Chemistry 139 General Chemistry Prep Chemical Nomenclature 8






































- Slides: 49

Chemistry& 139 General Chemistry Prep

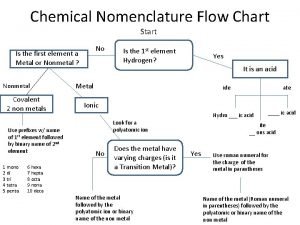
Chemical Nomenclature 8. 1 Classification of Compounds for Nomenclature Purposes 8. 2 Types of Binary Ionic Compounds 8. 3 Nomenclature for Binary Ionic Compounds 8. 4 Chemical Formulas for Polyatomic Ions 8. 5 Nomenclature for Ionic Compounds Containing Polyatomic Ions 8. 6 Nomenclature for Binary Molecular Compounds 8. 7 Nomenclature for Acids 8. 8 Systematic Procedures for Using Nomenclature Rules

Chemical Nomenclature 8. 1 Classification of Compounds for Nomenclature Purposes

Chemical Nomenclature is “the system used to distinguish compounds from each other and the rules used to devise their names” We now use rules developed by the IUPAC to devise names for compounds. Many common names continue to also be used e. g. water.

Ionic compounds are named according to different rules than molecular compounds. Typically: 1. Ionic compounds result from the combination of a metal with one or more nonmetals 2. Compounds resulting from combinations of a nonmetal with a other nonmetals results in molecular compounds. Na. Cl Ba. Cl 2 Mg. O NO 2 CH 4 ionic molecular

Chemical Nomenclature 8. 1 Classification of Compounds for Nomenclature Purposes IUPAC nomenclature rules allow us to devise names to distinguish compounds. Common names are also used for some compounds. Different rules are used to name ionic compounds than for molecular compounds. Ionic compounds are formed when a metal compounds with one or more nonmetals. Molecular compounds form when nonmetals combine.

Chemical Nomenclature 8. 2 Types of Binary Ionic Compounds

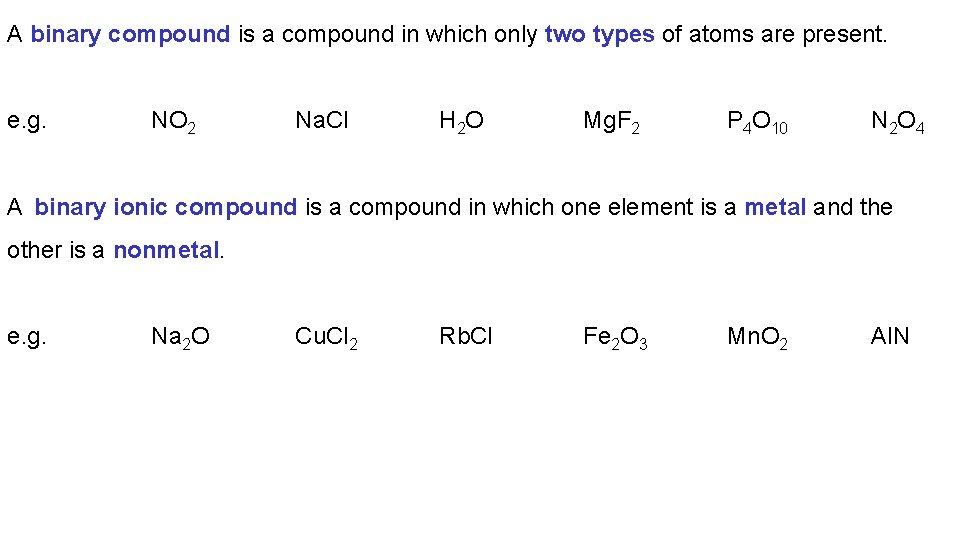
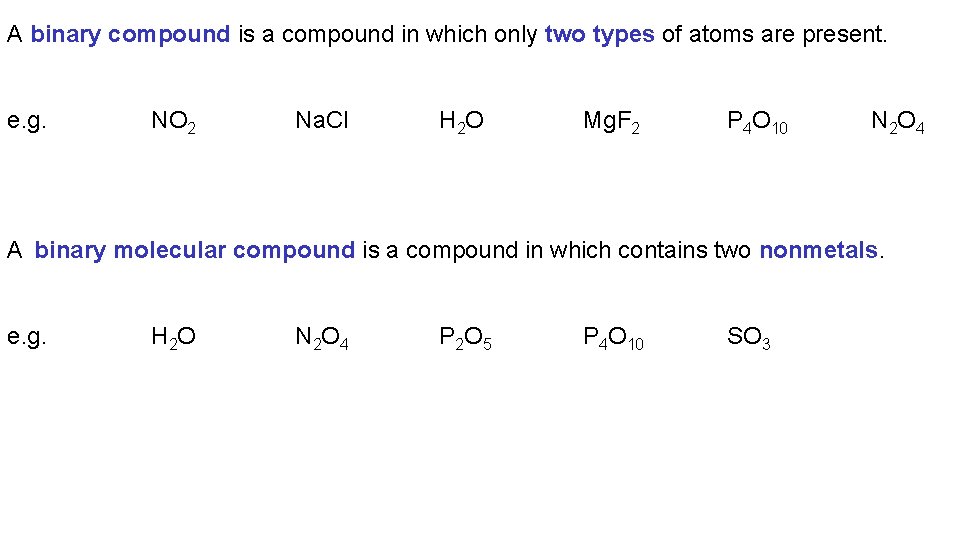
A binary compound is a compound in which only two types of atoms are present. e. g. NO 2 Na. Cl H 2 O Mg. F 2 P 4 O 10 N 2 O 4 A binary ionic compound is a compound in which one element is a metal and the other is a nonmetal. e. g. Na 2 O Cu. Cl 2 Rb. Cl Fe 2 O 3 Mn. O 2 Al. N

