GASTRIC TUMOURS Anatomy of the stomach Aetiology of



















































- Slides: 71

GASTRIC TUMOURS Anatomy of the stomach Aetiology of Gastric cancer Types of Gastric cancer Pathology of Gastric Cancer Evaluation of Gastric Cancer Treatment of Gastric Cancer

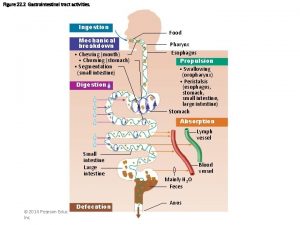
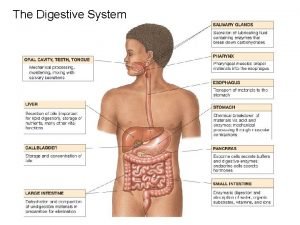
ANATOMY: The stomach J-shaped. The stomach has two surfaces (the anterior & posterior), two curvatures (the greater & lesser), two orifices (the cardia & pylorus). It has fundus, body and pyloric antrum.



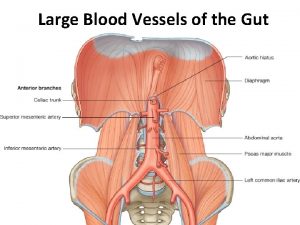
BLOOD SUPPLY: a. b. c. d. e. The left gastric artery Right gastro-epiploic artery Left gastro-epiploic artery Short gastric arteries The corresponding veins drain into portal system. The lymphatic drainage of the stomach corresponding its blood supply.




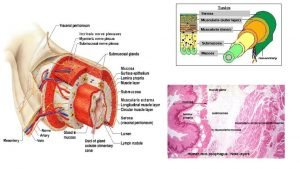
Anatomy • Stomach has five layers: – Mucosa • Epithelium, lamina propria, and muscularis mucosae* – Submucosa – Smooth muscle layer – Subserosa – Serosa


AETIOLOGY: Gastric cancer is the second most common fatal cancer in the world with high frequency in Japan. The disease presents most commonly in the 5 th and 6 th decades of life and affect males twice as often as females. Contn…

The cause of the disease multistep process but several predisposing factors attributed to cause the disease : a. b. c. d. Environment Diet Heredity Achlorhydria e. Atrophic gastritis f. Chronic gastric ulcer g. Adenomatous polyps h. Blood group A i. H. Pyloric colonisation

TYPES OF GASTRIC CANCER: A. Benign Tumours B. Malignant Tumours

TYPES OF GASTRIC CANCER: A. Benign Tumours B. Malignant Tumours

THE BENIGN TUMORS: Although benign tumors can occur in the stomach most gastric tumours are malignant.

The benign groups includes: - 1. Non-neoplastic gastric polyps 2. Adenomas 3. Neoplastic gastric polyps 4. Smooth muscles tumours benign (Leiomyomas) 5. Polyposis Syndrome (eg: - Polyposis coli, Juvenile polyps and P. J. Syndrome) 6. Other benign tumours are fibromas, and angiomas. neurofibromas, aberrat pancreas

PATHOLOGY OF GASTRIC (MALIGNANT) TUMOURS: The gastric cancer may arise in the antrum (50%), the gastric body (30%), the fundus or oesophago-gastric juntion (20%).

Types of Malignant Tumours: a. Adenocarcinoma b. Leiomyosarcoma c. Lymphomas d. Carcinoid Tumours

The macroscopic forms of gastric cancers are classified by (Bormann classification) into: - 1. Polypoid or Proliferative 2. Ulcerating 3. Ulcerating/Infiltrating 4. Diffuse Infiltrating (Linnitus. Plastica)

