Chapter 4 Reactions in Aqueous Solutions Copyright The



![p. H – A Measure of Acidity p. H describes the concentration of [H+] p. H – A Measure of Acidity p. H describes the concentration of [H+]](https://slidetodoc.com/presentation_image_h2/ba2eb3943b132c6d6c25d03ddd00ac93/image-33.jpg)

![Ion Molarity Problem What is the Molar concentration of the Potassium ion [K+] when Ion Molarity Problem What is the Molar concentration of the Potassium ion [K+] when](https://slidetodoc.com/presentation_image_h2/ba2eb3943b132c6d6c25d03ddd00ac93/image-65.jpg)
![Molarity Problem What is the Molar concentration of the Sodium ion [Na+] when 23. Molarity Problem What is the Molar concentration of the Sodium ion [Na+] when 23.](https://slidetodoc.com/presentation_image_h2/ba2eb3943b132c6d6c25d03ddd00ac93/image-67.jpg)


- Slides: 92

Chapter 4 Reactions in Aqueous Solutions Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

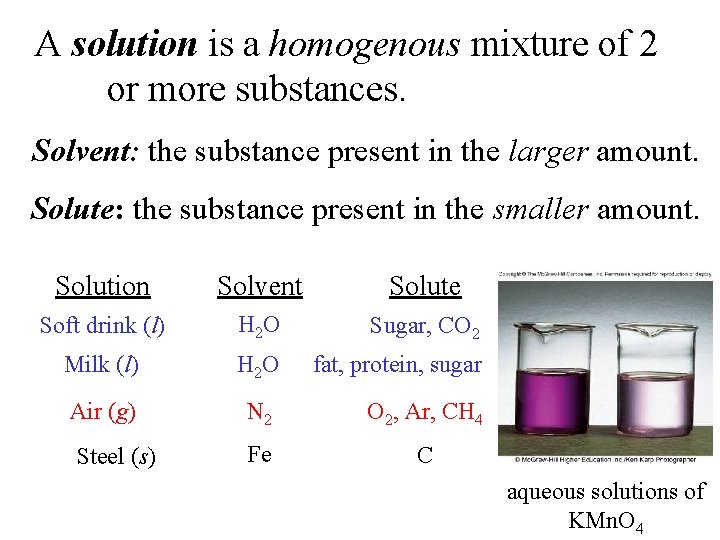
A solution is a homogenous mixture of 2 or more substances. Solvent: the substance present in the larger amount. Solute: the substance present in the smaller amount. Solution Solvent Solute Soft drink (l) H 2 O Sugar, CO 2 Milk (l) H 2 O fat, protein, sugar Air (g) N 2 O 2, Ar, CH 4 Steel (s) Fe C aqueous solutions of KMn. O 4

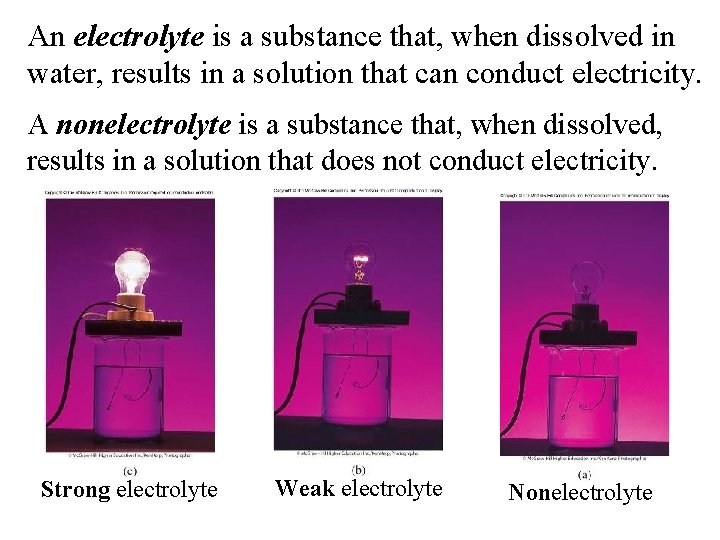
An electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. A nonelectrolyte is a substance that, when dissolved, results in a solution that does not conduct electricity. Strong electrolyte Weak electrolyte Nonelectrolyte

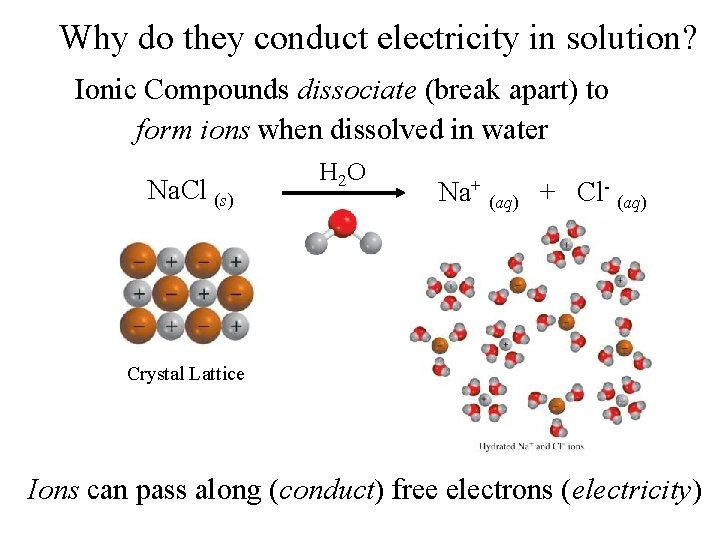
Why do they conduct electricity in solution? Ionic Compounds dissociate (break apart) to form ions when dissolved in water Na. Cl (s) H 2 O Na+ (aq) + Cl- (aq) Crystal Lattice Ions can pass along (conduct) free electrons (electricity)

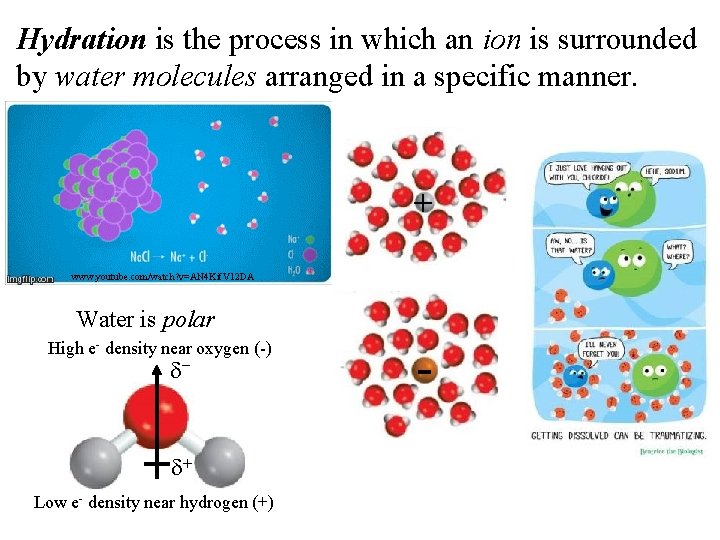
Hydration is the process in which an ion is surrounded by water molecules arranged in a specific manner. + www. youtube. com/watch? v=AN 4 Kif. V 12 DA Water is polar High e- density near oxygen (-) d- d+ Low e- density near hydrogen (+) -

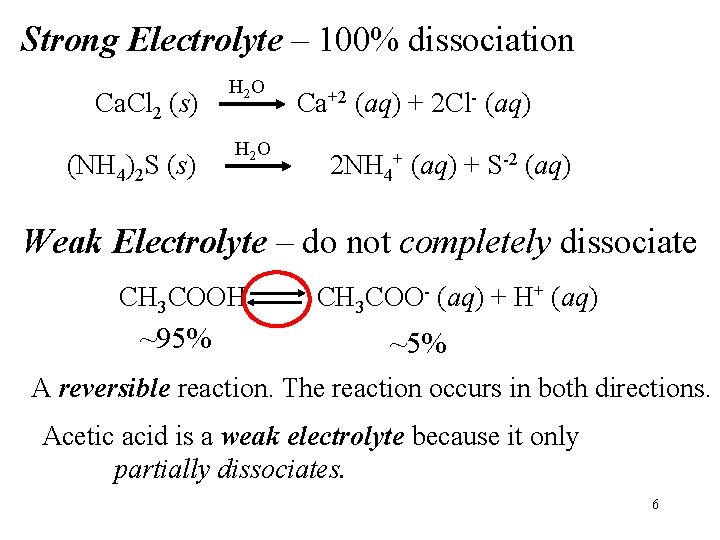
Strong Electrolyte – 100% dissociation Ca. Cl 2 (s) (NH 4)2 S (s) H 2 O Ca+2 (aq) + 2 Cl- (aq) 2 NH 4+ (aq) + S-2 (aq) Weak Electrolyte – do not completely dissociate CH 3 COOH ~95% CH 3 COO- (aq) + H+ (aq) ~5% A reversible reaction. The reaction occurs in both directions. Acetic acid is a weak electrolyte because it only partially dissociates. 6

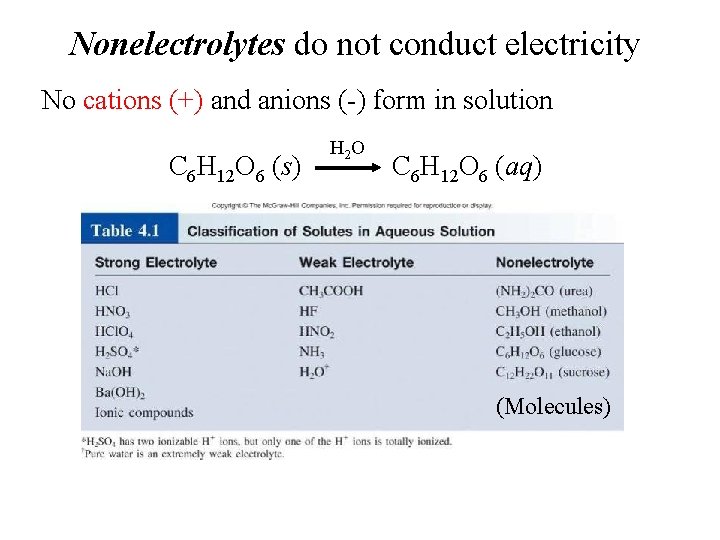
Nonelectrolytes do not conduct electricity No cations (+) and anions (-) form in solution C 6 H 12 O 6 (s) H 2 O C 6 H 12 O 6 (aq) (Molecules)

