CHM 1045 General Chemistry and Qualitative Analysis Unit







- Slides: 54

CHM 1045: General Chemistry and Qualitative Analysis Unit 2: Elements and Compounds: Atoms, Molecules & Ions Dr. Jorge L. Alonso Miami-Dade College – Kendall Campus Miami, FL Textbook References: • Modules #2 & 5 Atoms, Molecules, and Ions

The Early Development of the Atomic Theory Atoms, Molecules, and Ions

Ancient Atomic Theory Leucippus of Miletus & Democritus of Abdera (Gk. 5 th Cent BC) • More philosophical than experimental in origin. • Matter is made up of very small individual atomos objects that are indivisible. • Everything is made up of these atoms, which move around in a void (a vacuum). • The different physical properties -- color, taste, and so on -- of materials come about because atoms in Atoms, them are different shapes and/or arrangements Molecules, and Ions orientations with respect to each other.

Medieval Alchemy ( ﺍﻟﺨﻴﻤﻴﺎﺀ , al-khimia) Jabir ibn Hayyan Science as an early form of investigation, with occult philosophical and spiritual traditions. Merlin the Magician Principal aim of Alchemist: • the transmutation of common metals into gold or silver. Cinnabar (red powder) Hg Zn, Cu, Fe Au or Ag • the creation of a "panacea, " or the elixir of life, a remedy that supposedly Atoms, would cure all diseases and prolong Molecules, life indefinitely. and Ions

Chemistry in the Age of Enlightment Law of Conservation of Mass: (1743 - 1794) (s) 27 g (l) (g) 25 g + 2 g Atoms, Molecules, and Ions {Hg. O Movie}

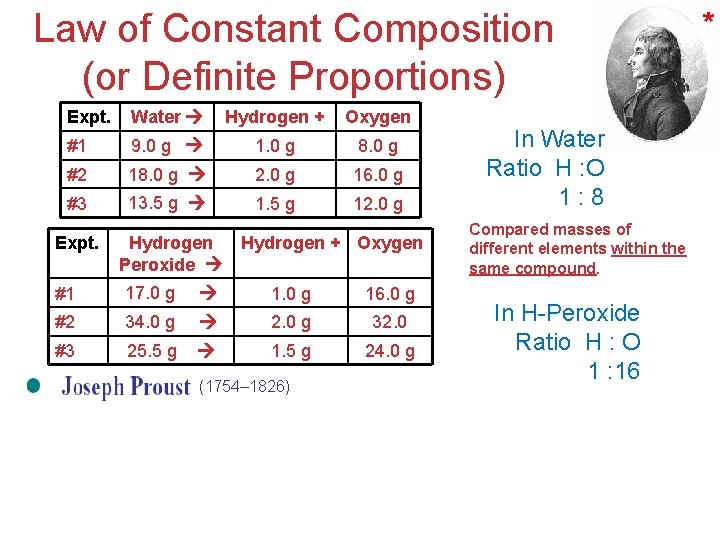
Law of Constant Composition (or Definite Proportions) Expt. Water Hydrogen + Oxygen #1 9. 0 g 1. 0 g 8. 0 g #2 18. 0 g 2. 0 g 16. 0 g #3 13. 5 g 1. 5 g 12. 0 g Expt. Hydrogen Peroxide Hydrogen + Oxygen #1 17. 0 g 16. 0 g #2 34. 0 g 2. 0 g 32. 0 #3 25. 5 g 1. 5 g 24. 0 g (1754– 1826) * In Water Ratio H : O 1: 8 Compared masses of different elements within the same compound. In H-Peroxide Ratio H : O 1 : 16 Atoms, Molecules, and Ions

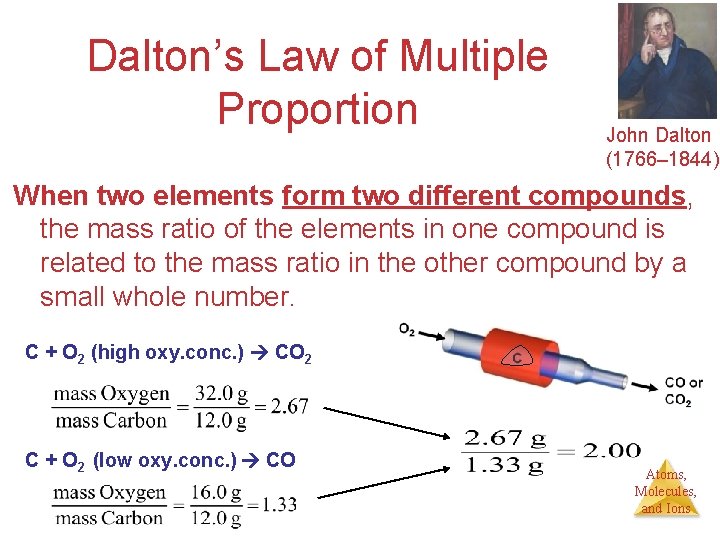
Dalton’s Law of Multiple Proportion John Dalton (1766– 1844). When two elements form two different compounds, the mass ratio of the elements in one compound is related to the mass ratio in the other compound by a small whole number. C + O 2 (high oxy. conc. ) CO 2 C + O 2 (low oxy. conc. ) CO Atoms, Molecules, and Ions

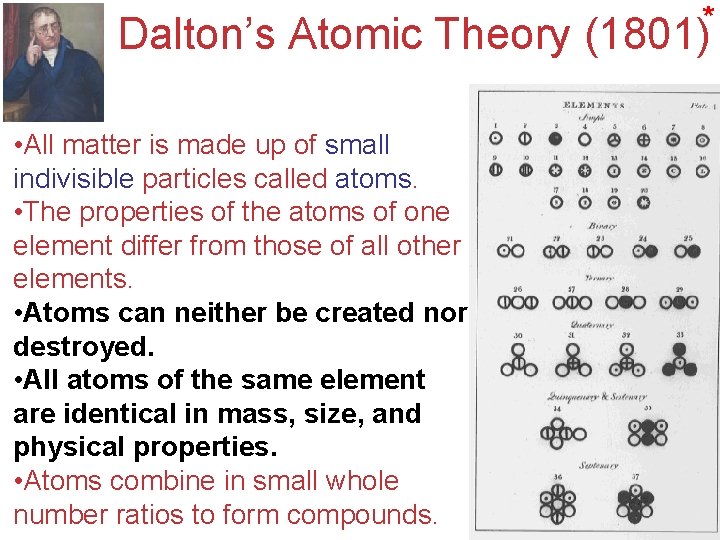
* Dalton’s Atomic Theory (1801) • Allmatter 1. matterisismadeupupofofsmall indivisible particles called atoms. • The 2. Thepropertiesofofthe theatomsofofone element differ from those of of all other element differ form those elements. other elements. • Atoms created nor 3. Atomscan canneither be created destroyed. • All element are 4. Allatomsof of the same element areidentical in mass, size, andand physicalproperties. • Atoms 5. Atomscombineininsmallwhole number ratios to to form compounds. number ratios form compounds. Atoms, Molecules, and Ions

The Electron Excess of electrons {Electroscope Rubber band rubs Movie} metals inside Electrically charged particles can be rubbed-out of many substances such as glass rods, hair, shoes, rubber tires and {Electroscope Movie*} shoes. Lack of electrons Rubber band Electric motor Van de Graaff Generator Atoms, Molecules, and Ions

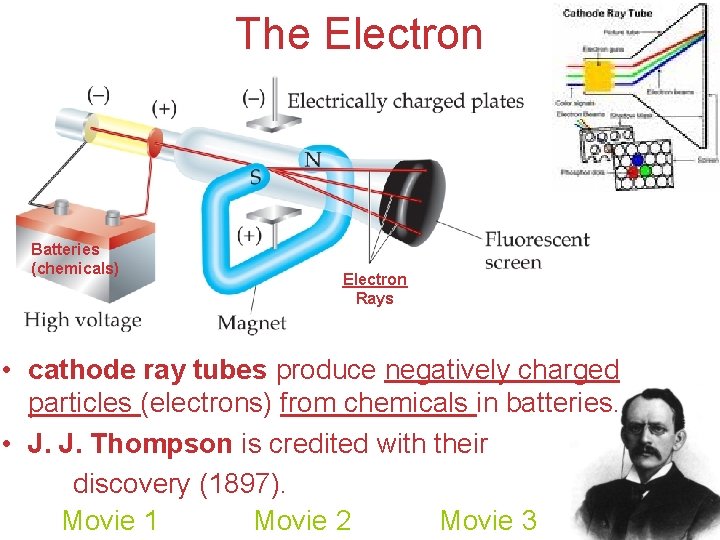
The Electron Batteries (chemicals) Electron Rays • cathode ray tubes produce negatively charged particles (electrons) from chemicals in batteries. • J. J. Thompson is credited with their discovery (1897). Movie 1 Movie 2 Movie 3 Atoms, Molecules, and Ions