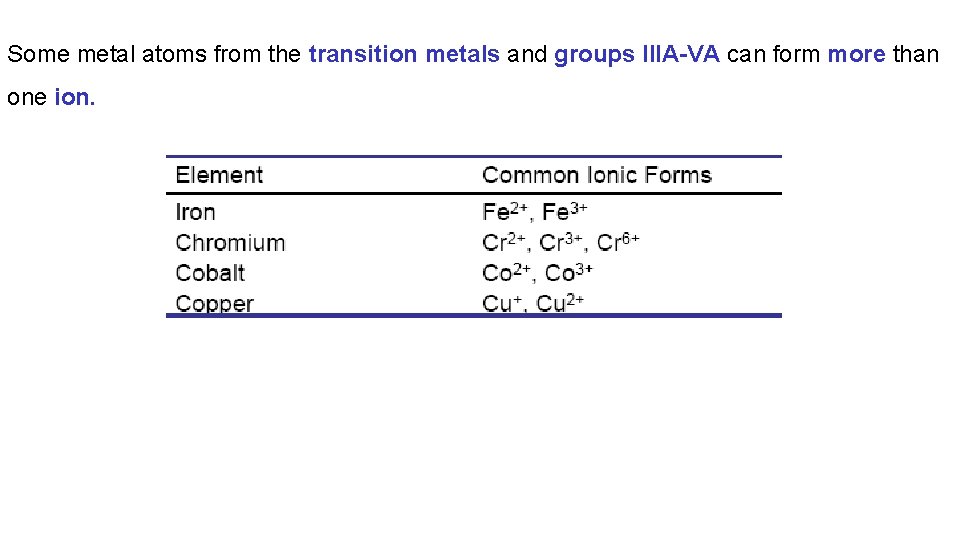
Binary ionic compounds can be grouped into two types: 1. Fixed-charge binary ionic compounds contain a metal that can only form one type of positive ion (group IA, group IIA, Al, Ga, Ag, Zn, Cd) 2. Variable-charge binary ionic compounds contain a metal that can form more than one type of positive ion (anything that is not in the list above).

Some metal atoms from the transition metals and groups IIIA-VA can form more than one ion.

Silver, Aluminum, Gallium, Zinc and Cadmium form only one ion: e. g. Ag+, Al 3+, Ga 3+, Cd 2+, Zn 2+

Chemical Nomenclature 8. 2 Types of Binary Ionic Compounds Binary ionic compounds contain a metal and a nonmetal. They may be divided into two classes; those containing a fixed charge metal ion (group IA, group IIA, Al, Ga, Cd, Zn, Ag) or a variable charge metal ion.

