CHM 1046 General Chemistry and Qualitative Analysis Unit










- Slides: 66

CHM 1046: General Chemistry and Qualitative Analysis Unit 12: Properties of Solutions Dr. Jorge L. Alonso Miami-Dade College – Kendall Campus Miami, FL Textbook Reference: • Chapter # 14 • Module # 2 Solutions

Solutions • Solutions (soln) are homogeneous mixtures of two or more pure substances. • The solvent (solv) is present in greatest abundance. • All other substances are solutes (solu). Volumetric flask Solutions {Prep. ASolu}

Solutions • Solutions are homogeneous mixtures of two or more pure substances. • In a solution, the solute (< 50%) is dispersed uniformly throughout the solvent (> 50%). Homogeneous Heterogeneous Solutions

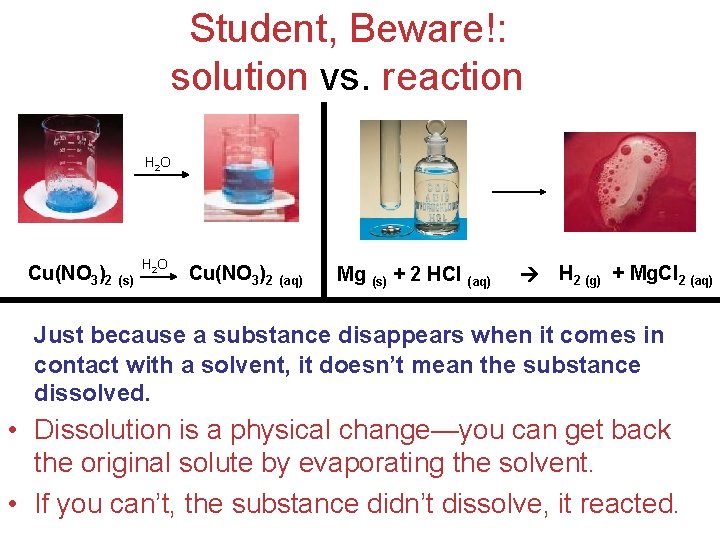
Student, Beware!: solution vs. reaction H 2 O Cu(NO 3)2 (s) H 2 O Cu(NO 3)2 (aq) Mg (s) + 2 HCl (aq) H 2 (g) + Mg. Cl 2 (aq) Just because a substance disappears when it comes in contact with a solvent, it doesn’t mean the substance dissolved. • Dissolution is a physical change—you can get back the original solute by evaporating the solvent. Solutions • If you can’t, the substance didn’t dissolve, it reacted.

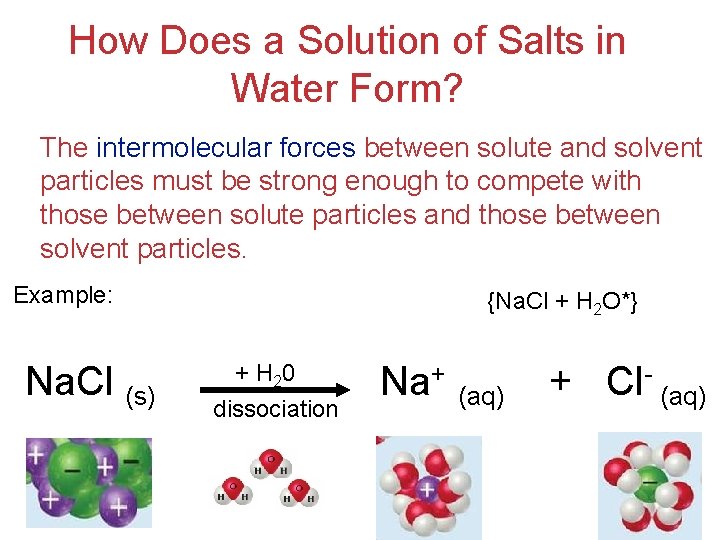
How Does a Solution of Salts in Water Form? The intermolecular forces between solute and solvent particles must be strong enough to compete with those between solute particles and those between solvent particles. Example: Na. Cl (s) {Na. Cl + H 2 O*} + H 20 dissociation Na+ (aq) + Cl- (aq) Solutions

Solubility in water Elements: mostly insoluble solids, liquids & gases. Covalent Compounds: many are insoluble gases, polar covalent are mostly soluble. Ionic Compounds: many are soluble. SOLUBILITY RULES: for Ionic Compounds (Salts) 1. All salts of alkali metals (IA) are soluble. 2. All NH 4+ salts are soluble. 3. All salts containing the anions: NO 3 -, Cl. O 4 -, (C 2 H 3 O 2 -) are soluble. 4. All Cl-, Br-, and I- are soluble except for Ag+, Pb 2+, and Hg 22+ salts. 5. All SO 42 - are soluble except for Pb 2+, Sr 2+, and Ba 2+. 6. All O 2 - are insoluble except for IA metals Ca 2+, Sr 2+, and Ba 2+ salts. HO {Soluble metal oxides form hydroxides: Ca. O 2 Ca 2+ + 2 OH-} 7. 6. 7. All OH- are insoluble except for IA metals, NH 4+ & slightly soluble Ca 2+ Ba 2+ & Sr 2+ All salts containing the anions: CO 32 -, PO 43 -, As. O 43 -, S 2 - and SO 32 - are insoluble except fro IA metals and NH 4+ salts. For salts containing the anions not mentioned above (e. g. , Cr. O 42 -, Cr 2 O 72 -Solutions , P 3 -, C 2 O 42 - etc. ) assume that they are insoluble except for IA metals and NH 4+ salts, unless, otherwise informed.

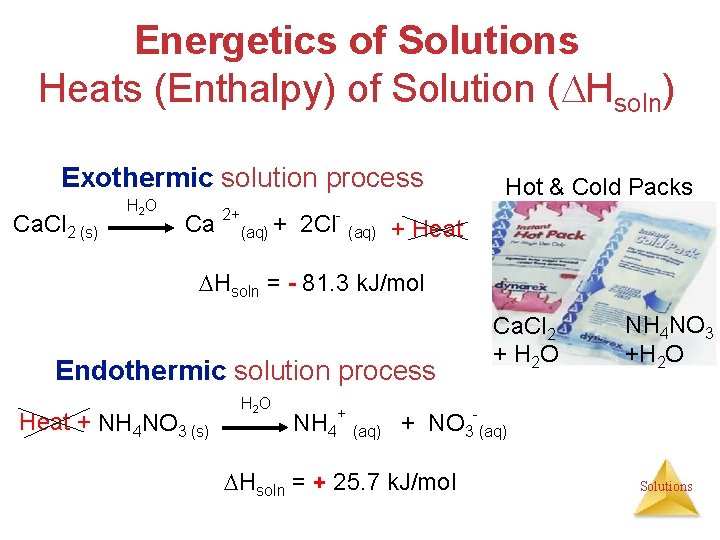
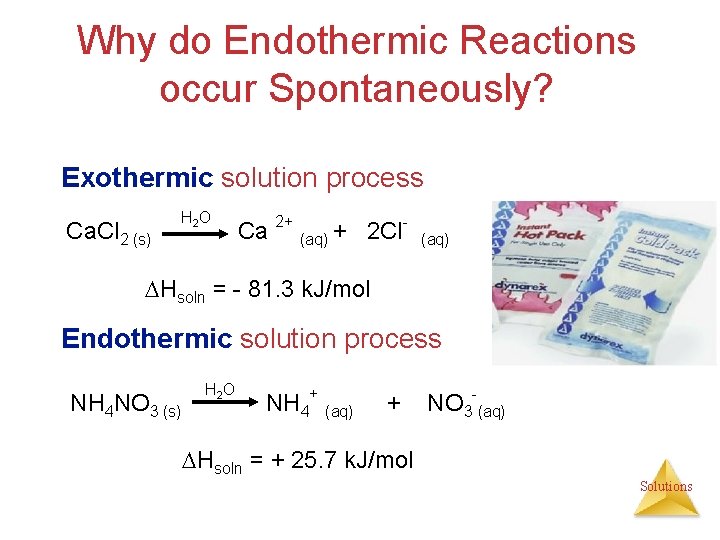
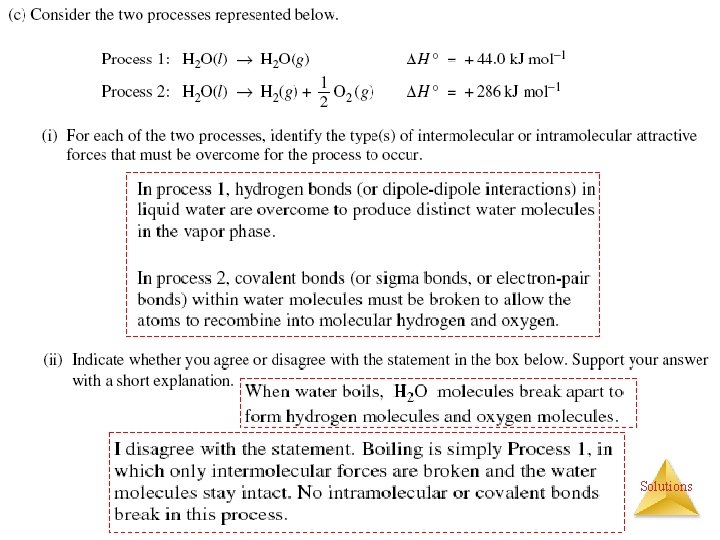
Energetics of Solutions Heats (Enthalpy) of Solution ( Hsoln) Exothermic solution process Ca. Cl 2 (s) H 2 O Hot & Cold Packs Ca 2+(aq) + 2 Cl- (aq) + Heat Hsoln = - 81. 3 k. J/mol Endothermic solution process Heat + NH 4 NO 3 (s) H 2 O Ca. Cl 2 + H 2 O NH 4 NO 3 +H 2 O NH 4+ (aq) + NO 3 -(aq) Hsoln = + 25. 7 k. J/mol Solutions

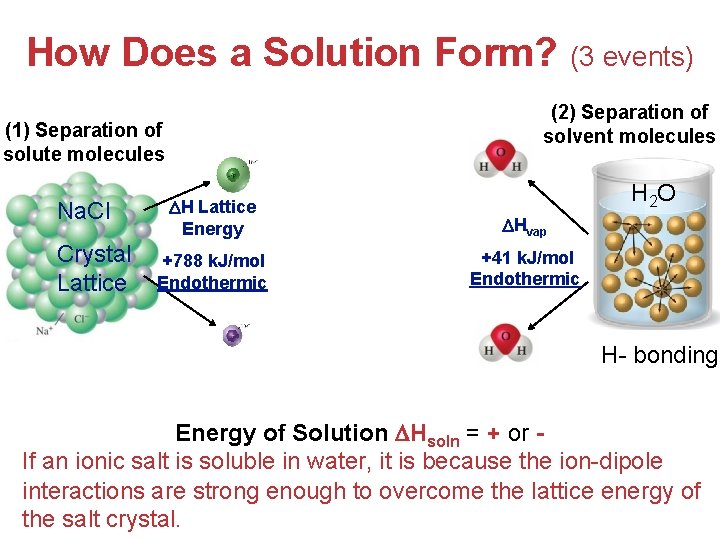
How Does a Solution Form? (3 events) (3) Formation of Ion-dipole interactions (1) Separation of solute molecules Na. Cl Crystal Lattice (2) Separation of solvent molecules H 2 O H Lattice Energy Hvap +788 k. J/mol Endothermic +41 k. J/mol Endothermic Exothermic H- bonding Energy of Solution Hsoln = + or If an ionic salt is soluble in water, it is because the ion-dipole Solutions interactions are strong enough to overcome the lattice energy of the salt crystal.

