Practice Test for CHM 151 final exam part




- Slides: 8

Practice Test for CHM 151 final exam part 2. The symbols in chemistry get in the way of getting to know elements and compounds. • H 2 O is water, true or false? (false) • The formula for water is H 2 O, true or false? (true) • What is the color of Au? (black) • What is the color of the element represented by Au? (gold or yellow) • Symbols make elements all look alike. For example (F, Cl, Br, and I) seem like the same size. However, which is the smallest? (fluorine) The largest? (I) Ca 2+ • Ca and Ca 2+ seem like the same size, however, draw them to show their relative sizes. Ca • O and O 2 - seem like the same size, however, draw them to show their relative sizes • CO 2 appears to have carbon on the left side and 2 oxygen atoms on the right side. Draw CO 2 using spheres for carbon and oxygen. Label them. H O O 2 - O C O • Draw the VSEPR structure of H 2 O. O H Chemistry uses the same symbol for many different things. • In 3 Liters + 2 Liters, what does the “+” mean? (It means to combine the quantities mathematically. ) • In 2 Ag + Cl 2 2 Ag. Cl, what does the “+” mean? (It means the two are mixed but not necessary combined. ) • In Ag+(aq) + Cl- Ag. Cl(s), what does the “+” mean by the Ag? (It means Ag has lost an electron giving it a positive 1 charge) • • • If the heat of a reaction ΔHrxn = +91 k. J, what does the “+” mean? (It means the products of the reaction has gained 91 kilojoules of energy. It is an endothermic reaction. ) If a drawing shows a “+” in a circle , what is that? (That is a proton or the nucleus of hydrogen. ) In “Bring temperature to +25°C. ” the “+” means what? (That means 25 degrees above zero. In “The change of temperature was +5. 5°C. ” what does the “+” mean? (The final temperature was 5°C higher. ) What could “m” mean in chemistry? (milli, meter, or molality (with italicized m). What could “M” mean in chemistry? Moles per liter (molarity), Mega, Molar Mass (with italicized M).

• • • DIMENSIONAL ANALYSIS. Where math classes relied more on solving an equation, chemistry will usually do dimensional analysis. For example, the equation for distance is d=vt (velocity x time). So this says multiply velocity times time and you get distance. In chemistry, you don’t think much about the equation but look at the units. If you see miles per hour and you see hours, you set it up to to get the final units to be a distance. 55 miles 4 hours = 220 miles hour In math, to find t (time), you may solve the equation with algebra to show that t=d/v. So you divide the distance by the speed. In chemistry, you look at the units. When given a distance of 700 meters per second a distance of 2100 meters, you look at the dimensions to tell you how to solve for time. . 1 sec 2100 meters = 3 seconds 700 meters If the speed was in meters per second but distance was 6 miles, you would do more conversions to get the answer to have the right unit. You don’t depend on a formula. You let the dimensions tell you what to do. Dimensional analysis is a series of multiplication problems that often involves multiplying by a rate (e. g. , 1. 6 km per mile). 1 sec 6 miles 1600 meters = 13. 7 seconds 700 meters • 1 mile Some problems are best done with a combination of algebra and dimensional analysis. Concentration x 2 drops of a 4% bleach solution is diluted to 1 quart. What is its new concentration in ppm (mg/Liter)? 4 g 100 m. L 4 g Volume = Concentration x Volume 2 drops 100 m. L 2 drops Liter mg quart = ? ? mg = quart Liter Multiply and divide both sides by the units on the unknown side to isolate unknown (? ) value Look to see what units cancel. If all don’t cancel, add conversion units to cancel all units. 4 g 100 m. L 2 drops Liter 1 m. L milli 1. 057 quarts mg quart 30 drops 0. 001 1 Liter = ? Our answer is 2. 8. If we wrote the original unknown concentration as mg/Liter that means the answer is 2. 8 mg/Liter, which is 2. 8 ppm.

