Organic Qualitative Analysis Determination of functional groups present



- Slides: 9

Organic Qualitative Analysis Determination of functional groups present in an unknown organic compound by their characteristic chemical reactions. You will be introduced to qualitative tests that indicate the presence (or absence if negative) of aryl and alkyl halides, unsaturated compounds (alkenes and alkynes), aromatics, alcohols and alkanes.

Solubility Tests (In water or sulfuric acid) Carefully place 1 m. L of the solvent (water or conc. H 2 SO 4) in a test tube. Add a drop of your unknown, and gently tap the test tube and swirl the solution. Solubility in Water: Mixing of the solutions indicates solubility. Separation into two layers indicates insolubility. Water solubility suggests a relatively low M. W. (< C 4) alcohol. Solubility in conc. H 2 SO 4: Sulfuric acid will solubilize alkenes (often with color change). It will not solubilize alkanes, alkyl halides or aromatics.

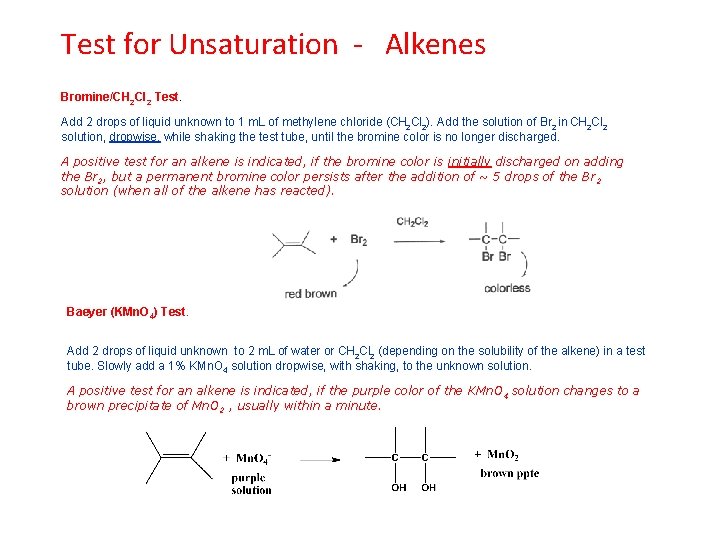
Test for Unsaturation - Alkenes Bromine/CH 2 Cl 2 Test. Add 2 drops of liquid unknown to 1 m. L of methylene chloride (CH 2 Cl 2). Add the solution of Br 2 in CH 2 Cl 2 solution, dropwise, while shaking the test tube, until the bromine color is no longer discharged. A positive test for an alkene is indicated, if the bromine color is initially discharged on adding the Br 2, but a permanent bromine color persists after the addition of ~ 5 drops of the Br 2 solution (when all of the alkene has reacted). Baeyer (KMn. O 4) Test. Add 2 drops of liquid unknown to 2 m. L of water or CH 2 Cl 2 (depending on the solubility of the alkene) in a test tube. Slowly add a 1% KMn. O 4 solution dropwise, with shaking, to the unknown solution. A positive test for an alkene is indicated, if the purple color of the KMn. O 4 solution changes to a brown precipitate of Mn. O 2 , usually within a minute.

Test for Unsaturation Aromatic compounds or Polyalkenes Ignition Test Place a small amount of the compound on a spatula and place in a hot Bunsen flame. A sooty yellow flame will indicate an aromatic compound or one with a high degree of unsaturation

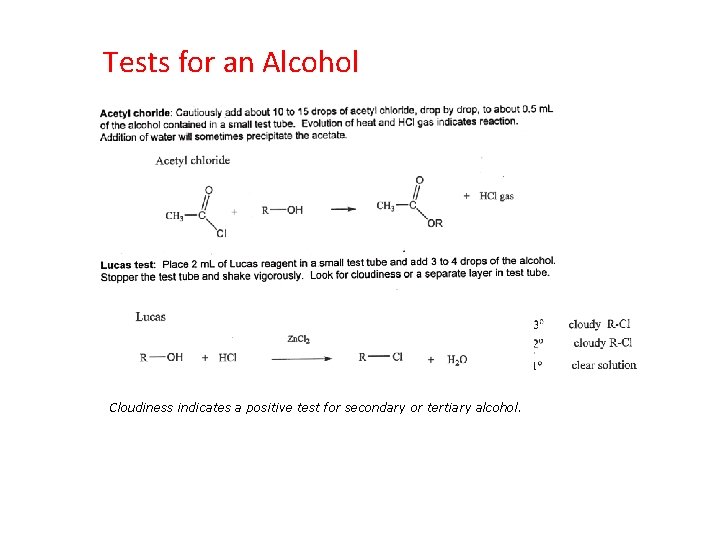
Tests for an Alcohol Chromic Acid Test Add 1 drop of liquid unknown to 1 m. L of acetone in a test tube. Add 1 drop of chromic acid reagent. A change from yellow-orange chromic acid to green Cr 2 O 3 (on reduction of the chromium(VI) to chromium(III))indicates a primary or secondary alcohol.