Chemical Nomenclature 8. 3 Nomenclature for Binary Ionic Compounds

We write the formula of a monatomic ion by first writing the element symbol followed by the charge of the ion as a superscript. e. g. Li+, Mg 2+, Al 3+, Fe 2+, Cu 2+, Cl-, S 2 -, O 2 -, etc Fixed-charge metal ions are named by giving the name of the parent metal atom and adding the word ion. e. g. Li+ ≡ lithium ion, Mg 2+ ≡ magnesium ion

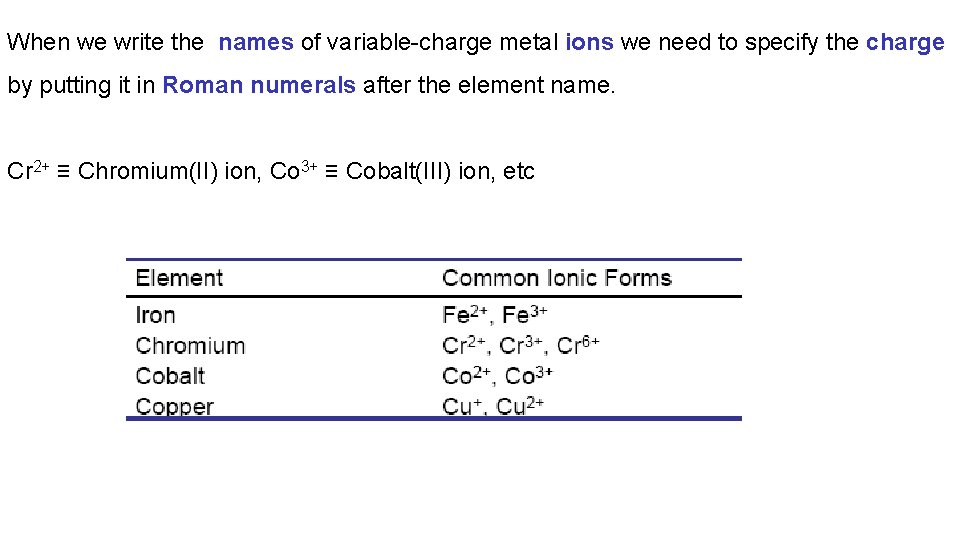
When we write the names of variable-charge metal ions we need to specify the charge by putting it in Roman numerals after the element name. Cr 2+ ≡ Chromium(II) ion, Co 3+ ≡ Cobalt(III) ion, etc

Sometimes we will see the lowest charged ion named by giving the Latin root followed by the suffix –ous and the highest charged ion is named by giving the Latin root and the suffix –ic. e. g. Cr 2+ = chromous ion Cr 3+ = chromic ion Cu+ = cuprous ion Cu 2+ = cupric ion Fe 2+ = ferrous ion Fe 3+ = ferric ion My Latin is fairly poor (except my pig Latin in which I’m fluent), so I tend not to use this system. BUT you will see it as you move through your chemistry sequence.

Monatomic anions are named by adding the suffix –ide to the “stem name” of the parent non-metal atom and adding the word ion. The stem name is typically the first syllable of the atom name. e. g Br- ≡ bromide ion, Cl- ≡ chloride ion, O 2 - ≡ oxide ion, S 2 - ≡ sulfide ion and P 3 - ≡ phosphide ion.

Binary ionic compounds are named by giving the name of the cation the anion and dropping all occurrences of the word ion. e. g. Fe. O ≡ Iron(II) oxide, Na. Cl ≡ Sodium chloride

Chemical Nomenclature 8. 3 Nomenclature for Binary Ionic Compounds Fixed-charge cations are named by appending the word ion to the name of the parent metal atom. Variable charge cations are named by giving the parent name of the atom, the charge of the ion in parenthesis followed by the word ion. Monatomic anions are named by giving the stem name of the parent atom, appended with the suffix –ide followed by the word ion. Binary ionic compounds are named by giving the name of the cation followed by the name of the anion and eliminating all occurrences of the word ion.

