GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION













- Slides: 34

GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION PETRUCCI HERRING MADURA BISSONNETTE Chemical Compounds 3 PHILIP DUTTON UNIVERSITY OF WINDSOR DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY Slide 3 - 1 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

Chemical Compounds Slide 3 - 2 CONTENTS 3 -1 Types of Chemical Compounds and Their Formulas 3 -2 The Mole Concept and Chemical Compounds 3 -3 Composition of Chemical Compounds 3 -4 Oxidation States: A Useful Tool in Describing Chemical Compounds 3 -5 Naming Compounds: Organic and Inorganic Compounds 3 -6 Names and Formulas of Inorganic Compounds General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

3 -1 Types of Chemical Compounds and Their Formulas Molecular Compounds Slide 3 - 3 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

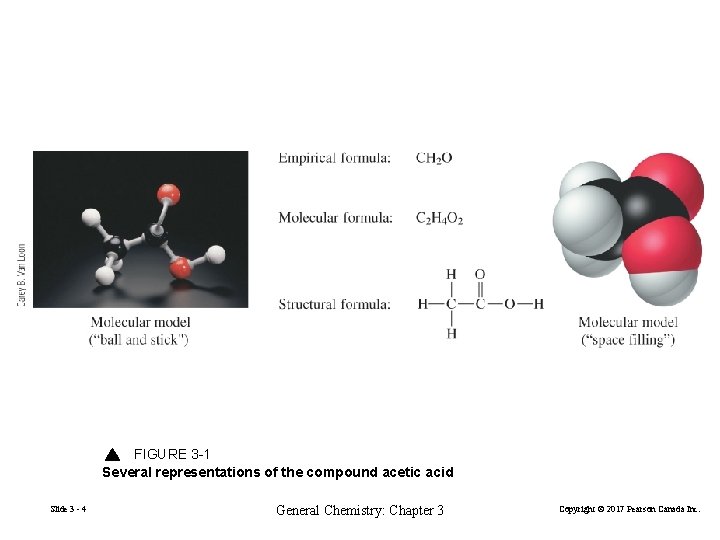
FIGURE 3 -1 Several representations of the compound acetic acid Slide 3 - 4 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

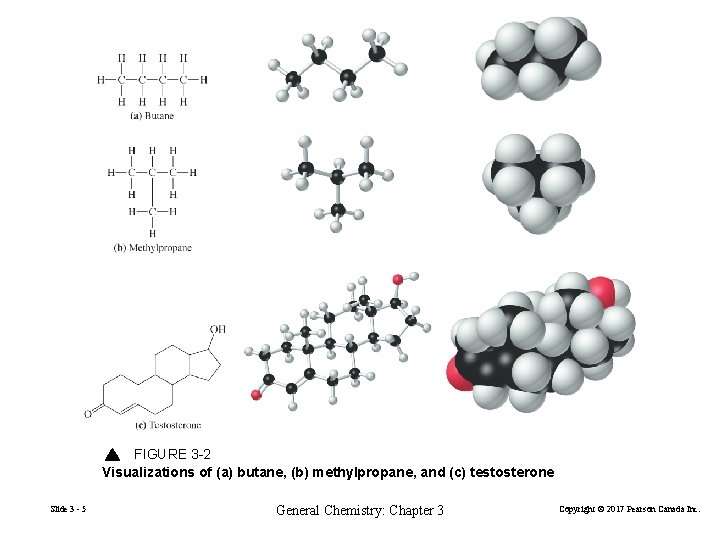
FIGURE 3 -2 Visualizations of (a) butane, (b) methylpropane, and (c) testosterone Slide 3 - 5 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

FIGURE 3 -3 Color scheme for use in molecular models Slide 3 - 6 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

Ionic Compounds • Atoms of almost all elements can gain or lose electrons to form charged species called ions. • Compounds composed of ions are known as ionic compounds. + Metals tend to lose electrons to form positively charged ions called cations. − Non-metals tend to gain electrons to form negatively charged ions called anions. Slide 3 - 7 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

FIGURE 3 -4 Portion of an ionic crystal and a formula unit of Na. Cl Slide 3 - 8 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