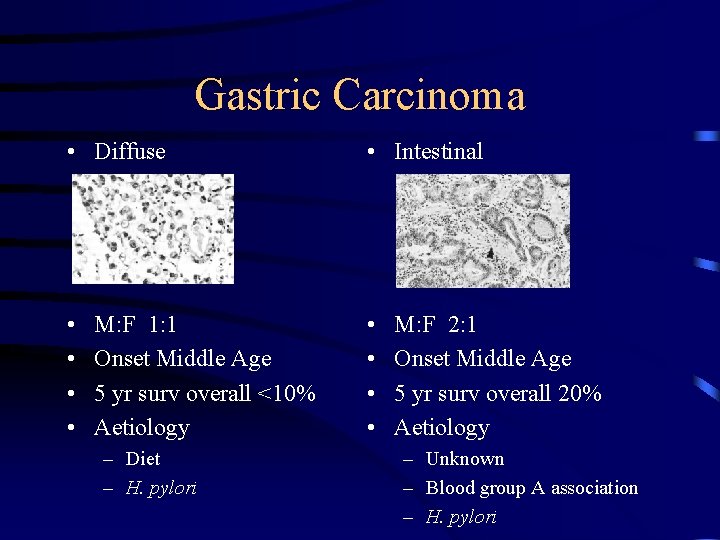
Microscopically the tumours commonly adenocarcinoma with range of differentiation. The most useful to clinician and epidemiologist is Lauren Histological Classification: a. Intestinal gastric cancer b. Diffuse gastric cancer

Gastric Carcinoma • Diffuse • Intestinal • • M: F 1: 1 Onset Middle Age 5 yr surv overall <10% Aetiology – Diet – H. pylori M: F 2: 1 Onset Middle Age 5 yr surv overall 20% Aetiology – Unknown – Blood group A association – H. pylori


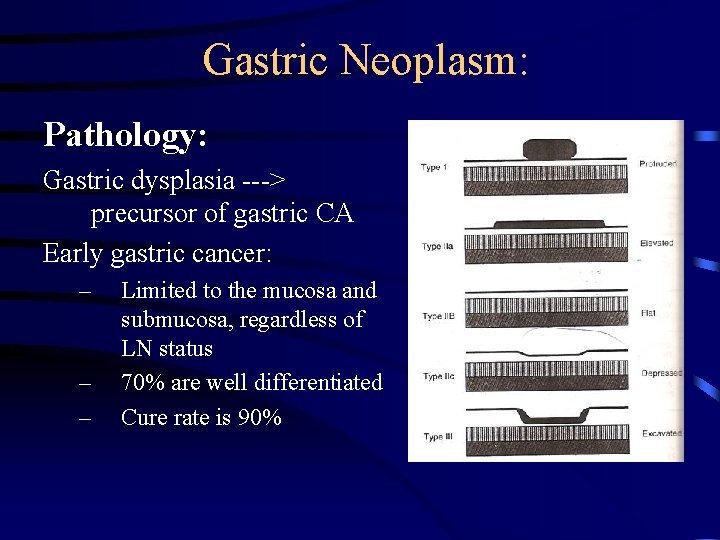
Gastric Neoplasm: Pathology: Gastric dysplasia ---> precursor of gastric CA Early gastric cancer: – – – Limited to the mucosa and submucosa, regardless of LN status 70% are well differentiated Cure rate is 90%

STAGING OF GASTRIC CANCER: a. TNM System b. CT Staging c. PHNS Staging System (Japanese) P-factor (Peritoneal dissemination) H-factor (The presence of hepatic metastases) N-factor (Lymphnodes involvement) S-factor (Serosal invasion)

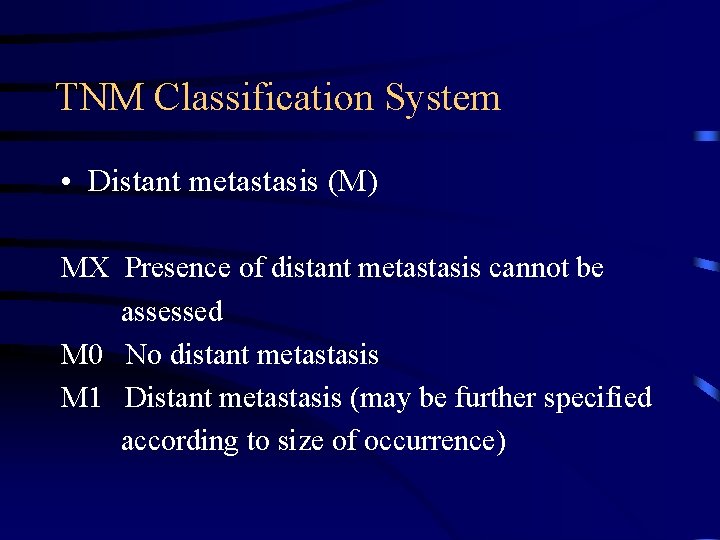
TNM Classification System • Distant metastasis (M) MX Presence of distant metastasis cannot be assessed M 0 No distant metastasis M 1 Distant metastasis (may be further specified according to size of occurrence)