MAKE IT REAL. Because chemistry relies heavily on symbols, it is easy to forget what is represented. When you come across a formula or name for a substance, do a search for images of that substance. • Know your Internet resources: – Google (or something similar) for finding Web articles. – Google (or something similar) for finding images. – www. wikipedia. com for encyclopedic articles. – www. dictionary. com for definitions and pronunciations. – www. webelements. com for information on elements. – http: //www. chemistrycoach. com/tutorial. htm for links to many tutorials. – http: //www. chemtutor. com/ for tutorials. – www. chemistryland. com your instructor’s website with many Power. Point presentations. • Another way to teach yourself about compounds is to build models of them or use chemistry modeling software. One site that has free computer modeling software is www. acdlabs. com. BE SKEPTICAL. It doesn’t help to learn a lot of chemistry and then let yourself get duped by bogus products or stories. To gain awareness of past and present myths and urban legends, visit these sites. – www. snopes. com – http: //hoaxbusters. ciac. org/ – www. greenfacts. org • Be more skeptical of information you find. If the site uses the following tactics, be skeptical: • 1. The use of technobabble (technical descriptions that are meaningless and have no basis in real science) • 2. Fantastic claims (When things sound too good to be true, then they probably aren't true. Fantastic health claims are the most common. ) • 3. Something is being sold (when money is at stake, there is more incentive to be deceptive) • 4. Scare tactics (sites that seem to dwell on danger and fear) • 5. Non-balanced discussion (When only one side is presented, then someone is probably biased and the whole truth isn't told)

GET INSPIRED: • Chemistry (and science in general) is really amazing. You should always look for ways to be inspired and to round out your knowledge of current developments. • Get subscription to science magazines or visit their websites – Discover – Popular Science – Popular Mechanics – Scientific American – New Scientist • Watch DVDs or television programs about science – Discovery channel: Mythbusters, Alien Planet, I Shouldn’t be Alive, etc. – Nova series: The Elegant Universe, World in Balance, The Miracle of Life, The Best Mind since Einstein – The Learning Channel: Honey We’re Killing the Kids. • Visit Science Museums, attend conferences, attend presentations and other events. – Arizona Science Center – American Chemical Society – Presenters at ASU, MCC, or elsewhere in the valley. – Star gazing events. • Job Shadowing – Find someone who is in the career that you are aiming for and spend time with them. • Bring it home – Get a chemistry kit or testing kits. Look into do it yourself projects (making soap, biodiesel, alcohol, slime, etc) – Make your kids “toys” more educational. Microscopes are reasonably priced. Infrared thermometer. – Visit the science kits online stores.

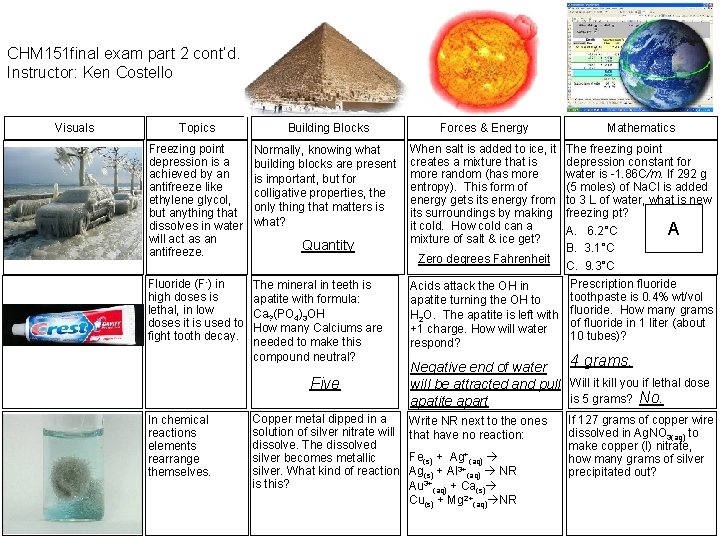
CHM 151 final exam part 2 cont’d. Instructor: Ken Costello Visuals Topics Building Blocks Forces & Energy Freezing point depression is a achieved by an antifreeze like ethylene glycol, but anything that dissolves in water will act as an antifreeze. Normally, knowing what building blocks are present is important, but for colligative properties, the only thing that matters is what? When salt is added to ice, it creates a mixture that is more random (has more entropy). This form of energy gets its energy from its surroundings by making it cold. How cold can a mixture of salt & ice get? Fluoride (F-) in high doses is lethal, in low doses it is used to fight tooth decay. The mineral in teeth is apatite with formula: Ca? (PO 4)3 OH How many Calciums are needed to make this compound neutral? Quantity Five In chemical reactions elements rearrange themselves. Copper metal dipped in a solution of silver nitrate will dissolve. The dissolved silver becomes metallic silver. What kind of reaction is this? Mathematics The freezing point depression constant for water is -1. 86 C/m. If 292 g (5 moles) of Na. Cl is added to 3 L of water, what is new freezing pt? A A. 6. 2°C B. 3. 1°C Zero degrees Fahrenheit C. 9. 3°C Prescription fluoride Acids attack the OH in toothpaste is 0. 4% wt/vol apatite turning the OH to H 2 O. The apatite is left with fluoride. How many grams of fluoride in 1 liter (about +1 charge. How will water 10 tubes)? respond? 4 grams. Negative end of water will be attracted and pull Will it kill you if lethal dose is 5 grams? No. apatite apart Write NR next to the ones that have no reaction: Fe(s) + Ag+(aq) Ag(s) + Al 3+(aq) NR Au 3+(aq) + Ca(s) Cu(s) + Mg 2+(aq) NR If 127 grams of copper wire dissolved in Ag. NO 3(aq) to make copper (I) nitrate, how many grams of silver precipitated out?