Tests for an Alcohol Cloudiness indicates a positive test for secondary or tertiary alcohol.

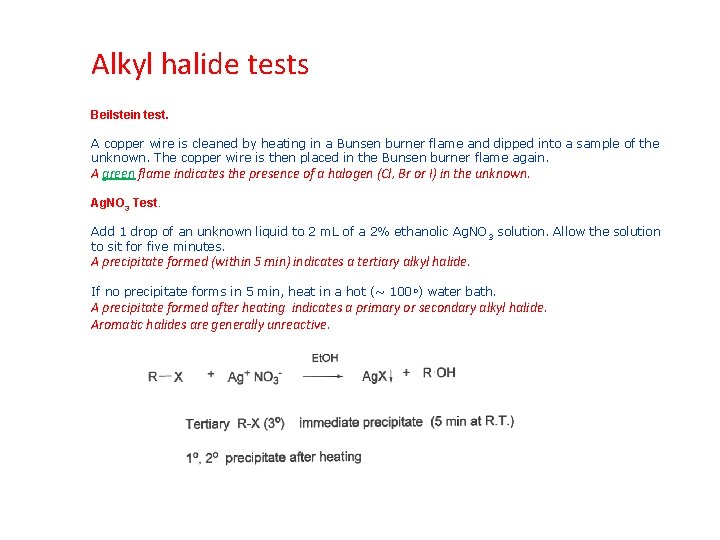
Alkyl halide tests Beilstein test. A copper wire is cleaned by heating in a Bunsen burner flame and dipped into a sample of the unknown. The copper wire is then placed in the Bunsen burner flame again. A green flame indicates the presence of a halogen (Cl, Br or I) in the unknown. Ag. NO 3 Test. Add 1 drop of an unknown liquid to 2 m. L of a 2% ethanolic Ag. NO 3 solution. Allow the solution to sit for five minutes. A precipitate formed (within 5 min) indicates a tertiary alkyl halide. If no precipitate forms in 5 min, heat in a hot (~ 100 o) water bath. A precipitate formed after heating indicates a primary or secondary alkyl halide. Aromatic halides are generally unreactive.

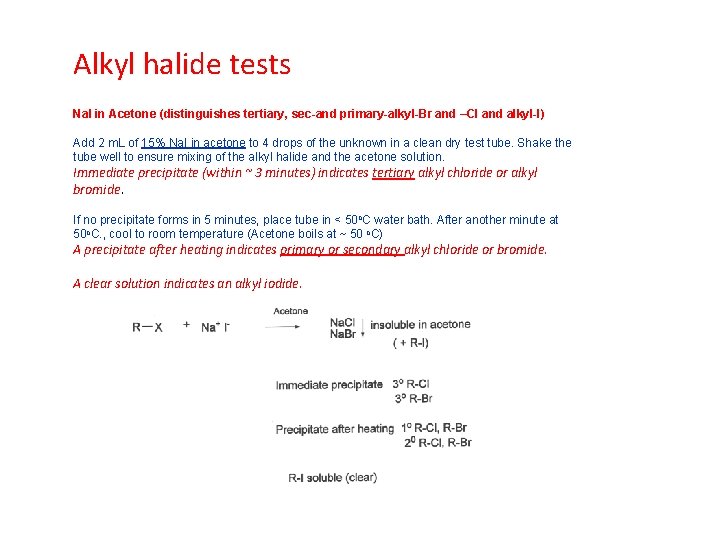
Alkyl halide tests Na. I in Acetone (distinguishes tertiary, sec-and primary-alkyl-Br and –Cl and alkyl-I) Add 2 m. L of 15% Na. I in acetone to 4 drops of the unknown in a clean dry test tube. Shake the tube well to ensure mixing of the alkyl halide and the acetone solution. Immediate precipitate (within ~ 3 minutes) indicates tertiary alkyl chloride or alkyl bromide. If no precipitate forms in 5 minutes, place tube in < 50 o. C water bath. After another minute at 50 o. C. , cool to room temperature (Acetone boils at ~ 50 o. C) A precipitate after heating indicates primary or secondary alkyl chloride or bromide. A clear solution indicates an alkyl iodide.

Infrared Spectrum and Refractive Index q When you have completed your qualitative analysis tests, measure the refractive index of your unknown. Correct the value observed at room temperature to a corresponding n. D 20 value. q Record an IR spectrum of you unknown. q This is the Qualitative Analysis lab, and your lab report should reflect this. Irrespective of the information obtained from IR and refractive index, your report should indicate the evidence obtained by qualitative analysis (positive and negative) that supports your identification.