SPREAD OF GASTRIC CANCER: The diffuse type spreads rapidly through the submucosal and serosal lymphatic and penetrates the gastric wall at early stage, the intestinal variety remains localized for a while and has less tendency to disseminate. The spread by: 1. 2. 3. 4. Direct (loco regional) Lymphatic Blood (Haematogenous) Transcoelomic

Clinical Manifestation: 1. Weight loss due to anorexia and early satiety is the most common symptoms 2. Abdominal pain (not severe) common 3. Nausea / vomiting 4. Chronic occult blood loss is common; GIT bleeding (5%) 5. Dysphagia (cardia involvement)

Clinical Manifestation: 6. Paraneoplastic syndromes ( Trousseau’s syndrome – thrombophlebitis; acanthosis nigricans – hyperpigmentation of axilla and groin; peripheral neuropathy) 7. Signs of distant metastasis: a. b. c. d. e. Hepatomegally / ascites Krukenbergs tumor Blummers shelf (drop metastasis) Virchow’s node Sister Joseph node (pathognomonic of advances dse)

SUMMARY: Often asymptomatic until late stage. Marked weight loss Anorexia Feeling of abdominal fullness or discomfort Epigastric mass Iron Deficiency Anaemia Left supraclavicular mass (Troisier’s Sign) Obstructive Jaundice (Secondary in porta hepatitis) Pelvic mass (Krukenberg)

EVALUATION OF GASTRIC CANCER: History Clinical Examination Investigations The clinical features of gastric cancer may arise from local disease, its complications or its metastases.

INVESTIGATIONS: A. Upper gastero intestinal endoscopy with multiple biopsy and brush cytology B. Radiology: CT Scan of the chest and abdomen USS upper abdomen Barium meal C. Diagnostic laparoscopy

Diagnosis: 1. UGIS (double contrast) 2. Endoscopy (Biopsy / Ultrasound) • • GOLD STANDARD Best pre-operative staging Needle aspiration of LN w/ ultrasound guidance Can even give preop neoadjuvant tx 3. CT scan (intravenous and oral contrast): • For pre-operative staging 4. Whole body Positron Emission Tomography scanning (PET): • Tumor cell preferentially accumulate positronemitting 18 F fluorodeoxyglucose.

Laboratory • Assists in determining optimal therapy. • CBC identifies anemia, with may be caused by bleeding, liver dysfunction, or poor nutrition. • 30% have anemia. • Electrolyte panels and LFTs are also essential to better characterize patients clinical state.

Investigations for patients with gastric cancer • Endoscopy & biopsy • Performance status • Physiological assessment – Cardio-pulmonary function • CT chest & abdomen • EUS (endoscopic ultrasound) • Laparoscopy

CT scanning • Technique – Spiral CT of chest and abdomen

Laparoscopy • Inspect peritoneal surfaces, liver surface. • Identification of advanced disease avoids non-therapeutic laparotomy in 25%. • Patients with small volume metastases in peritoneum or liver have a life expectancy of 3 -9 months, thus rarely benefit from palliative resection.

Screening of Gastric Cancer • Patients at risk for gastric CA should undergo yearly endoscopy and biopsy: 1. 2. 3. 4. 5. 6. Familial adenomatous polyposis Hereditary nonpolyposis colorectal cancer Gastric adenomas Menetrier’s disease Intestinal metaplasia or dysplasia Remote gastrectomy or gastrojejunostomy