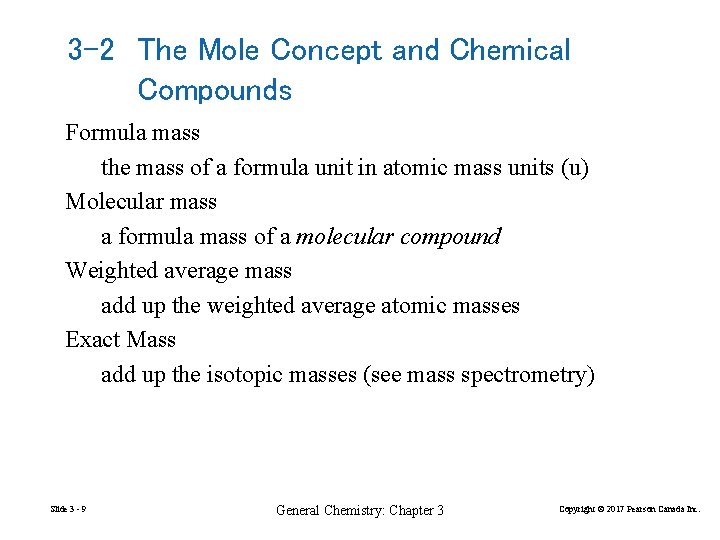
3 -2 The Mole Concept and Chemical Compounds Formula mass the mass of a formula unit in atomic mass units (u) Molecular mass a formula mass of a molecular compound Weighted average mass add up the weighted average atomic masses Exact Mass add up the isotopic masses (see mass spectrometry) Slide 3 - 9 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

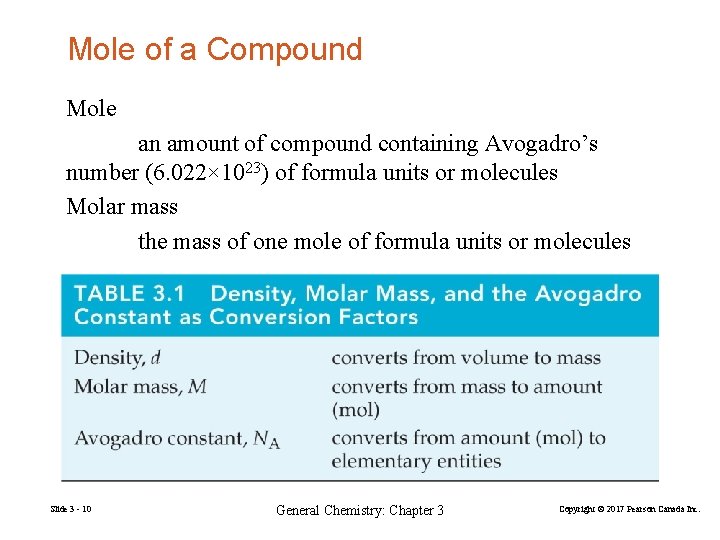
Mole of a Compound Mole an amount of compound containing Avogadro’s number (6. 022× 1023) of formula units or molecules Molar mass the mass of one mole of formula units or molecules Slide 3 - 10 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

Mole of an Element – A Second Look FIGURE 3 -5 Molecular forms of elemental sulfur and phosphorus Slide 3 - 11 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

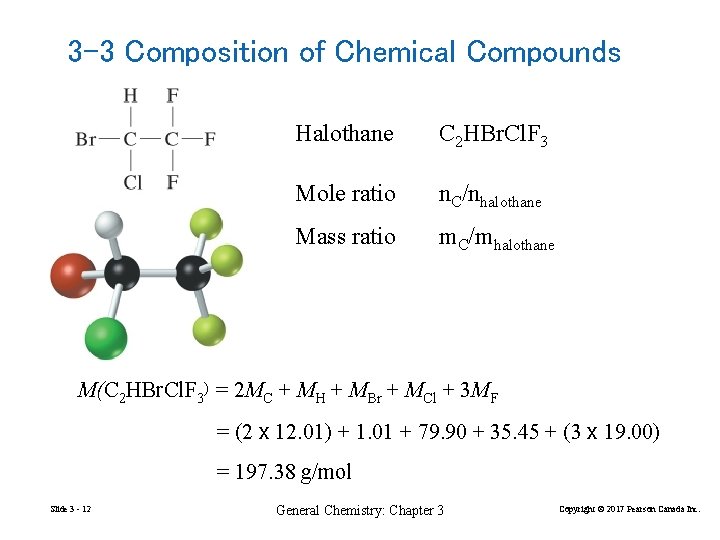
3 -3 Composition of Chemical Compounds Halothane C 2 HBr. Cl. F 3 Mole ratio n. C/nhalothane Mass ratio m. C/mhalothane M(C 2 HBr. Cl. F 3) = 2 MC + MH + MBr + MCl + 3 MF = (2 x 12. 01) + 1. 01 + 79. 90 + 35. 45 + (3 x 19. 00) = 197. 38 g/mol Slide 3 - 12 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