Chemical Nomenclature 8. 4 Chemical Formulas for Polyatomic Ions

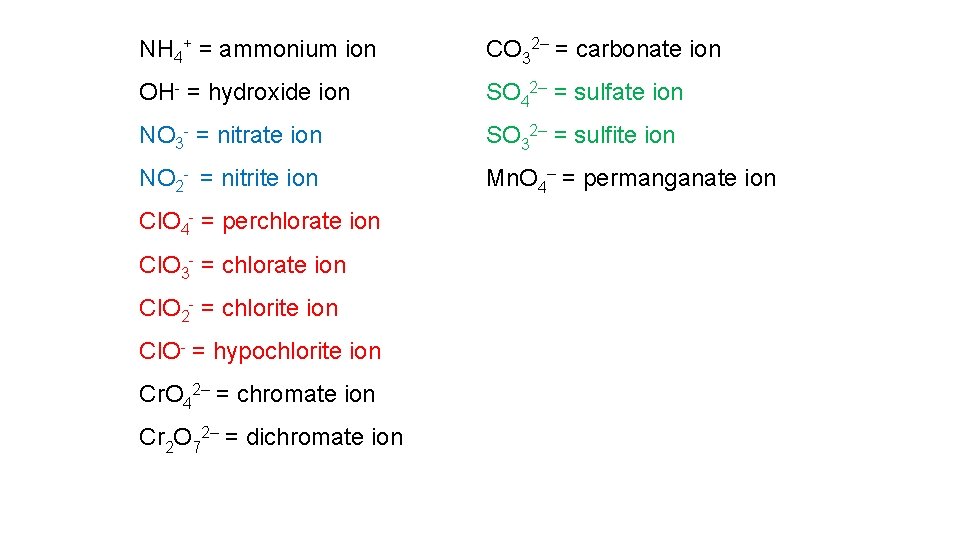
A polyatomic ion is formed from a group of atoms (held together by covalent bonds) that has lost or gained one or more electrons. The common polyatomic cations are shown below. Polyatomic cations NH 4+ = ammonium ion Hg 22+ = mercury(I) ion H 3 O+ = hydronium ion

There are many more polyatomic anions: Cr. O 42– = chromate ion CO 32– = carbonate ion Cr 2 O 72– = dichromate ion HCO 3– = hydrogen carbonate ion SO 42– = sulfate ion Mn. O 4– = permanganate ion SO 32– = sulfite ion

Most polyatomic anions are oxo-anions containing a non-metal covalently bound to one or more oxygen atoms. Frequently a non-metal atom will form several oxo-anions. e. g. Chlorine forms a “family” of four oxo-anions. Cl. O– = hypochlorite ion Cl. O 2– = chlorite ion Cl. O 3– = chlorate ion Cl. O 4– = perchlorate ion

Fortunately there are some rules to help us remember the names of members of “families” of oxo-anions. With two oxoanions in the family: • The ion with the most O atoms takes the nonmetal root and the suffix –ate • The ion with the least O atoms takes the nonmetal root and the suffix –ite e. g. NO 3 - = nitrate ion NO 2 - = nitrite ion

With four oxoanions in the family: e. g. Cl. O 4 -, Cl. O 3 -, Cl. O 2 -, Cl. O- • The ion with the most O atoms has the prefix per-, the nonmetal root, and the suffix –ate e. g. Cl. O 4 - = perchlorate ion • The ion with one fewer O atoms takes the nonmetal root and the suffix ‑ate e. g. Cl. O 3 - = chlorate ion

• The ion with two fewer O atoms takes the nonmetal root and the suffix ‑ite e. g. Cl. O 2 - = chlorite ion • The ion with the least O atoms has the prefix hypo-, the nonmetal root, and the suffix –ite e. g. Cl. O- = chlorite ion • For a nonmetal with the root X the sequence of names in order of decreasing oxygen content would be. per-X-ate X-ite hypo-X-ite

NH 4+ = ammonium ion CO 32– = carbonate ion OH- = hydroxide ion SO 42– = sulfate ion NO 3 - = nitrate ion SO 32– = sulfite ion NO 2 - = nitrite ion Mn. O 4– = permanganate ion Cl. O 4 - = perchlorate ion Cl. O 3 - = chlorate ion Cl. O 2 - = chlorite ion Cl. O- = hypochlorite ion Cr. O 42– = chromate ion Cr 2 O 72– = dichromate ion