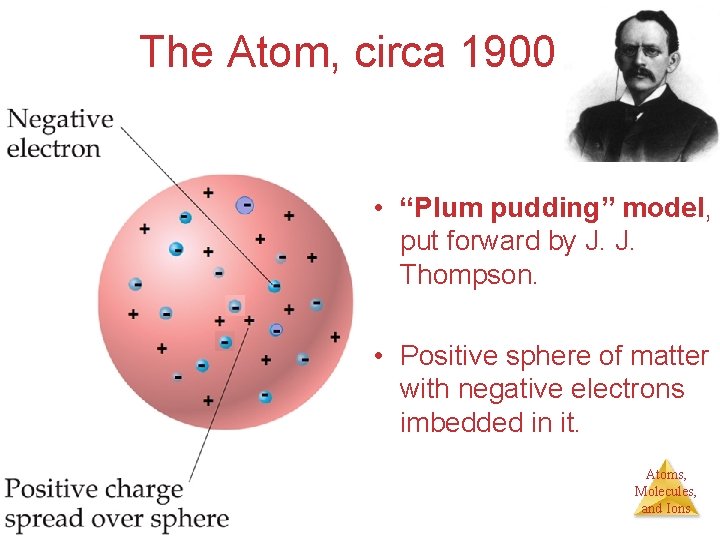
The Atom, circa 1900 • “Plum pudding” model, put forward by J. J. Thompson. • Positive sphere of matter with negative electrons imbedded in it. Atoms, Molecules, and Ions

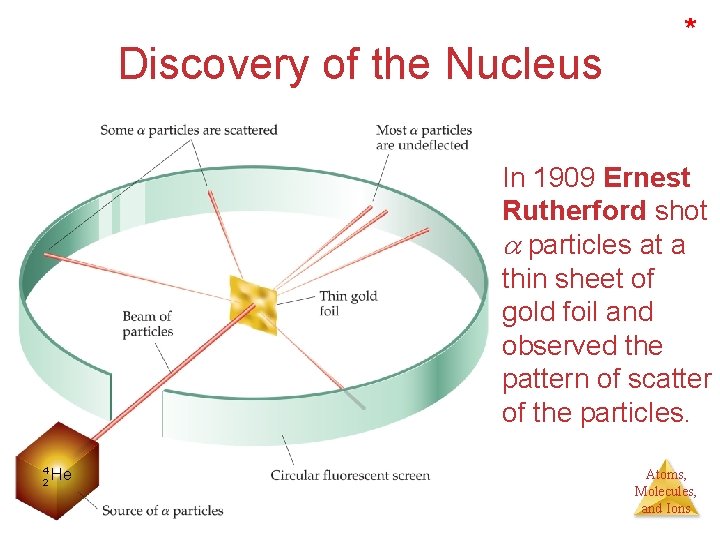
Discovery of the Nucleus * In 1909 Ernest Rutherford shot particles at a thin sheet of gold foil and observed the pattern of scatter of the particles. 4 2 He Atoms, Molecules, and Ions

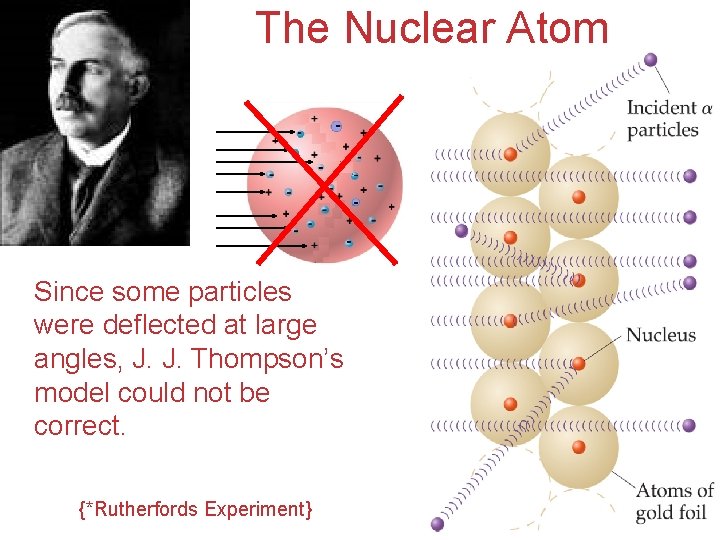
The Nuclear Atom Since some particles were deflected at large angles, J. J. Thompson’s model could not be correct. {*Rutherfords Experiment} Atoms, Molecules, and Ions

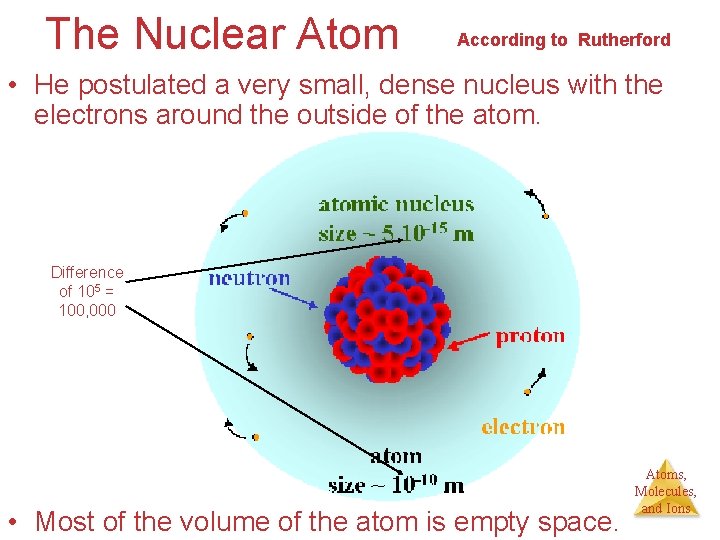
The Nuclear Atom According to Rutherford • He postulated a very small, dense nucleus with the electrons around the outside of the atom. Difference of 105 = 100, 000 • Most of the volume of the atom is empty space. Atoms, Molecules, and Ions

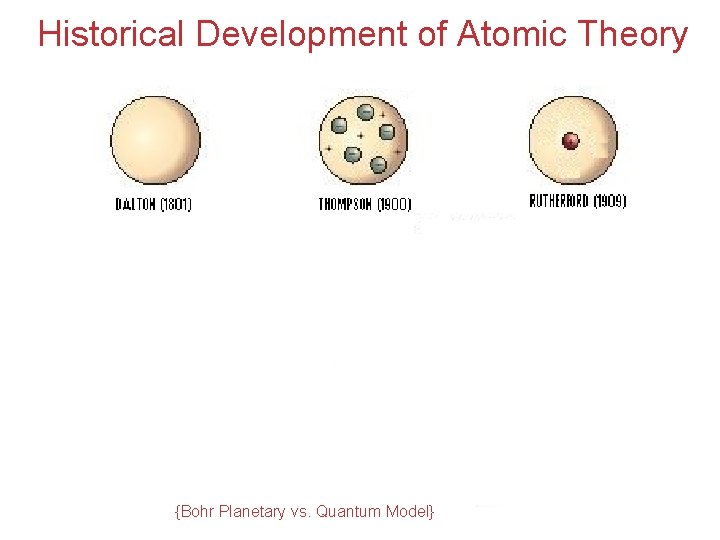
Historical Development of Atomic Theory {Bohr Planetary vs. Quantum Model} Atoms, Molecules, and Ions

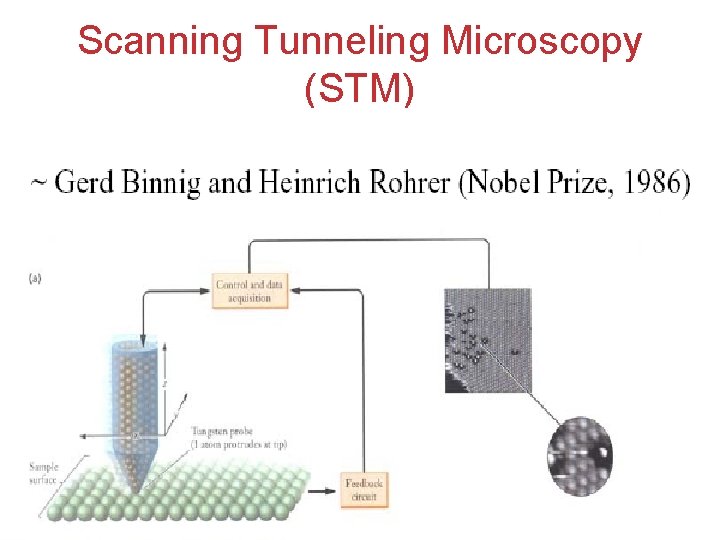
Scanning Tunneling Microscopy (STM) Atoms, Molecules, and Ions