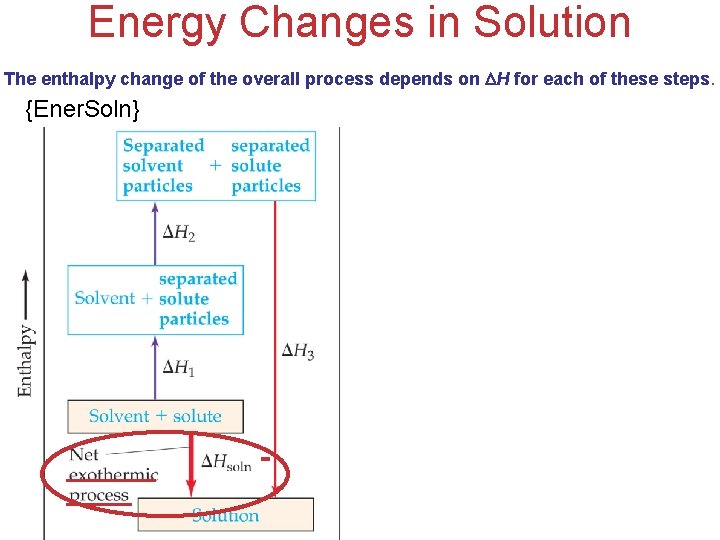
Energy Changes in Solution The enthalpy change of the overall process depends on H for each of these steps. {Ener. Soln} - + Solutions

Why do Endothermic Reactions occur Spontaneously? Exothermic solution process Ca. Cl 2 (s) H 2 O Ca 2+ (aq) + 2 Cl- (aq) Hsoln = - 81. 3 k. J/mol Endothermic solution process NH 4 NO 3 (s) H 2 O NH 4+ (aq) + NO 3 -(aq) Hsoln = + 25. 7 k. J/mol Solutions

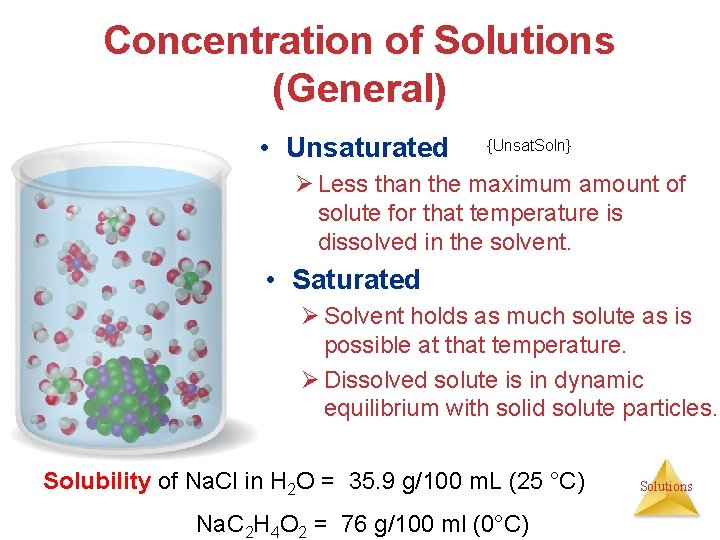
Concentration of Solutions (General) • Unsaturated {Unsat. Soln} Ø Less than the maximum amount of solute for that temperature is dissolved in the solvent. • Saturated Ø Solvent holds as much solute as is possible at that temperature. Ø Dissolved solute is in dynamic equilibrium with solid solute particles. Solubility of Na. Cl in H 2 O = 35. 9 g/100 m. L (25 °C) Na. C 2 H 4 O 2 = 76 g/100 ml (0°C) Solutions

Concentration of Solutions {*Super. Sat 1} {*Super. Sat 2} (General) • Supersaturated Ø Solvent holds more solute than is normally possible at that temperature. Ø These solutions are unstable; crystallization can usually be stimulated by adding a “seed crystal” or scratching the side of the flask. Solutions

Factors Affecting Solubility A. Solids and Liquids: 1. Polarity 2. Temperature B. Gases : 1. Molar Mass 2. Pressure – Henry’s Law 3. Temperature Solutions

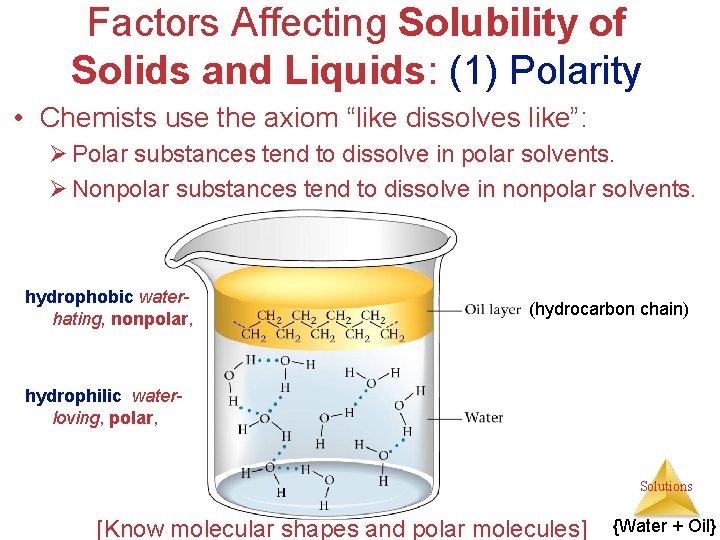
Factors Affecting Solubility of Solids and Liquids: (1) Polarity • Chemists use the axiom “like dissolves like”: Ø Polar substances tend to dissolve in polar solvents. Ø Nonpolar substances tend to dissolve in nonpolar solvents. hydrophobic waterhating, nonpolar, (hydrocarbon chain) hydrophilic waterloving, polar, Solutions [Know molecular shapes and polar molecules] {Water + Oil}

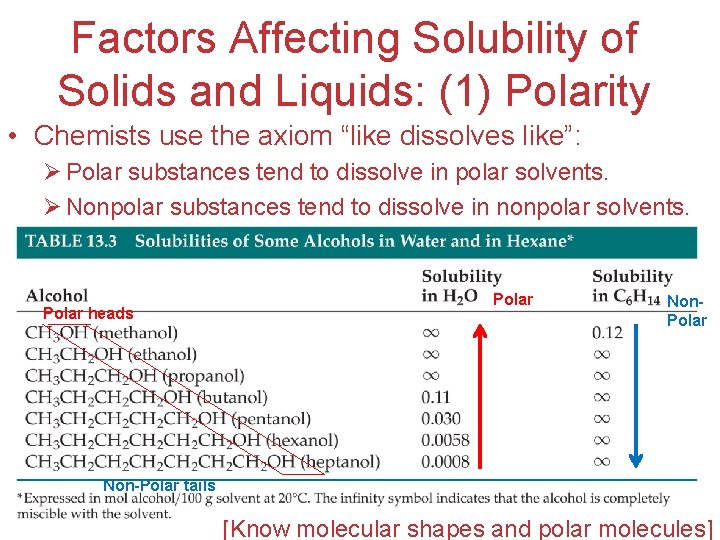
Factors Affecting Solubility of Solids and Liquids: (1) Polarity • Chemists use the axiom “like dissolves like”: Ø Polar substances tend to dissolve in polar solvents. Ø Nonpolar substances tend to dissolve in nonpolar solvents. Polar heads Non-Polar tails Polar Non. Polar Solutions [Know molecular shapes and polar molecules]

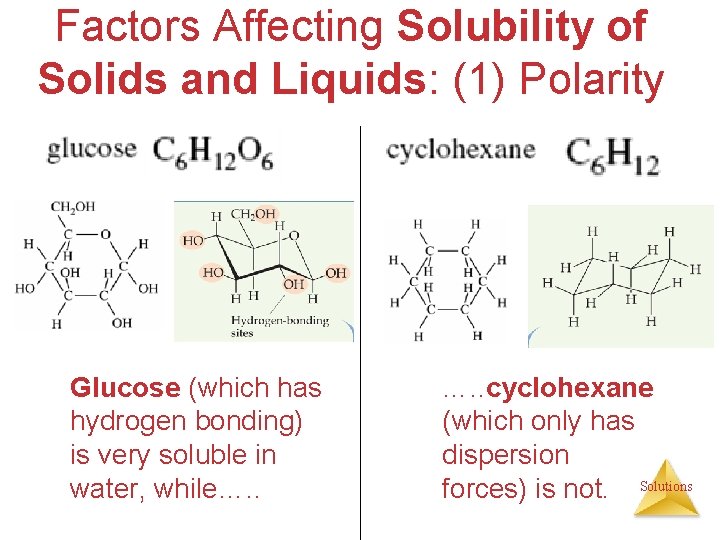
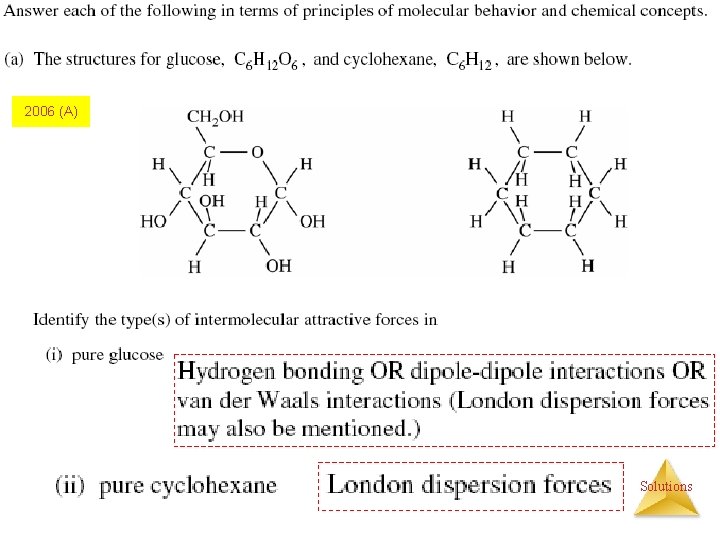
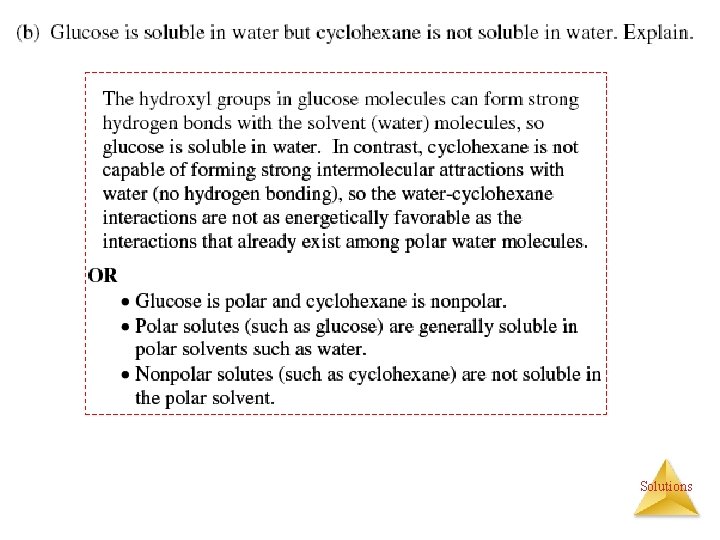
Factors Affecting Solubility of Solids and Liquids: (1) Polarity Glucose (which has hydrogen bonding) is very soluble in water, while…. . cyclohexane (which only has dispersion forces) is not. Solutions

