CHM 1046 General Chemistry and Qualitative Analysis Unit

![The Common-Ion Effect [H 3 O+] [F−] Ka = [HF] = X= What is The Common-Ion Effect [H 3 O+] [F−] Ka = [HF] = X= What is](https://slidetodoc.com/presentation_image_h/10107fdafcc87f41217a1f0b99227693/image-4.jpg)
![[ ] Acids and Bases [ ] Acids and Bases](https://slidetodoc.com/presentation_image_h/10107fdafcc87f41217a1f0b99227693/image-6.jpg)
![Add base (OH-) Buffers [HF] + H 2 O OH- + HF H 2 Add base (OH-) Buffers [HF] + H 2 O OH- + HF H 2](https://slidetodoc.com/presentation_image_h/10107fdafcc87f41217a1f0b99227693/image-8.jpg)
![[ ] Ka = [ ] [H 3 O+] [A−] [HA] [base] p. H [ ] Ka = [ ] [H 3 O+] [A−] [HA] [base] p. H](https://slidetodoc.com/presentation_image_h/10107fdafcc87f41217a1f0b99227693/image-12.jpg)
![p. H= p. Ka + log [base] [acid] Acids and Bases p. H= p. Ka + log [base] [acid] Acids and Bases](https://slidetodoc.com/presentation_image_h/10107fdafcc87f41217a1f0b99227693/image-13.jpg)
![Addition of Strong Acid or Base to a Buffer Add base (OH-) [HA] + Addition of Strong Acid or Base to a Buffer Add base (OH-) [HA] +](https://slidetodoc.com/presentation_image_h/10107fdafcc87f41217a1f0b99227693/image-15.jpg)



- Slides: 37

CHM 1046: General Chemistry and Qualitative Analysis Unit 18 Acid-Base Equilibria: Buffers & Hydrolysis Dr. Jorge L. Alonso Miami-Dade College – Kendall Campus Miami, FL Textbook Reference: • Chapter 19 (sec. 1 -4) • Modules # 7 -8 Acids and Bases

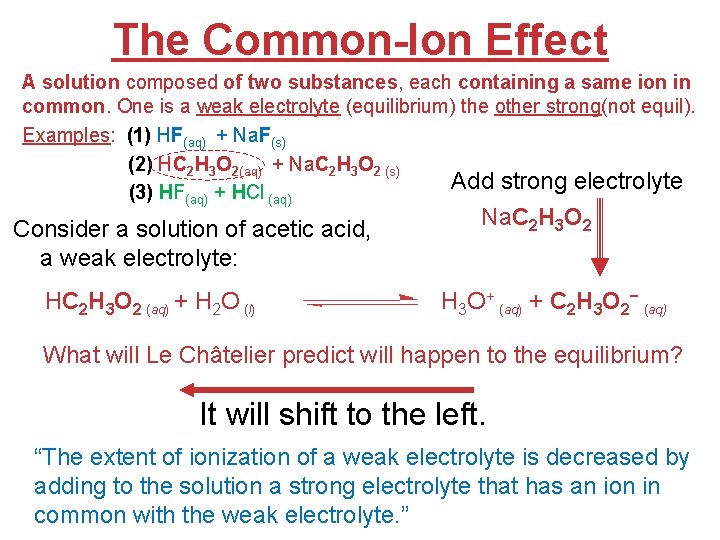
The Common-Ion Effect A solution composed of two substances, each containing a same ion in common. One is a weak electrolyte (equilibrium) the other strong(not equil). Examples: (1) HF(aq) + Na. F(s) (2) HC 2 H 3 O 2(aq) + Na. C 2 H 3 O 2 (s) Add strong electrolyte (3) HF(aq) + HCl (aq) Consider a solution of acetic acid, a weak electrolyte: HC 2 H 3 O 2 (aq) + H 2 O (l) Na. C 2 H 3 O 2 H 3 O+ (aq) + C 2 H 3 O 2− (aq) What will Le Châtelier predict will happen to the equilibrium? It will shift to the left. “The extent of ionization of a weak electrolyte is decreased. Acids by adding to the solution a strong electrolyte that has an ion inand Bases common with the weak electrolyte. ”

The Common-Ion Effect Problem: Calculate the fluoride ion concentration and p. H of a solution that is 0. 20 M in HF and 0. 10 M in HCl. Ka for HF is 6. 8 10− 4. HF(aq) + H 2 O(l) H 3 O+(aq) + F−(aq) Because HCl, a strong acid, is also present, the initial [H 3 O+] is not 0, but rather 0. 10 M. [H 3 O+] [F−] = 6. 8 10 -4 Ka = [HF] Initially Change At Equilibrium [HF], M 0. 20 −x 0. 20 − x 0. 20 [H 3 O+], M 0. 10 +x 0. 10 + x 0. 10 [F−], M 0 Acids +x and x. Bases
![The CommonIon Effect H 3 O F Ka HF X What is The Common-Ion Effect [H 3 O+] [F−] Ka = [HF] = X= What is](https://slidetodoc.com/presentation_image_h/10107fdafcc87f41217a1f0b99227693/image-4.jpg)
The Common-Ion Effect [H 3 O+] [F−] Ka = [HF] = X= What is [F−] ? And p. H? (0. 10+x) (x) = (0. 20 -x) (0. 20) (6. 8 10− 4) (0. 10) 6. 8 10− 4 x = 1. 4 10− 3 Therefore, [F−] = x = 1. 4 10− 3 [H 3 O+] = 0. 10 + x = 0. 10 + 1. 4 10− 3 = 0. 10 M • So, p. H = −log (0. 10) p. H = 1. 00 Acids and Bases

Acids and Bases
![Acids and Bases [ ] Acids and Bases](https://slidetodoc.com/presentation_image_h/10107fdafcc87f41217a1f0b99227693/image-6.jpg)
[ ] Acids and Bases