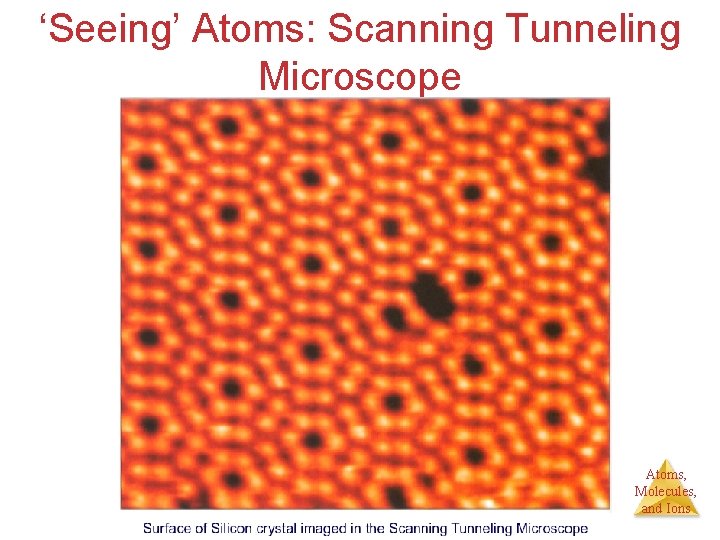
‘Seeing’ Atoms: Scanning Tunneling Microscope Atoms, Molecules, and Ions

‘Seeing’ Atoms: Scanning Tunneling Microscope Atoms, Molecules, and Ions

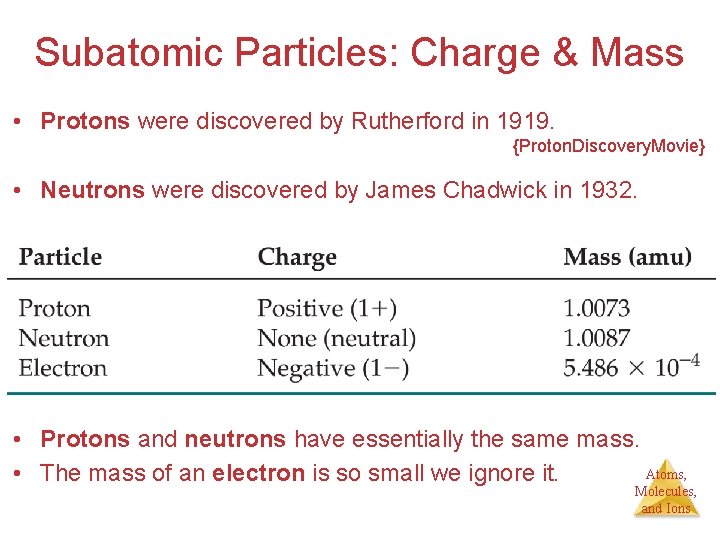
Subatomic Particles: Charge & Mass • Protons were discovered by Rutherford in 1919. {Proton. Discovery. Movie} • Neutrons were discovered by James Chadwick in 1932. • Protons and neutrons have essentially the same mass. Atoms, • The mass of an electron is so small we ignore it. Molecules, and Ions

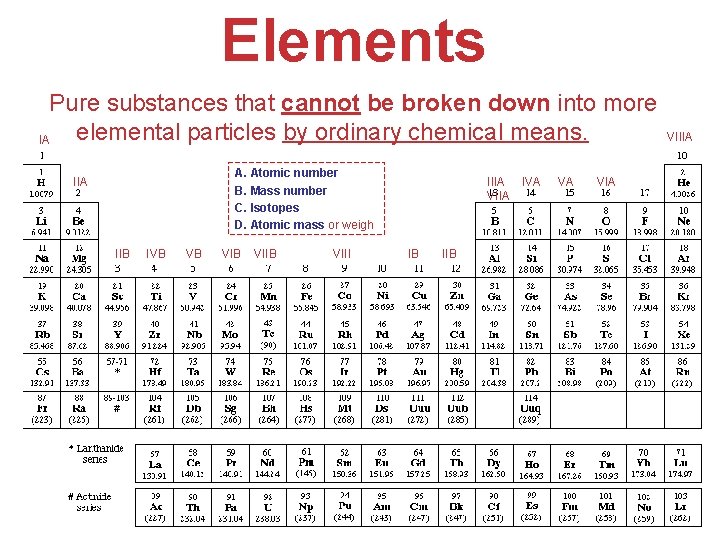
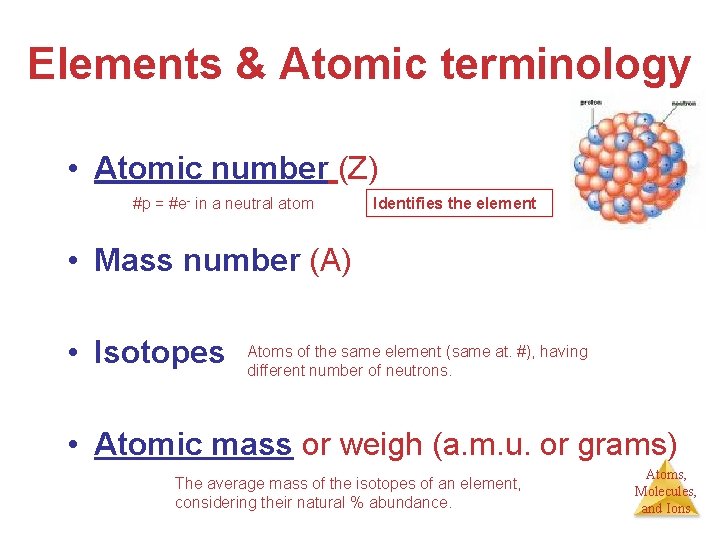
Elements Pure substances that cannot be broken down into more elemental particles by ordinary chemical means. IA A. Atomic number B. Mass number C. Isotopes D. Atomic mass or weigh IIA IIB IVB VB VIIB VIII IIIA IVA VIIA IB VA VIIIA VIA IIB Atoms, Molecules, and Ions

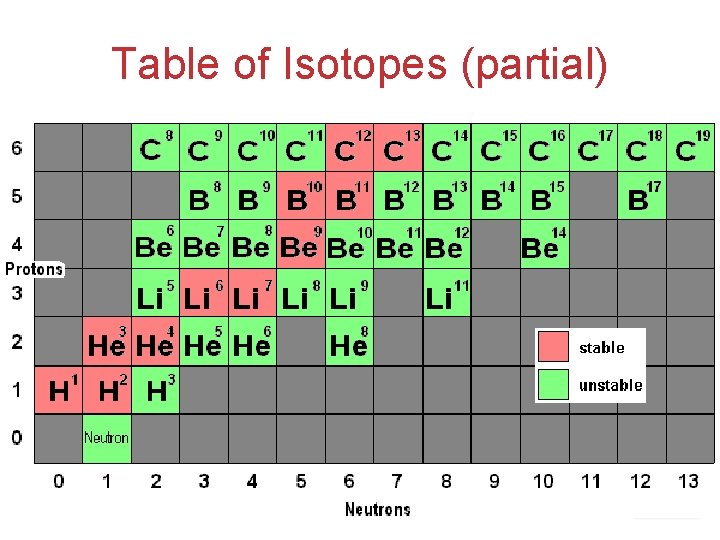
Elements & Atomic terminology • Atomic number (Z) = #p #p = #e- in a neutral atom Identifies the element • Mass number (A) = (#p+) + (#no) A = Z + N of the same element (same at. #), having • Isotopes Atoms different number of neutrons. • Atomic mass or weigh (a. m. u. or grams) The average mass of the isotopes of an element, considering their natural % abundance. Atoms, Molecules, and Ions

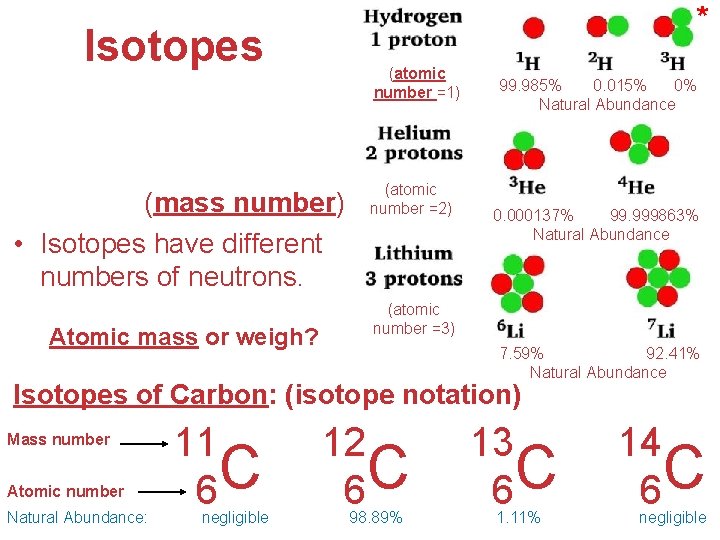
* Isotopes (atomic number =1) • Atoms of the same element with different masses (mass number) • Isotopes have different numbers of neutrons. Atomic mass or weigh? (atomic number =2) 99. 985% 0. 015% 0% Natural Abundance 0. 000137% 99. 999863% Natural Abundance (atomic number =3) 7. 59% 92. 41% Natural Abundance Isotopes of Carbon: (isotope notation) Mass number Atomic number Natural Abundance: 11 C 6 negligible 12 C 6 98. 89% 13 C 6 1. 11% 14 C 6 Atoms, Molecules, and Ions negligible