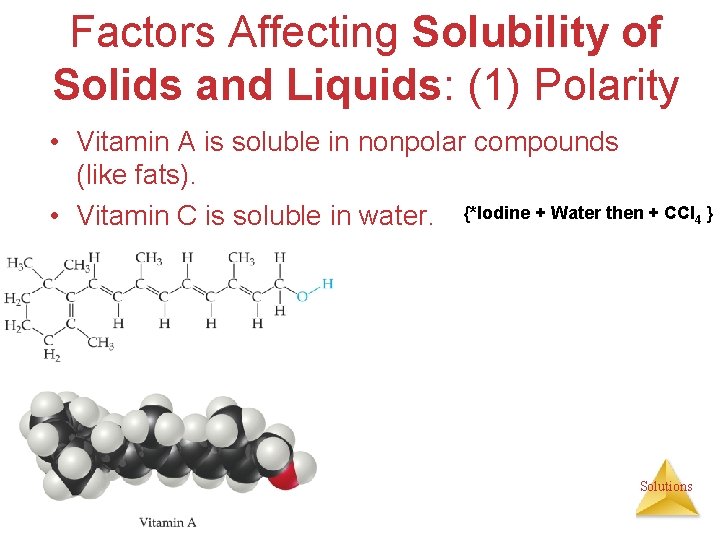
Factors Affecting Solubility of Solids and Liquids: (1) Polarity • Vitamin A is soluble in nonpolar compounds (like fats). • Vitamin C is soluble in water. {*Iodine + Water then + CCl 4 } Solutions

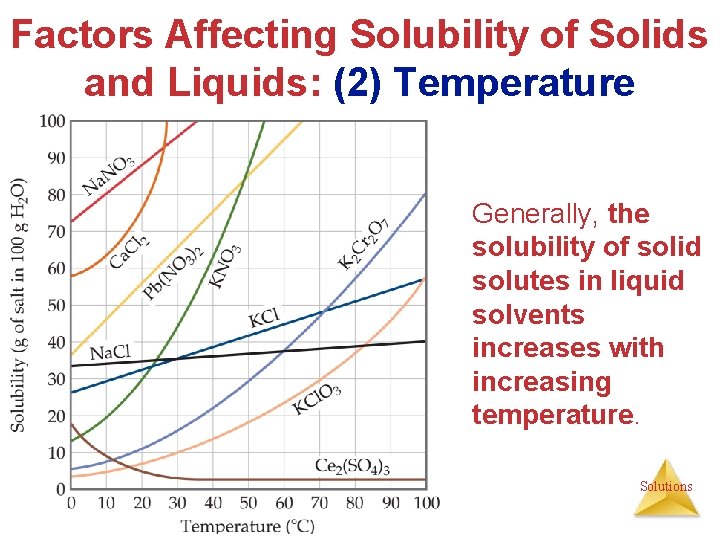
Factors Affecting Solubility of Solids and Liquids: (2) Temperature Generally, the solubility of solid solutes in liquid solvents increases with increasing temperature. Solutions

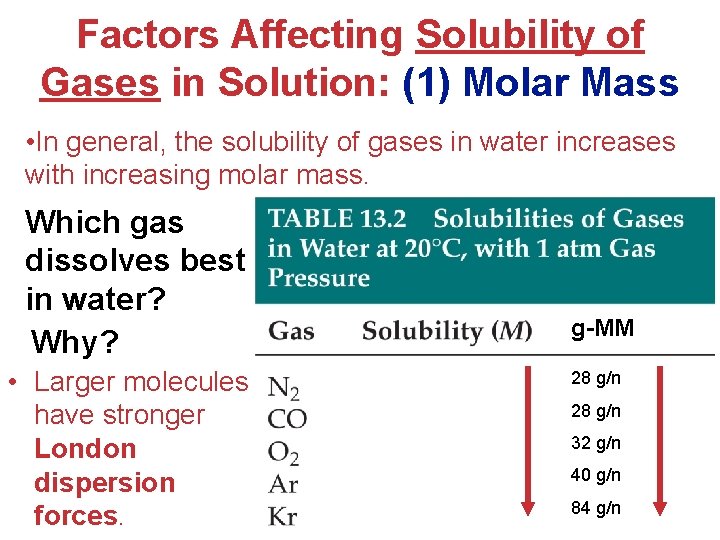
Factors Affecting Solubility of Gases in Solution: (1) Molar Mass • In general, the solubility of gases in water increases with increasing molar mass. Which gas dissolves best in water? Why? • Larger molecules have stronger London dispersion forces. g-MM 28 g/n 32 g/n 40 g/n 84 g/n Solutions

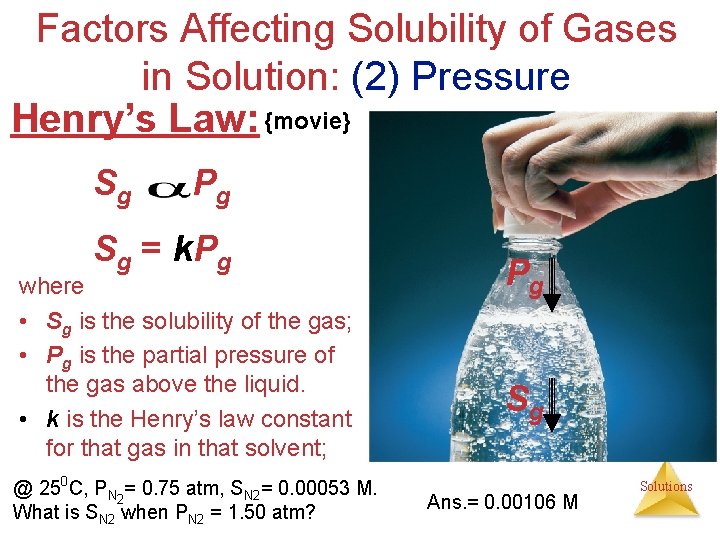
Factors Affecting Solubility of Gases in Solution: (2) Pressure • The solubility of liquids and solids does not change appreciably with pressure. • The solubility of a gas (Sg) in a liquid is directly proportional to its pressure (Pg). {Henrys Law} Sg Pg Solutions

Factors Affecting Solubility of Gases in Solution: (2) Pressure Henry’s Law: {movie} Sg Pg Sg = k. Pg where • Sg is the solubility of the gas; • Pg is the partial pressure of the gas above the liquid. • k is the Henry’s law constant for that gas in that solvent; @ 250 C, PN 2= 0. 75 atm, SN 2= 0. 00053 M. What is SN 2 when PN 2 = 1. 50 atm? Pg Sg Ans. = 0. 00106 M Solutions

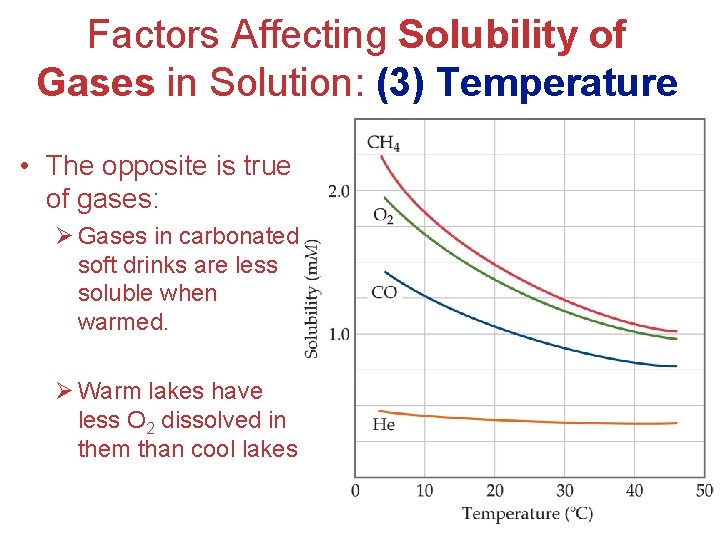
Factors Affecting Solubility of Gases in Solution: (3) Temperature • The opposite is true of gases: Ø Gases in carbonated soft drinks are less soluble when warmed. Ø Warm lakes have less O 2 dissolved in them than cool lakes. Solutions

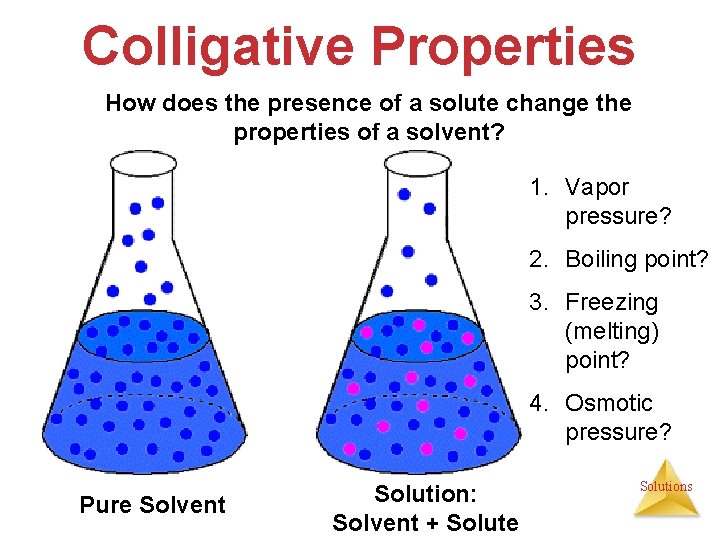
Colligative Properties How does the presence of a solute change the properties of a solvent? 1. Vapor pressure? 2. Boiling point? 3. Freezing (melting) point? 4. Osmotic pressure? Pure Solvent Solution: Solvent + Solute Solutions