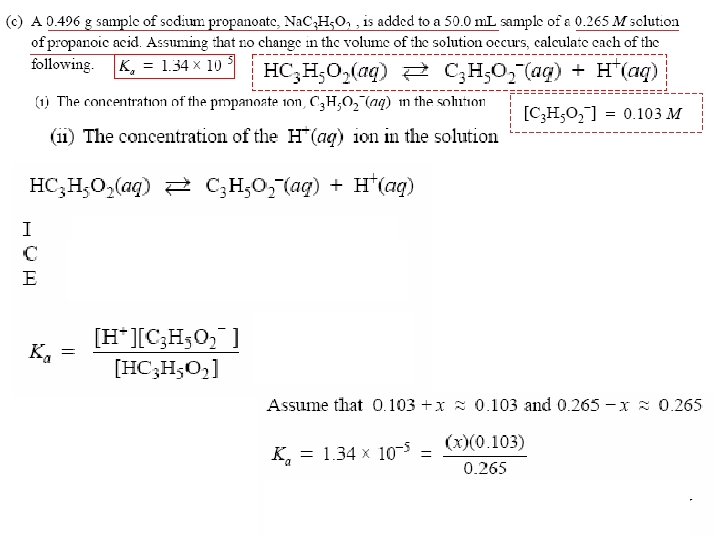
Buffers • Solutions that are particularly resistant to p. H changes, even when strong acid or base is added. • Buffers are solutions of a weak acid mixed with a salt of its conjugate base (a weak conjugate acidbase pair solution). i. e. , acetic acid + sodium acetate [ HC 2 H 3 O 2(aq)] + H 2 O(l) H 3 O+(aq) +[C 2 H 3 O 2−Acids (aq)] Which is less acidic, buffer (WA + Salt of c. base) or same conc. of weak acid alone? and Bases
![Add base OH Buffers HF H 2 O OH HF H 2 Add base (OH-) Buffers [HF] + H 2 O OH- + HF H 2](https://slidetodoc.com/presentation_image_h/10107fdafcc87f41217a1f0b99227693/image-8.jpg)
Add base (OH-) Buffers [HF] + H 2 O OH- + HF H 2 O + F- If an acid is added, the F− reacts with H+ to form HF and water. Add acid (H 3 O+) H 3 O+ + [F- ] HF H 3 O+ + F- If a hydroxide is added to an Acids equimolar solution of HF in Na. F, and for example, the HF reacts with Bases − − the OH to make F and water.

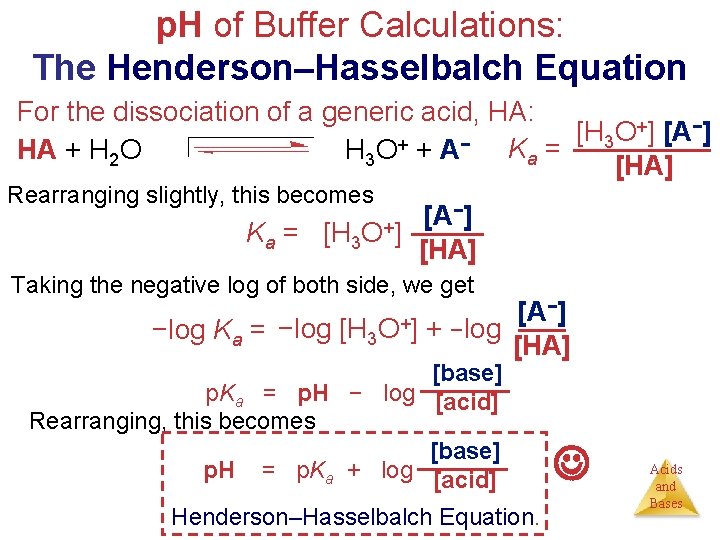
p. H of Buffer Calculations: The Henderson–Hasselbalch Equation For the dissociation of a generic acid, HA: +] [A−] [H O 3 HA + H 2 O H 3 O + + A − K a = [HA] Rearranging slightly, this becomes −] [A Ka = [H 3 O+] [HA] Taking the negative log of both side, we get −] [A −log Ka = −log [H 3 O+] + −log [HA] [base] p. Ka = p. H − log [acid] Rearranging, this becomes [base] p. H = p. Ka + log [acid] Henderson–Hasselbalch Equation. Acids and Bases

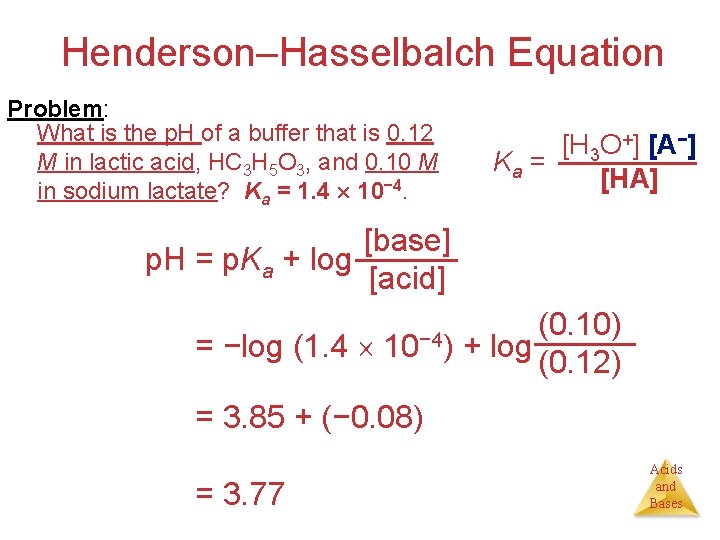
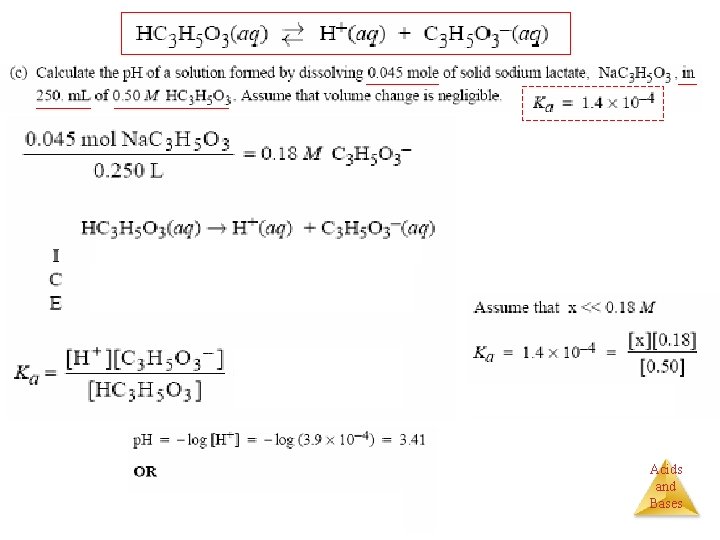
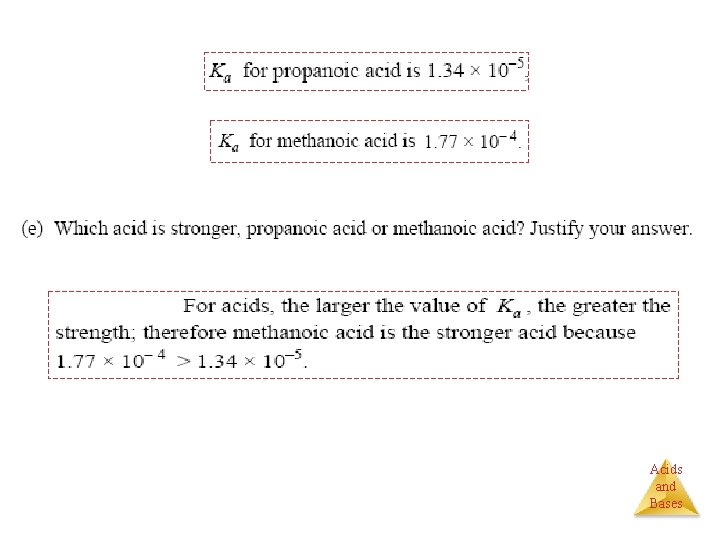
Henderson–Hasselbalch Equation Problem: What is the p. H of a buffer that is 0. 12 M in lactic acid, HC 3 H 5 O 3, and 0. 10 M in sodium lactate? Ka = 1. 4 10− 4. [H 3 O+] [A−] Ka = [HA] [base] p. H = p. Ka + log [acid] p. H = −log (1. 4 10− 4) (0. 10) + log (0. 12) p. H = 3. 85 + (− 0. 08) p. H = 3. 77 Acids and Bases

