∆Hº = +90. 25 k. J mol– 1 NO(g) + ½O 2(g) → NO 2(g) ∆Hº = – 57. 07 k. J mol– 1 ½N 2(g) + O 2(g) → NO 2(g) ∆Hº = +33. 18 k. J mol– 1 If a process occurs in stages or steps (even hypothetically), the enthalpy change for the overall process is the sum of the enthalpy changes for the individual steps Slide 7 - 43 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

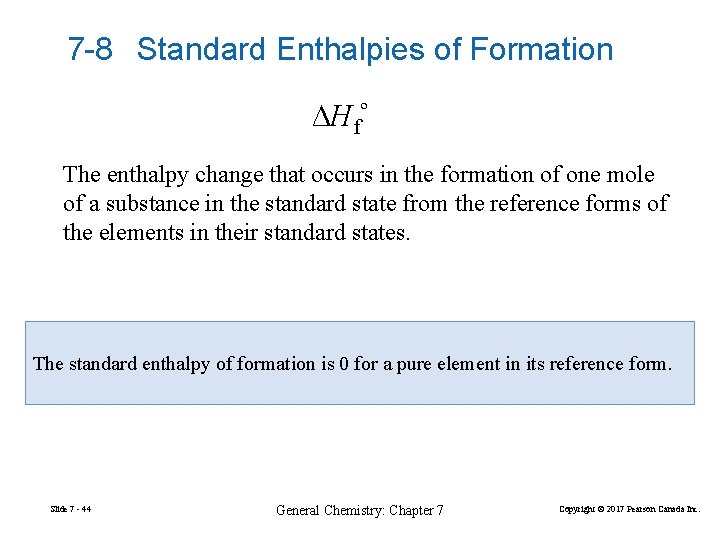
7 -8 Standard Enthalpies of Formation ∆Hfº The enthalpy change that occurs in the formation of one mole of a substance in the standard state from the reference forms of the elements in their standard states. The standard enthalpy of formation is 0 for a pure element in its reference form. Slide 7 - 44 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

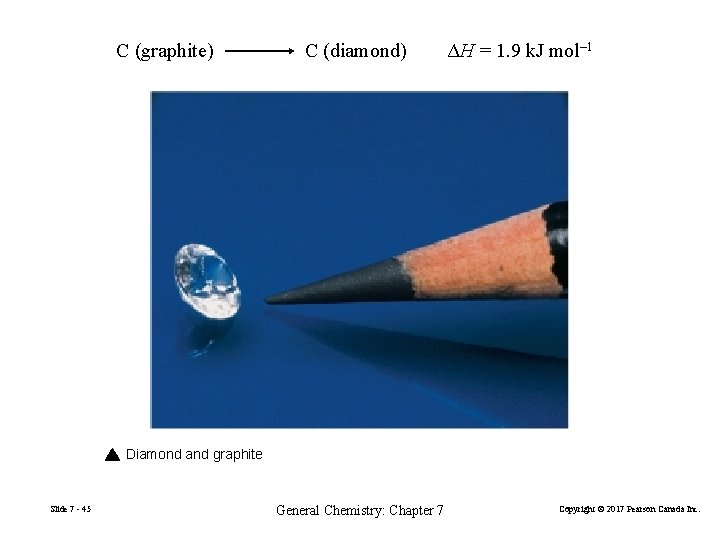
C (graphite) C (diamond) ΔH = 1. 9 k. J mol– 1 Diamond and graphite Slide 7 - 45 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

Br 2(l) Br 2(g) DHfº = 30. 91 k. J Liquid bromine vaporizing Slide 7 - 46 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