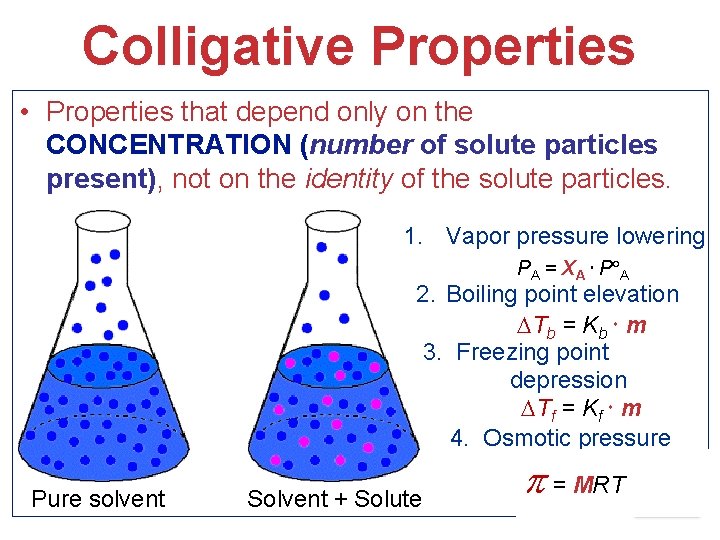
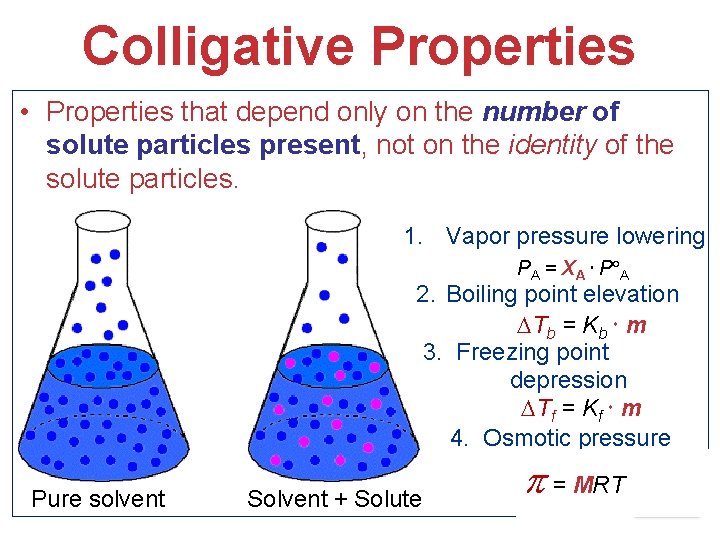
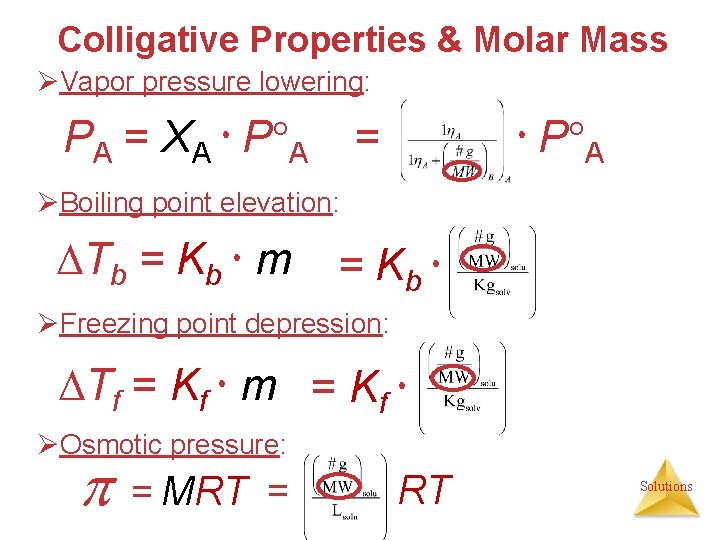
Colligative Properties • Properties that depend only on the CONCENTRATION (number of solute particles present), not on the identity of the solute particles. 1. Vapor pressure lowering PA = XA P A 2. Boiling point elevation Tb = Kb m 3. Freezing point depression Tf = Kf m 4. Osmotic pressure Pure solvent Solvent + Solute = MRT Solutions

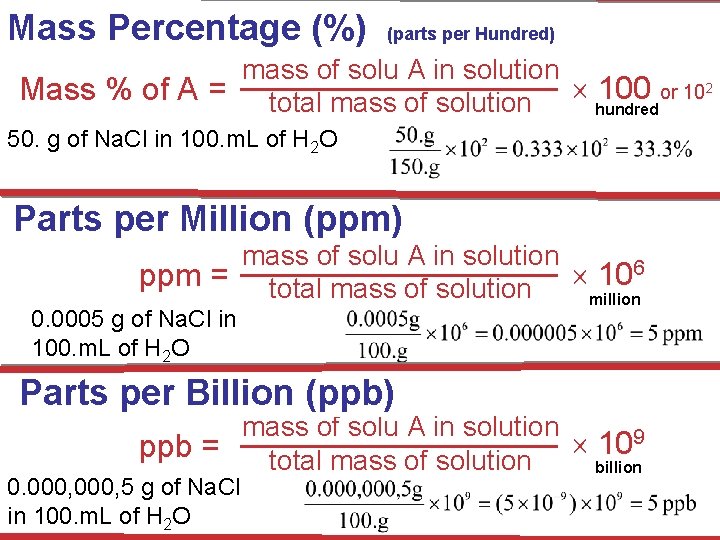
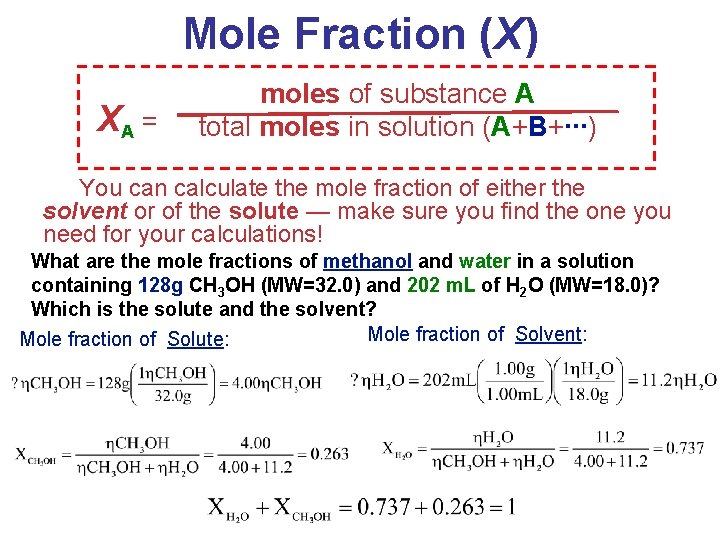
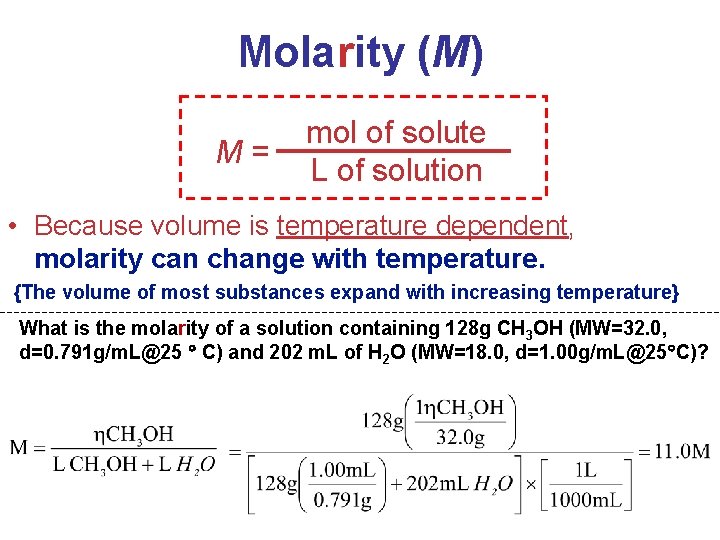
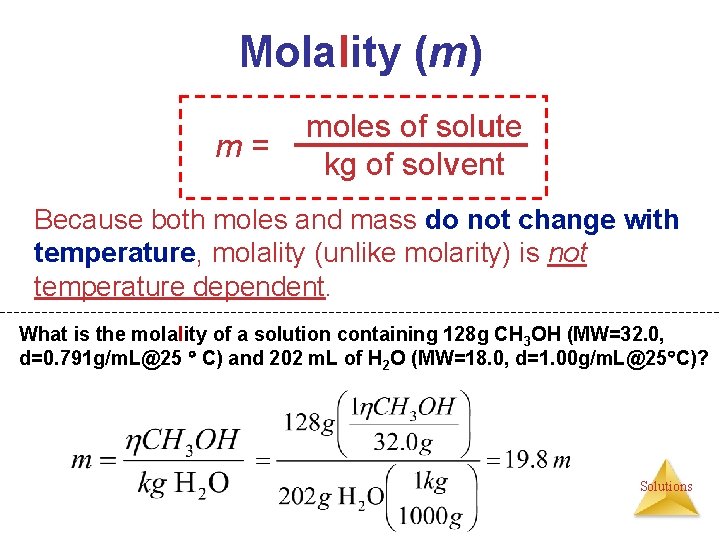
Ways of Expressing Concentrations of Solutions (1) Mass Percentage (%) (2) Parts per Million (ppm) & Parts per Billion (ppb) (3) Mole Fraction (X) (4) Molarity (M) (5) Molality (m) Solutions

Mass Percentage (%) (parts per Hundred) mass of solu A in solution 100 or 102 Mass % of A = total mass of solution hundred 50. g of Na. Cl in 100. m. L of H 2 O Parts per Million (ppm) mass of solu A in solution ppm = total mass of solution 106 million 0. 0005 g of Na. Cl in 100. m. L of H 2 O Parts per Billion (ppb) mass of solu A in solution 109 ppb = total mass of solution billion 0. 000, 5 g of Na. Cl in 100. m. L of H 2 O Solutions