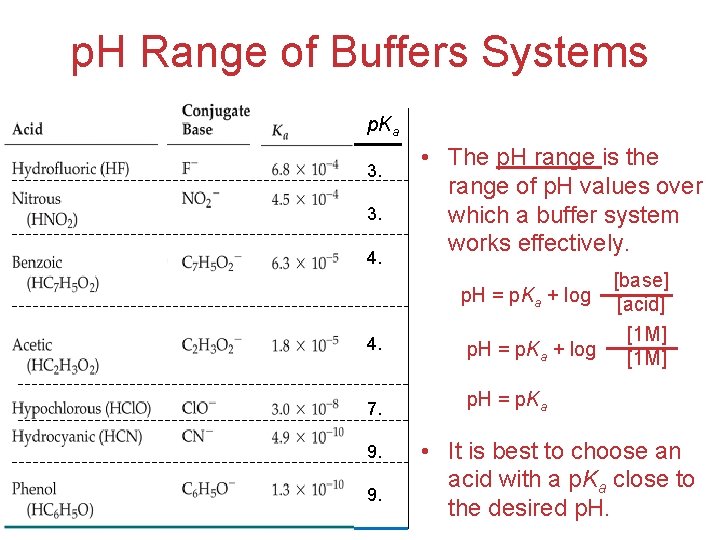
p. H Range of Buffers Systems p. Ka 3. 3. 4. 7. 9. 9. • The p. H range is the range of p. H values over which a buffer system works effectively. [base] p. H = p. Ka + log [acid] [1 M] p. H = p. Ka + log [1 M] p. H = p. Ka • It is best to choose an Acids acid with a p. Ka close andto Bases the desired p. H.
![Ka H 3 O A HA base p H [ ] Ka = [ ] [H 3 O+] [A−] [HA] [base] p. H](https://slidetodoc.com/presentation_image_h/10107fdafcc87f41217a1f0b99227693/image-12.jpg)
[ ] Ka = [ ] [H 3 O+] [A−] [HA] [base] p. H = p. Ka + log [acid] Acids and Bases
![p H p Ka log base acid Acids and Bases p. H= p. Ka + log [base] [acid] Acids and Bases](https://slidetodoc.com/presentation_image_h/10107fdafcc87f41217a1f0b99227693/image-13.jpg)
p. H= p. Ka + log [base] [acid] Acids and Bases

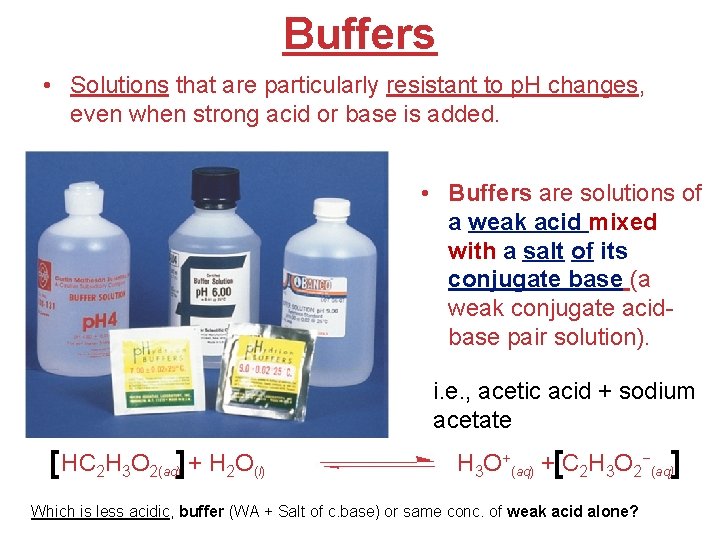
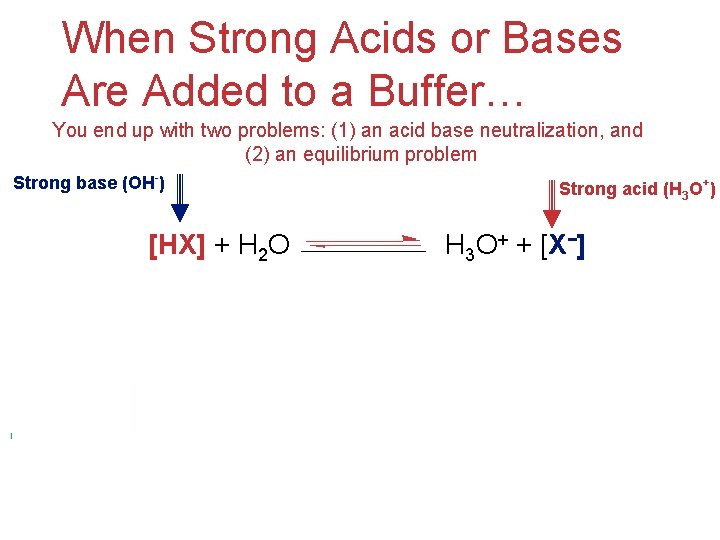
When Strong Acids or Bases Are Added to a Buffer… You end up with two problems: (1) an acid base neutralization, and (2) an equilibrium problem Strong base (OH-) [HX] + H 2 O Strong acid (H 3 O+) H 3 O+ + [X−] Acids and Bases
![Addition of Strong Acid or Base to a Buffer Add base OH HA Addition of Strong Acid or Base to a Buffer Add base (OH-) [HA] +](https://slidetodoc.com/presentation_image_h/10107fdafcc87f41217a1f0b99227693/image-15.jpg)
Addition of Strong Acid or Base to a Buffer Add base (OH-) [HA] + H 2 O Add some OH- What are the new equilibrium conc. after acid or base is added? Add some H 3 O + Add acid (H 3 O+) H 3 O+ + [A−] (HA + OH- A- + H 2 O) (H 3 O+ + A- HA + H 2 O) (1) Neutralization is NOT an equilibrium reaction. (2) However, it affects the amounts of the weak acid [HA] and its conjugate base [A-] left over, and a new equilibrium will be established. (1) Use: Mole ICEnd Table: *used for neutralization Rx* HA A- H 3 O+ or OH- η η +η Change (H 3 O+ or OH-) +η +η -η End (after)reaction η η 0η Mole (η) Initially Moles (η) Molarity (η/L) For a buffer: (2) p. H [base] = p. Ka + log [acid] Acids and Bases