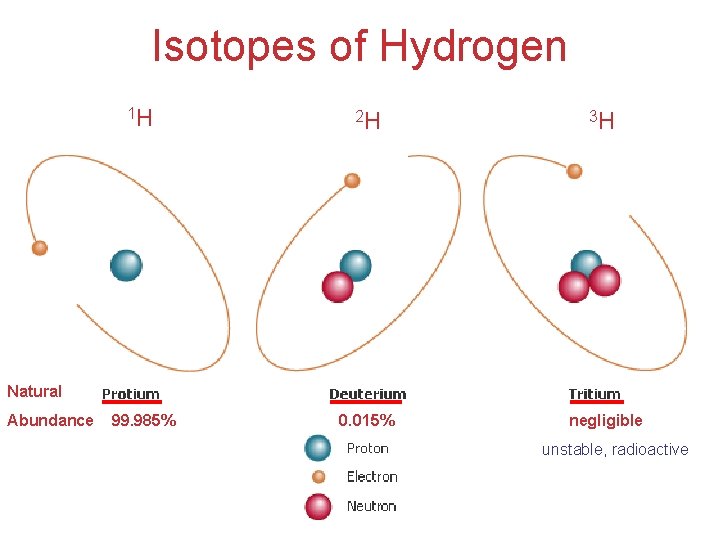
Isotopes of Hydrogen 1 H 2 H 3 H 99. 985% 0. 015% negligible Natural Abundance unstable, radioactive Atoms, Molecules, and Ions

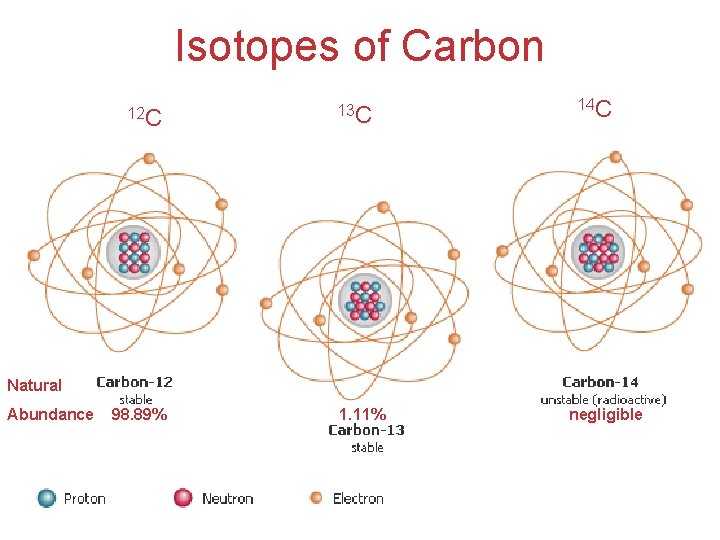
Isotopes of Carbon 12 C 13 C 14 C Natural Abundance 98. 89% 1. 11% negligible Atoms, Molecules, and Ions

Table of Isotopes (partial) Atoms, Molecules, and Ions

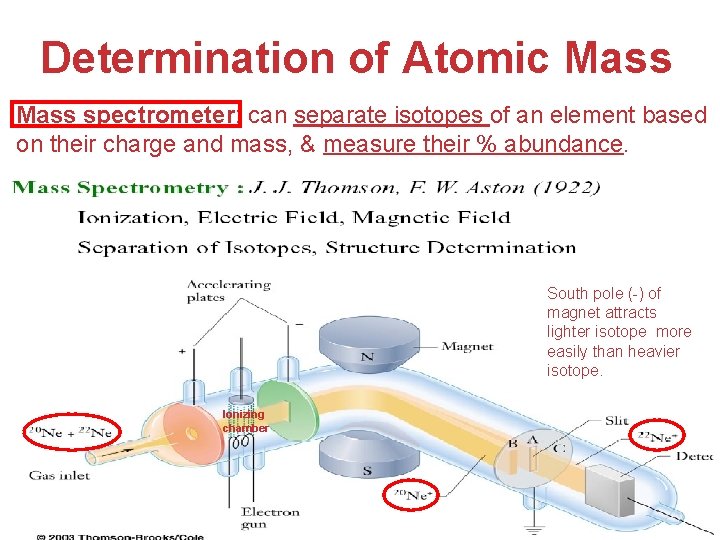
Determination of Atomic Mass spectrometer: can separate isotopes of an element based on their charge and mass, & measure their % abundance. South pole (-) of magnet attracts lighter isotope more easily than heavier isotope. Ionizing chamber Atoms, Molecules, and Ions

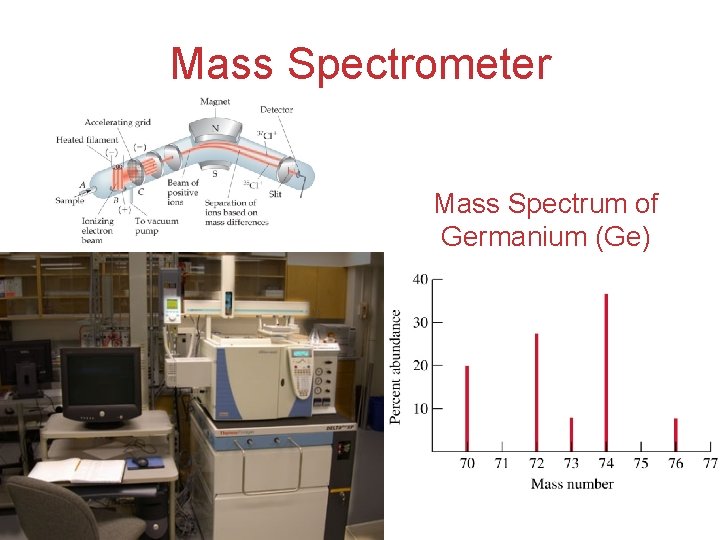
Mass Spectrometer Mass Spectrum of Germanium (Ge) Atoms, Molecules, and Ions

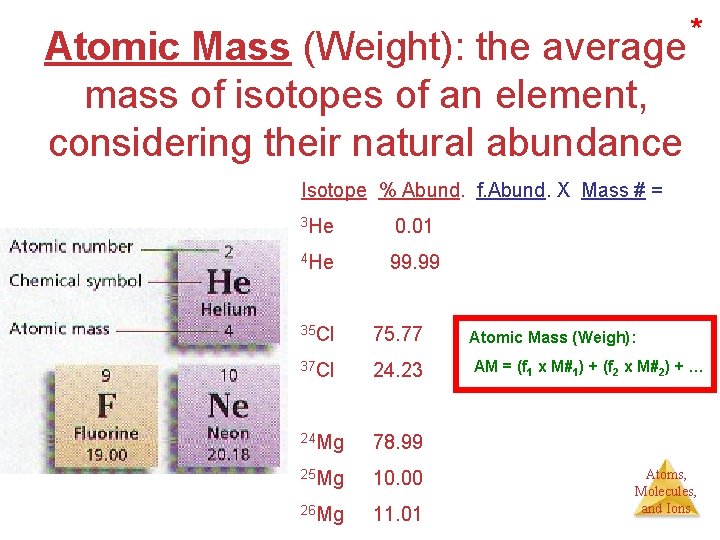
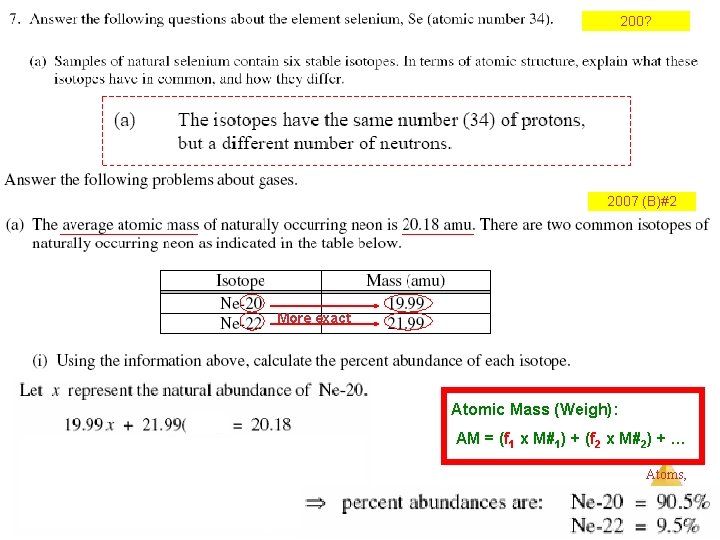
Atomic Mass (Weight): the average mass of isotopes of an element, considering their natural abundance * Isotope % Abund. f. Abund. X Mass # = 3 He 0. 01 (0. 0001 x 3) = 0. 0003 4 He 99. 99 (0. 9999 x 4) = +3. 9996 4. 000 35 Cl 75. 77 Atomic Mass (Weigh): 37 Cl 24. 23 AM = (f 1 x M#1) + (f 2 x M#2) + … 24 Mg 78. 99 25 Mg 10. 00 26 Mg 11. 01 Atoms, Molecules, and Ions

200? 2007 (B)#2 More exact Atomic Mass (Weigh): AM = (f 1 x M#1) + (f 2 x M#2) + … Atoms, Molecules, and Ions