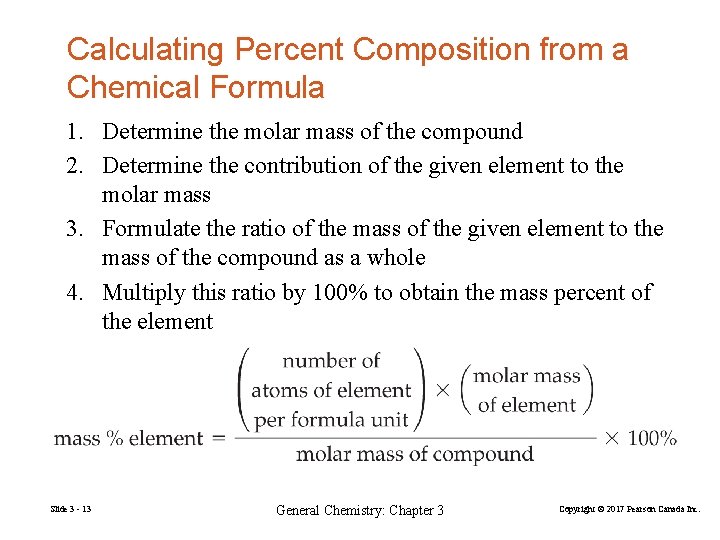
Calculating Percent Composition from a Chemical Formula 1. Determine the molar mass of the compound 2. Determine the contribution of the given element to the molar mass 3. Formulate the ratio of the mass of the given element to the mass of the compound as a whole 4. Multiply this ratio by 100% to obtain the mass percent of the element Slide 3 - 13 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

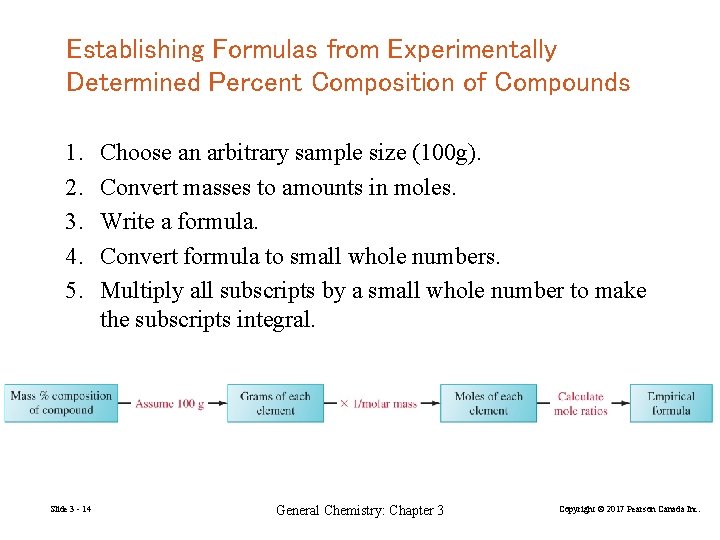
Establishing Formulas from Experimentally Determined Percent Composition of Compounds 1. 2. 3. 4. 5. Slide 3 - 14 Choose an arbitrary sample size (100 g). Convert masses to amounts in moles. Write a formula. Convert formula to small whole numbers. Multiply all subscripts by a small whole number to make the subscripts integral. General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