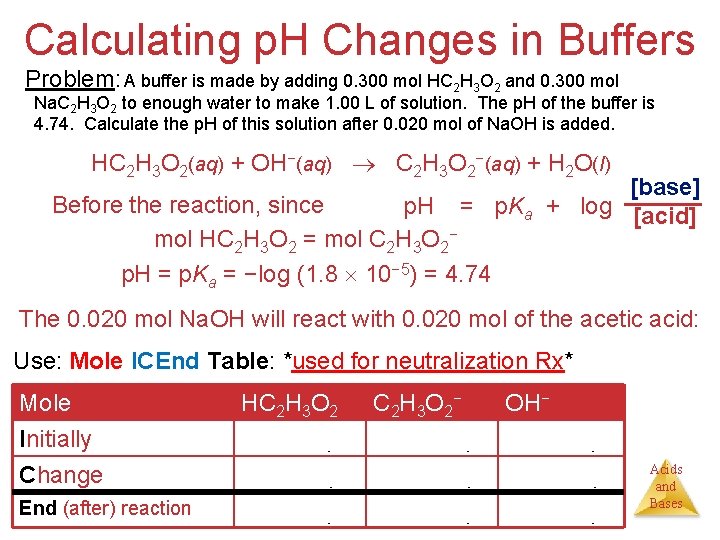
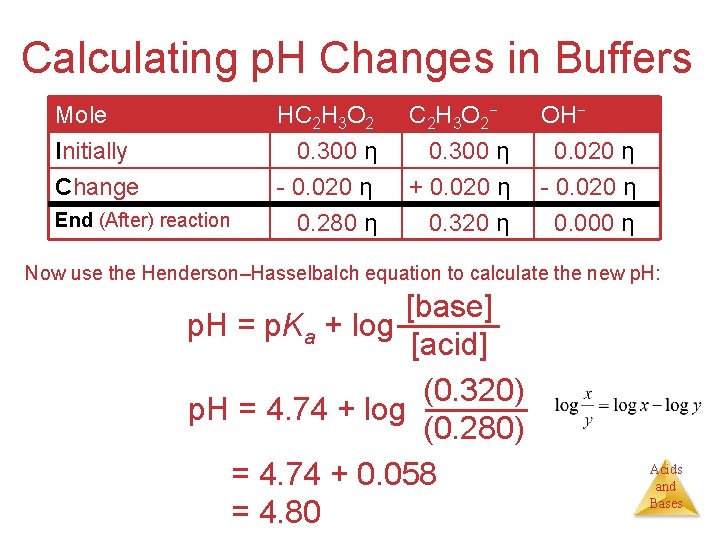
Calculating p. H Changes in Buffers Problem: A buffer is made by adding 0. 300 mol HC 2 H 3 O 2 and 0. 300 mol Na. C 2 H 3 O 2 to enough water to make 1. 00 L of solution. The p. H of the buffer is 4. 74. Calculate the p. H of this solution after 0. 020 mol of Na. OH is added. HC 2 H 3 O 2(aq) + OH−(aq) C 2 H 3 O 2−(aq) + H 2 O(l) [base] = p. Ka + log [acid] Before the reaction, since p. H mol HC 2 H 3 O 2 = mol C 2 H 3 O 2− p. H = p. Ka = −log (1. 8 10− 5) = 4. 74 The 0. 020 mol Na. OH will react with 0. 020 mol of the acetic acid: Use: Mole ICEnd Table: *used for neutralization Rx* Mole Initially Change End (after) reaction HC 2 H 3 O 2 0. 300 η - 0. 020 η 0. 280 η C 2 H 3 O 2− 0. 300 η + 0. 020 η 0. 320 η OH− 0. 020 η - 0. 020 η 0. 000 η Acids and Bases

Calculating p. H Changes in Buffers Mole Initially Change End (After) reaction HC 2 H 3 O 2 0. 300 η - 0. 020 η 0. 280 η C 2 H 3 O 2− 0. 300 η + 0. 020 η 0. 320 η OH− 0. 020 η - 0. 020 η 0. 000 η Now use the Henderson–Hasselbalch equation to calculate the new p. H: [base] p. H = p. Ka + log [acid] (0. 320) p. H = 4. 74 + log (0. 280) p. H = 4. 74 + 0. 058 p. H = 4. 80 Acids and Bases