Atoms, Molecules, and Ions

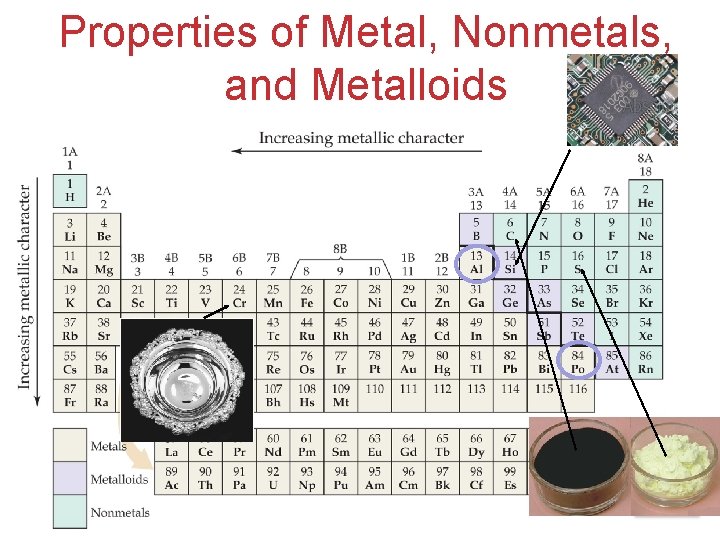
Properties of Metal, Nonmetals, and Metalloids Atoms, Molecules, and Ions

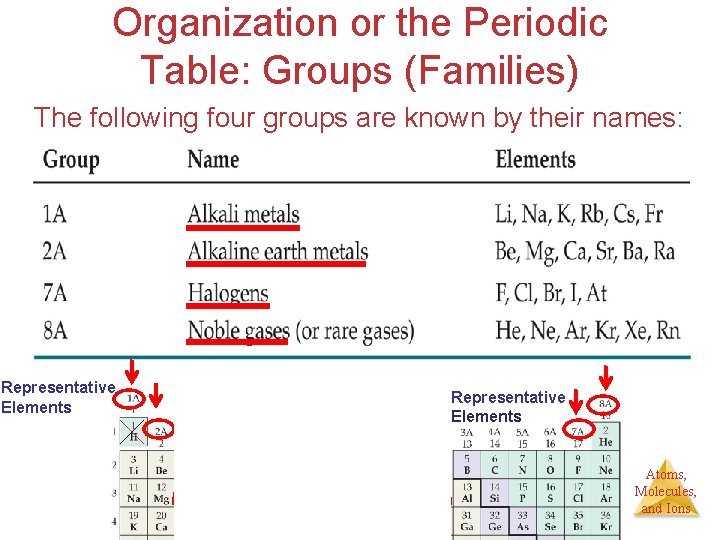
Organization or the Periodic Table: Groups (Families) The following four groups are known by their names: Representative Elements Transition Metals Representative Elements Atoms, Molecules, and Ions

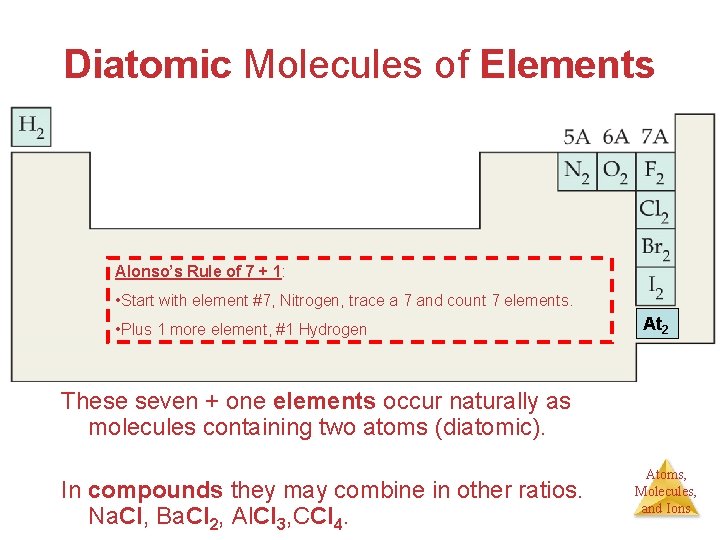
Diatomic Molecules of Elements Alonso’s Rule of 7 + 1: • Start with element #7, Nitrogen, trace a 7 and count 7 elements. • Plus 1 more element, #1 Hydrogen At 2 These seven + one elements occur naturally as molecules containing two atoms (diatomic). In compounds they may combine in other ratios. Na. Cl, Ba. Cl 2, Al. Cl 3, CCl 4. Atoms, Molecules, and Ions

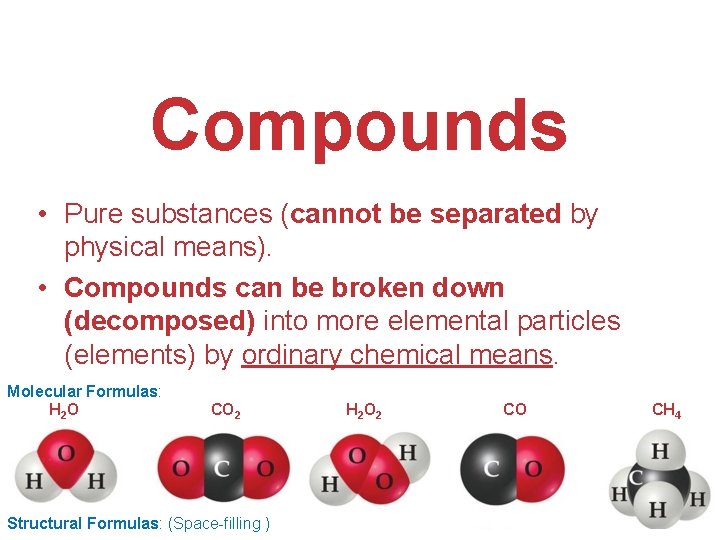
Compounds • Pure substances (cannot be separated by physical means). • Compounds can be broken down (decomposed) into more elemental particles (elements) by ordinary chemical means. Molecular Formulas: H 2 O CO 2 Structural Formulas: (Space-filling ) H 2 O 2 CO CH 4 Atoms, Molecules, and Ions

Classification of Compounds Covalent (Molecular) Compounds: Non-Metals + Non-Metals. Acids: Hydrogen + Nonmetals (polar covalent) Ionic Compounds Salts: Metal + Non-Metal Bases: Metals + Hydroxide Ion (OH-) Acid Salts: Metal + Acid (Hydrogen + Nonmetal) Organic Compounds: covalent compounds containing carbon (C) atom chains, with mostly H & O atoms attached Atoms, to the Molecules, chain. and Ions

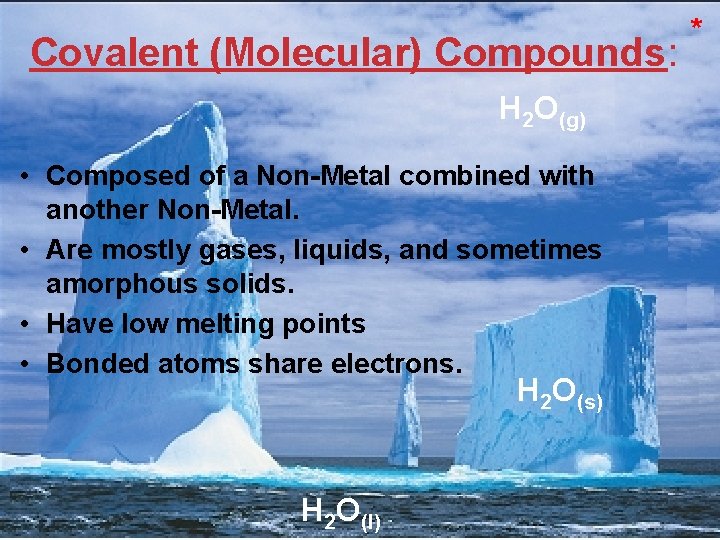
Covalent (Molecular) Compounds: * H 2 O(g) • Composed of a Non-Metal combined with another Non-Metal. • Are mostly gases, liquids, and sometimes amorphous solids. • Have low melting points • Bonded atoms share electrons. H 2 O(s) H 2 O(l) Atoms, Molecules, and Ions

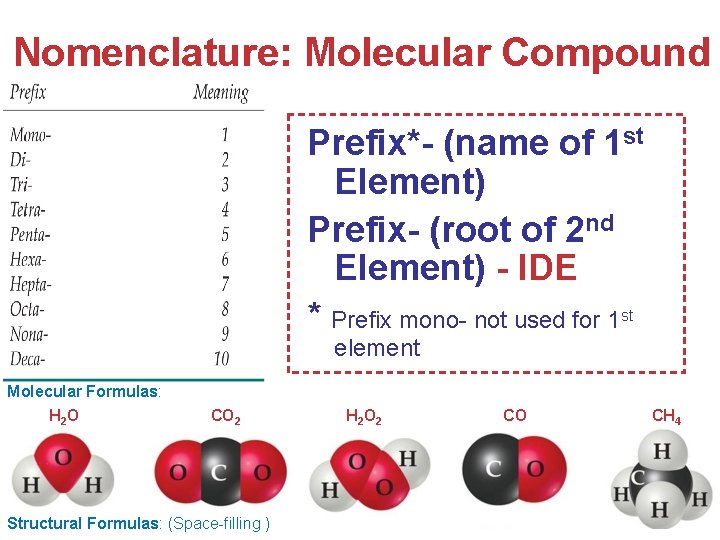
Nomenclature: Molecular Compound Prefix*- (name of 1 st Element) Prefix- (root of 2 nd Element) - IDE * Prefix mono- not used for 1 st element Molecular Formulas: H 2 O CO 2 Structural Formulas: (Space-filling ) H 2 O 2 CO CH 4 Atoms, Molecules, and Ions