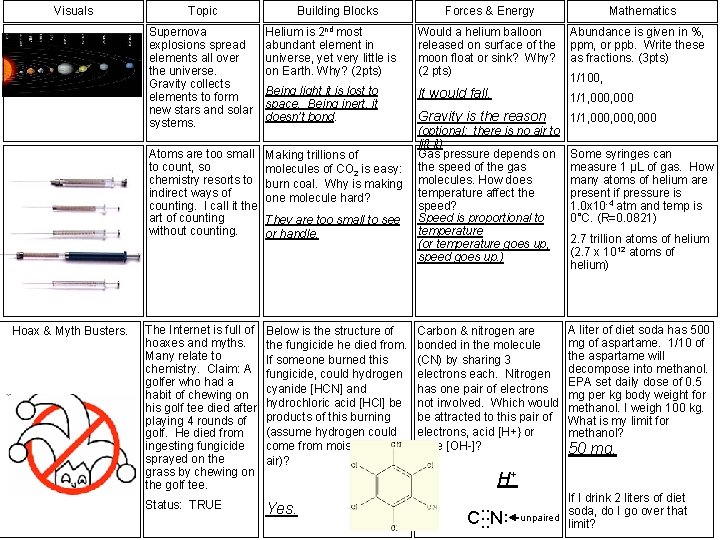
Visuals Topic Building Blocks Supernovas are responsible for creating the 100+ elements we know of. Looking at the atomic weights, what element needs to fuse with oxygen to make silicon? Carbon. Forces & Energy Millions of degrees are needed for elements to fuse together. Why? To overcome electron to electron & proton to proton repulsions Mathematics The remains of a supernova are neutrons at density of 454 billion g/cm 3. A teaspoon (5 cc) of this weighs how many lbs. ? A. 17 battleships (5 billion lbs) B. 9 locomotives (1 million lbs) C. 454 billion lbs. A D. 1 million grams. The Periodic Table of the Elements organizes elements in rows and columns. If sodium had been the building block, find an alternative with similar properties. One of these: Potassium (K), Lithium (Li), Rubidium (Rb), or any from Group 1 A. These seven nonmetals combine to make poisonous gases. Knowing which gases are lighter or heavier than air could save your life. Name this compound P 3 O 5 (2 pts) Triphosphorus pentaoxide Which column in the Periodic Table has elements that pull the hardest on electrons? 7 A Electronegativity measures attraction to electrons: F=4. 0, Na=0. 93, K=0. 82, Cl=3. 16. Which has stronger bond, Na. F or KCl? Na. F Or 17 Air inside a hot air balloon is a mixture of nitrogen, oxygen, carbon dioxide, water vapor, and carbon monoxide. What force is causing the hot air balloon to rise? Gravity. To compare densities of gases, compare molecular weight. Which is more dense, nitrogen monoxide or carbon monoxide? Nitrogen monoxide.