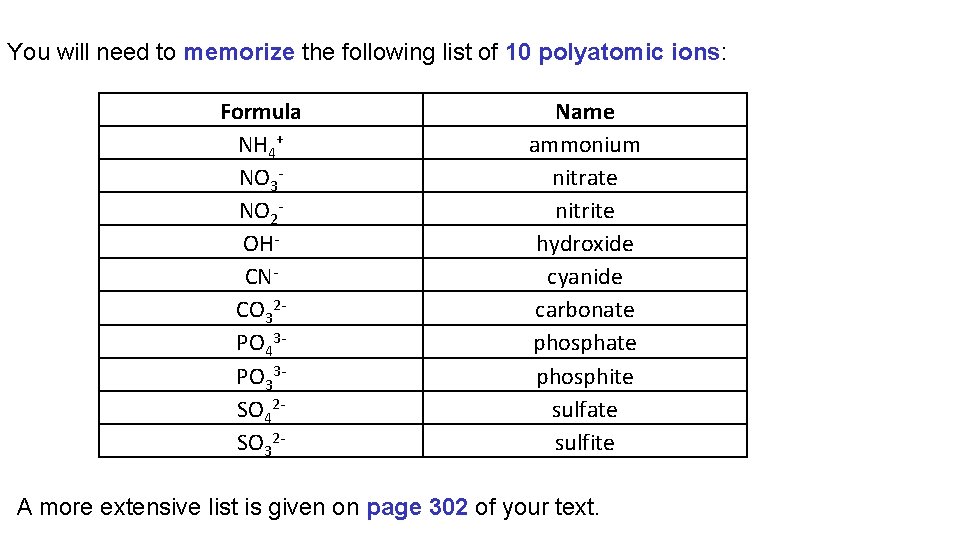
You will need to memorize the following list of 10 polyatomic ions: Formula NH 4+ NO 3 NO 2 OHCNCO 32 PO 43 PO 33 SO 42 SO 32 - Name ammonium nitrate nitrite hydroxide cyanide carbonate phosphite sulfate sulfite A more extensive list is given on page 302 of your text.

Chemical Nomenclature 8. 4 Chemical Formulas for Polyatomic Ions Polyatomic ions are groups of atoms that have lost or gained electrons. There are no systematic names for polyatomic ions. There are many more negative polyatomic ions than positive polyatomic ions.

Chemical Nomenclature 8. 5 Nomenclature for Ionic Compounds Containing Polyatomic Ions

When writing the formula of an ionic compound that contains more than one of a polyatomic ion we put the ion in brackets then add the subscript. e. g. (NH 4)2 S, Ca(NO 2)2, Fe 3(PO 4)2 The cation is always written first. To name the compound write then name of cation followed by the name of the anion and eliminate all occurrences of the word ion.

Chemical Nomenclature 8. 5 Nomenclature for Ionic Compounds Containing Polyatomic Ions Parenthesis are used to indicate more than one of a polyatomic ion is present in the formula unit of an ionic compound. The names of compounds containing polyatomic ions are produced by writing the name of the cation, followed by the name of the anion and eliminating all occurrences of the word ion

Chemical Nomenclature 8. 6 Nomenclature for Binary Molecular Compounds

A binary compound is a compound in which only two types of atoms are present. e. g. NO 2 Na. Cl H 2 O Mg. F 2 P 4 O 10 N 2 O 4 A binary molecular compound is a compound in which contains two nonmetals. e. g. H 2 O N 2 O 4 P 2 O 5 P 4 O 10 SO 3

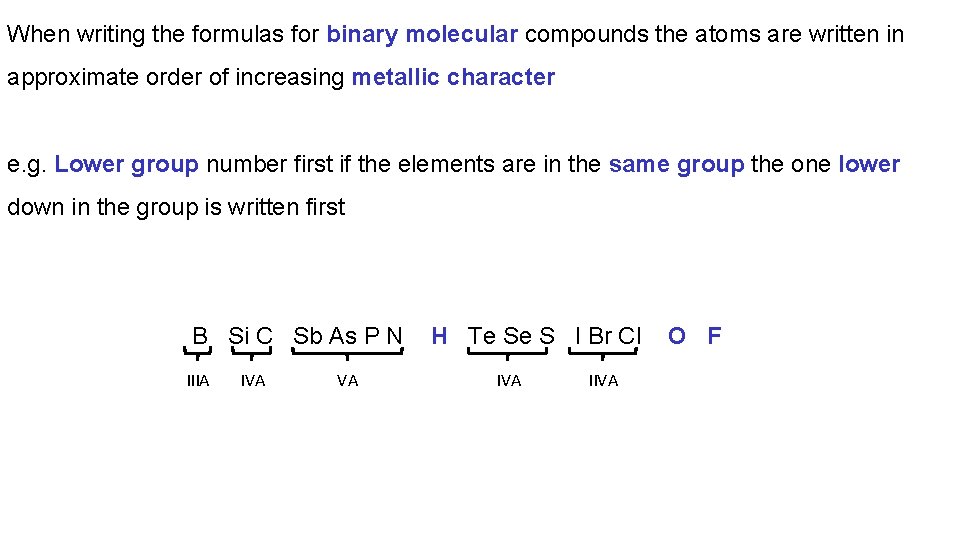
When writing the formulas for binary molecular compounds the atoms are written in approximate order of increasing metallic character e. g. Lower group number first if the elements are in the same group the one lower down in the group is written first B Si C Sb As P N IIIA IVA VA H Te Se S I Br Cl IVA IIVA O F