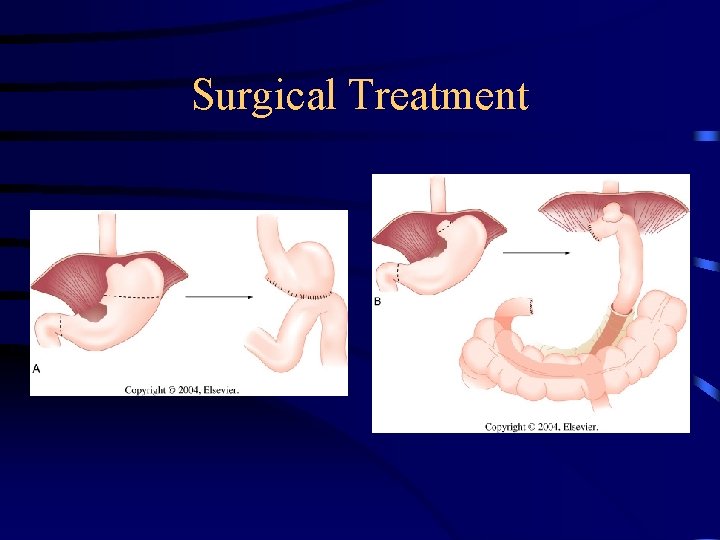
TREATMENTS OF GASTRIC CANCER: Surgery (Early or Advanced Cancer) Distal tumours which involve the partial gasterectomy). lower ½ (sub-total or

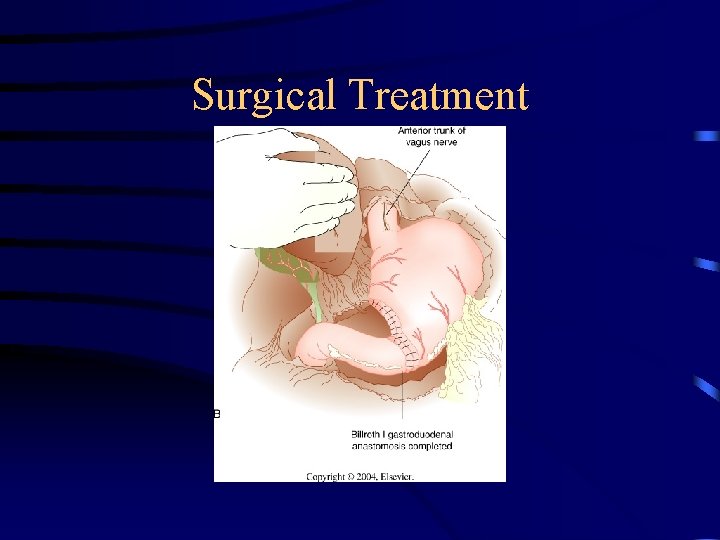
Surgical Treatment

TREATMENT: SURGERY: – The only curative tx for gastric cancer – Except: 1. Can’t tolerate abdominal surgery 2. Overwhelming metastasis – Palliation is poor w/ non-resective operations – GOAL: resect all tumors, w/ negative margins (5 cm) and adequate lymphadenectomy (need for RFS) – Enbloc resection of adjacent organ is done if needed.