Slide 7 - 47 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

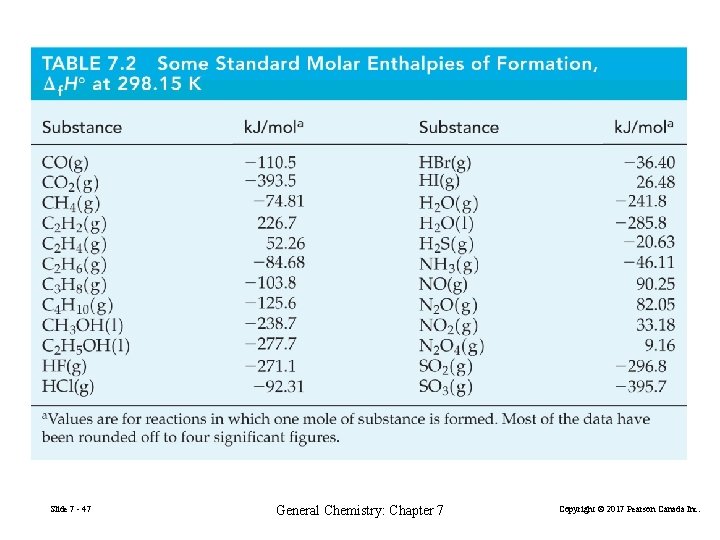
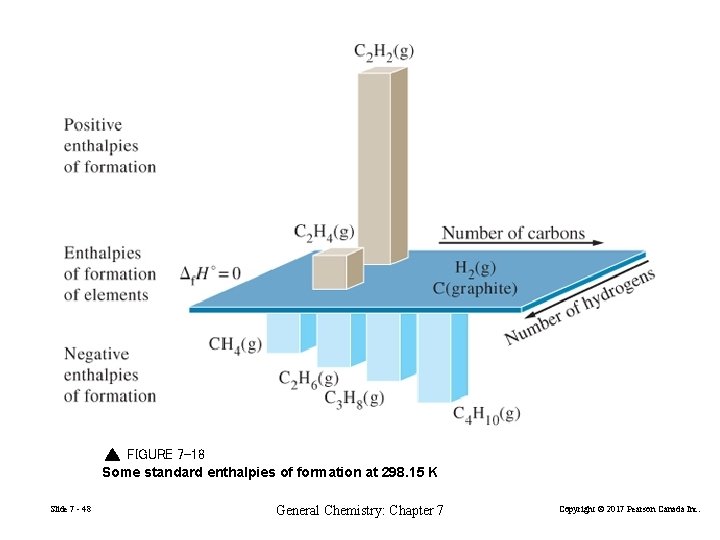
FIGURE 7 -18 Some standard enthalpies of formation at 298. 15 K Slide 7 - 48 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

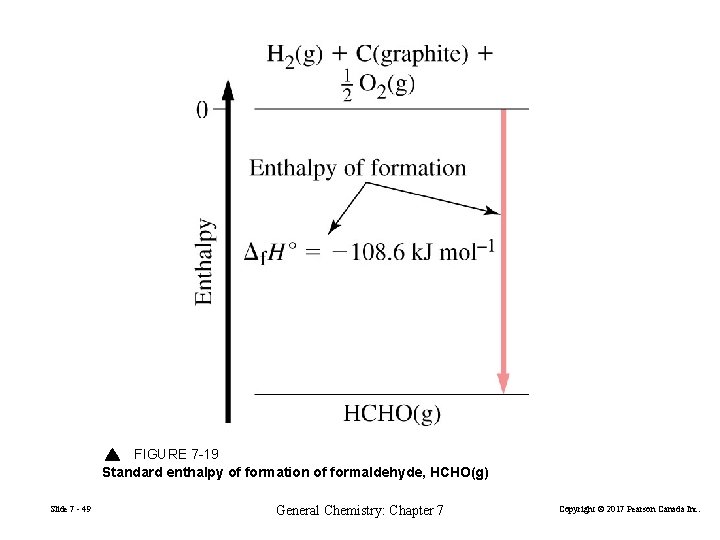
FIGURE 7 -19 Standard enthalpy of formation of formaldehyde, HCHO(g) Slide 7 - 49 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