Two nonmetals can combine to form a binary covalent compound. The rules for naming binary ionic compounds are: a. The name of the first element in the formula is given unaltered. b. The second element in the formula is named with its root and the suffix –ide d. Greek prefixes are used to indicate the number of atoms of each element present.

You will need to memorize the first ten Greek numerical prefixes. Number Greek Prefix 1 mono 2 di 3 tri 4 tetra 5 penta 6 hexa 7 hepta 8 octa 9 nona 10 deca- Memorizing these is not a total waste of time. You can impress friends at parties “I just drank a hexa-pack” sounds quite sophisticated.

If there is one of the first element do not give it a prefix. e. g. CS 2 = carbon disulfide CO 2 = carbon dioxide CO = carbon monoxide PCl 5 = phosphorus pentachloride NO 2 = nitrogen dioxide The formula of a compound follows the same sequence of elements as the name.

If hydrogen is the first nonmetal listed in the formula name the compound as though it were an ionic compound (Greek prefixes are omitted) e. g. Hydrogen chloride (HCl), hydrogen fluoride (HF), hydrogen sulfide (H 2 S) etc.

Chemical Nomenclature 8. 6 Nomenclature for Binary Molecular Compounds Binary ionic compounds are named by given the name of the first element that appears in the formula followed by the stem name of the second element to which the suffix –ide has been appended, the number of each atom in the formula is then indicated using Greek prefixes. If there is one of the first atom the prefix mono is omitted. In compounds where hydrogen is the first atom in the formula Greek prefixes are omitted.

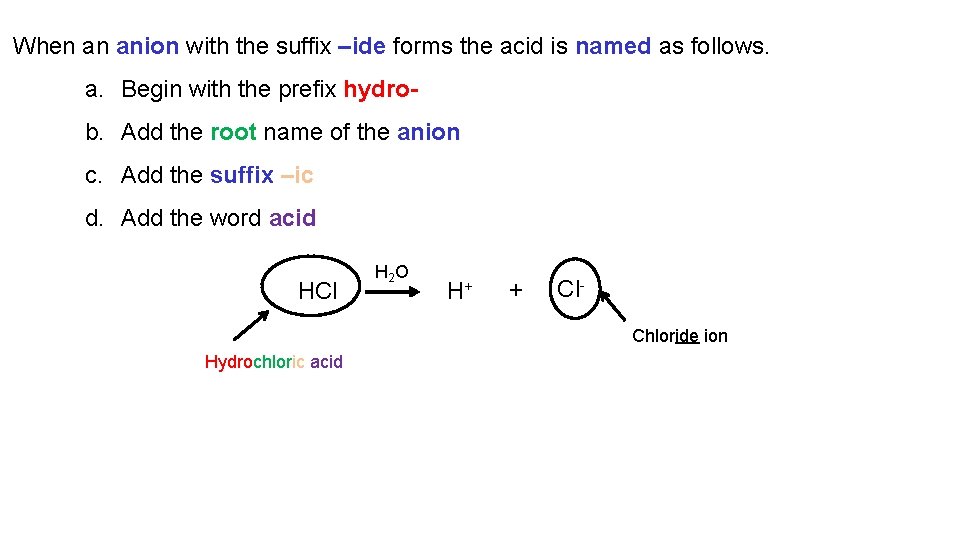
Chemical Nomenclature 8. 7 Nomenclature for Acids