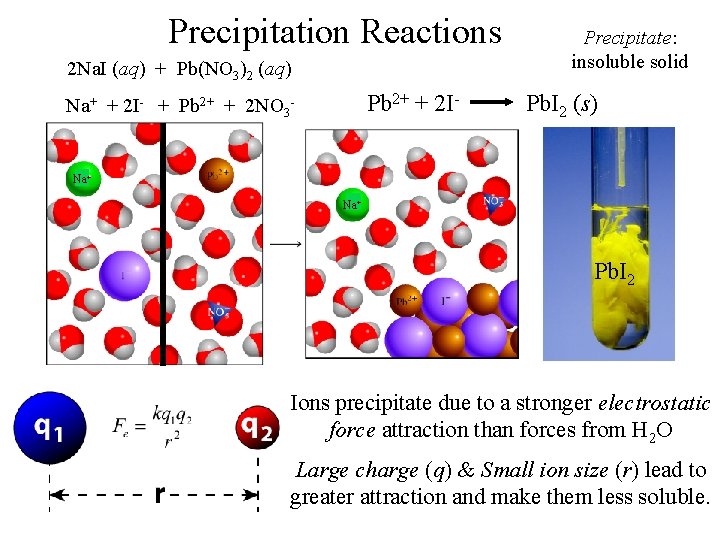
Precipitation Reactions 2 Na. I (aq) + Pb(NO 3)2 (aq) Pb 2+ + 2 I- Na+ + 2 I- + Pb 2+ + 2 NO 3 - Precipitate: insoluble solid Pb. I 2 (s) Na+ Pb. I 2 Ions precipitate due to a stronger electrostatic force attraction than forces from H 2 O Large charge (q) & Small ion size (r) lead to greater attraction and make them less soluble.

Examples of Insoluble Compounds Cd. S Pb. S Ni(OH)2 Al(OH)3 9

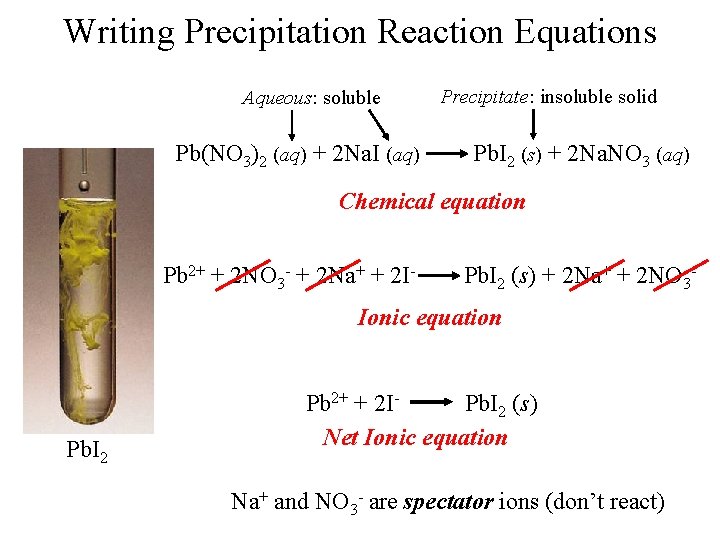
Writing Precipitation Reaction Equations Aqueous: soluble Pb(NO 3)2 (aq) + 2 Na. I (aq) Precipitate: insoluble solid Pb. I 2 (s) + 2 Na. NO 3 (aq) Chemical equation Pb 2+ + 2 NO 3 - + 2 Na+ + 2 I- Pb. I 2 (s) + 2 Na+ + 2 NO 3 - Ionic equation Pb. I 2 Pb 2+ + 2 IPb. I 2 (s) Net Ionic equation Na+ and NO 3 - are spectator ions (don’t react)

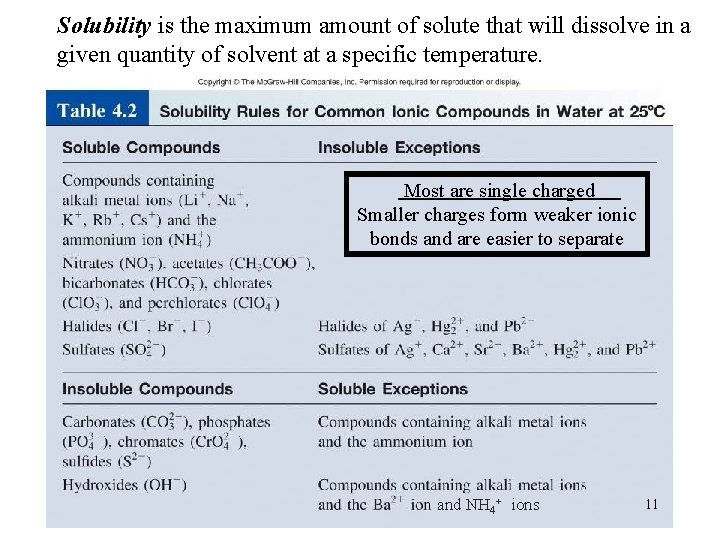
Solubility is the maximum amount of solute that will dissolve in a given quantity of solvent at a specific temperature. Most are single charged Smaller charges form weaker ionic bonds and are easier to separate and NH 4+ ions 11

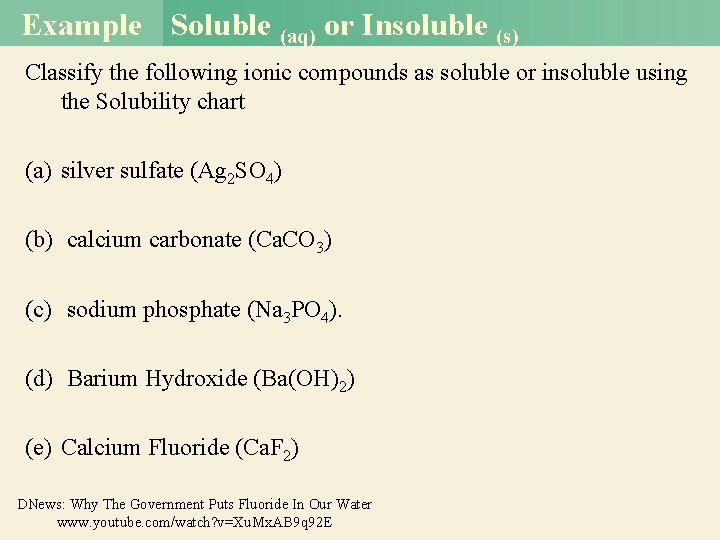
Example Soluble (aq) or Insoluble (s) Classify the following ionic compounds as soluble or insoluble using the Solubility chart (a) silver sulfate (Ag 2 SO 4) (a) Ag 2 SO 4 (s) is insoluble. (b) calcium carbonate (Ca. CO 3) (b) This is a carbonate and Ca is a Group 2 A metal. Therefore, Ca. CO 3 (s) is insoluble. (c) sodium phosphate (Na 3 PO 4). (c) Sodium is an alkali metal (Group 1 A) so Na 3 PO 4 (aq) is soluble. (d) Barium Hydroxide (Ba(OH)2) (e) Calcium Fluoride (Ca. F 2) (d) Hydroxides are normally insoluble, but Barium is one of the exceptions along with all alkali metals making it soluble (aq). (e) Neither Ca+2 nor F-1 are found listed as soluble, so it assumed the compound is DNews: Why The Government Puts Fluoride In Our Water insoluble (s) www. youtube. com/watch? v=Xu. Mx. AB 9 q 92 E

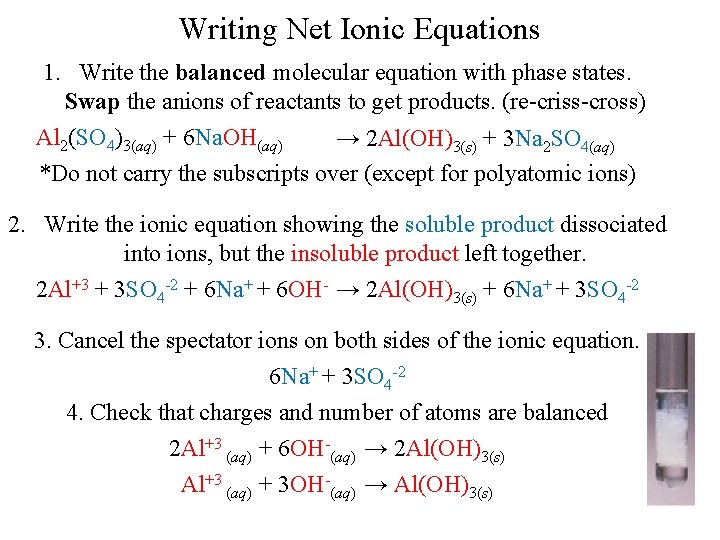
Writing Net Ionic Equations 1. Write the balanced molecular equation with phase states. Swap the anions of reactants to get products. (re-criss-cross) Al 2(SO 4)3(aq) + 6 Na. OH(aq) → 2 Al(OH)3(s) + 3 Na 2 SO 4(aq) *Do not carry the subscripts over (except for polyatomic ions) 2. Write the ionic equation showing the soluble product dissociated into ions, but the insoluble product left together. 2 Al+3 + 3 SO 4 -2 + 6 Na+ + 6 OH- → 2 Al(OH)3(s) + 6 Na+ + 3 SO 4 -2 3. Cancel the spectator ions on both sides of the ionic equation. 6 Na+ + 3 SO 4 -2 4. Check that charges and number of atoms are balanced 2 Al+3 (aq) + 6 OH-(aq) → 2 Al(OH)3(s) Al+3 (aq) + 3 OH-(aq) → Al(OH)3(s)