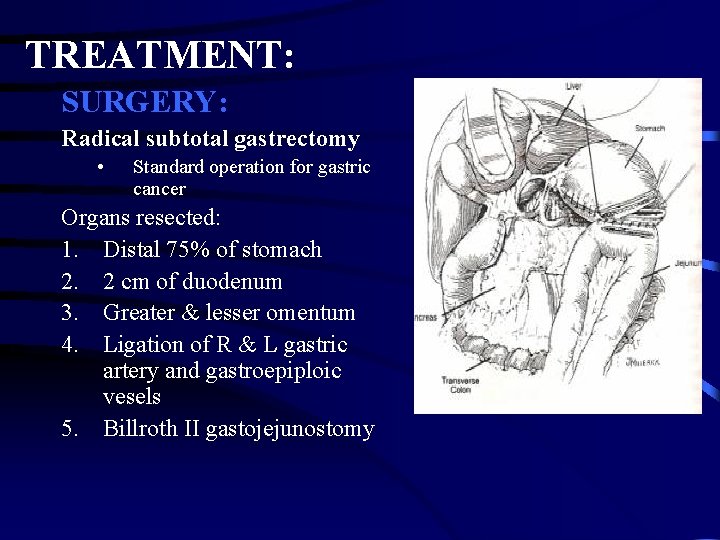
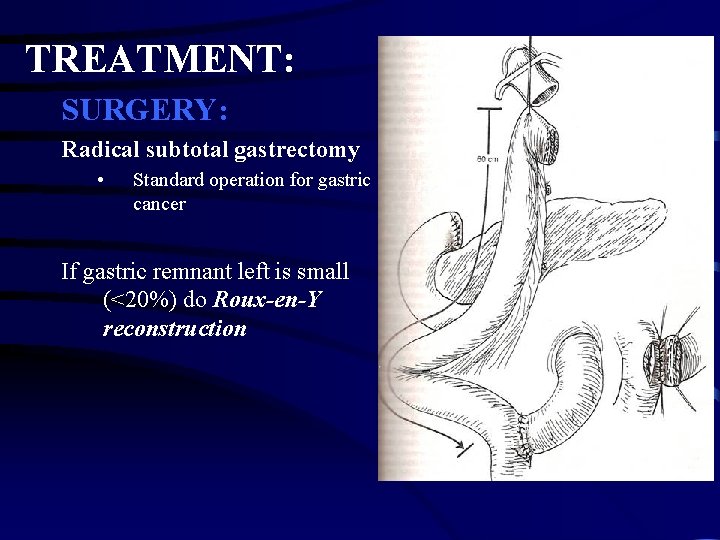
TREATMENT: SURGERY: Radical subtotal gastrectomy • Standard operation for gastric cancer Organs resected: 1. Distal 75% of stomach 2. 2 cm of duodenum 3. Greater & lesser omentum 4. Ligation of R & L gastric artery and gastroepiploic vesels 5. Billroth II gastojejunostomy

TREATMENT: SURGERY: Radical subtotal gastrectomy • Standard operation for gastric cancer If gastric remnant left is small (<20%) do Roux-en-Y reconstruction

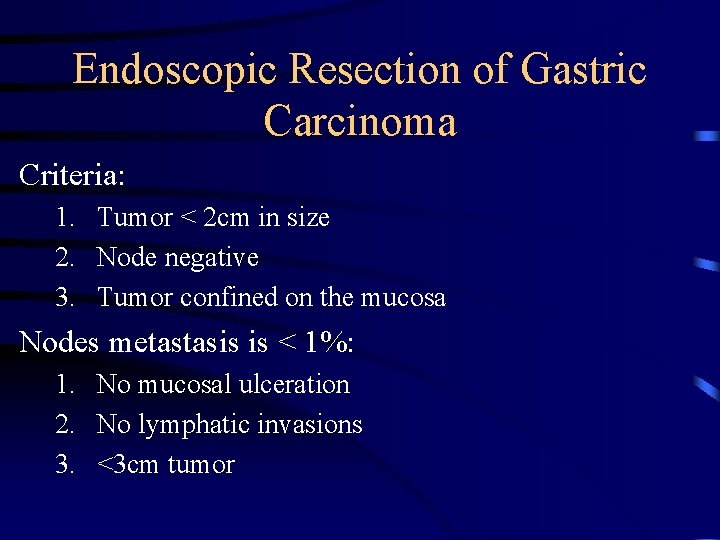
Endoscopic Resection of Gastric Carcinoma Criteria: 1. Tumor < 2 cm in size 2. Node negative 3. Tumor confined on the mucosa Nodes metastasis is < 1%: 1. No mucosal ulceration 2. No lymphatic invasions 3. <3 cm tumor

Treatment of gastric cancer • Endoscopic treatment – EMR (endoscopic mucosal resection) – ablation • Surgery • Multimodal treatment – Neo-adjuvant – Adjuvant • Palliative treatment

Endsocopic mucosal resection • T 1 mucosal disease – Minimal risk of LN metastases • Various techniques • Specimen obtained

Distal Pancreatectomy • Associated with marked increase in morbidity & mortality with or without splenectomy • Indications for pancreatectomy: – Direct invasion of the tail of the pancreas – Likelihood of splenic artery nodal involvement

Surgical Treatment


Indications for Splenectomy • If macroscopic disease can be resected & the operation is potentially curative then en bloc splenectomy or pancreaticosplenectomy is worthwhile. • If it is more palliative then this benefit must be weighed against the potential complications of splenectomy and more extensive operation

Chemotherapy for gastric cancer (Pre-operatve & post-operative) Radiotherapy (Pre-intra & post-operatively)

Adjuvant Therapy • Rationale is to provide additional locoregional control. • Radiotherapy- studies show improved survival, lower rates of local recurrence when compared to surgery alone. • In unresectable patients, higher 4 year survival with mutimodal tx, in comparison to chemo alone.