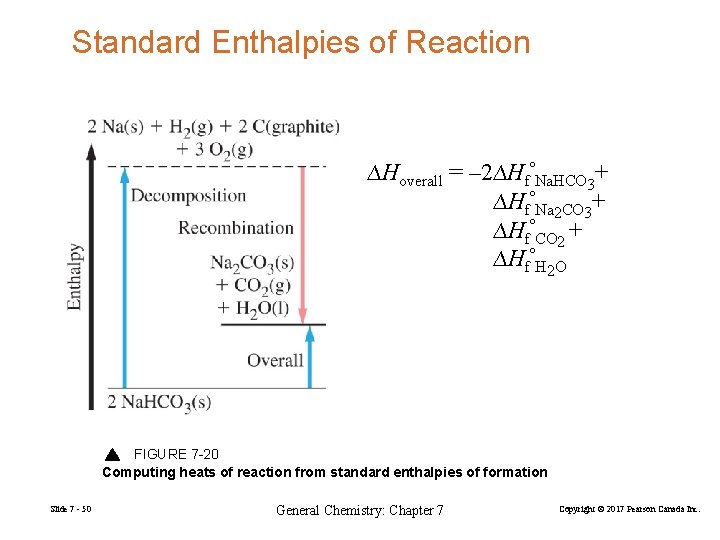
Standard Enthalpies of Reaction ∆Hoverall = – 2∆HfºNa. HCO 3+ ∆HfºNa 2 CO 3+ ∆HfºCO 2 + ∆HfºH 2 O FIGURE 7 -20 Computing heats of reaction from standard enthalpies of formation Slide 7 - 50 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

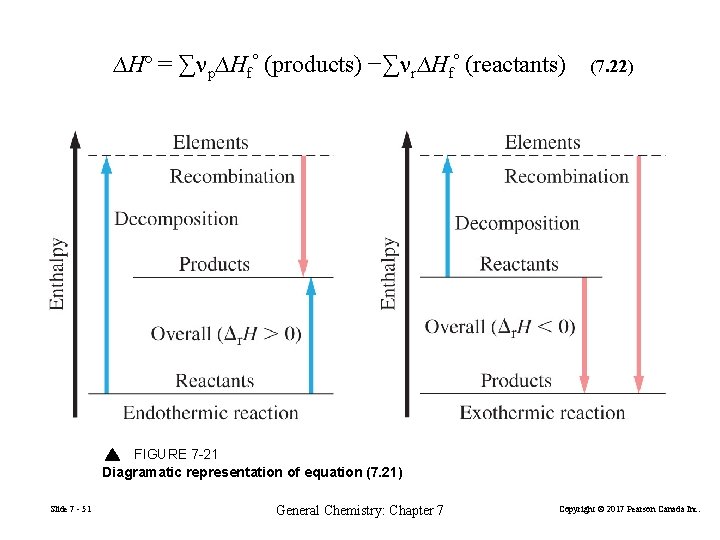
∆Hº = ∑np∆Hfº (products) −∑nr∆Hfº (reactants) (7. 22) FIGURE 7 -21 Diagramatic representation of equation (7. 21) Slide 7 - 51 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

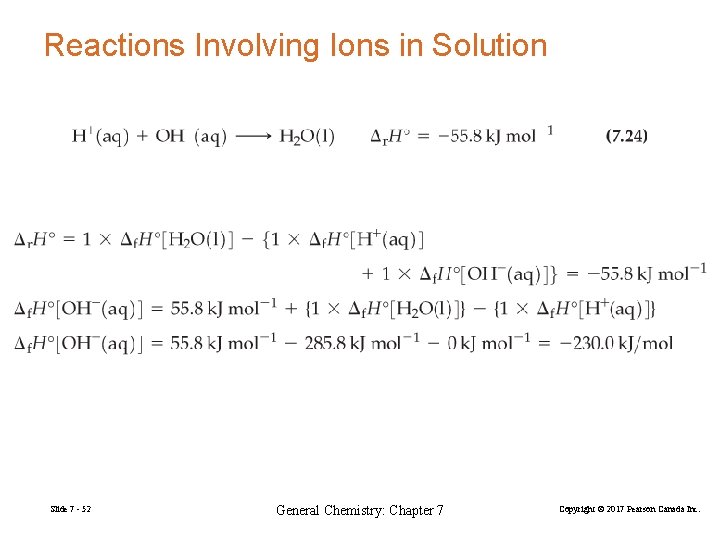
Reactions Involving Ions in Solution Slide 7 - 52 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