Mole Fraction (X) XA = moles of substance A total moles in solution (A+B+∙∙∙) You can calculate the mole fraction of either the solvent or of the solute — make sure you find the one you need for your calculations! What are the mole fractions of methanol and water in a solution containing 128 g CH 3 OH (MW=32. 0) and 202 m. L of H 2 O (MW=18. 0)? Which is the solute and the solvent? Mole fraction of Solvent: Mole fraction of Solute: Solutions

Molarity (M) M= mol of solute L of solution • Because volume is temperature dependent, molarity can change with temperature. {The volume of most substances expand with increasing temperature} What is the molarity of a solution containing 128 g CH 3 OH (MW=32. 0, d=0. 791 g/m. L@25 C) and 202 m. L of H 2 O (MW=18. 0, d=1. 00 g/m. L@25 C)? Solutions

Molality (m) m= moles of solute kg of solvent Because both moles and mass do not change with temperature, molality (unlike molarity) is not temperature dependent. What is the molality of a solution containing 128 g CH 3 OH (MW=32. 0, d=0. 791 g/m. L@25 C) and 202 m. L of H 2 O (MW=18. 0, d=1. 00 g/m. L@25 C)? Solutions

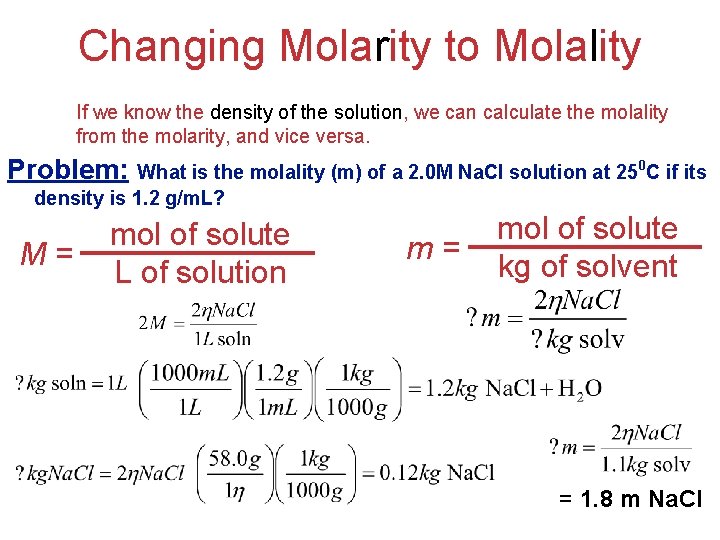
Changing Molarity to Molality If we know the density of the solution, we can calculate the molality from the molarity, and vice versa. Problem: What is the molality (m) of a 2. 0 M Na. Cl solution at 250 C if its density is 1. 2 g/m. L? M= mol of solute L of solution m= mol of solute kg of solvent Solutions = 1. 8 m Na. Cl

Colligative Properties • Properties that depend only on the number of solute particles present, not on the identity of the solute particles. 1. Vapor pressure lowering PA = XA P A 2. Boiling point elevation Tb = Kb m 3. Freezing point depression Tf = Kf m 4. Osmotic pressure Pure solvent Solvent + Solute = MRT Solutions

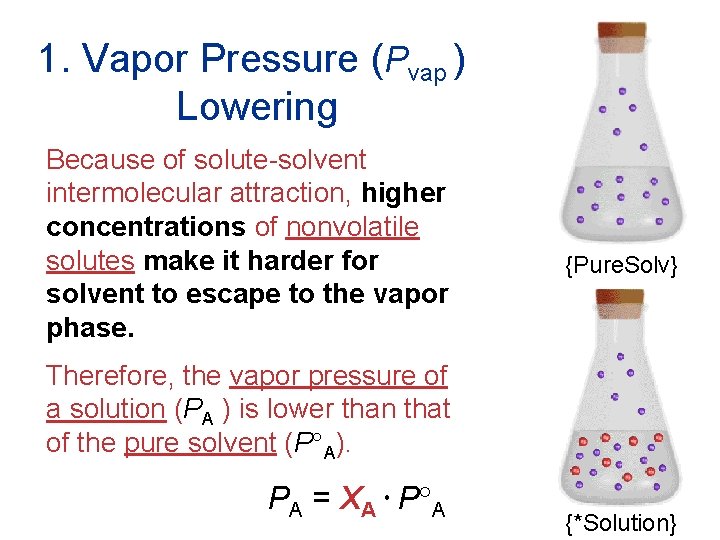
1. Vapor Pressure (Pvap ) Lowering Because of solute-solvent intermolecular attraction, higher concentrations of nonvolatile solutes make it harder for solvent to escape to the vapor phase. {Pure. Solv} Therefore, the vapor pressure of a solution (PA ) is lower than that of the pure solvent (P A). PA = XA P A Solutions {*Solution}

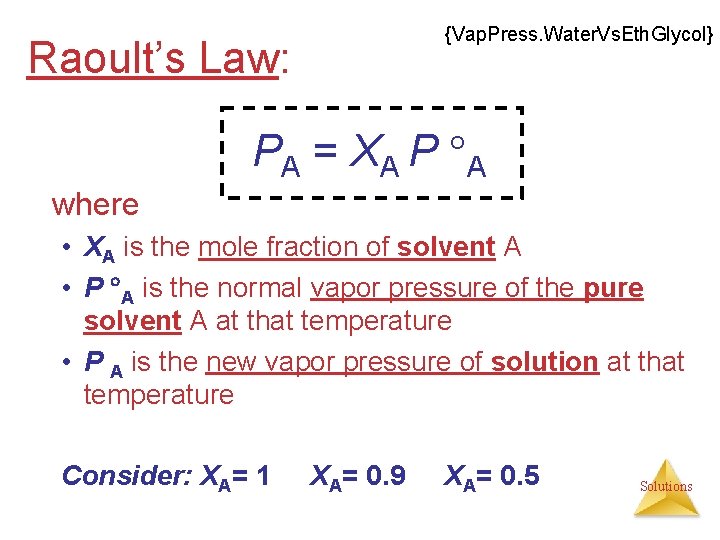
{Vap. Press. Water. Vs. Eth. Glycol} Raoult’s Law: PA = XA P A where • XA is the mole fraction of solvent A • P A is the normal vapor pressure of the pure solvent A at that temperature • P A is the new vapor pressure of solution at that temperature Consider: XA= 1 XA= 0. 9 XA= 0. 5 Solutions

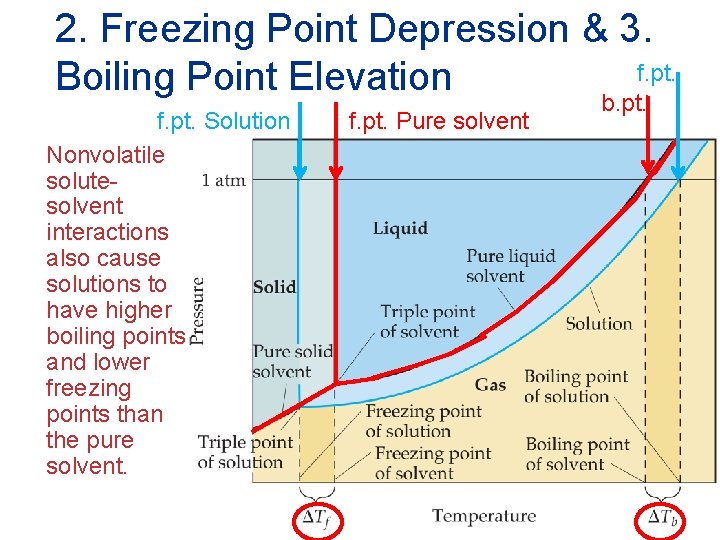
2. Freezing Point Depression & 3. f. pt. Boiling Point Elevation f. pt. Solution Nonvolatile solutesolvent interactions also cause solutions to have higher boiling points and lower freezing points than the pure solvent. f. pt. Pure solvent b. pt. Solutions

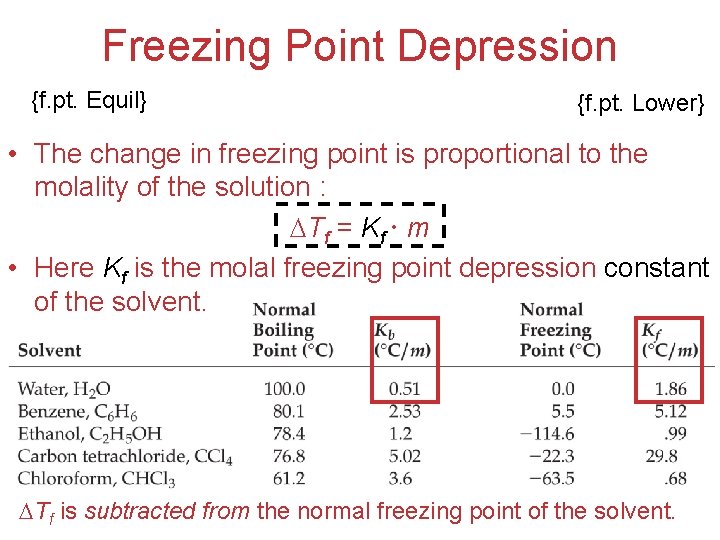
Freezing Point Depression {f. pt. Equil} {f. pt. Lower} • The change in freezing point is proportional to the molality of the solution : Tf = Kf m • Here Kf is the molal freezing point depression constant of the solvent. Solutions Tf is subtracted from the normal freezing point of the solvent.

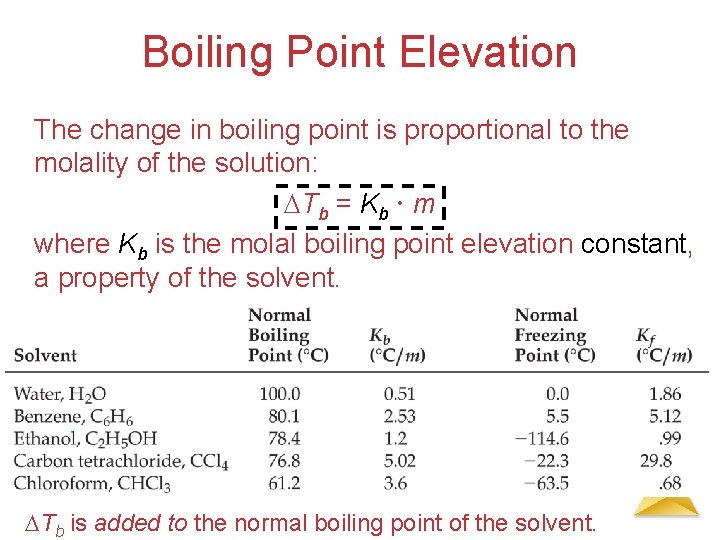
Boiling Point Elevation The change in boiling point is proportional to the molality of the solution: Tb = Kb m where Kb is the molal boiling point elevation constant, a property of the solvent. Solutions Tb is added to the normal boiling point of the solvent.