Acids and Bases

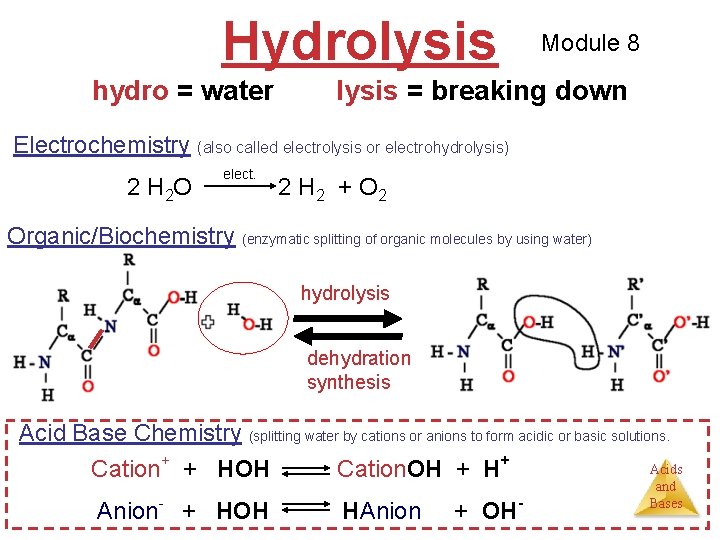
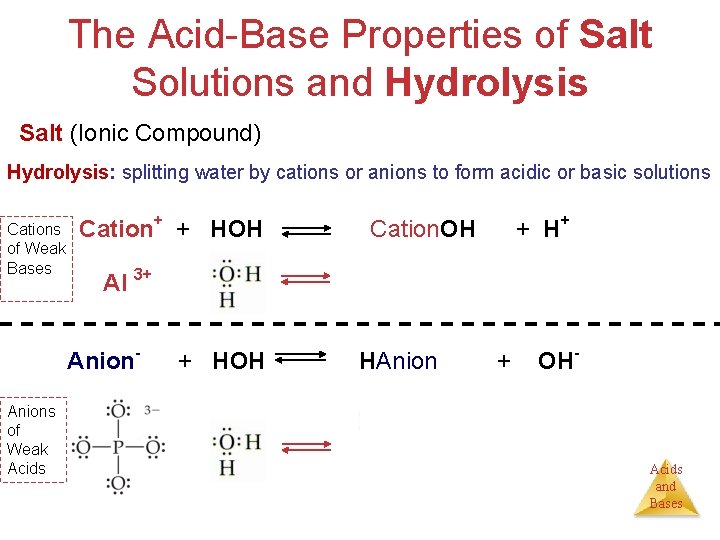
Hydrolysis hydro = water Module 8 lysis = breaking down Electrochemistry (also called electrolysis or electrohydrolysis) 2 H 2 O elect. 2 H 2 + O 2 Organic/Biochemistry (enzymatic splitting of organic molecules by using water) hydrolysis dehydration synthesis Acid Base Chemistry (splitting water by cations or anions to form acidic or basic solutions. Acids Cation+ + HOH Cation. OH + H+ Anion- + HOH HAnion + OH- and Bases

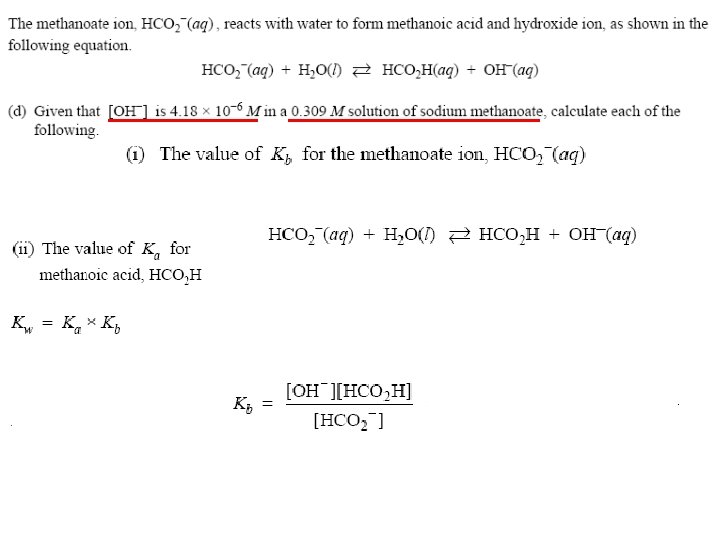
The Acid-Base Properties of Salt Solutions and Hydrolysis Salt (Ionic Compound) = Metal + Nonmetal = Cation+ + Anion. Hydrolysis: splitting water by cations or anions to form acidic or basic solutions Cations of Weak Bases Cation+ + HOH Al 3+ Anion- Anions of Weak Acids + H+ Cation. OH Al 3+ + HOH HAnion + OH- Acids and Bases

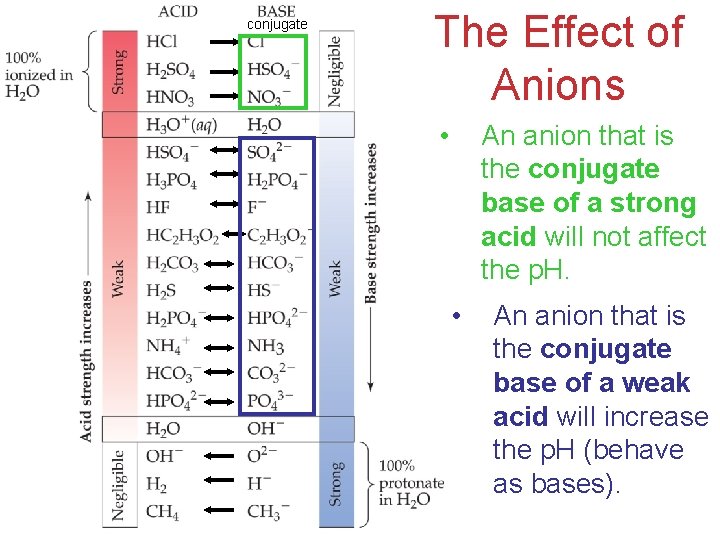
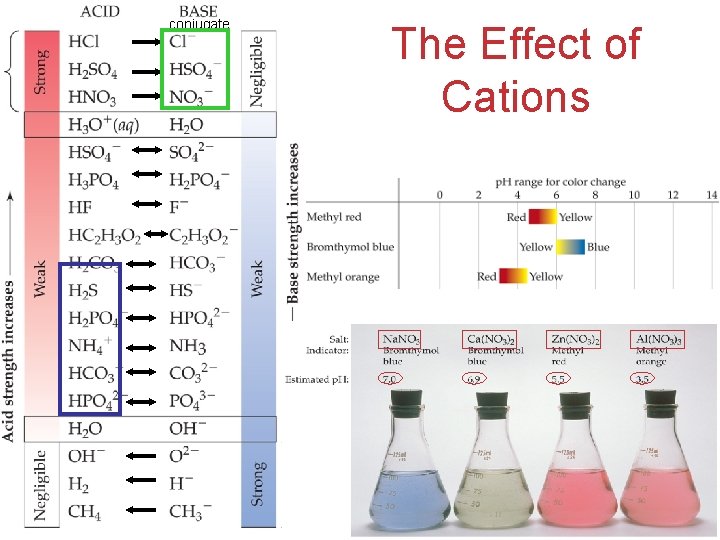
conjugate The Effect of Anions • An anion that is the conjugate base of a strong acid will not affect the p. H. • An anion that is the conjugate base of a weak acid will increase the p. H (behave Acids as bases). and Bases