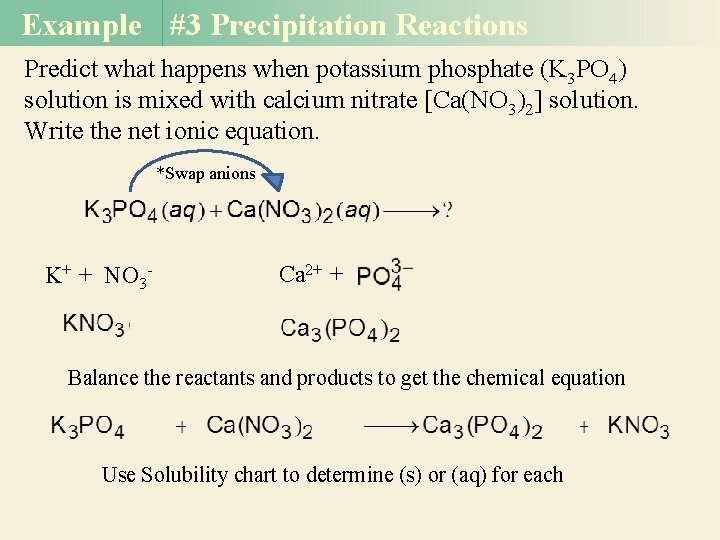
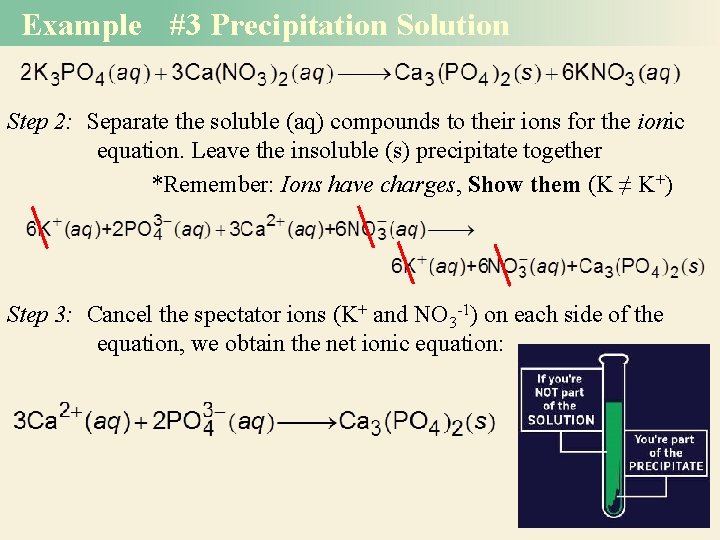
Example #3 Precipitation Reactions Predict what happens when potassium phosphate (K 3 PO 4) solution is mixed with calcium nitrate [Ca(NO 3)2] solution. Write the net ionic equation. *Swap anions K+ + NO 3 - Ca 2+ + Balance the reactants and products to get the chemical equation Use Solubility chart to determine (s) or (aq) for each

Example #3 Precipitation Solution Step 2: Separate the soluble (aq) compounds to their ions for the ionic equation. Leave the insoluble (s) precipitate together *Remember: Ions have charges, Show them (K ≠ K+) Step 3: Cancel the spectator ions (K+ and NO 3 -1) on each side of the equation, we obtain the net ionic equation:

Writing Net Ionic Equations Practice Pb(NO 3)2 and Li. Cl Mg. SO 4 and Co. Br Al 2(SO 4)3 and NH 4 OH Li 2 CO 3 and Ca(NO 3)2 Crash Course: Precipitation Reactions: www. youtube. com/watch? v=IIu 16 dy 3 Th. I Argyria: Silver ingestion Beautiful Reactions: Precipitation https: //vimeo. com/106810691

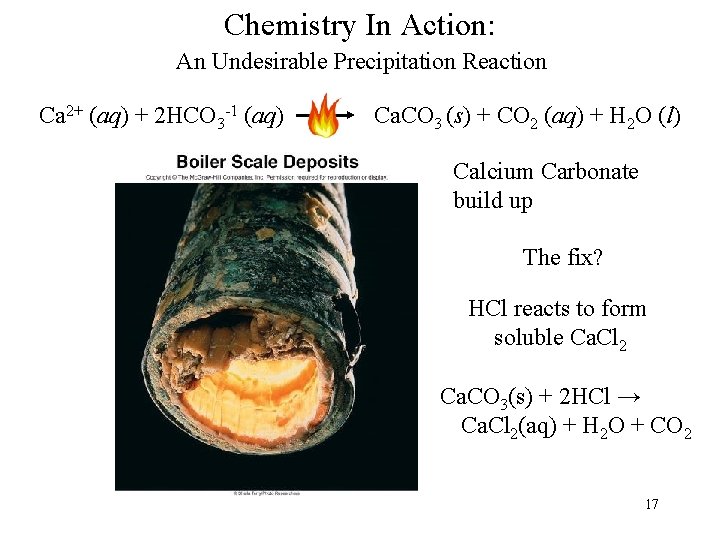
Chemistry In Action: An Undesirable Precipitation Reaction Ca 2+ (aq) + 2 HCO 3 -1 (aq) Ca. CO 3 (s) + CO 2 (aq) + H 2 O (l) Calcium Carbonate build up The fix? HCl reacts to form soluble Ca. Cl 2 Ca. CO 3(s) + 2 HCl → Ca. Cl 2(aq) + H 2 O + CO 2 17

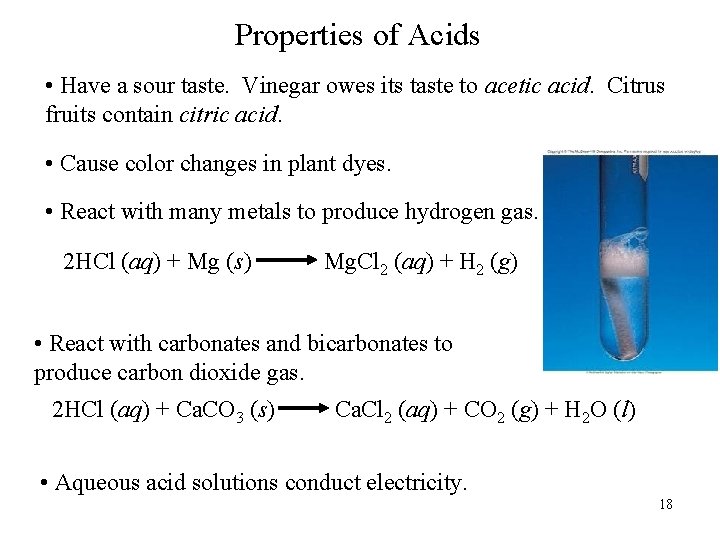
Properties of Acids • Have a sour taste. Vinegar owes its taste to acetic acid. Citrus fruits contain citric acid. • Cause color changes in plant dyes. • React with many metals to produce hydrogen gas. 2 HCl (aq) + Mg (s) Mg. Cl 2 (aq) + H 2 (g) • React with carbonates and bicarbonates to produce carbon dioxide gas. 2 HCl (aq) + Ca. CO 3 (s) Ca. Cl 2 (aq) + CO 2 (g) + H 2 O (l) • Aqueous acid solutions conduct electricity. 18

Properties of Bases • Have a bitter taste. • Feel slippery. Many soaps contain bases. • Cause color changes in plant dyes. • Aqueous base solutions conduct electricity. Examples: 19

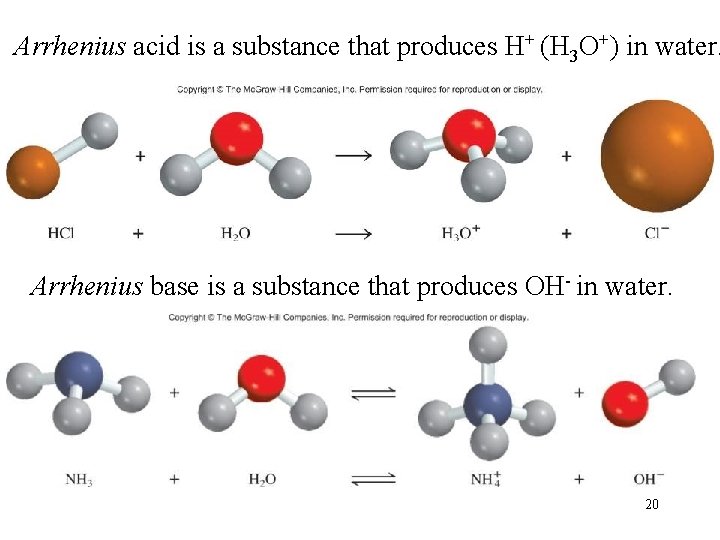
Arrhenius acid is a substance that produces H+ (H 3 O+) in water. Arrhenius base is a substance that produces OH- in water. 20

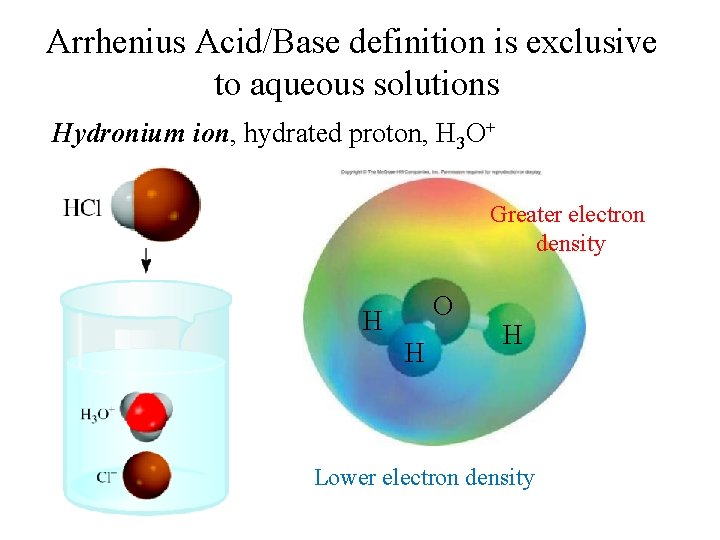
Arrhenius Acid/Base definition is exclusive to aqueous solutions Hydronium ion, hydrated proton, H 3 O+ Greater electron density O H H H Lower electron density