* Ionic Compounds Atoms, Molecules, and Ions

Ionic Compounds (Salts): • Composed of a Metal ion (cation, M+) combined with an Non. Metal ion (anion, N-); atoms exchange transfer electrons. • Are Crystalline Solids. • Have high melting points • Smallest component particle is called a formula unit, not a molecule. Cations Atoms, Molecules, and Ions Anions

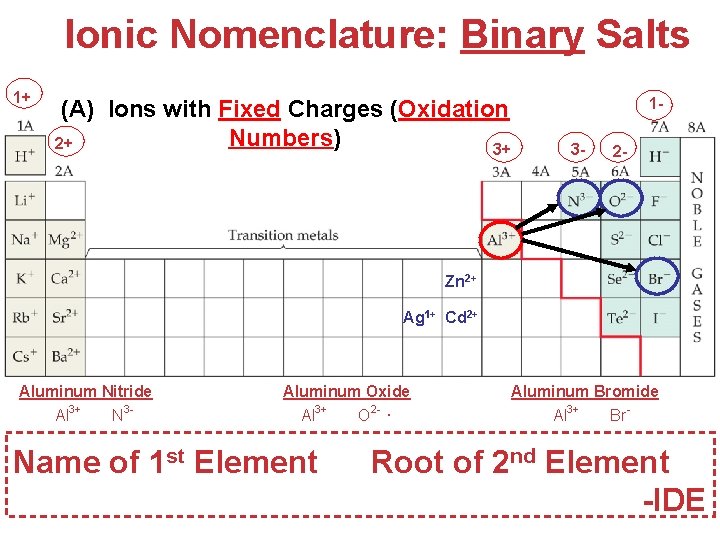
Ionic Nomenclature: Binary Salts 1+ (A) Ions with Fixed Charges (Oxidation Numbers) 2+ 3+ 13 - 2 - Zn 2+ Ag 1+ Cd 2+ Aluminum Nitride Al 3+ N 3 - Al. N Aluminum Oxide Al 3+ O 2 - Al 2 O 3 Name of 1 st Element Aluminum Bromide Al 3+ Br- Al. Br 3 Root of 2 nd Element Atoms, Molecules, -IDE and Ions

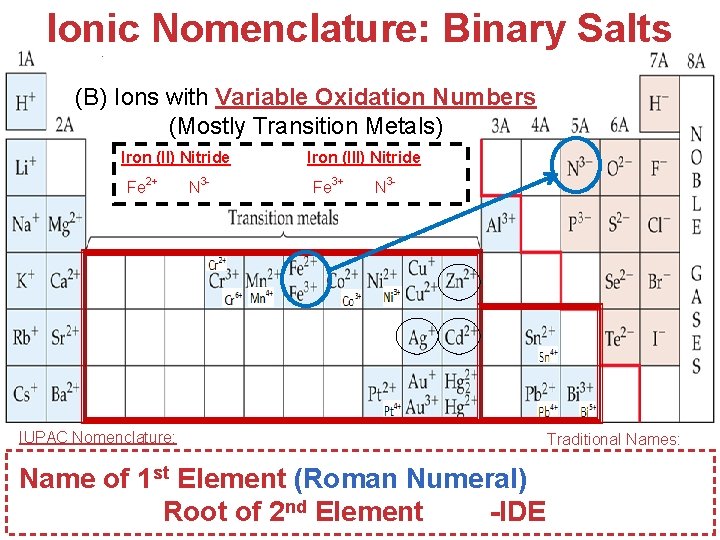
Ionic Nomenclature: Binary Salts (B) Ions with Variable Oxidation Numbers (Mostly Transition Metals) Iron (II) Nitride Fe 2+ IUPAC Nomenclature: N 3 - Fe 3 N 2 Iron (III) Nitride Fe 3+ N 3 - Fe. N Traditional Names: Atoms, Name of 1 st Element (Roman Numeral) (or –ous, -ic) Molecules, and Ions Root of 2 nd Element -IDE

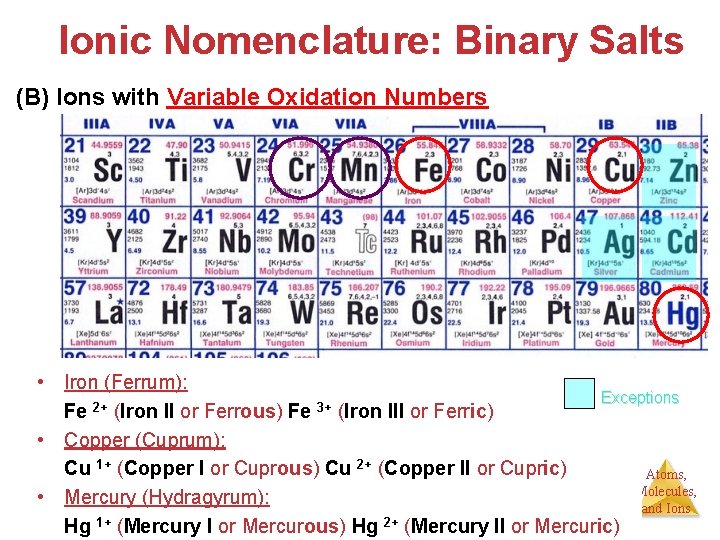
Ionic Nomenclature: Binary Salts (B) Ions with Variable Oxidation Numbers • Iron (Ferrum): Exceptions 2+ 3+ Fe (Iron II or Ferrous) Fe (Iron III or Ferric) • Copper (Cuprum): Cu 1+ (Copper I or Cuprous) Cu 2+ (Copper II or Cupric) Atoms, Molecules, • Mercury (Hydragyrum): and Ions 1+ 2+ Hg (Mercury I or Mercurous) Hg (Mercury II or Mercuric)

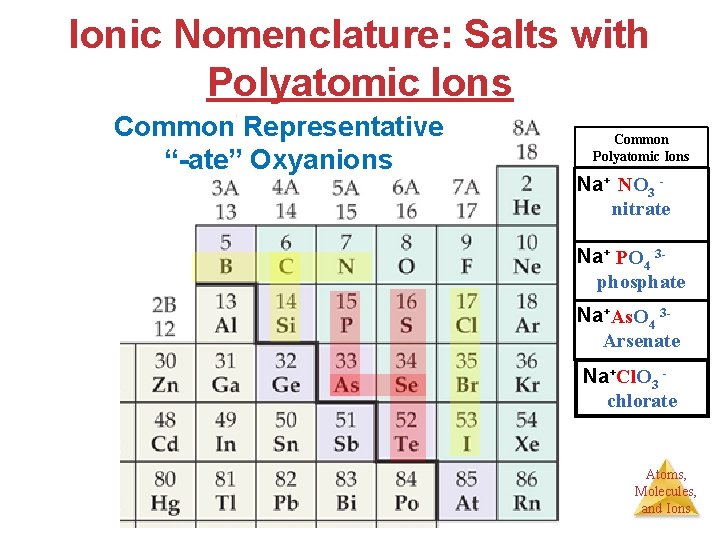
Ionic Nomenclature: Salts with Polyatomic Ions Common Representative “-ate” Oxyanions Common Polyatomic Ions Na+ NO 3 nitrate Na+ PO 4 3 phosphate Na+As. O 4 3 Arsenate Na+Cl. O 3 chlorate Atoms, Molecules, and Ions

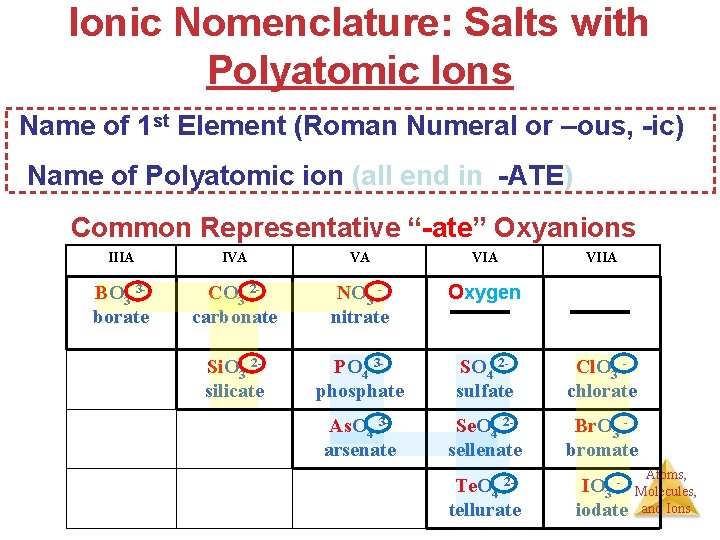
Ionic Nomenclature: Salts with Polyatomic Ions Name of 1 st Element (Roman Numeral or –ous, -ic) Name of Polyatomic ion (all end in -ATE) Common Representative “-ate” Oxyanions IIIA IVA VA VIIA BO 3 3 borate CO 3 2 carbonate NO 3 nitrate Oxygen Si. O 3 2 silicate PO 4 3 phosphate SO 4 2 sulfate Cl. O 3 chlorate As. O 4 3 arsenate Se. O 4 2 sellenate Br. O 3 bromate Te. O 4 2 tellurate IO 3 iodate Atoms, Molecules, and Ions