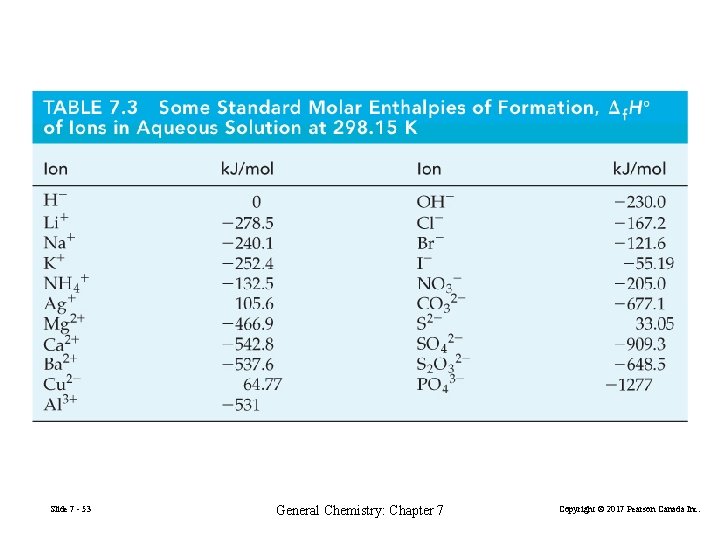
Slide 7 - 53 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

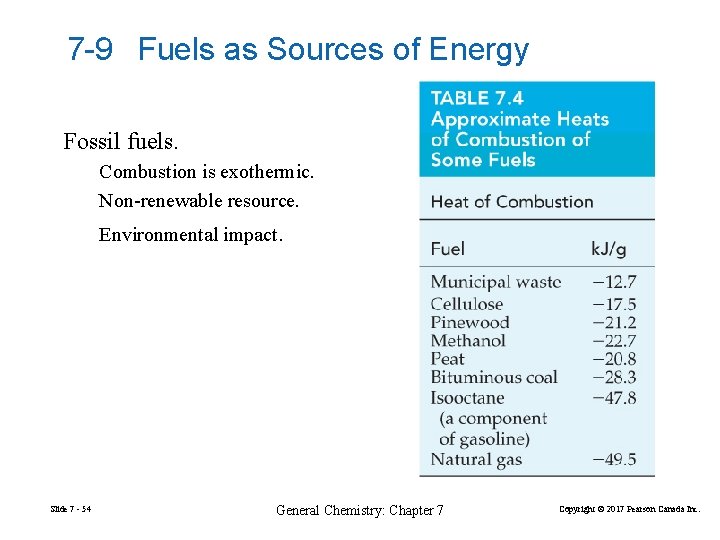
7 -9 Fuels as Sources of Energy Fossil fuels. Combustion is exothermic. Non-renewable resource. Environmental impact. Slide 7 - 54 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

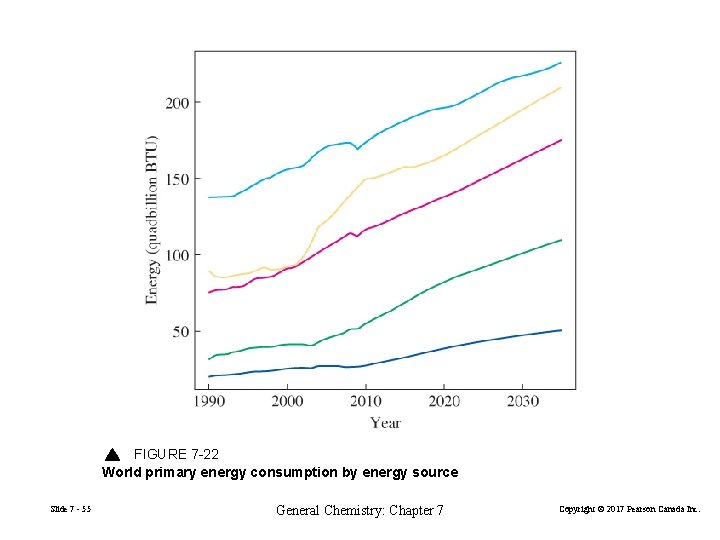
FIGURE 7 -22 World primary energy consumption by energy source Slide 7 - 55 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