Chemotherapy • Numerous randomized clinical trials comparing combination chemotherapy in the adjuvant setting to surgery alone did not demonstrate a consistent survival benefit. • The most widely used regimen is 5 -FU, doxorubicin, and mitomycin-c. The addition of leukovorin did not increase response rates.

Advanced Unresectable Disease • Surgery is for palliation, pain, allowing oral intake • Radiation provides relief from bleeding, obstruction and pain in 50 -75%. Median duration of palliation is 4 -18 months

Outcome • 5 -year survival for a curative resection is 30 -50% for stage II disease, 10 -25% for stage III disease. • Adjuvant therapy because of high incidence of local and systemic failure. • A recent Intergroup 0116 randomized study offers evidence of a survival benefit associated with postoperative chemoradiotherapy

Complications • Mortality 1 -2% • Anastamotic leak, bleeding, ileus, transit failure, cholecystitis, pancreatitis, pulmonary infections, and thromboembolism. • Late complications include dumping syndrome, vitamin B-12 deficiency, reflux esophagitis, osteoporosis.

OTHER GASTRIC TUMOURS: Gastric Lymphomas: Primary lymphomas of the stomach of (NHL). The symptoms are similar to those of gastric cancer (adenocarcinoma). The diagnosis is made principally from endoscopic examination with biopsy and cytology. CT Scanning is important in staging the disease. the non Hodgkin’s type

Treatment: - Well-localized disease should be treated with resection (surgery) followed by radiotherapy or chemotherapy. - Extensive disease by adjuvant chemotherapy & radiotherapy than surgery.

Leiomyosarcoma: Arise in the stomach representing 1% of gastric tumors.

Stromal tumours • GIST (Gastro-Intestinal Stromal Tumour) – Presentation • Incidental • Bleeding – Pathology • • • Blend sheets of spindle cells Previously mistaken for leiomyomata Origin cell – interstitial cell of Cahal C-kit +ve Actin -ve

Stromal tumours • Prognostic factors – Size (>4 cm) – Resection margins – Mitoses – Vacuoles on EUS

Stromal tumours • Surgical Treatment – Excision with clear margins – No lymphadenectomy required • Non –surgical treatment – Glivec (imatinib) – Recurrence / inoperable – ? Neoadjuvant / adjuvant

Gastric Carcinoid Tumour: Are very rare. There is established gastritis & carcinoid & pernicious Gastric carcinoids are best treated by by endoscopic resection. association between atrophic anemia. local resection. If very small

Gastric Carcinoid Tumours • • <1% of gastric tumours 4 -41% of GIT carcinoid tumours Most ECL/argyrophil cell origin (80%) 3 clinico-pathological subtypes: – Type 1, 2 & 3

Gastric Carcinoid Tumours • Type 1 : Hypergastrinaemia with Autoimmune chronic atrophic gastritis (Type A) – Pernicious anaemia • Type 2 : Hypergastrinaemia with hypertrophic gastropathy – Zollinger-Ellison syndrome • Type 3 : Sporadic, no relation to hypergastrinaemia