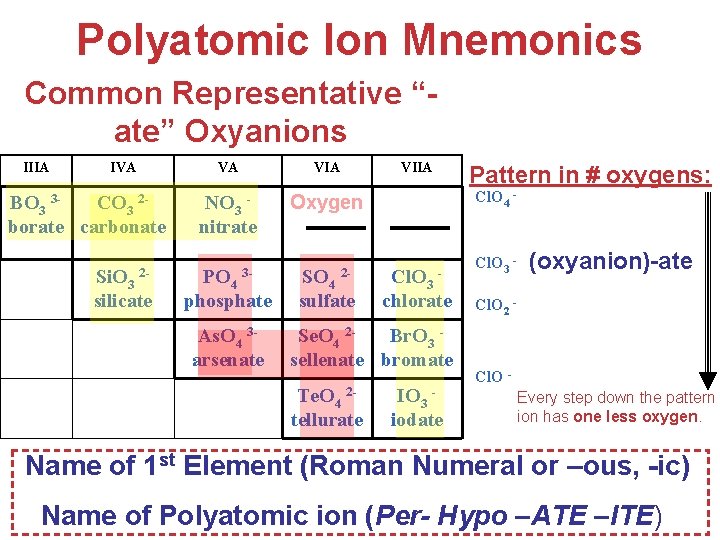
Polyatomic Ion Mnemonics Common Representative “ate” Oxyanions IIIA IVA BO 3 3 CO 3 2 borate carbonate Si. O 3 2 silicate VA VIA NO 3 nitrate Oxygen PO 4 3 phosphate As. O 4 3 arsenate SO 4 2 sulfate VIIA Cl. O 4 - Per-(oxyanion)-ate Cl. O 3 chlorate Se. O 4 2 Br. O 3 sellenate bromate Te. O 4 2 tellurate Pattern in # oxygens: IO 3 iodate Cl. O 3 - (oxyanion)-ate Cl. O 2 - (oxyanion)- ite Hypo-(oxyanion)-ite Cl. O Every step down the pattern ion has one less oxygen. Name of 1 st Element (Roman Numeral or –ous, Atoms, -ic) Molecules, and Ions Name of Polyatomic ion (Per- Hypo –ATE –ITE)

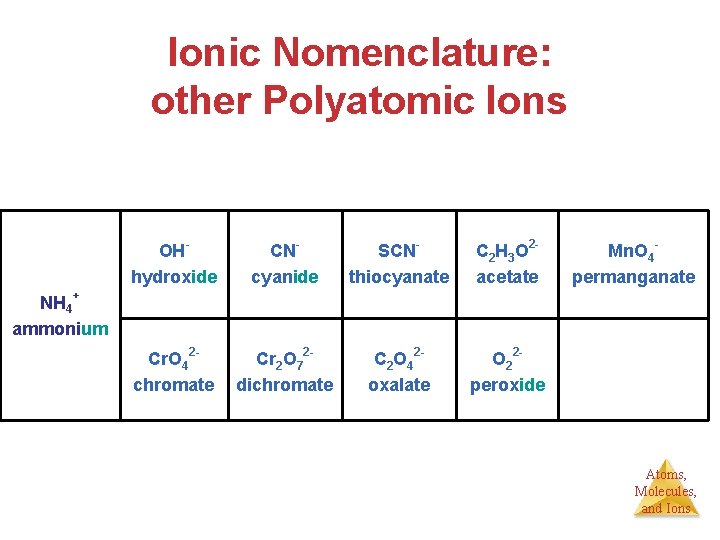
Ionic Nomenclature: other Polyatomic Ions OHhydroxide CNcyanide SCNthiocyanate C 2 H 3 O 2 acetate Cr. O 42 chromate Cr 2 O 72 dichromate C 2 O 42 oxalate O 22 peroxide Mn. O 4 permanganate NH 4+ ammonium Atoms, Molecules, and Ions

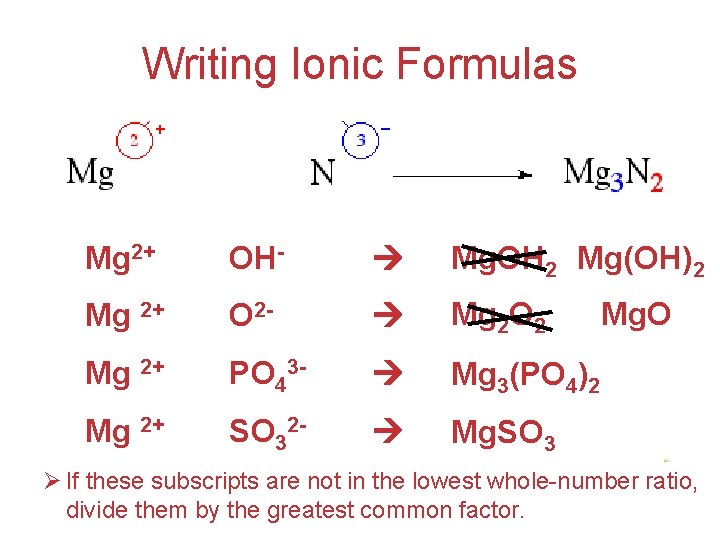
Writing Ionic Formulas Mg 2+ OH- Mg. OH 2 Mg(OH)2 Mg 2+ O 2 - Mg 2 O 2 Mg 2+ PO 43 - Mg 3(PO 4)2 Mg 2+ SO 32 - Mg. SO 3 Mg. O Atoms, Ø If these subscripts are not in the lowest whole-number. Molecules, ratio, and Ions divide them by the greatest common factor.

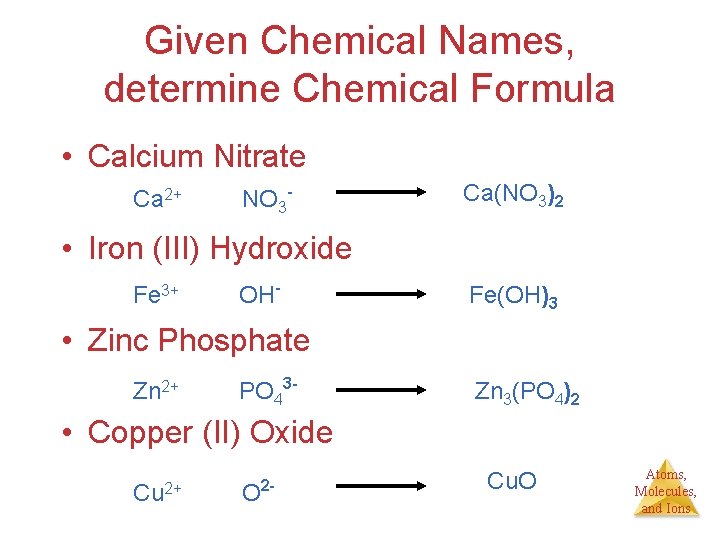
Given Chemical Names, determine Chemical Formula • Calcium Nitrate Ca 2+ NO 3 - Ca(NO 3)2 • Iron (III) Hydroxide Fe 3+ OH- Fe(OH)3 • Zinc Phosphate Zn 2+ PO 43 - Zn 3(PO 4)2 • Copper (II) Oxide Cu 2+ O 2 - Cu. O Atoms, Molecules, and Ions

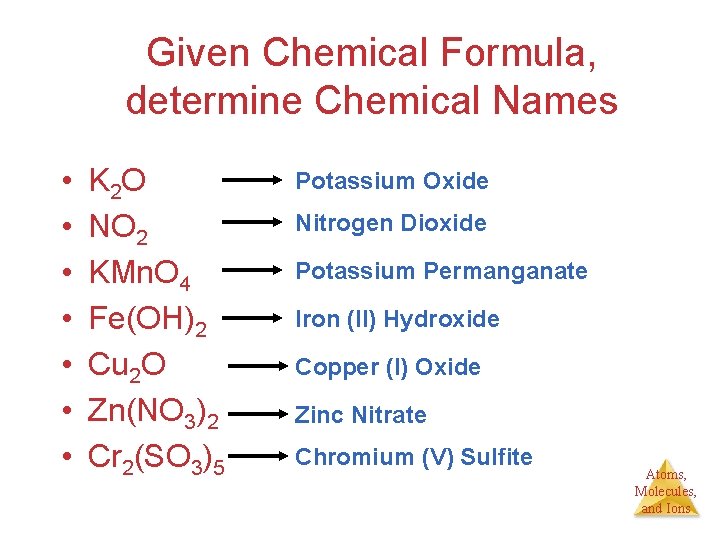
Given Chemical Formula, determine Chemical Names • • K 2 O NO 2 KMn. O 4 Fe(OH)2 Cu 2 O Zn(NO 3)2 Cr 2(SO 3)5 Potassium Oxide Nitrogen Dioxide Potassium Permanganate Iron (II) Hydroxide Copper (I) Oxide Zinc Nitrate Chromium (V) Sulfite Atoms, Molecules, and Ions