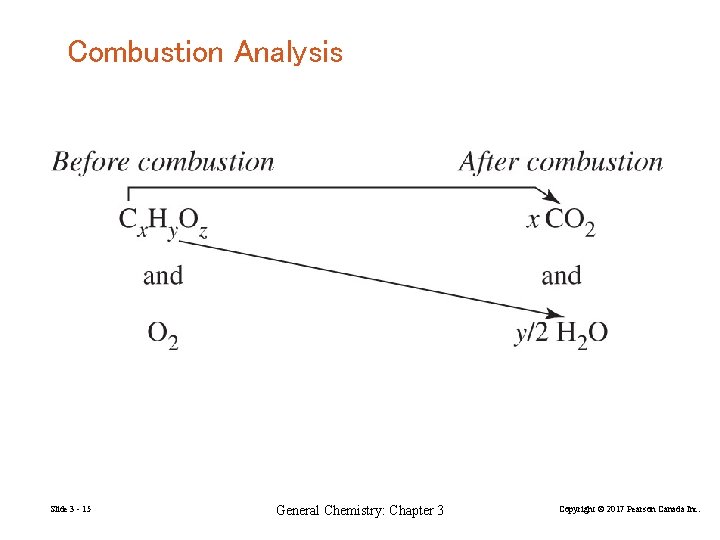
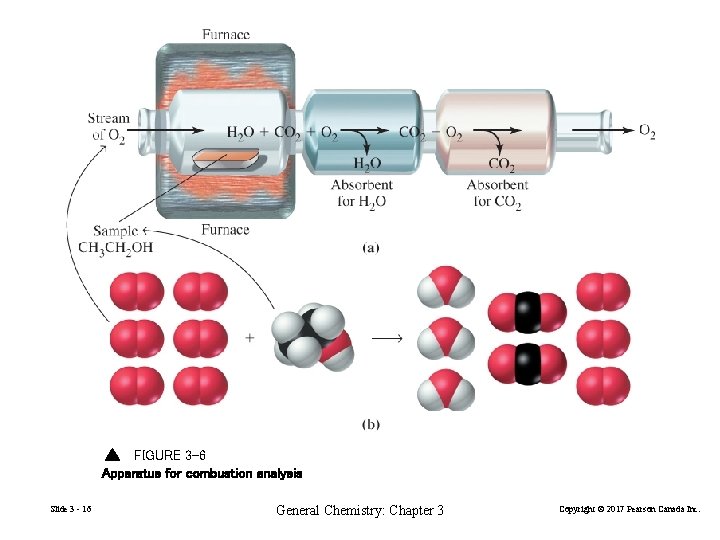
Combustion Analysis Slide 3 - 15 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

FIGURE 3 -6 Apparatus for combustion analysis Slide 3 - 16 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

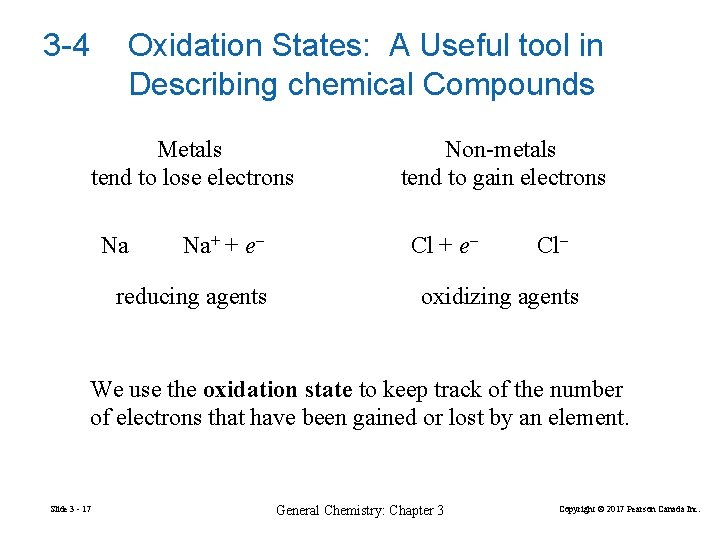
3 -4 Oxidation States: A Useful tool in Describing chemical Compounds Metals tend to lose electrons Na Na+ + e– reducing agents Non-metals tend to gain electrons Cl + e– Cl– oxidizing agents We use the oxidation state to keep track of the number of electrons that have been gained or lost by an element. Slide 3 - 17 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