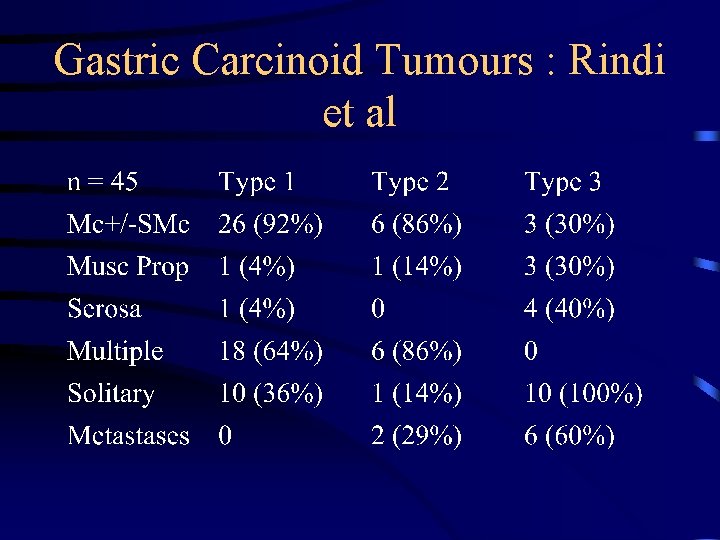
Gastric Carcinoid Tumours : Rindi et al

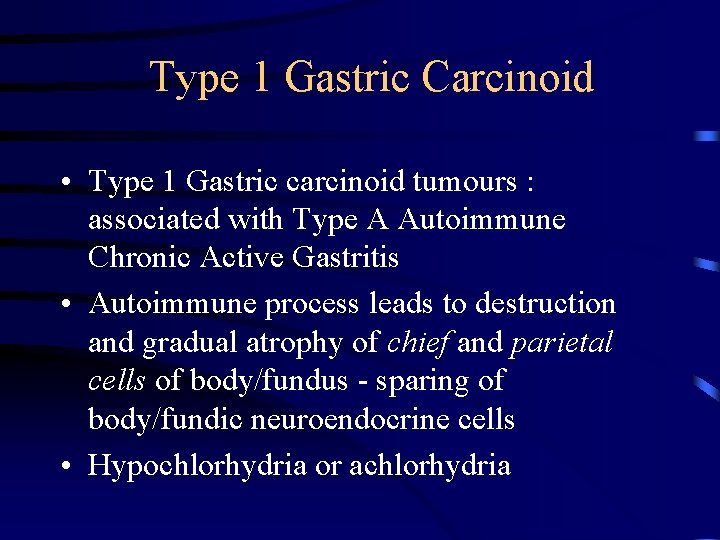
Type 1 Gastric Carcinoid • Type 1 Gastric carcinoid tumours : associated with Type A Autoimmune Chronic Active Gastritis • Autoimmune process leads to destruction and gradual atrophy of chief and parietal cells of body/fundus - sparing of body/fundic neuroendocrine cells • Hypochlorhydria or achlorhydria

Gastric Carcinoid Tumours • Hyperplastic precursor sequence • Hypergastrinaemia -- Neuroendocrine hyperplasia -- Dysplasia -- Neoplasia • Pernicious anaemia only present in 20 -46% of patients (latent effect) • Natural history : most probably remain stationary; some regress and some metastasize

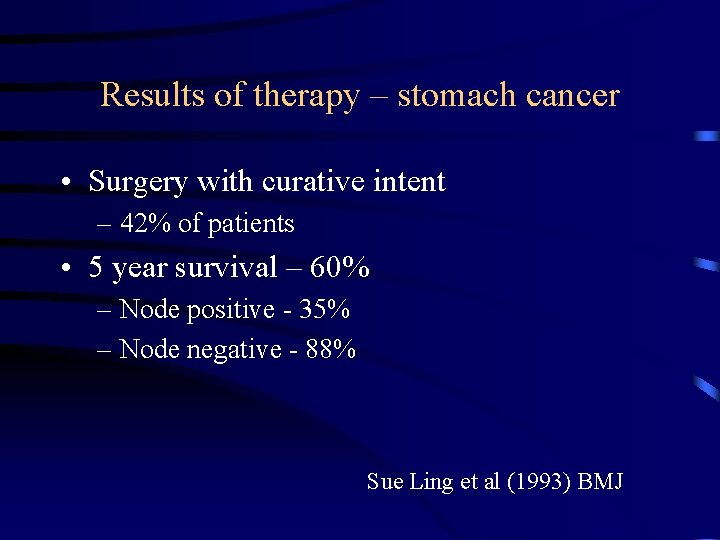
Results of therapy – stomach cancer • Surgery with curative intent – 42% of patients • 5 year survival – 60% – Node positive - 35% – Node negative - 88% Sue Ling et al (1993) BMJ