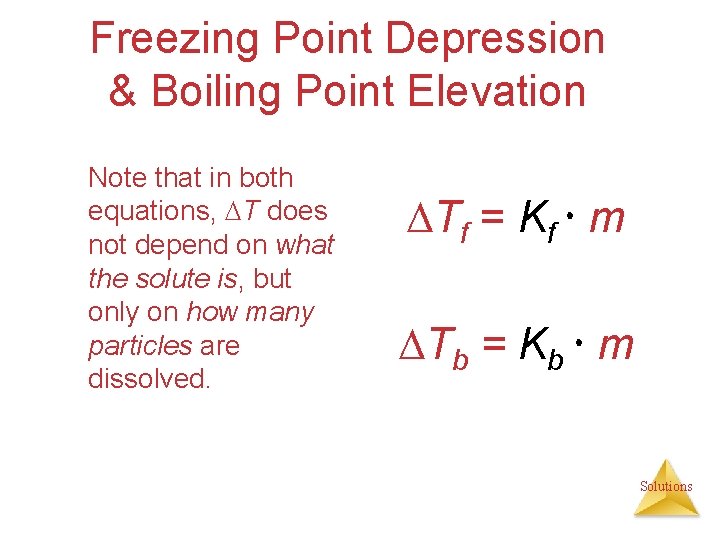
Freezing Point Depression & Boiling Point Elevation Note that in both equations, T does not depend on what the solute is, but only on how many particles are dissolved. Tf = Kf m Tb = Kb m Solutions

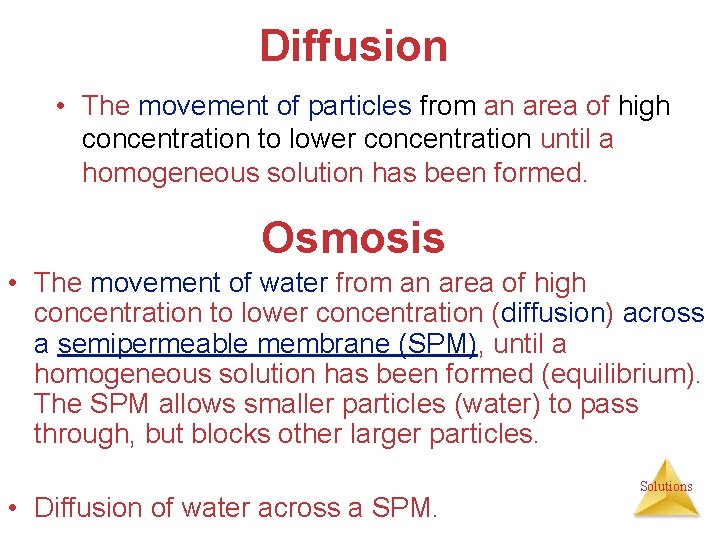
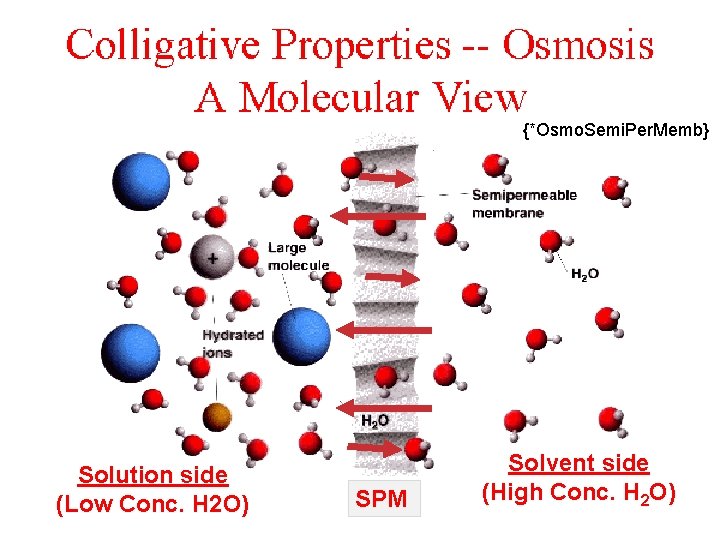
Diffusion • The movement of particles from an area of high concentration to lower concentration until a homogeneous solution has been formed. Osmosis • The movement of water from an area of high concentration to lower concentration (diffusion) across a semipermeable membrane (SPM), until a homogeneous solution has been formed (equilibrium). The SPM allows smaller particles (water) to pass through, but blocks other larger particles. • Diffusion of water across a SPM. Solutions

Osmosis In osmosis, there is net movement of solvent from the area of higher solvent concentration (lower solute concentration) to the area of lower solvent concentration (higher solute concentration). Solution {*Osmo. Press} Solvent Solutions

{*Osmo. Semi. Per. Memb} Solution side (Low Conc. H 2 O) SPM Solvent side (High Conc. HSolutions 2 O)

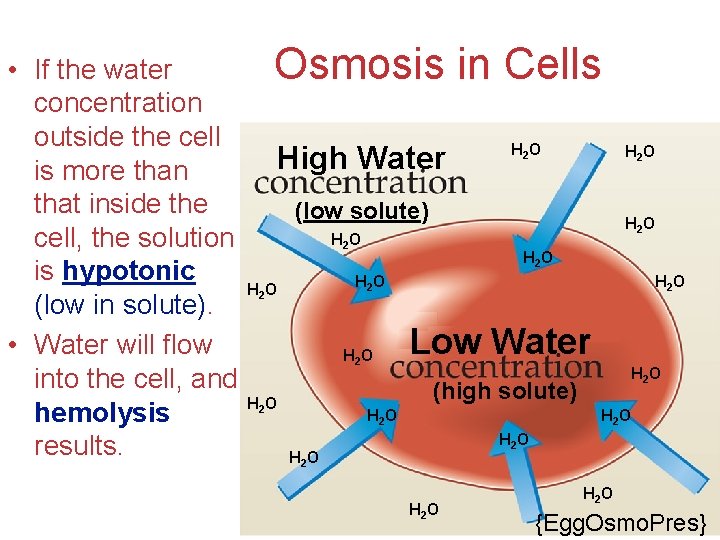
• If the water concentration outside the cell is more than that inside the cell, the solution is hypotonic (low in solute). • Water will flow into the cell, and hemolysis results. Osmosis in Cells High Water H 2 O (low solute) H 2 O H 2 O Low Water H 2 O (high solute) H 2 O H 2 O Solutions {Egg. Osmo. Pres}

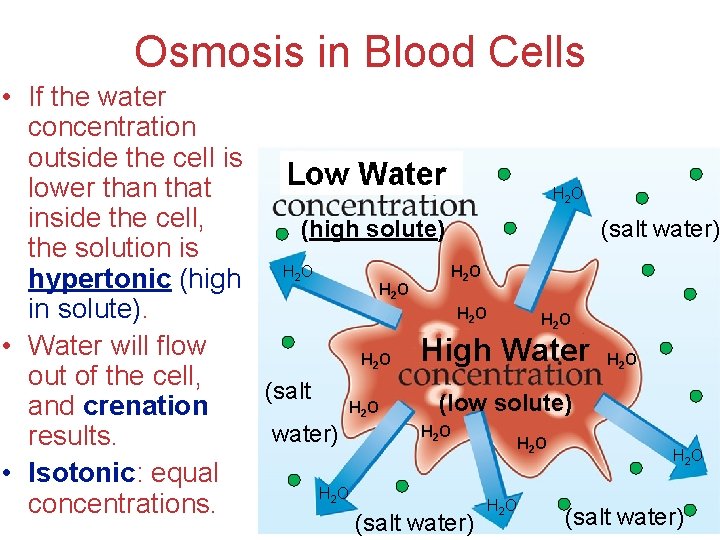
Osmosis in Blood Cells • If the water concentration outside the cell is lower than that HO inside the cell, (high solute) (salt water) the solution is HO hypertonic (high H O HO in solute). HO HO • Water will flow High Water H O HO out of the cell, (salt (low solute) HO and crenation HO water) results. HO HO • Isotonic: equal Solutions HO HO concentrations. (salt water) 2 2 2 2 (salt water) 2

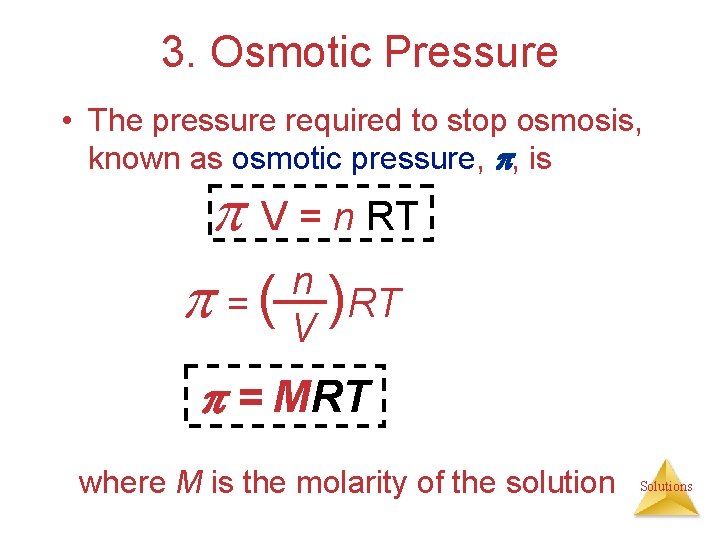
3. Osmotic Pressure • The pressure required to stop osmosis, known as osmotic pressure, , is V = n RT n = ( V )RT = MRT where M is the molarity of the solution Solutions