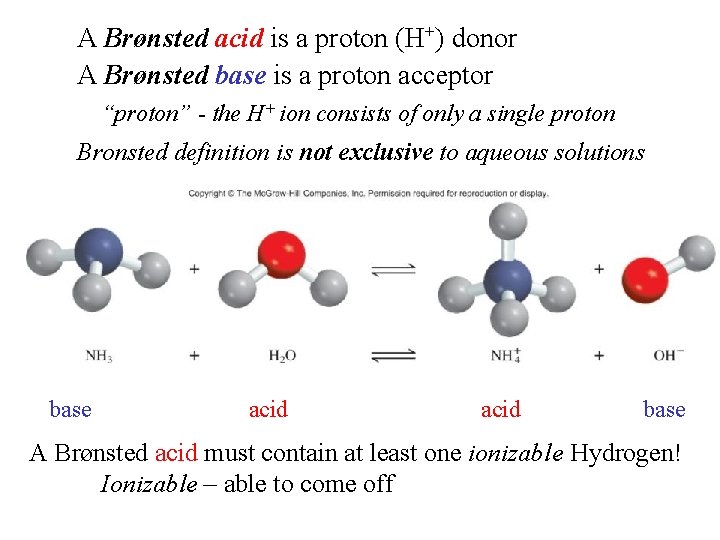
A Brønsted acid is a proton (H+) donor A Brønsted base is a proton acceptor “proton” - the H+ ion consists of only a single proton Bronsted definition is not exclusive to aqueous solutions base acid base A Brønsted acid must contain at least one ionizable Hydrogen! Ionizable – able to come off

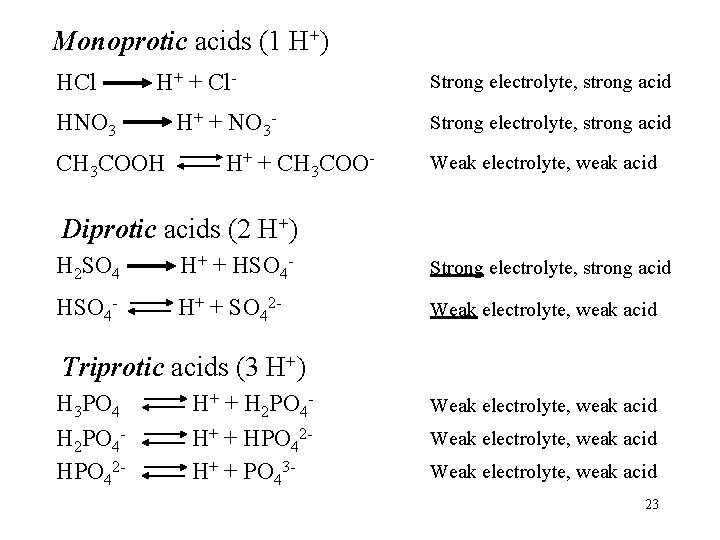
Monoprotic acids (1 H+) HCl H+ + Cl- HNO 3 CH 3 COOH H+ + NO 3 H+ + CH 3 COO- Strong electrolyte, strong acid Weak electrolyte, weak acid Diprotic acids (2 H+) H 2 SO 4 H+ + HSO 4 - Strong electrolyte, strong acid HSO 4 - H+ + SO 42 - Weak electrolyte, weak acid Triprotic acids (3 H+) H 3 PO 4 H 2 PO 4 HPO 42 - H+ + H 2 PO 4 H+ + HPO 42 H+ + PO 43 - Weak electrolyte, weak acid 23

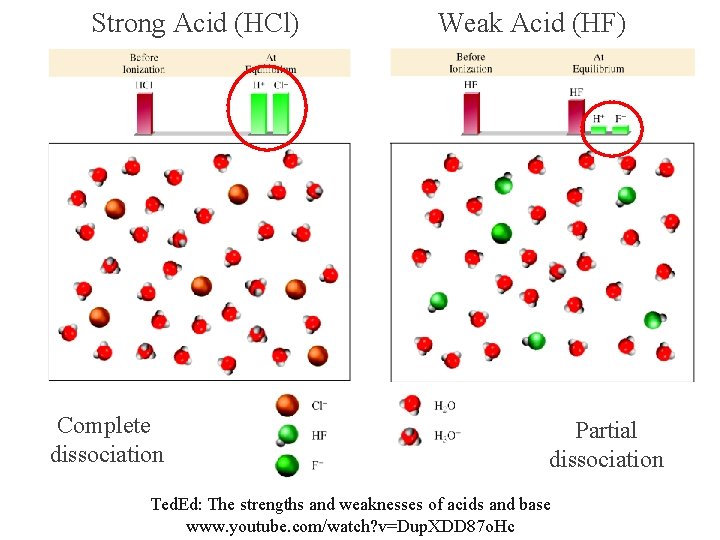
Strong Acid (HCl) Complete dissociation Weak Acid (HF) Partial dissociation Ted. Ed: The strengths and weaknesses of acids and base www. youtube. com/watch? v=Dup. XDD 87 o. Hc

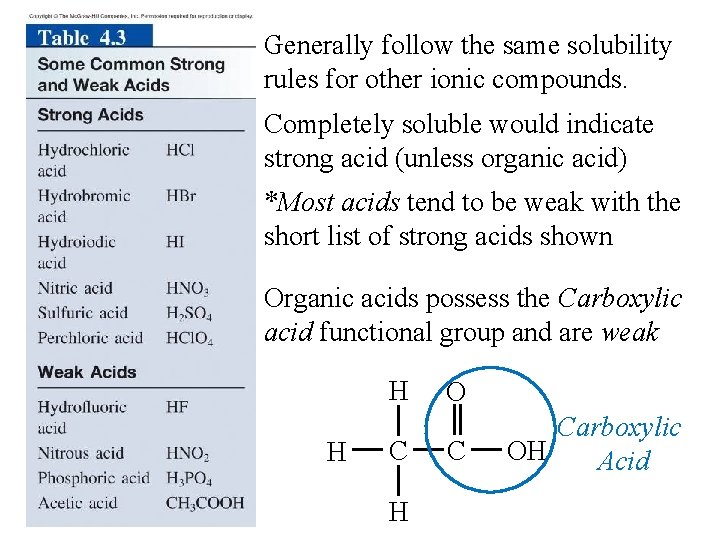
Generally follow the same solubility rules for other ionic compounds. Completely soluble would indicate strong acid (unless organic acid) *Most acids tend to be weak with the short list of strong acids shown Organic acids possess the Carboxylic acid functional group and are weak H H C H O C Carboxylic OH Acid

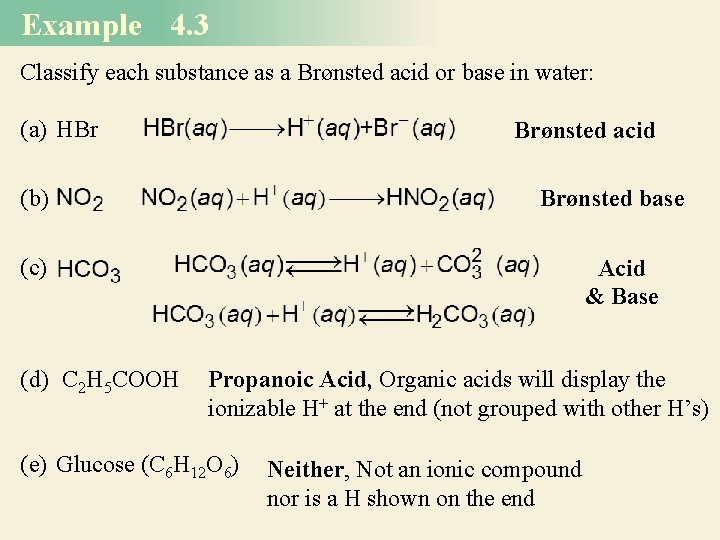
Example 4. 3 Classify each substance as a Brønsted acid or base in water: (a) HBr Brønsted acid (b) Brønsted base (c) (d) C 2 H 5 COOH Acid & Base Propanoic Acid, Organic acids will display the ionizable H+ at the end (not grouped with other H’s) (e) Glucose (C 6 H 12 O 6) Neither, Not an ionic compound nor is a H shown on the end

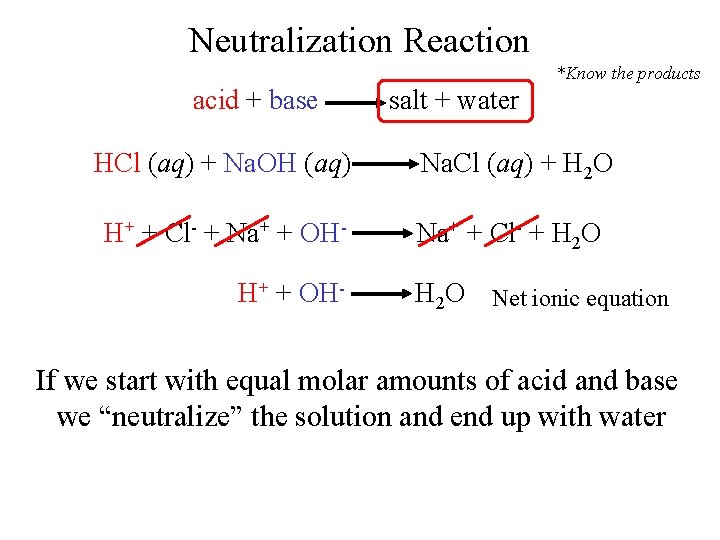
Neutralization Reaction *Know the products acid + base salt + water HCl (aq) + Na. OH (aq) Na. Cl (aq) + H 2 O H+ + Cl- + Na+ + OH- Na+ + Cl- + H 2 O H+ + OH- H 2 O Net ionic equation If we start with equal molar amounts of acid and base we “neutralize” the solution and end up with water