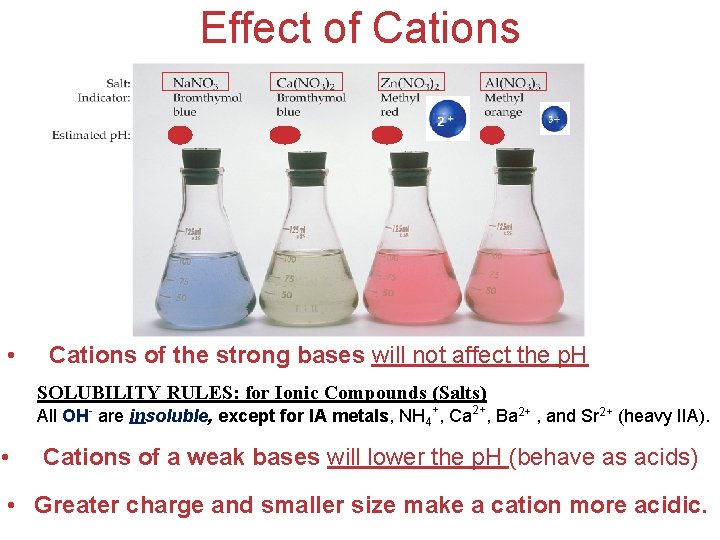
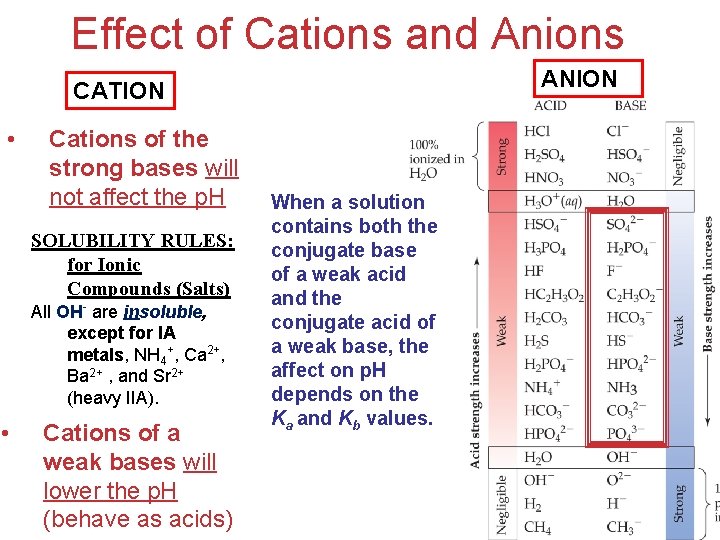
Effect of Cations • Cations of the strong bases will not affect the p. H SOLUBILITY RULES: for Ionic Compounds (Salts) All OH- are insoluble, except for IA metals, NH 4+, Ca 2+, Ba 2+ , and Sr 2+ (heavy IIA). • Cations of a weak bases will lower the p. H (behave as acids) Acids and Bases • Greater charge and smaller size make a cation more acidic.

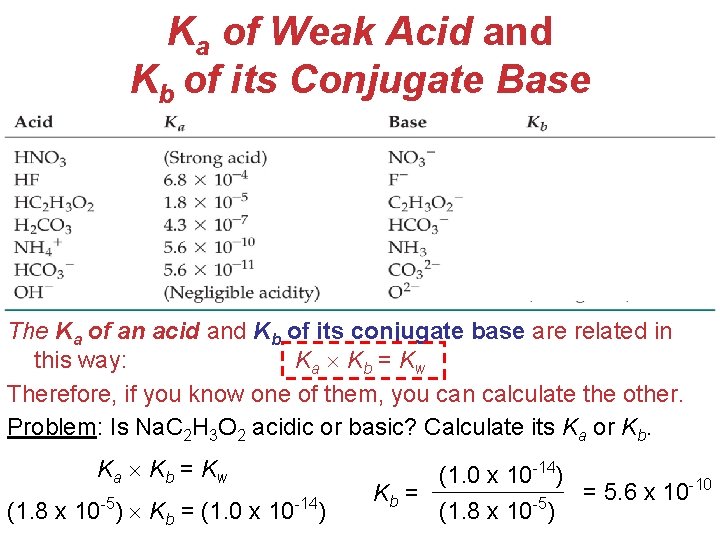
Ka of Weak Acid and Kb of its Conjugate Base The Ka of an acid and Kb of its conjugate base are related in this way: Ka Kb = Kw Therefore, if you know one of them, you can calculate the other. Problem: Is Na. C 2 H 3 O 2 acidic or basic? Calculate its Ka or Kb. Ka Kb = Kw (1. 8 x 10 -5) Kb = (1. 0 x 10 -14) Acids (1. 0 x 10 -14) and -10 = 5. 6 x 10 Kb = Bases -5 (1. 8 x 10 )

Effect of Cations and Anions ANION CATION • Cations of the strong bases will not affect the p. H SOLUBILITY RULES: for Ionic Compounds (Salts) All OH- are insoluble, except for IA metals, NH 4+, Ca 2+, Ba 2+ , and Sr 2+ (heavy IIA). • Cations of a weak bases will lower the p. H (behave as acids) When a solution contains both the conjugate base of a weak acid and the conjugate acid of a weak base, the affect on p. H depends on the Ka and Kb values. Acids and Bases