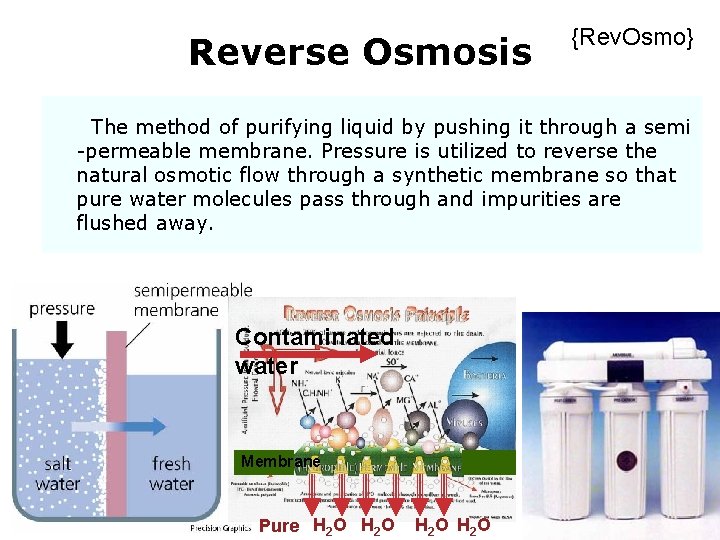
Reverse Osmosis {Rev. Osmo} The method of purifying liquid by pushing it through a semi -permeable membrane. Pressure is utilized to reverse the natural osmotic flow through a synthetic membrane so that pure water molecules pass through and impurities are flushed away. Contaminated water Membrane Solutions Pure H 2 O H 2 O

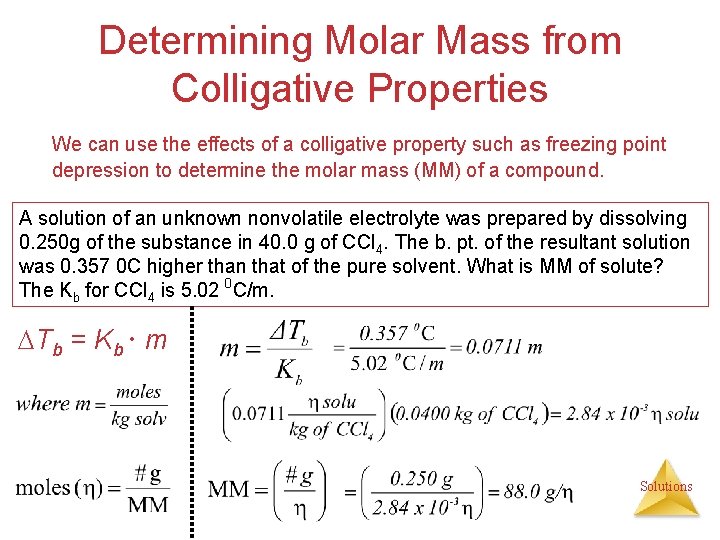
Determining Molar Mass from Colligative Properties We can use the effects of a colligative property such as freezing point depression to determine the molar mass (MM) of a compound. A solution of an unknown nonvolatile electrolyte was prepared by dissolving 0. 250 g of the substance in 40. 0 g of CCl 4. The b. pt. of the resultant solution was 0. 357 0 C higher than that of the pure solvent. What is MM of solute? The Kb for CCl 4 is 5. 02 0 C/m. Tb = Kb m Solutions

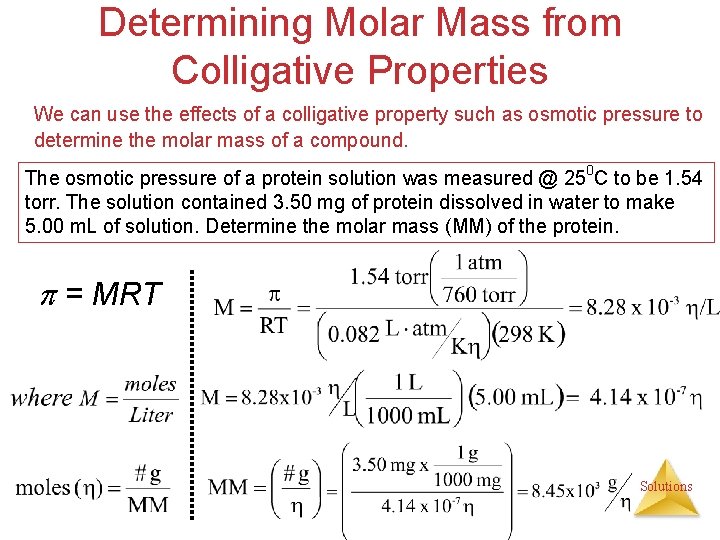
Determining Molar Mass from Colligative Properties We can use the effects of a colligative property such as osmotic pressure to determine the molar mass of a compound. The osmotic pressure of a protein solution was measured @ 250 C to be 1. 54 torr. The solution contained 3. 50 mg of protein dissolved in water to make 5. 00 m. L of solution. Determine the molar mass (MM) of the protein. = MRT Solutions

Colligative Properties & Molar Mass ØVapor pressure lowering: PA = XA P A = ØBoiling point elevation: Tb = Kb m = Kb ØFreezing point depression: Tf = Kf m = Kf ØOsmotic pressure: = MRT = RT Solutions

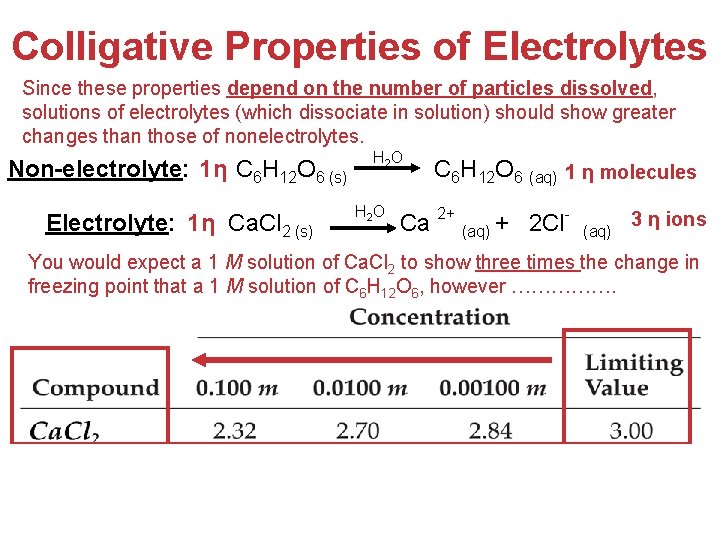
Colligative Properties of Electrolytes Since these properties depend on the number of particles dissolved, solutions of electrolytes (which dissociate in solution) should show greater changes than those of nonelectrolytes. Non-electrolyte: 1η C 6 H 12 O 6 (s) Electrolyte: 1η Ca. Cl 2 (s) H 2 O C 6 H 12 O 6 (aq) 1 η molecules Ca 2+ (aq) + 2 Cl- (aq) 3 η ions You would expect a 1 M solution of Ca. Cl 2 to show three times the change in freezing point that a 1 M solution of C 6 H 12 O 6, however ……………. Solutions

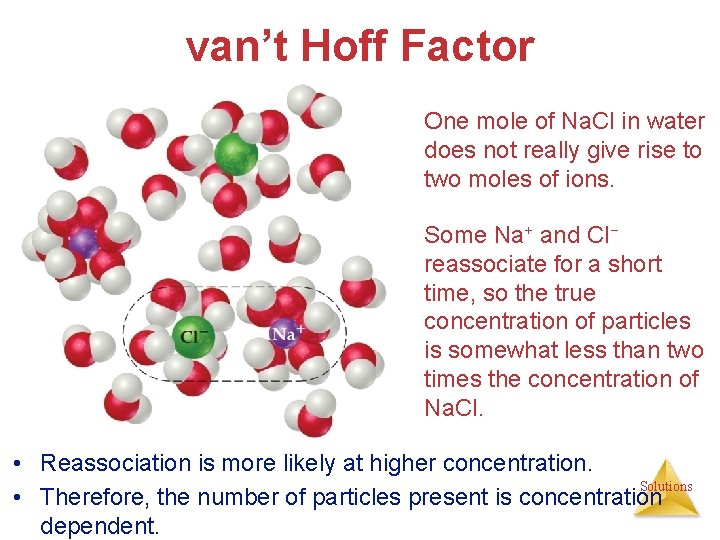
van’t Hoff Factor One mole of Na. Cl in water does not really give rise to two moles of ions. Some Na+ and Cl− reassociate for a short time, so the true concentration of particles is somewhat less than two times the concentration of Na. Cl. • Reassociation is more likely at higher concentration. Solutions • Therefore, the number of particles present is concentration dependent.

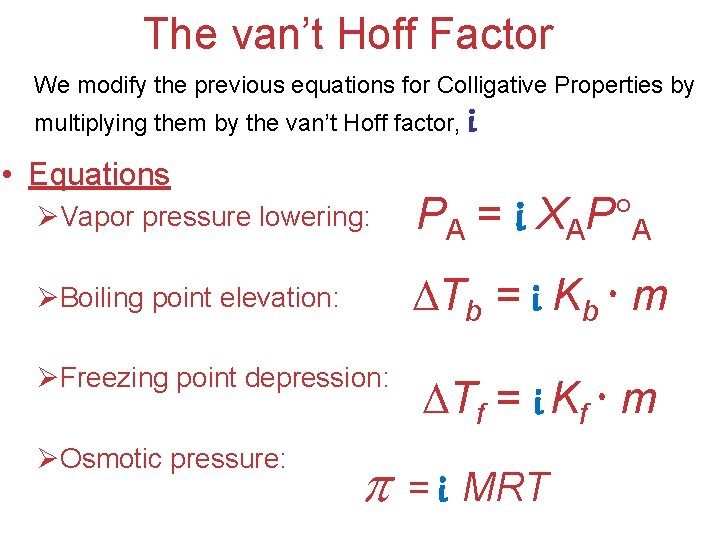
The van’t Hoff Factor We modify the previous equations for Colligative Properties by multiplying them by the van’t Hoff factor, • Equations i ØVapor pressure lowering: PA = i XAP A ØBoiling point elevation: Tb = i Kb m ØFreezing point depression: ØOsmotic pressure: Tf = i Kf m = i MRT Solutions

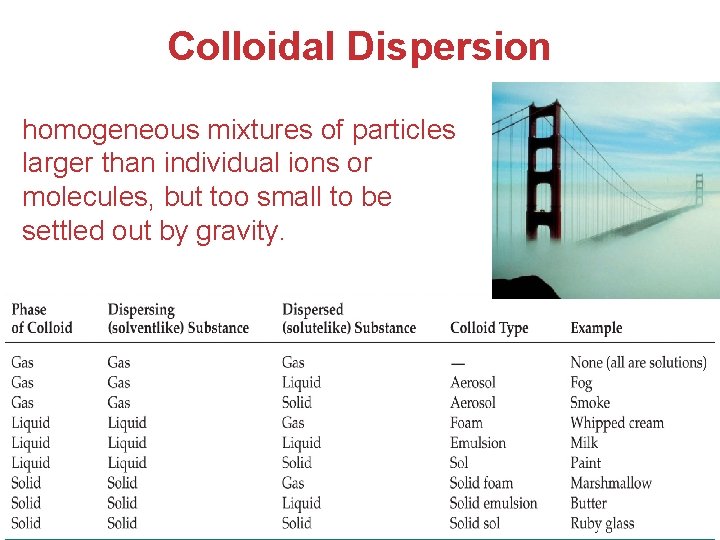
Colloidal Dispersion homogeneous mixtures of particles larger than individual ions or molecules, but too small to be settled out by gravity. Solutions