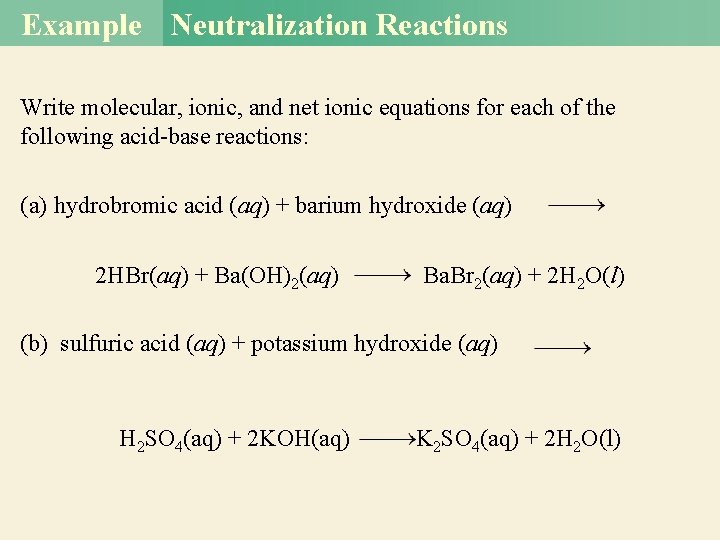
Example Neutralization Reactions Write molecular, ionic, and net ionic equations for each of the following acid-base reactions: (a) hydrobromic acid (aq) + barium hydroxide (aq) 2 HBr(aq) + Ba(OH)2(aq) Ba. Br 2(aq) + 2 H 2 O(l) (b) sulfuric acid (aq) + potassium hydroxide (aq) H 2 SO 4(aq) + 2 KOH(aq) K 2 SO 4(aq) + 2 H 2 O(l)

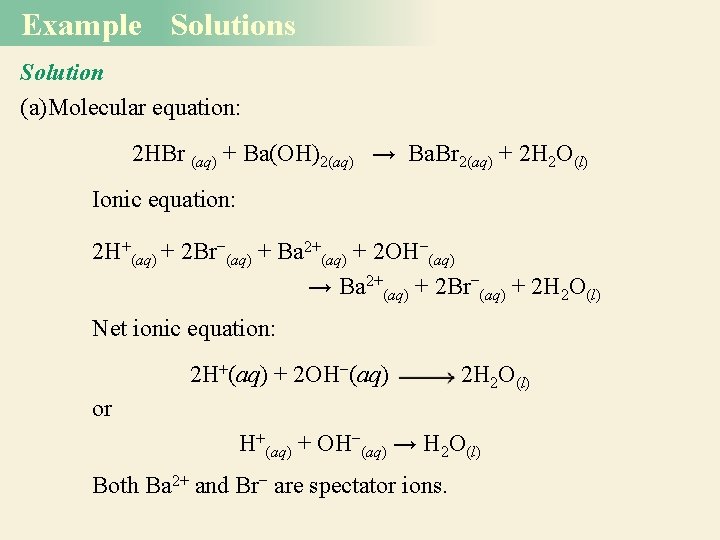
Example Solutions Solution (a)Molecular equation: 2 HBr (aq) + Ba(OH)2(aq) → Ba. Br 2(aq) + 2 H 2 O(l) Ionic equation: 2 H+(aq) + 2 Br−(aq) + Ba 2+(aq) + 2 OH−(aq) → Ba 2+(aq) + 2 Br−(aq) + 2 H 2 O(l) Net ionic equation: 2 H+(aq) + 2 OH−(aq) 2 H 2 O(l) or H+(aq) + OH−(aq) → H 2 O(l) Both Ba 2+ and Br− are spectator ions.

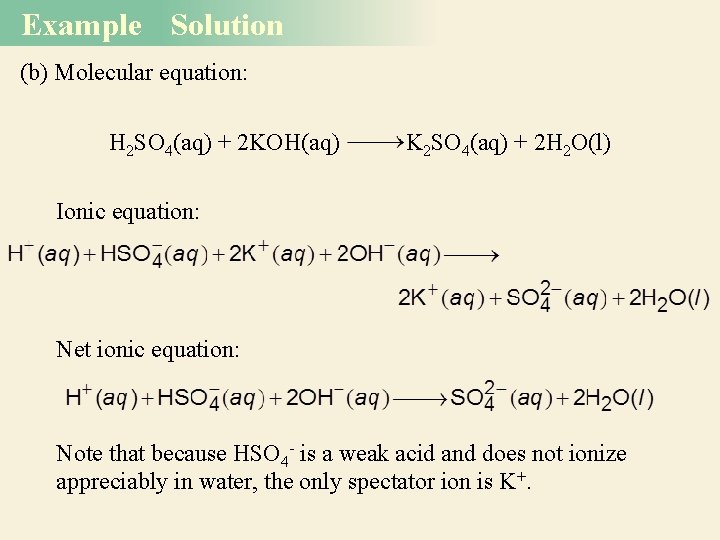
Example Solution (b) Molecular equation: H 2 SO 4(aq) + 2 KOH(aq) K 2 SO 4(aq) + 2 H 2 O(l) Ionic equation: Net ionic equation: Note that because HSO 4 - is a weak acid and does not ionize appreciably in water, the only spectator ion is K+.

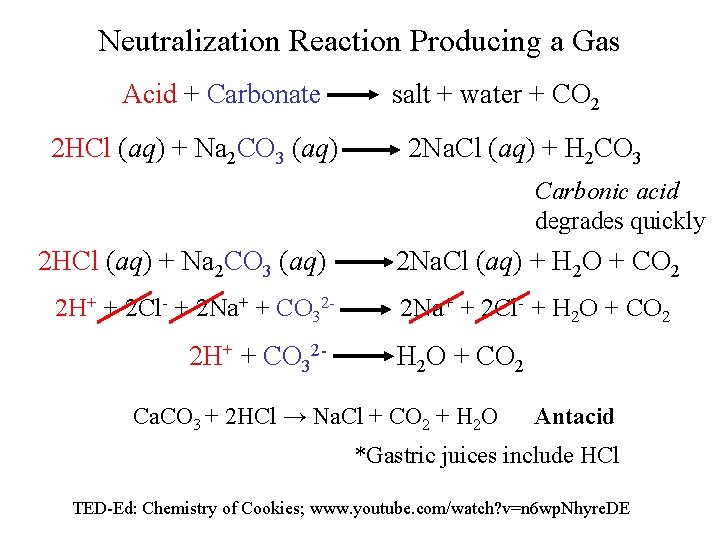
Neutralization Reaction Producing a Gas Acid + Carbonate 2 HCl (aq) + Na 2 CO 3 (aq) salt + water + CO 2 2 Na. Cl (aq) + H 2 CO 3 Carbonic acid degrades quickly 2 HCl (aq) + Na 2 CO 3 (aq) 2 H+ + 2 Cl- + 2 Na+ + CO 32 - 2 H+ + CO 32 - 2 Na. Cl (aq) + H 2 O + CO 2 2 Na+ + 2 Cl- + H 2 O + CO 2 Ca. CO 3 + 2 HCl → Na. Cl + CO 2 + H 2 O Antacid *Gastric juices include HCl TED-Ed: Chemistry of Cookies; www. youtube. com/watch? v=n 6 wp. Nhyre. DE

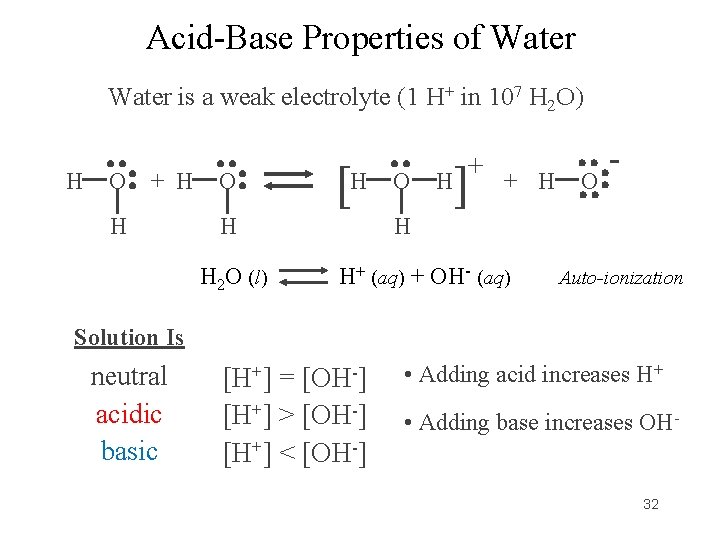
Acid-Base Properties of Water is a weak electrolyte (1 H+ in 107 H 2 O) H O + H H O [H H H 2 O (l) O + ] H + H O - H H+ (aq) + OH- (aq) Auto-ionization Solution Is neutral acidic basic [H+] = [OH-] [H+] > [OH-] [H+] < [OH-] • Adding acid increases H+ • Adding base increases OH 32
![p H A Measure of Acidity p H describes the concentration of H p. H – A Measure of Acidity p. H describes the concentration of [H+]](https://slidetodoc.com/presentation_image_h2/ba2eb3943b132c6d6c25d03ddd00ac93/image-33.jpg)
p. H – A Measure of Acidity p. H describes the concentration of [H+] on a logarithmic scale. p. H = -log [H+] Remember: A log scale is base 10: p. H 6 is 10 x more acidic than p. H 7 p. H 5 is 100 x more acidic than p. H 7 p. H 4 is 103 x more acidic than p. H 7 Solution Is neutral [H+] = [OH-] [H+] = 1. 0 x 10 -7 p. H = 7 acidic [H+] > [OH-] [H+] > 1. 0 x 10 -7 p. H < 7 basic [H+] < [OH-] [H+] < 1. 0 x 10 -7 p. H > 7 Practice: Compare the acidity of p. H 8 to p. H 10.