Slide 3 - 18 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

3 -5 Naming Compounds: Organic and Inorganic Compounds Lead (IV) oxide Lead (II) oxide FIGURE 3 -7 Two oxides of lead Slide 3 - 19 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

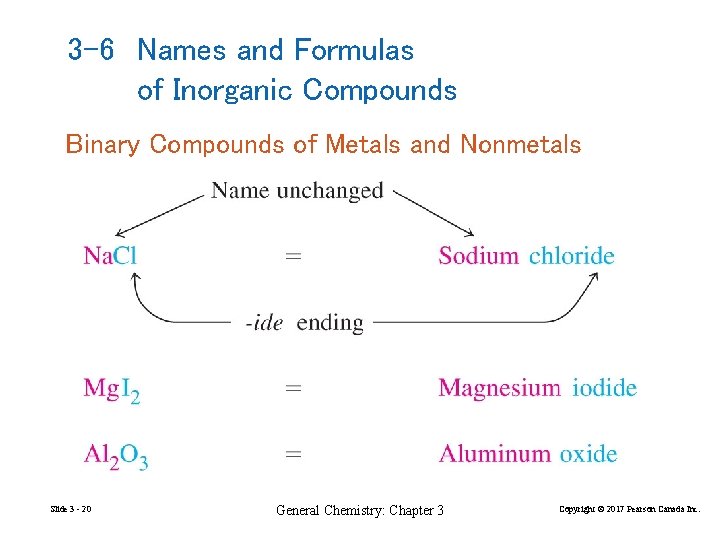
3 -6 Names and Formulas of Inorganic Compounds Binary Compounds of Metals and Nonmetals Slide 3 - 20 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

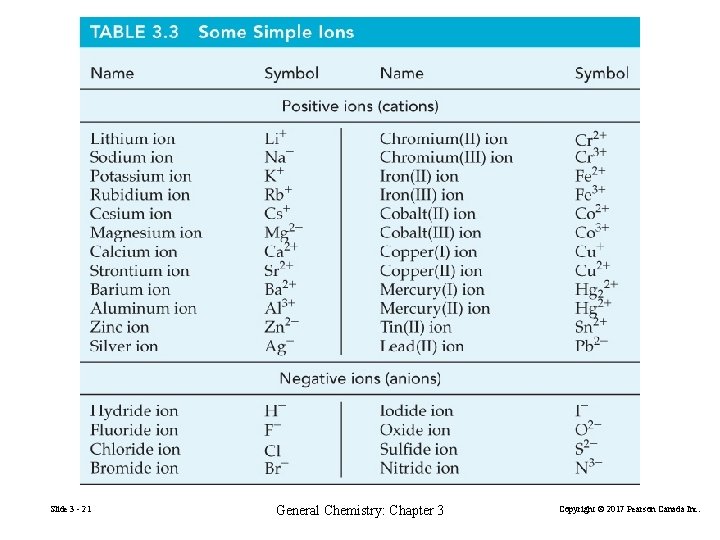
Slide 3 - 21 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

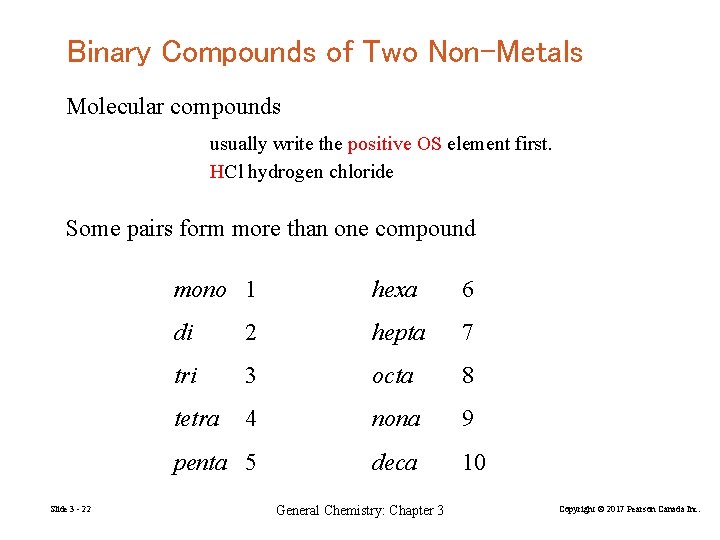
Binary Compounds of Two Non-Metals Molecular compounds usually write the positive OS element first. HCl hydrogen chloride Some pairs form more than one compound Slide 3 - 22 mono 1 hexa 6 di 2 hepta 7 tri 3 octa 8 tetra 4 nona 9 penta 5 deca 10 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