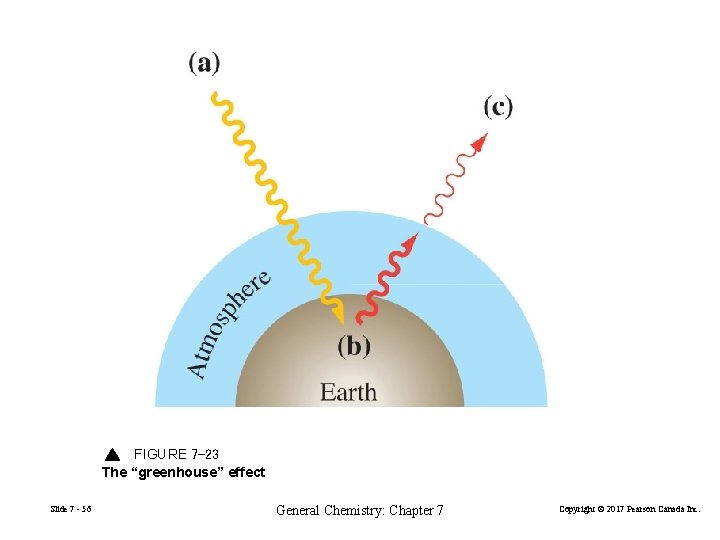
FIGURE 7 -23 The “greenhouse” effect Slide 7 - 56 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

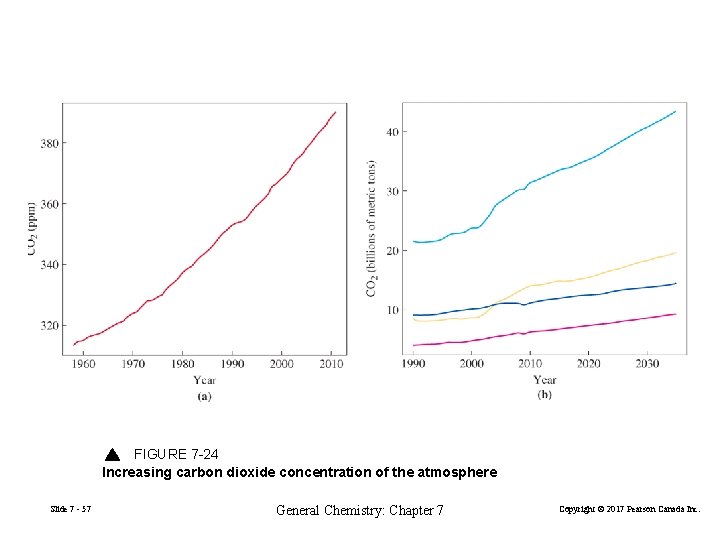
FIGURE 7 -24 Increasing carbon dioxide concentration of the atmosphere Slide 7 - 57 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

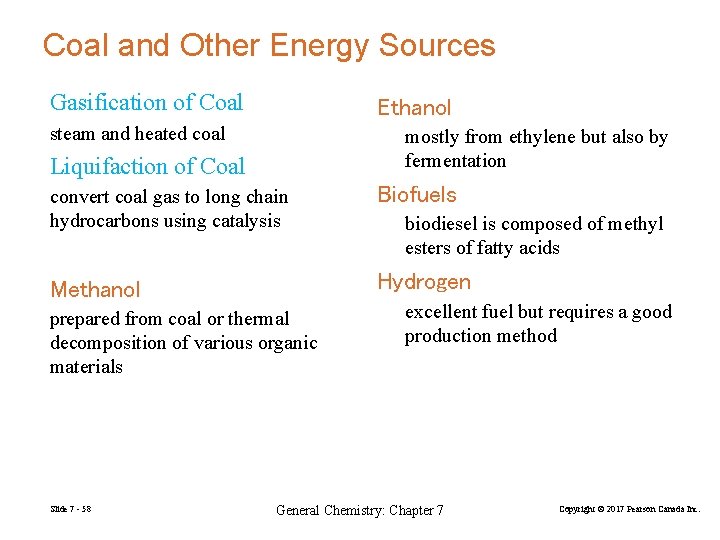
Coal and Other Energy Sources Gasification of Coal Ethanol steam and heated coal mostly from ethylene but also by fermentation Liquifaction of Coal convert coal gas to long chain hydrocarbons using catalysis Biofuels Methanol Hydrogen prepared from coal or thermal decomposition of various organic materials Slide 7 - 58 biodiesel is composed of methyl esters of fatty acids excellent fuel but requires a good production method General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