p. H Meter H+ is an electrolyte p. H meters measure electrical conductance to determine concentration and then calculate p. H

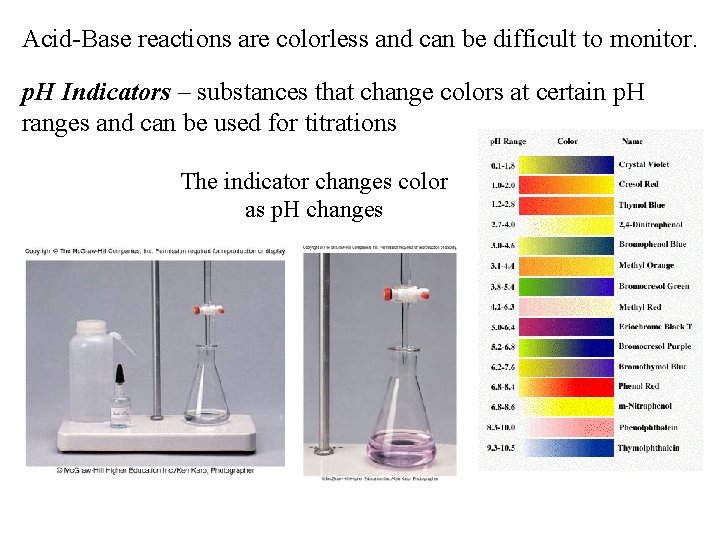
Acid-Base reactions are colorless and can be difficult to monitor. p. H Indicators – substances that change colors at certain p. H ranges and can be used for titrations The indicator changes color as p. H changes

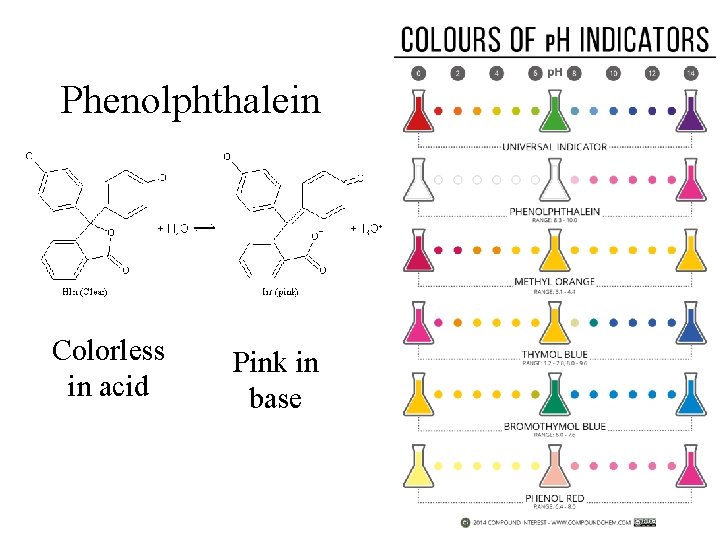
Phenolphthalein Colorless in acid Pink in base

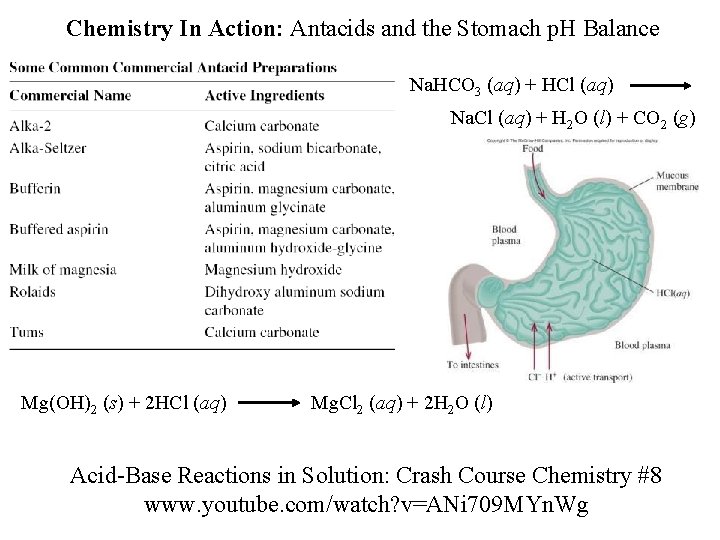
Chemistry In Action: Antacids and the Stomach p. H Balance Na. HCO 3 (aq) + HCl (aq) Na. Cl (aq) + H 2 O (l) + CO 2 (g) Mg(OH)2 (s) + 2 HCl (aq) Mg. Cl 2 (aq) + 2 H 2 O (l) Acid-Base Reactions in Solution: Crash Course Chemistry #8 www. youtube. com/watch? v=ANi 709 MYn. Wg

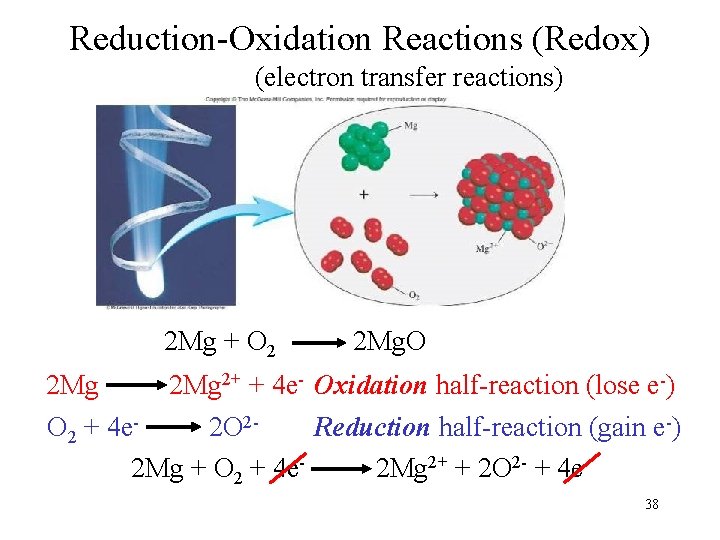
Reduction-Oxidation Reactions (Redox) (electron transfer reactions) 2 Mg + O 2 2 Mg. O 2 Mg 2+ + 4 e- Oxidation half-reaction (lose e-) O 2 + 4 e 2 O 2 Reduction half-reaction (gain e-) 2 Mg + O 2 + 4 e 2 Mg 2+ + 2 O 2 - + 4 e 38

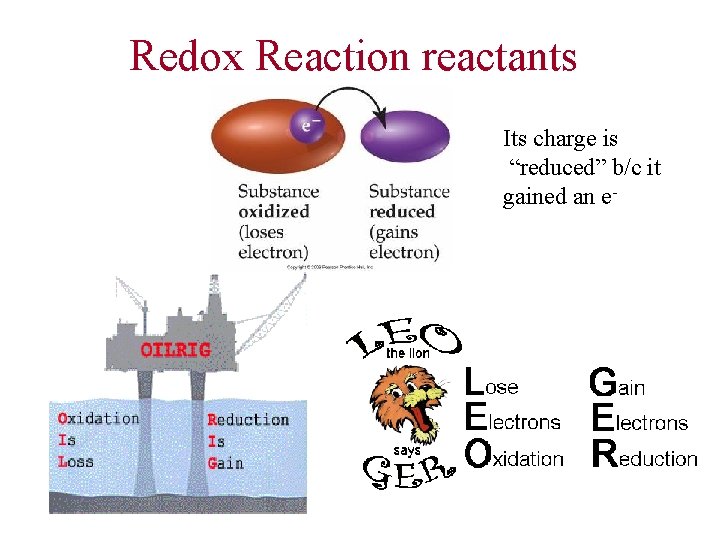
Redox Reaction reactants Its charge is “reduced” b/c it gained an e-

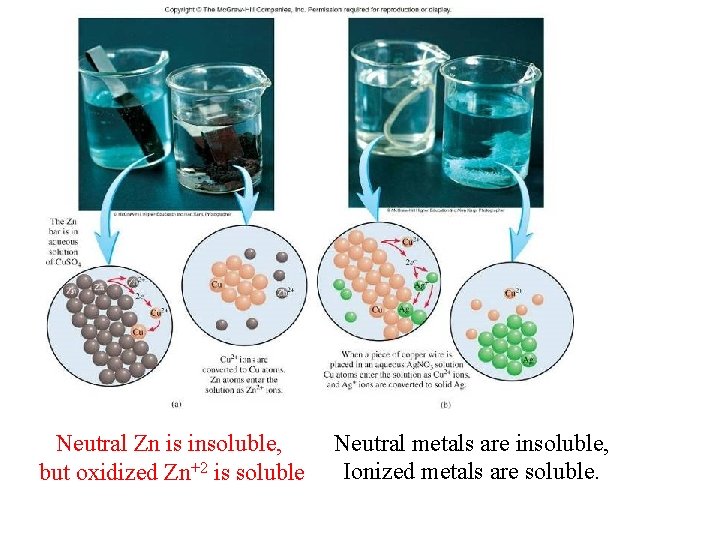
Neutral Zn is insoluble, but oxidized Zn+2 is soluble Neutral metals are insoluble, Ionized metals are soluble.