Slide 3 - 23 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

Binary Acids produce H+ when dissolved in water. They are compounds that ionize in water. The symbol (aq) signifies aqueous solution. HF(aq) = hydrofluoric acid HBr(aq) = hydrobromic acid HCl(aq) = hydrochloric acid HI(aq) = hydroiodic acid H 2 S(aq) = hydrosulfuric acid Slide 3 - 24 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

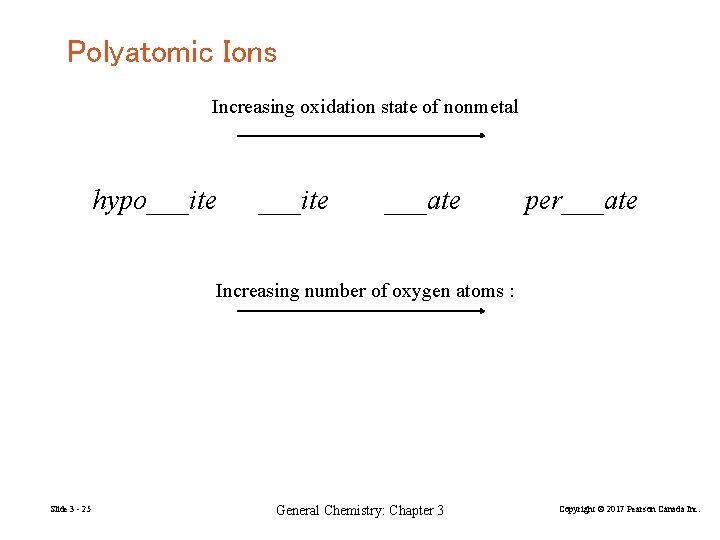
Polyatomic Ions Increasing oxidation state of nonmetal hypo___ite ___ate per___ate Increasing number of oxygen atoms : Slide 3 - 25 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

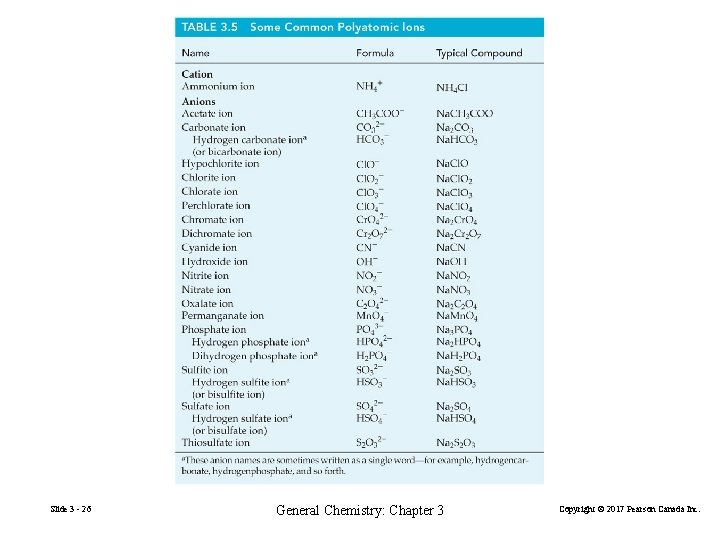
Slide 3 - 26 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