Acids and Bases

Acids and Bases

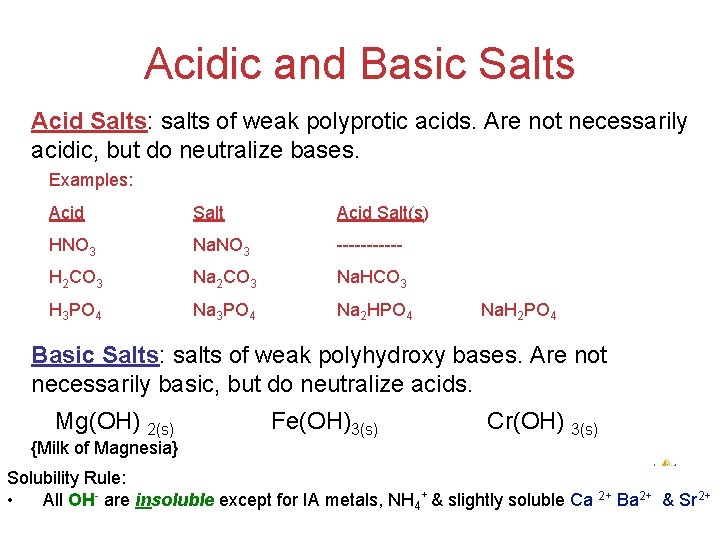
Acidic and Basic Salts Acid Salts: salts of weak polyprotic acids. Are not necessarily acidic, but do neutralize bases. Examples: Acid Salt(s) HNO 3 Na. NO 3 ------ H 2 CO 3 Na. HCO 3 H 3 PO 4 Na 2 HPO 4 Na. H 2 PO 4 Basic Salts: salts of weak polyhydroxy bases. Are not necessarily basic, but do neutralize acids. Mg(OH) 2(s) {Milk of Magnesia} Fe(OH)3(s) Cr(OH) 3(s) Acids Solubility Rule: and + 2+ 2+ • All OH are insoluble except for IA metals, NH 4 & slightly soluble Ca Ba Bases & Sr 2+

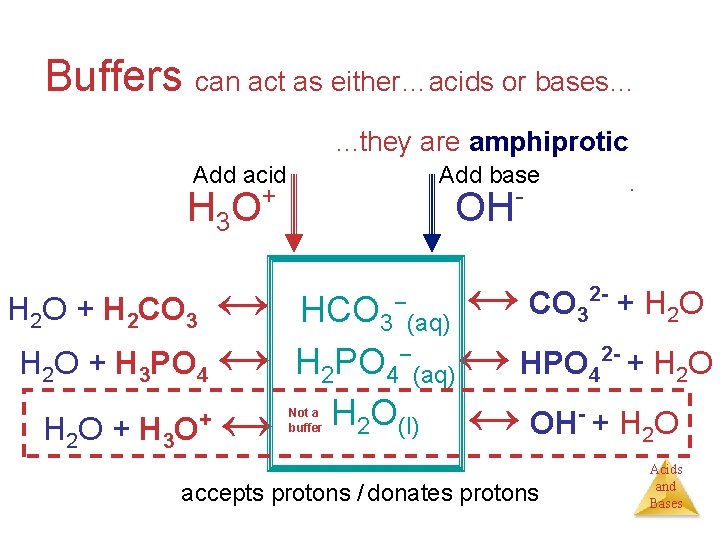
Buffers can act as either…acids or bases…. . . they are amphiprotic Add acid H 3 O + OH H 2 O + H 3 PO 4 + . - ↔ HCO 3 (aq) ↔ CO + H O ↔ H 2 PO 4−(aq) ↔ HPO + H O ↔ OH + H O 2 (l) ↔ − H 2 O + H 2 CO 3 H 2 O + H 3 O Add base 3 2 - 4 Not a buffer accepts protons / donates protons - 2 2 Acids and Bases

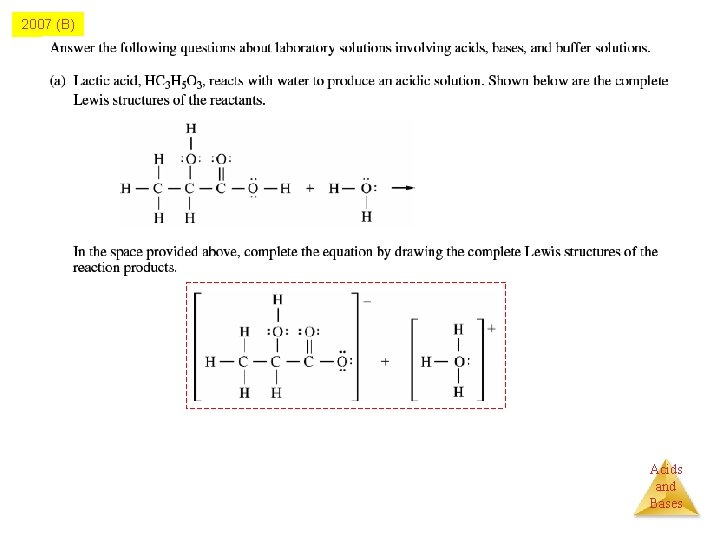
2007 (B) Acids and Bases

Making Solution Molarity Acids and Bases

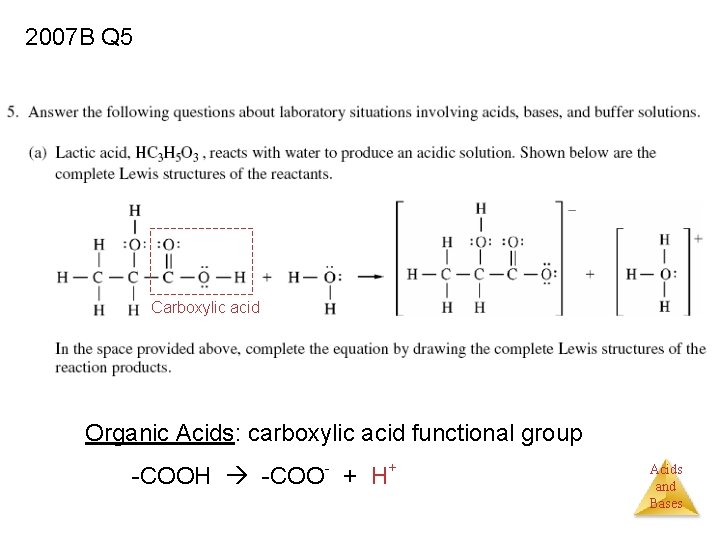
2007 B Q 5 Carboxylic acid Organic Acids: carboxylic acid functional group -COOH -COO- + H+ Acids and Bases