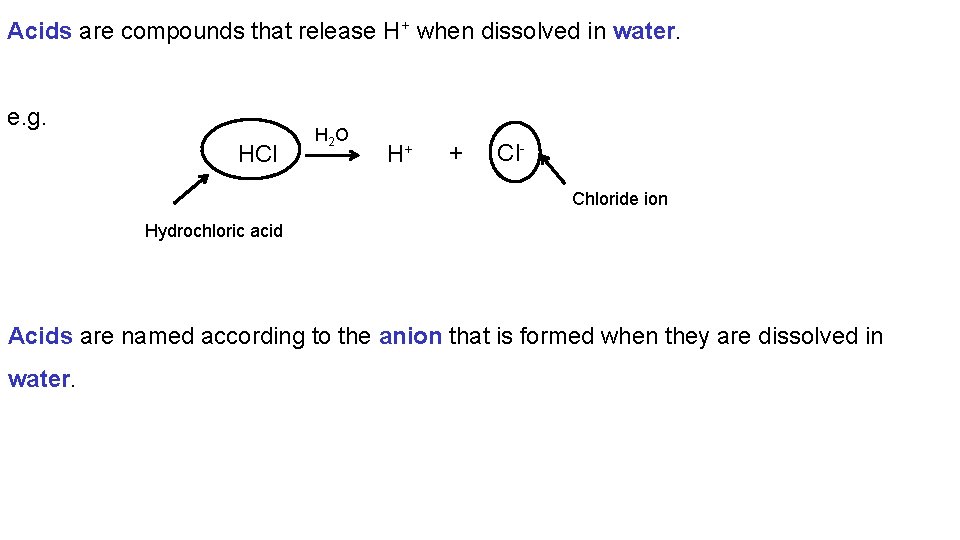
Acids are compounds that release H+ when dissolved in water. e. g. HCl H 2 O H+ + Cl. Chloride ion Hydrochloric acid Acids are named according to the anion that is formed when they are dissolved in water.

When an anion with the suffix –ide forms the acid is named as follows. a. Begin with the prefix hydrob. Add the root name of the anion c. Add the suffix –ic d. Add the word acid HCl H 2 O H+ + Cl. Chloride ion Hydrochloric acid

For molecular compounds containing hydrogen and a nonmetal other than oxygen the compound is only named as an acid when dissolved in water. HF = hydrogen fluoride HF(aq) = hydrofluoric acid HCl = hydrogen chloride HCl(aq) = hydrochloric acid HBr = hydrogen bromide HBr(aq) = hydrobromic acid

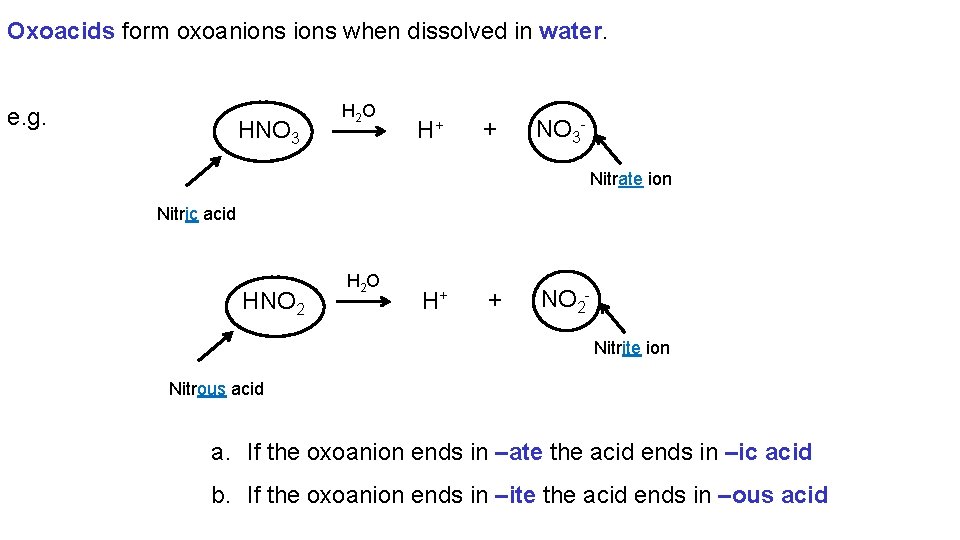
Oxoacids form oxoanions when dissolved in water. e. g. HNO 3 H 2 O H+ + NO 3 Nitrate ion Nitric acid HNO 2 H 2 O H+ + NO 2 Nitrite ion Nitrous acid a. If the oxoanion ends in –ate the acid ends in –ic acid b. If the oxoanion ends in –ite the acid ends in –ous acid