7 -10 Spontaneous and Nonspontaneous Processes: An Introduction spontaneous process occurs in a system left to itself; once started, no external action is necessary to make the process continue. nonspontaneous process will not occur unless some external action is continuously applied. Slide 7 - 59 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

spontaneous processes may be endothermic or exothermic Slide 7 - 60 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

If a process is spontaneous, the reverse process is nonspontaneous Both spontaneous and nonspontaneous processes are possible, but only spontaneous processes will occur without intervention. Some spontaneous processes occur very slowly, and others occur rather rapidly. Some spontaneous processes are exothermic, and others are endothermic. Slide 7 - 61 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.

End of Chapter Slide 7 - 62 General Chemistry: Chapter 7 Copyright © 2017 Pearson Canada Inc.
 Management eleventh edition stephen p robbins
Management eleventh edition stephen p robbins Management 11th edition by stephen p robbins
Management 11th edition by stephen p robbins Management eleventh edition
Management eleventh edition Management eleventh edition
Management eleventh edition General chemistry
General chemistry Bhore committee
Bhore committee Eleventh 5 year plan
Eleventh 5 year plan 11th five year plan
11th five year plan For his eleventh birthday elvis presley
For his eleventh birthday elvis presley Introduction to genetic analysis tenth edition
Introduction to genetic analysis tenth edition No slip condition
No slip condition Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Modern systems analysis and design 7th edition
Modern systems analysis and design 7th edition Susanna s epp
Susanna s epp Using mis (10th edition) 10th edition
Using mis (10th edition) 10th edition Report
Report Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Electrical engineering
Electrical engineering Learning principles and applications
Learning principles and applications Applications of nuclear chemistry
Applications of nuclear chemistry Irradiated food
Irradiated food Modern labor economics 12th edition solution
Modern labor economics 12th edition solution Modern labor economics 12th edition pdf
Modern labor economics 12th edition pdf Modern real estate practice in pennsylvania
Modern real estate practice in pennsylvania Modern database management 12th edition ppt
Modern database management 12th edition ppt Modern database management 12th edition ppt
Modern database management 12th edition ppt Modern operating systems 3rd edition
Modern operating systems 3rd edition Tanenbaum structured computer organization
Tanenbaum structured computer organization Modern database management 8th edition
Modern database management 8th edition University physics with modern physics fifteenth edition
University physics with modern physics fifteenth edition Modern database management 11th edition
Modern database management 11th edition Modern labor economics 12th edition
Modern labor economics 12th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Expert systems: principles and programming, fourth edition
Expert systems: principles and programming, fourth edition Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Pericyclic
Pericyclic Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein Introductory chemistry 4th edition
Introductory chemistry 4th edition Prefix multipliers
Prefix multipliers Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Chemistry: the central science chapter 14 answers
Chemistry: the central science chapter 14 answers Organic chemistry (3rd) edition chapter 1 problem 20s
Organic chemistry (3rd) edition chapter 1 problem 20s Nomenclature of ethers
Nomenclature of ethers Zumdahl chemistry, 9th edition notes
Zumdahl chemistry, 9th edition notes Organic chemistry third edition david klein
Organic chemistry third edition david klein Lesson 81 drop in molecular views
Lesson 81 drop in molecular views Chemistry by raymond chang 10th edition
Chemistry by raymond chang 10th edition Democritus atomic model diagram
Democritus atomic model diagram Halohydrin
Halohydrin Thermodynamic control
Thermodynamic control Organic chemistry
Organic chemistry Properties of modern materials
Properties of modern materials Modern chemistry textbook answers chapter 9
Modern chemistry textbook answers chapter 9 Chemical formulas and chemical compounds chapter 7 review
Chemical formulas and chemical compounds chapter 7 review Modern chemistry chapter 15
Modern chemistry chapter 15 Chapter 14 acids and bases
Chapter 14 acids and bases Chapter 13 ions in aqueous solutions
Chapter 13 ions in aqueous solutions Modern chemistry chapter 12 test
Modern chemistry chapter 12 test Modern chemistry chapter 4
Modern chemistry chapter 4 Modern chemistry solutions
Modern chemistry solutions