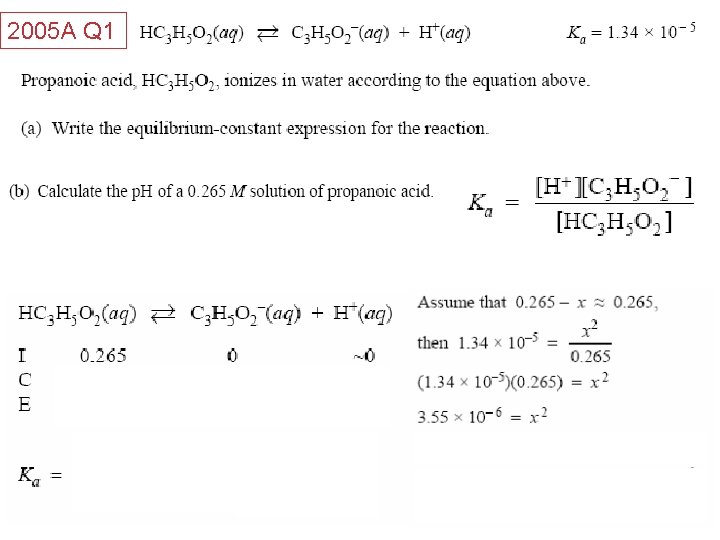
2005 A Q 1 Acids and Bases

Acids and Bases

Review of Strong Acid & Bases Strong acids have very weak conjugate bases HX + acid H 2 O base H 3 O + + conj. a X Has no affinity for H+ - conj. b Strong bases have very weak conjugate acids MOH M+ base conj. a + OH- Has no affinity for OH- conj. b Salts of Strong acids & bases have very weak conjugate acids & bases MX salt + H 2 O M+(aq) + conj. a Example: HCl X- (aq) conj. b Example: Na. OH Example: Na. Cl Have no Acids and affinity for Bases H+ or OH

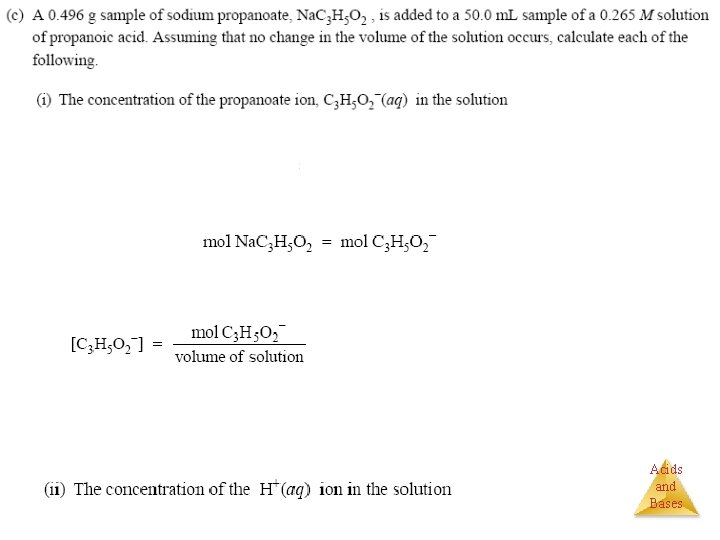
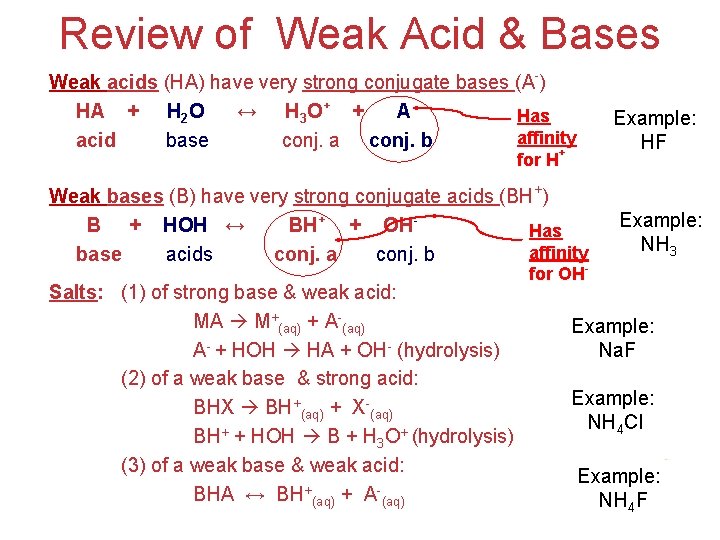
Review of Weak Acid & Bases Weak acids (HA) have very strong conjugate bases (A-) HA + H 2 O ↔ H 3 O+ + AHas affinity acid base conj. a conj. b Example: HF Weak bases (B) have very strong conjugate acids (BH+) B + HOH ↔ BH+ + OHHas affinity base acids conj. a conj. b Example: NH 3 for H+ Salts: (1) of strong base & weak acid: MA M+(aq) + A-(aq) A- + HOH HA + OH- (hydrolysis) (2) of a weak base & strong acid: BHX BH+(aq) + X-(aq) BH+ + HOH B + H 3 O+ (hydrolysis) (3) of a weak base & weak acid: BHA ↔ BH+(aq) + A-(aq) for OH- Example: Na. F Example: NH 4 Cl Acids Example: and NH 4 F Bases

Reactions of Cations with Water • Cations with acidic protons (like NH 4+) will lower the p. H of a solution: NH 4+ + H 2 O H 3 O+ + NH 3 H O H • Most metal cations that are hydrated in solution also lower the p. H of the solution, acting as Lewis acids: Ø The attraction between nonbonding electrons on waters’ oxygen and the metal cation causes a shift of the electron density in water. Ø This makes the O-H bond more polar and the water more acidic. Greater charge and smaller size Acids and make a cation more acidic. Bases

conjugate The Effect of Cations Acids and Bases