Chemical Nomenclature 8. 7 Nomenclature for Acids are molecular compounds that when dissolved in water form hydrogen ions and an anion. Acids are named according to the anion formed when they are dissolved in water. Acids which produced anions with names of the form X–ide and are named as “Hydro-Xic acid”. Acids which produce anions with names of the form X–ate are named as “X-ic acid”. Acids which produce anions with names of the form X–ite are named as “X-ous acids. ”

Chemical Nomenclature 8. 8 Systematic Procedures for Using Nomenclature Rules

In naming compounds it is best to use the following systematic procedure: 1. Decide if the compound is ionic or molecular (look for a metal of NH 4+) 2. If ionic name according to the rules for ionic compounds 3. If molecular decide if an acid or non-acidic and name accordingly.

Chemical Nomenclature 8. 8 Systematic Procedures for Using Nomenclature Rules Nomenclature rules are used to name chemical compounds. Different rules exist for ionic compounds, molecular compounds and acids. The first task in deciding the name of a compound is to identify what class of compound it is. If the compound contains a polyatomic ion (or forms one when dissolved in water) then the name of the polyatomic ion will need to have been memorized.
 General chemistry nomenclature
General chemistry nomenclature Chemical nomenclature
Chemical nomenclature Priority of functional groups
Priority of functional groups Organic chemistry nomenclature
Organic chemistry nomenclature Organic chemistry nomenclature
Organic chemistry nomenclature Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Empirical formula pogil
Empirical formula pogil Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 1 chemical changes
Section 1 chemical changes Love formula in chemistry
Love formula in chemistry Are kc and kp equal
Are kc and kp equal Five chemical changes
Five chemical changes 5 general types of chemical reactions
5 general types of chemical reactions What are the five general types of chemical reactions
What are the five general types of chemical reactions Planos en cinematografia
Planos en cinematografia Where did general lee surrender to general grant?
Where did general lee surrender to general grant? Molekülerite nedir
Molekülerite nedir Pixl knowit gcse chemistry quantitative
Pixl knowit gcse chemistry quantitative Chemistry grade 11 unit 4 chemical kinetics
Chemistry grade 11 unit 4 chemical kinetics Chapter 8 review chemical equations and reactions section 2
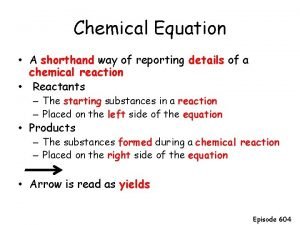
Chapter 8 review chemical equations and reactions section 2 A chemist shorthand
A chemist shorthand Chapter 9 chemical names and formulas answer key
Chapter 9 chemical names and formulas answer key Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Equilibrium
Equilibrium Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Section 139(9)
Section 139(9) Psalm 139:13–24
Psalm 139:13–24 Tu fuiste quien formo todo mi cuerpo
Tu fuiste quien formo todo mi cuerpo Salmos 139:23-24
Salmos 139:23-24 Article 139
Article 139 Section 139(7) of the constitution
Section 139(7) of the constitution Tu mi scruti e mi conosci salmo
Tu mi scruti e mi conosci salmo Sal 139
Sal 139 Lied 139
Lied 139 Cu tine nu sunt singur versuri
Cu tine nu sunt singur versuri Psalm 139:1-24
Psalm 139:1-24 The wheel sverige
The wheel sverige Psaume 139 en creole
Psaume 139 en creole Psalm 50 gnt
Psalm 50 gnt Psalm 145:1-21
Psalm 145:1-21 Psalm 139 16
Psalm 139 16 Ley orgánica de las fuerzas armadas 2013
Ley orgánica de las fuerzas armadas 2013 Rpe meaning
Rpe meaning 139
139 Salmo 139:7-10
Salmo 139:7-10 Transportation research board
Transportation research board ü
ü Psalm 1 gnt
Psalm 1 gnt 139 x 3
139 x 3