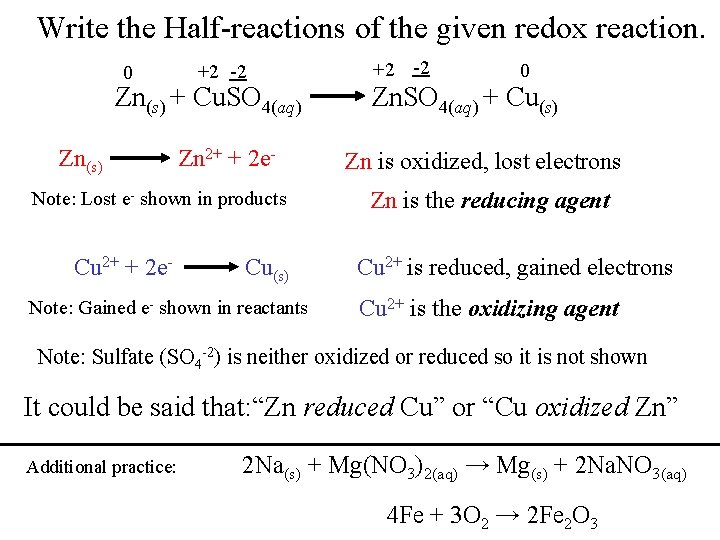
Write the Half-reactions of the given redox reaction. 0 +2 -2 Zn(s) + Cu. SO 4(aq) Zn(s) Zn 2+ + 2 e- Note: Lost e- shown in products Cu 2+ + 2 e- Cu(s) Note: Gained e- shown in reactants +2 -2 0 Zn. SO 4(aq) + Cu(s) Zn is oxidized, lost electrons Zn is the reducing agent Cu 2+ is reduced, gained electrons Cu 2+ is the oxidizing agent Note: Sulfate (SO 4 -2) is neither oxidized or reduced so it is not shown It could be said that: “Zn reduced Cu” or “Cu oxidized Zn” Additional practice: 2 Na(s) + Mg(NO 3)2(aq) → Mg(s) + 2 Na. NO 3(aq) 4 Fe + 3 O 2 → 2 Fe 2 O 3

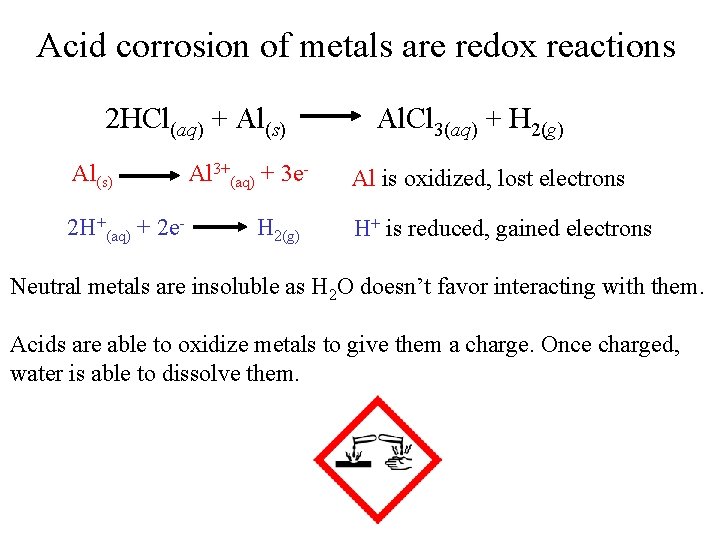
Acid corrosion of metals are redox reactions 2 HCl(aq) + Al(s) 2 H+(aq) + 2 e- Al 3+(aq) + 3 e. H 2(g) Al. Cl 3(aq) + H 2(g) Al is oxidized, lost electrons H+ is reduced, gained electrons Neutral metals are insoluble as H 2 O doesn’t favor interacting with them. Acids are able to oxidize metals to give them a charge. Once charged, water is able to dissolve them.

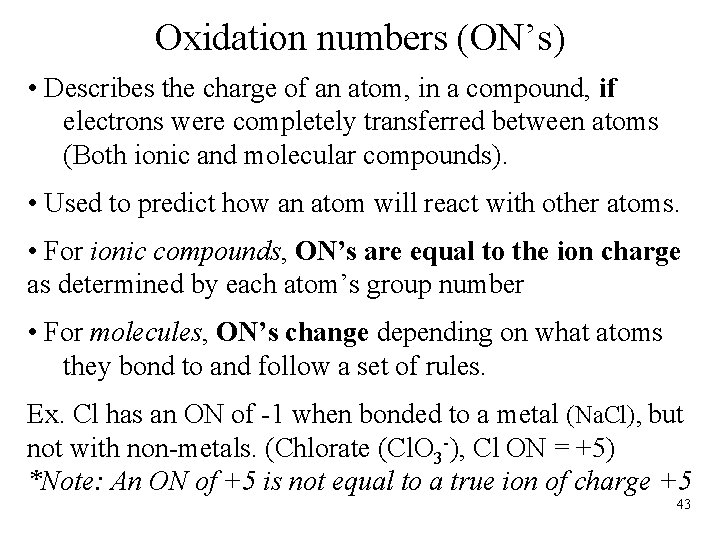
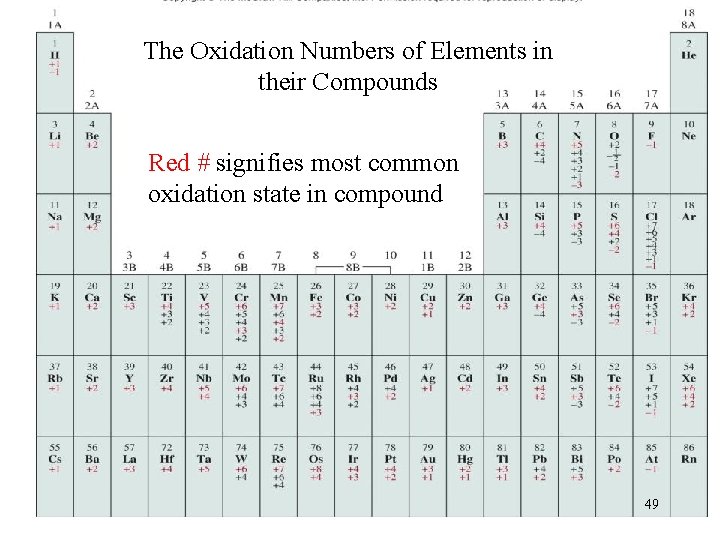
Oxidation numbers (ON’s) • Describes the charge of an atom, in a compound, if electrons were completely transferred between atoms (Both ionic and molecular compounds). • Used to predict how an atom will react with other atoms. • For ionic compounds, ON’s are equal to the ion charge as determined by each atom’s group number • For molecules, ON’s change depending on what atoms they bond to and follow a set of rules. Ex. Cl has an ON of -1 when bonded to a metal (Na. Cl), but not with non-metals. (Chlorate (Cl. O 3 -), Cl ON = +5) *Note: An ON of +5 is not equal to a true ion of charge +5 43

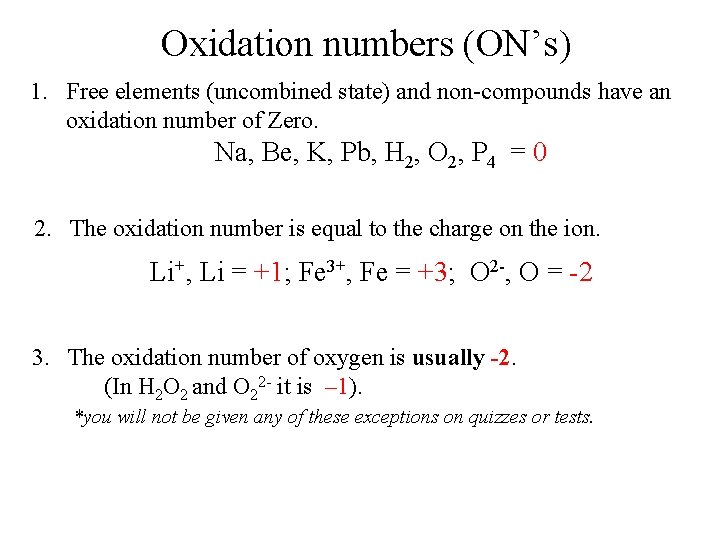
Oxidation numbers (ON’s) 1. Free elements (uncombined state) and non-compounds have an oxidation number of Zero. Na, Be, K, Pb, H 2, O 2, P 4 = 0 2. The oxidation number is equal to the charge on the ion. Li+, Li = +1; Fe 3+, Fe = +3; O 2 -, O = -2 3. The oxidation number of oxygen is usually -2. (In H 2 O 2 and O 22 - it is – 1). *you will not be given any of these exceptions on quizzes or tests.

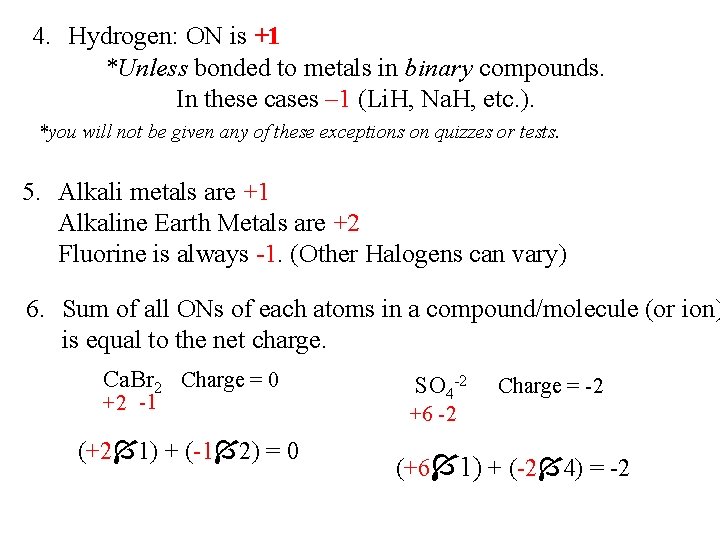
4. Hydrogen: ON is +1 *Unless bonded to metals in binary compounds. In these cases – 1 (Li. H, Na. H, etc. ). *you will not be given any of these exceptions on quizzes or tests. 5. Alkali metals are +1 Alkaline Earth Metals are +2 Fluorine is always -1. (Other Halogens can vary) 6. Sum of all ONs of each atoms in a compound/molecule (or ion) is equal to the net charge. Ca. Br 2 Charge = 0 +2 -1 (+2 1) + (-1 2) = 0 SO 4 -2 Charge = -2 +6 -2 (+6 1) + (-2 4) = -2