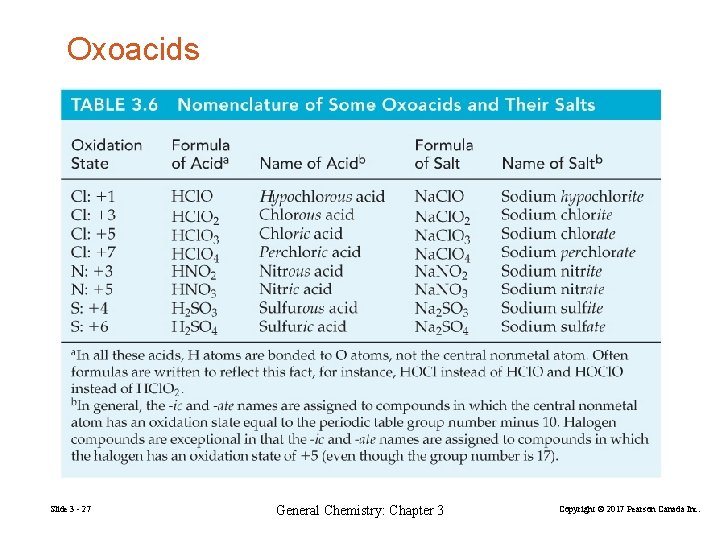
Oxoacids Slide 3 - 27 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

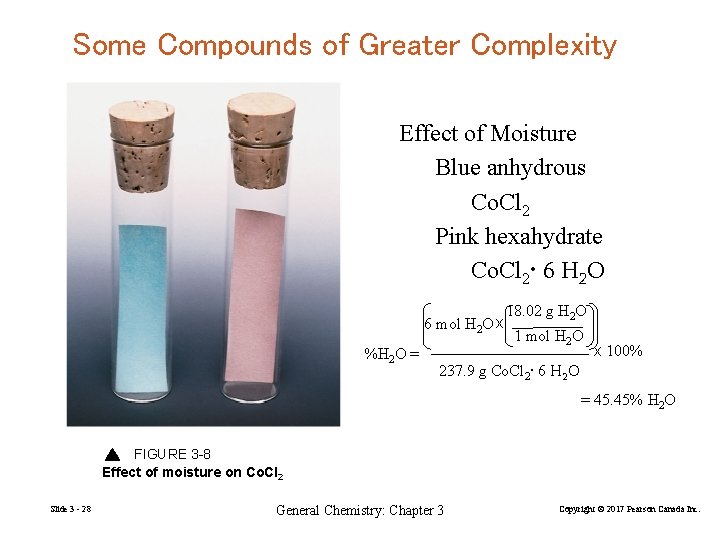
Some Compounds of Greater Complexity Effect of Moisture Blue anhydrous Co. Cl 2 Pink hexahydrate Co. Cl 2 • 6 H 2 O 6 mol H 2 O x %H 2 O = 18. 02 g H 2 O 1 mol H 2 O x 100% 237. 9 g Co. Cl 2 • 6 H 2 O = 45. 45% H 2 O FIGURE 3 -8 Effect of moisture on Co. Cl 2 Slide 3 - 28 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

3 -7 Names and Formulas of Organic Compounds Organic compounds abound in nature Fats, carbohydrates and proteins are foods. Propane, gasoline, kerosene, oil are fuels. Drugs and plastics are produced by chemical industries. Carbon atoms form chains and rings and act as the framework of molecules. Slide 3 - 29 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

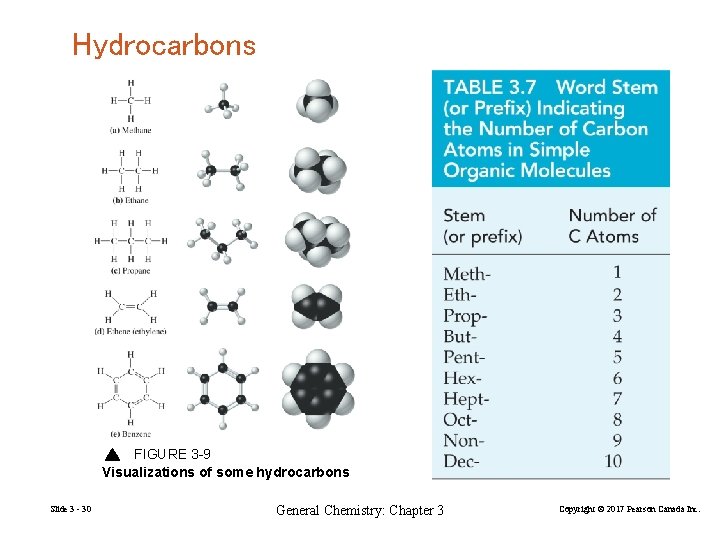
Hydrocarbons FIGURE 3 -9 Visualizations of some hydrocarbons Slide 3 - 30 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