Visuals Topic Groups 1 A, & 2 A are called alkali metals & alkali Earth metals. The name “alkali” is Arabic for the saltwort plant. Building Blocks The ashes of the Al Kali plant contain hydroxides (OH -) of groups 1 A & 1 B. These neutralize acids (H+) to make what? Mathematics Two moles of Ca(OH)2 is mixed with 3 moles of H 2 SO 4. How many moles of water is produced? 4 moles (optional) It may boil and splash. Water absorbs Draw a water molecule. heat well, which Label atoms and show is why it’s used in where partial charges are. . car radiators. + H O Lab students are warned about adding acids (H+) to bases (OH-). These neutralize each other, so what’s the problem? A lot of heat is produced. Water H Forces & Energy What causes water to have a How many calories is higher than expected boiling needed to raise 53 gallons (200 Liters) of water in a point? water heater from 20°C The + & - ends of water (room temp) to 70°C? pull on each other. A. 10, 000 calories B. 100, 000 calories C. 10, 000 calories C Living things are built from 6 elements with a few metals needed for certain proteins. What are those 6 elements? In living things, energy from 6 pts sugar makes ATP (adenosine triphosphate) Hydrogen which provides energy for Carbon reactions. How many Oxygen phosphate groups are in ATP? Nitrogen Sulfur 3 Energy from ribose comes from this reaction. Balance it. C 5 H 10 O 5 + O 2 CO 2 + H 2 O C 5 H 10 O 5 + 5 O 2 5 CO 2 + 5 H 2 O

Visuals Hoax & Myth Busters. Topic Building Blocks Forces & Energy Mathematics Supernova explosions spread elements all over the universe. Gravity collects elements to form new stars and solar systems. Helium is 2 nd most abundant element in universe, yet very little is on Earth. Why? (2 pts) Would a helium balloon released on surface of the moon float or sink? Why? (2 pts) Being light it is lost to space. Being inert, it doesn’t bond. It would fall. 1/1, 000 Gravity is the reason 1/1, 000, 000 Atoms are too small to count, so chemistry resorts to indirect ways of counting. I call it the art of counting without counting. Making trillions of molecules of CO 2 is easy: burn coal. Why is making one molecule hard? The Internet is full of hoaxes and myths. Many relate to chemistry. Claim: A golfer who had a habit of chewing on his golf tee died after playing 4 rounds of golf. He died from ingesting fungicide sprayed on the grass by chewing on the golf tee. Below is the structure of the fungicide he died from. If someone burned this fungicide, could hydrogen cyanide [HCN] and hydrochloric acid [HCl] be products of this burning (assume hydrogen could come from moisture in the air)? Status: TRUE Yes. They are too small to see or handle. (optional: there is no air to lift it) Gas pressure depends on the speed of the gas molecules. How does temperature affect the speed? Speed is proportional to temperature (or temperature goes up, speed goes up. ) Carbon & nitrogen are bonded in the molecule (CN) by sharing 3 electrons each. Nitrogen has one pair of electrons not involved. Which would be attracted to this pair of electrons, acid [H+} or base [OH-]? Abundance is given in %, ppm, or ppb. Write these as fractions. (3 pts) 1/100, Some syringes can measure 1 μL of gas. How many atoms of helium are present if pressure is 1. 0 x 10 -4 atm and temp is 0°C. (R=0. 0821) 2. 7 trillion atoms of helium (2. 7 x 1012 atoms of helium) A liter of diet soda has 500 mg of aspartame. 1/10 of the aspartame will decompose into methanol. EPA set daily dose of 0. 5 mg per kg body weight for methanol. I weigh 100 kg. What is my limit for methanol? 50 mg. H+ . . … If I drink 2 liters of diet soda, do I go over that unpaired limit? C N