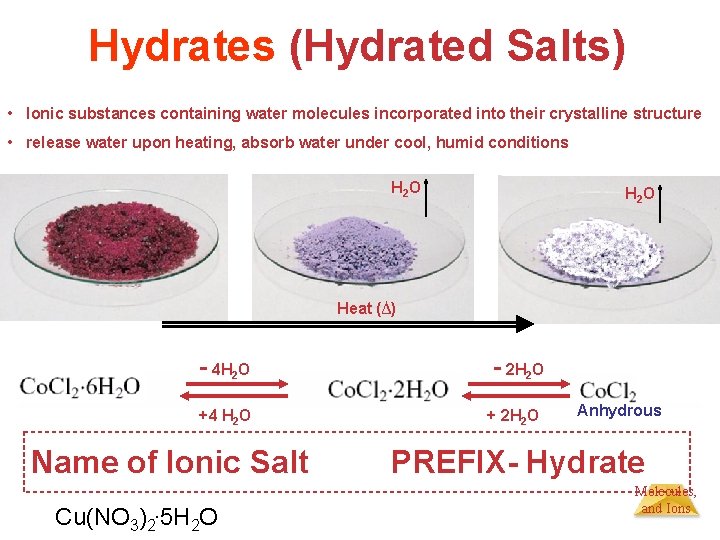
Hydrates (Hydrated Salts) • Ionic substances containing water molecules incorporated into their crystalline structure • release water upon heating, absorb water under cool, humid conditions H 2 O Heat (∆) - 4 H 2 O - 2 H 2 O +4 H 2 O + 2 H 2 O Name of Ionic Salt Cu(NO 3)2. 5 H 2 O Anhydrous PREFIX- Hydrate. Atoms, Molecules, and Ions

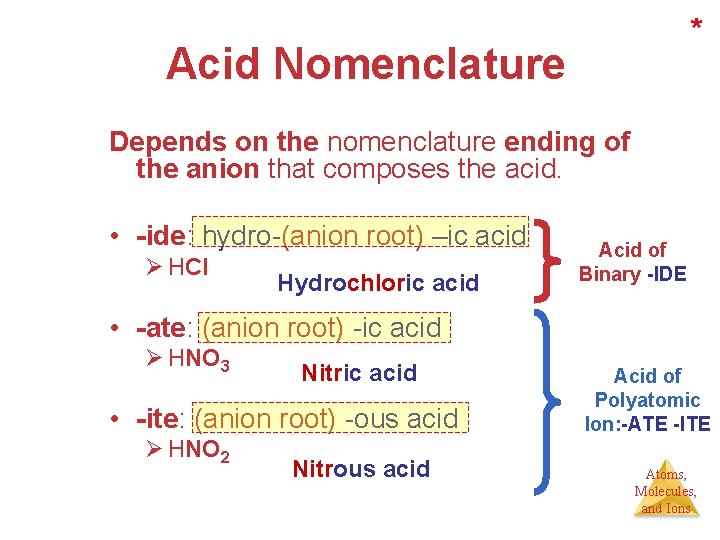
* Acid Nomenclature Depends on the nomenclature ending of the anion that composes the acid. • -ide: hydro-(anion root) –ic acid Ø HCl Hydrochloric acid Acid of Binary -IDE • -ate: (anion root) -ic acid Ø HNO 3 Nitric acid • -ite: (anion root) -ous acid Ø HNO 2 Nitrous acid Acid of Polyatomic Ion: -ATE -ITE Atoms, Molecules, and Ions

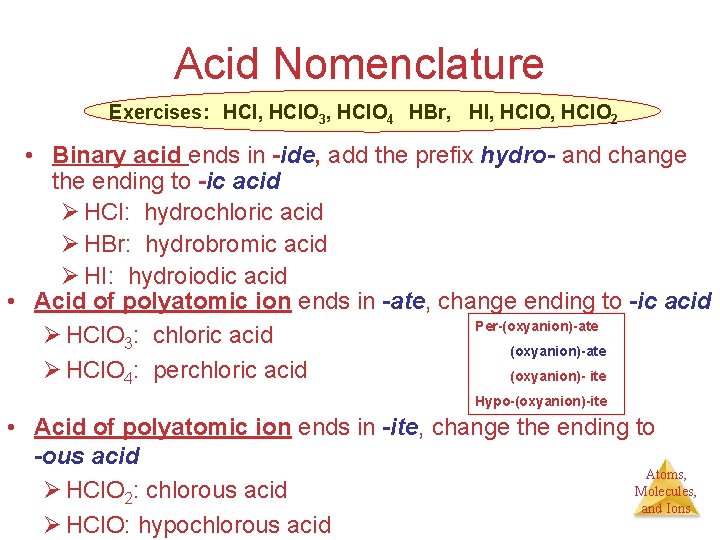
Acid Nomenclature Exercises: HCl, HCl. O 3, HCl. O 4 HBr, HI, HCl. O 2 • Binary acid ends in -ide, add the prefix hydro- and change the ending to -ic acid Ø HCl: hydrochloric acid Ø HBr: hydrobromic acid Ø HI: hydroiodic acid • Acid of polyatomic ion ends in -ate, change ending to -ic acid Per-(oxyanion)-ate Ø HCl. O 3: chloric acid (oxyanion)-ate Ø HCl. O 4: perchloric acid (oxyanion)- ite Hypo-(oxyanion)-ite • Acid of polyatomic ion ends in -ite, change the ending to -ous acid Atoms, Molecules, Ø HCl. O 2: chlorous acid and Ions Ø HCl. O: hypochlorous acid

Acidic Salts and Basic Salts Acid Salts: salts of weak polyprotic acids. Are not necessarily acidic, but do neutralize bases. Examples: Acid Salt(s) HNO 3 Na. NO 3 ------ H 2 CO 3 Na. HCO 3 sodium hydrogen carbonate H 3 PO 4 Na 2 HPO 4 sodium mono-hydrogen phosphate Na. H 2 PO 4 sodium di-hydrogen phosphate Basic Salts: salts of weak polyhydroxy bases. They do not dissolve well in water and thus exists mostly in the undissociated solid salt state instead of the dissolved basic state. They do neutralize acids. Mg(OH) 2(s) (Milk of Magnesia) Fe(OH)3(s) Cr(OH) 3(s)) solid salt ↔ Cr 3+ (aq) + 3 OH- (aq) dissolved (aq) base Atoms, Molecules, and Ions Solubility Rule: • All OH- are insoluble except for IA metals, NH 4+ & slightly soluble Ca 2+ Ba 2+ & Sr 2+

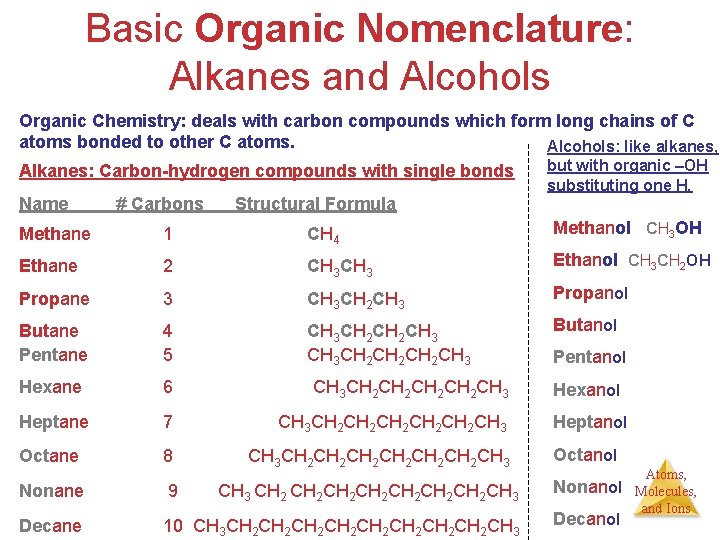
Basic Organic Nomenclature: Alkanes and Alcohols Organic Chemistry: deals with carbon compounds which form long chains of C atoms bonded to other C atoms. Alcohols: like alkanes, Alkanes: Carbon-hydrogen compounds with single bonds Name # Carbons Structural Formula but with organic –OH substituting one H. Methane 1 CH 4 Methanol CH 3 OH Ethane 2 CH 3 Ethanol CH 3 CH 2 OH Propane 3 CH 3 CH 2 CH 3 Propanol Butane Pentane 4 5 CH 3 CH 2 CH 3 CH 2 CH 2 CH 3 Butanol Hexane 6 CH 3 CH 2 CH 2 CH 3 Heptane 7 CH 3 CH 2 CH 2 CH 2 CH 3 Octane 8 CH 3 CH 2 CH 2 CH 2 CH 3 Nonane 9 CH 3 CH 2 CH 2 CH 3 Decane 10 CH 3 CH 2 CH 2 CH 3 Pentanol Hexanol Heptanol Octanol Atoms, Nonanol Molecules, and Ions Decanol