Isomers have the same molecular formula but have different arrangements of atoms in space. Butane and methylpropane are isomers. Slide 3 - 31 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

Functional Groups FIGURE 3 -10 Visualizations of some alcohols Slide 3 - 32 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

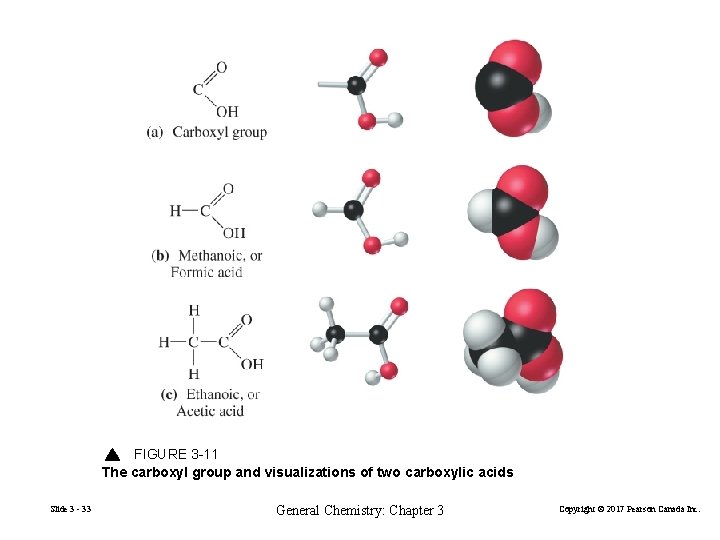
FIGURE 3 -11 The carboxyl group and visualizations of two carboxylic acids Slide 3 - 33 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.

End of Chapter Slide 3 - 34 General Chemistry: Chapter 3 Copyright © 2017 Pearson Canada Inc.
 Management eleventh edition
Management eleventh edition Management eleventh edition
Management eleventh edition Management eleventh edition
Management eleventh edition Management eleventh edition
Management eleventh edition General chemistry
General chemistry Chadha committee
Chadha committee Eleventh 5 year plan
Eleventh 5 year plan Eleventh plan
Eleventh plan For his eleventh birthday elvis presley
For his eleventh birthday elvis presley Human genetics concepts and applications 10th edition
Human genetics concepts and applications 10th edition Fluid mechanics fundamentals and applications
Fluid mechanics fundamentals and applications Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Modern systems analysis and design 7th edition
Modern systems analysis and design 7th edition A computer programming team has 13 members
A computer programming team has 13 members Mis chapter 6
Mis chapter 6 Using mis 10th edition
Using mis 10th edition Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Electrical engineering
Electrical engineering Learning principles and applications
Learning principles and applications 25 m/s
25 m/s Applications of nuclear chemistry
Applications of nuclear chemistry Modern labor economics 12th edition solution
Modern labor economics 12th edition solution Modern labor economics 12th edition
Modern labor economics 12th edition Modern real estate practice in pennsylvania 14th edition
Modern real estate practice in pennsylvania 14th edition Modern database management 12th edition ppt
Modern database management 12th edition ppt Modern database management 12th edition ppt
Modern database management 12th edition ppt Modern operating systems 3rd edition
Modern operating systems 3rd edition Modern operating systems tanenbaum 5th edition
Modern operating systems tanenbaum 5th edition Modern database management 8th edition
Modern database management 8th edition University physics with modern physics fifteenth edition
University physics with modern physics fifteenth edition Transaction cannot be subdivided
Transaction cannot be subdivided Modern labor economics 12th edition
Modern labor economics 12th edition Computer security principles and practice 4th edition
Computer security principles and practice 4th edition