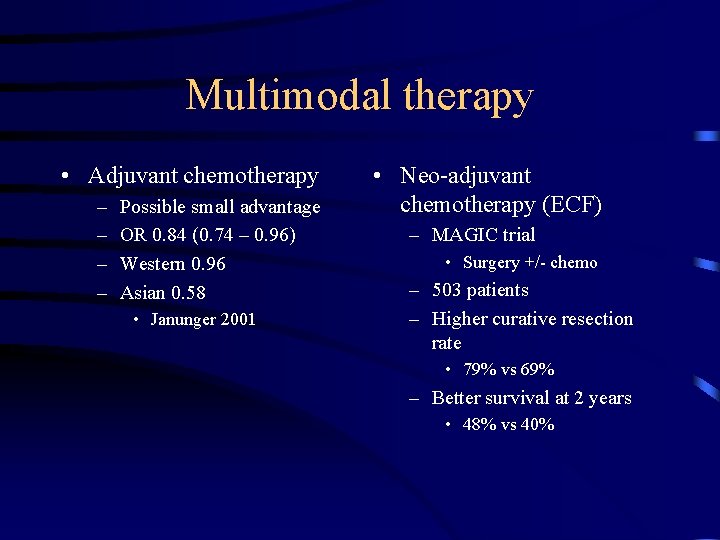
Multimodal therapy • Adjuvant chemotherapy – – Possible small advantage OR 0. 84 (0. 74 – 0. 96) Western 0. 96 Asian 0. 58 • Janunger 2001 • Neo-adjuvant chemotherapy (ECF) – MAGIC trial • Surgery +/- chemo – 503 patients – Higher curative resection rate • 79% vs 69% – Better survival at 2 years • 48% vs 40%

Palliative chemotherapy • Median survival benefit 3 – 6 months • Combination therapy superior • 50% gain improvement in QOL
 Tongue rough edges
Tongue rough edges Kelling madlener procedure
Kelling madlener procedure Gastric folds
Gastric folds Pud triple therapy
Pud triple therapy Stomach anatomy
Stomach anatomy Ruminant stomach
Ruminant stomach Gastric emptying ppt
Gastric emptying ppt Microscopic anatomy of the stomach
Microscopic anatomy of the stomach Stomach blood supply anatomy
Stomach blood supply anatomy Lower esophageal sphincter
Lower esophageal sphincter Transpyloric plane level
Transpyloric plane level Stomach borders
Stomach borders Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Lời thề hippocrates
Lời thề hippocrates Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Bổ thể
Bổ thể Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phản ứng thế ankan
Phản ứng thế ankan Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập điện thế nghỉ
điện thế nghỉ Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Chó sói
Chó sói Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Số nguyên tố là số gì
Số nguyên tố là số gì Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Hệ hô hấp
Hệ hô hấp Tư thế ngồi viết
Tư thế ngồi viết Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Tư thế worms-breton
Tư thế worms-breton ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Thể thơ truyền thống
Thể thơ truyền thống Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Ng-html
Ng-html Giọng cùng tên là
Giọng cùng tên là 101012 bằng
101012 bằng Chúa yêu trần thế
Chúa yêu trần thế Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Gastric pit
Gastric pit Sondajul gastric
Sondajul gastric Regulation of stomach emptying
Regulation of stomach emptying Ulcer bulbar definitie
Ulcer bulbar definitie Harris drip enema
Harris drip enema Subhepatic prefix
Subhepatic prefix Where is the left gastric artery
Where is the left gastric artery Sbo axr
Sbo axr Vomiting case presentation
Vomiting case presentation Gastric
Gastric What are peristaltic movements
What are peristaltic movements Spire gastric band
Spire gastric band Gastric pit
Gastric pit Which bacteria gives protection against gastric injury
Which bacteria gives protection against gastric injury Glandular epithelial cell
Glandular epithelial cell Her2 gastric
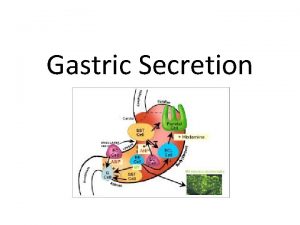
Her2 gastric Secretion of gastric juice
Secretion of gastric juice Gastric lavage for afb
Gastric lavage for afb