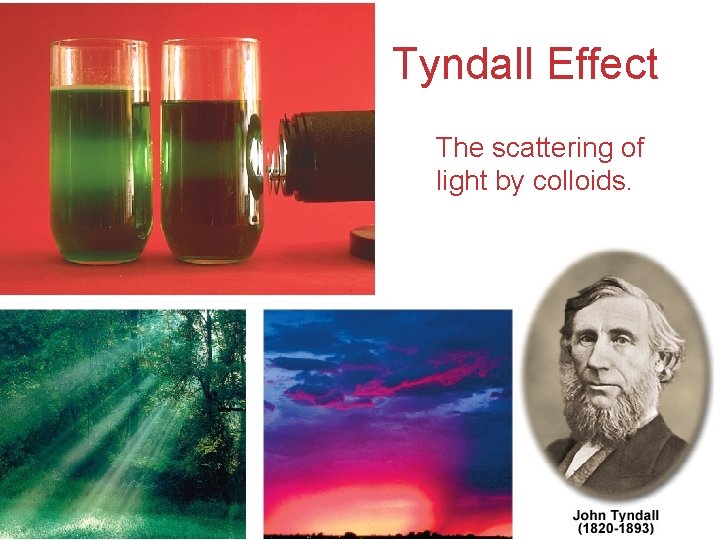
Tyndall Effect The scattering of light by colloids. Solutions

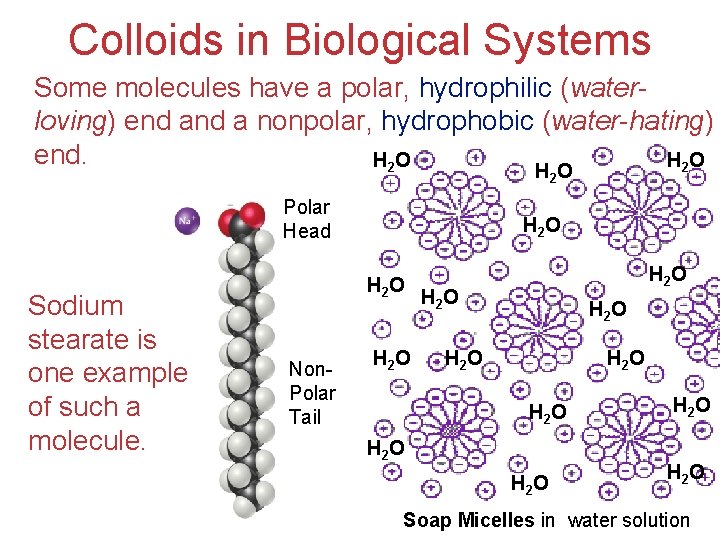
Colloids in Biological Systems Some molecules have a polar, hydrophilic (waterloving) end a nonpolar, hydrophobic (water-hating) end. H 2 O HO 2 Polar Head Sodium stearate is one example of such a molecule. H 2 O Non. Polar Tail H 2 O H 2 O H 2 O Solutions Soap Micelles in water solution

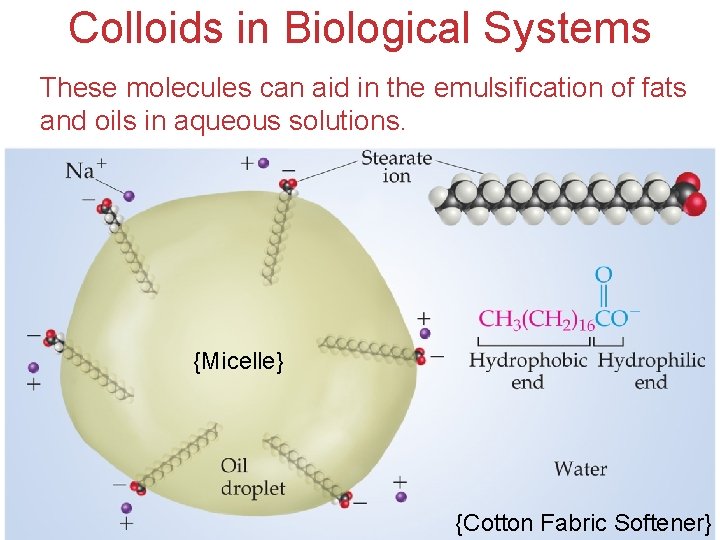
Colloids in Biological Systems These molecules can aid in the emulsification of fats and oils in aqueous solutions. {Micelle} Solutions {Cotton Fabric Softener}

Suspension: heterogenous fluid containing solid particles that are sufficiently large for sedimentation. Usually they must be larger than 1 micrometre. [1] The internal phase (solid) is dispersed throughout the external phase (fluid) through mechanical agitation, with the use of certain excipients or suspending agents. Solutions

Solutions

Solutions

Solutions

Solutions

Solutions

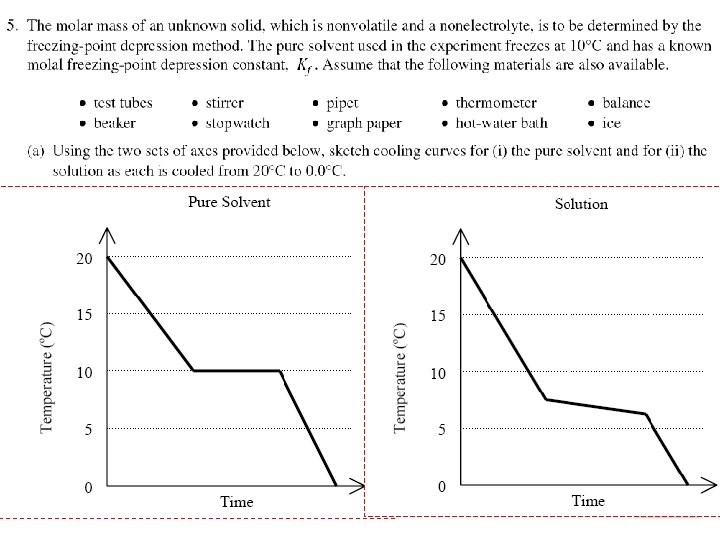
2006 (A) Solutions

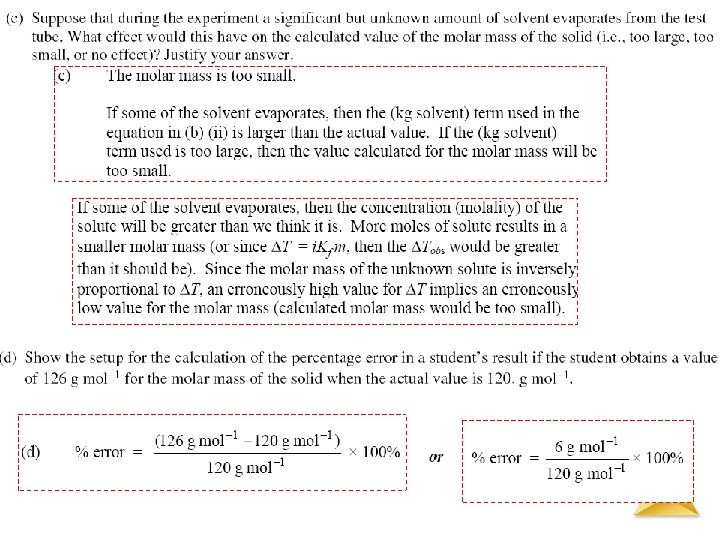
Solutions

Solutions

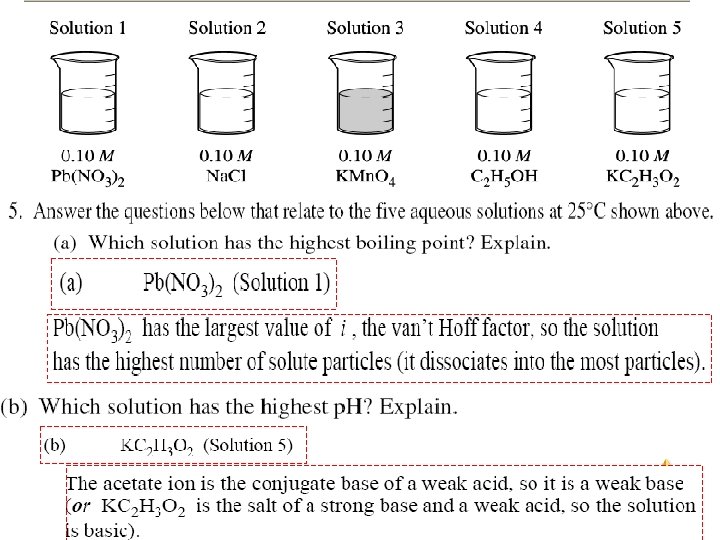
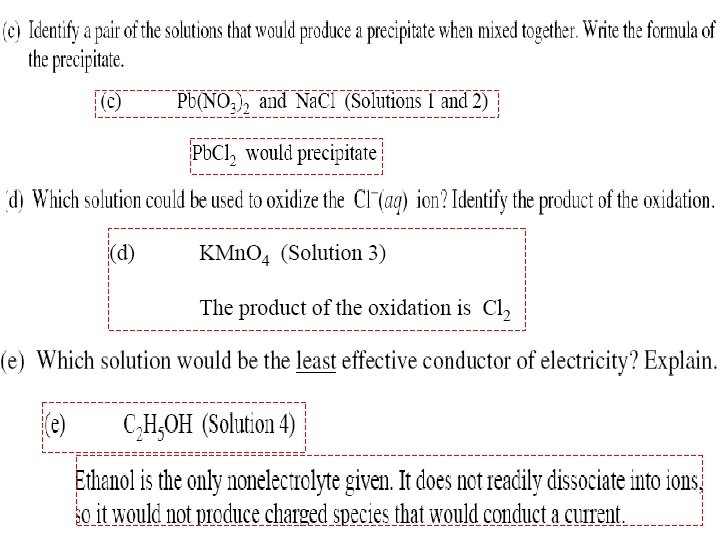
Solutions

2003 A Solutions

Energetics of Solutions Heats of Solution ( Hsoln) Recyclable Hot Pack: Super Saturated Solution of Sodium Acetate Na + (aq) + C 2 H 3 O 2 _ crystallization (aq) heating Na. C 2 H 3 O 2(s) ∆Hsoln = - 67 k. J/n {Hot. Pack Na Acet} Solutions