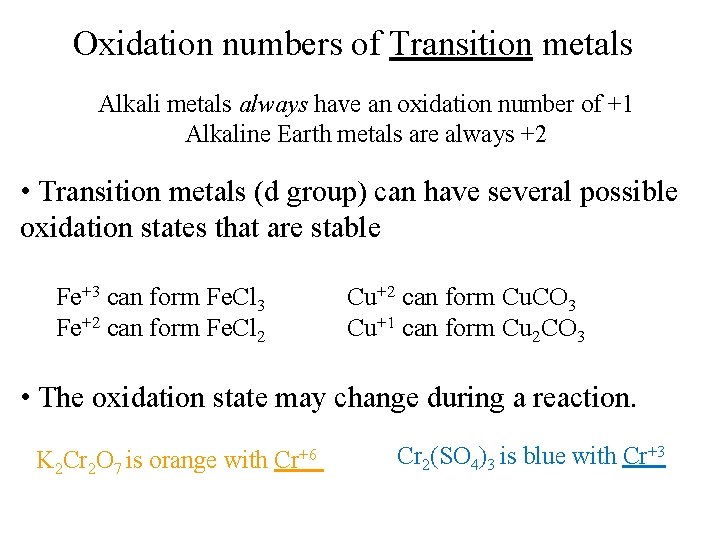
Oxidation numbers of Transition metals Alkali metals always have an oxidation number of +1 Alkaline Earth metals are always +2 • Transition metals (d group) can have several possible oxidation states that are stable Fe+3 can form Fe. Cl 3 Fe+2 can form Fe. Cl 2 Cu+2 can form Cu. CO 3 Cu+1 can form Cu 2 CO 3 • The oxidation state may change during a reaction. K 2 Cr 2 O 7 is orange with Cr+6 Cr 2(SO 4)3 is blue with Cr+3

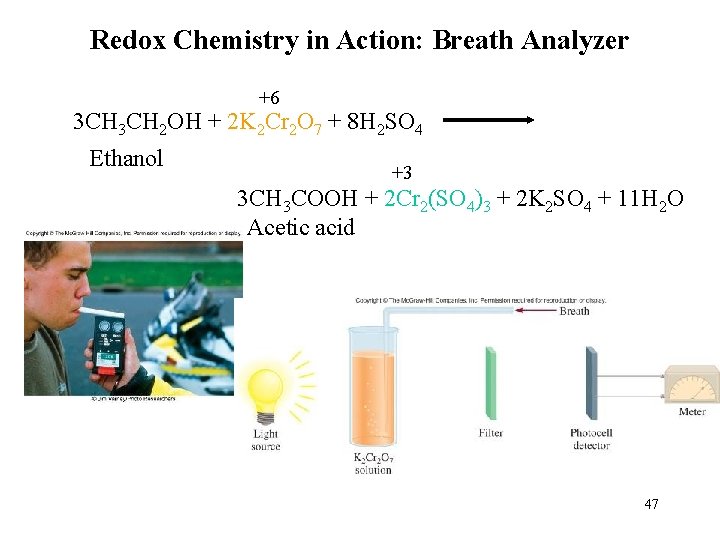
Redox Chemistry in Action: Breath Analyzer +6 3 CH 2 OH + 2 K 2 Cr 2 O 7 + 8 H 2 SO 4 Ethanol +3 3 CH 3 COOH + 2 Cr 2(SO 4)3 + 2 K 2 SO 4 + 11 H 2 O Acetic acid 47

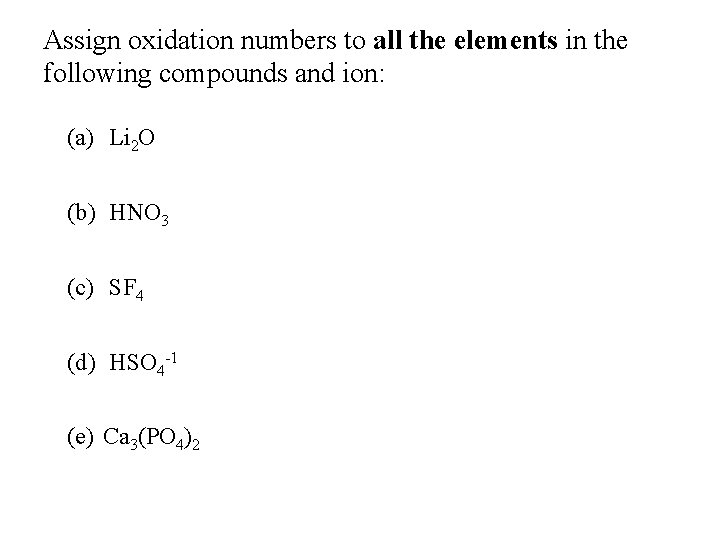
Assign oxidation numbers to all the elements in the following compounds and ion: +1 -2 (a) Li 2 O Oxygen is always -2 and Li is +1 in a compound (+1) • 2 + (-2) • 1 = 0 +1 +5 -2 (b) HNO 3 +4 Oxygen is always -2 and H is +1 in a compound. (+1) • 1 + (N) • 1 + (-2) • 3 = 0 ; N must = +5 -1 (c) SF 4 Fluorine is always -1 and the sum must equal 0 (S) • 1 + (-1) • 4 = 0 ; S must = +4 +1 +6 -2 (d) HSO 4 -1 +2 Oxygen is always -2 and H is +1 in a compound. (+1) • 1 + (S) • 1 + (-2) • 4 = -1 ; S must = +6 +5 -2 (e) Ca 3(PO 4)2 Oxygen is always -2 and Ca is +2 in a compound. (+2) • 3 + (P) • 2 + (-2) • 8 = 0 ; P must = +5

The Oxidation Numbers of Elements in their Compounds Red # signifies most common oxidation state in compound 49

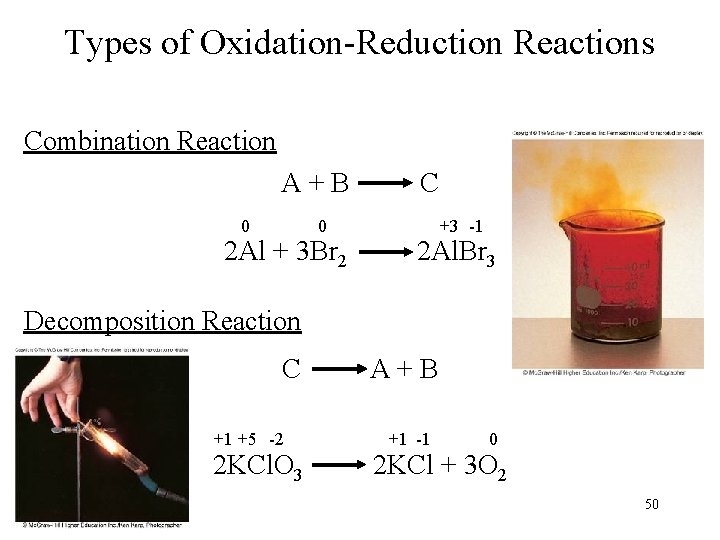
Types of Oxidation-Reduction Reactions Combination Reaction A+B 0 0 2 Al + 3 Br 2 C +3 -1 2 Al. Br 3 Decomposition Reaction C +1 +5 -2 2 KCl. O 3 A+B +1 -1 0 2 KCl + 3 O 2 50

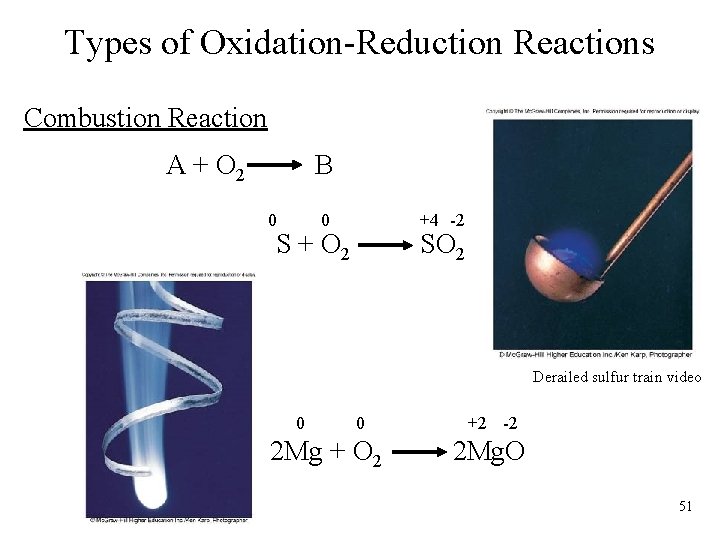
Types of Oxidation-Reduction Reactions Combustion Reaction A + O 2 B 0 +4 -2 0 S + O 2 SO 2 Derailed sulfur train video 0 0 2 Mg + O 2 +2 -2 2 Mg. O 51

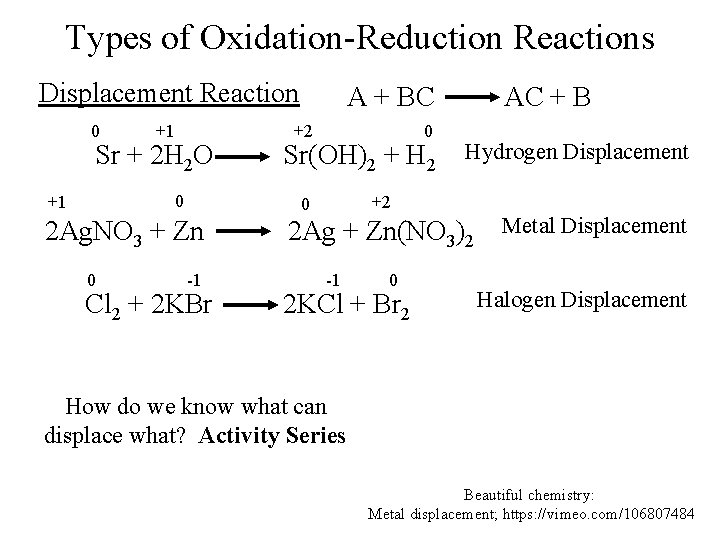
Types of Oxidation-Reduction Reactions Displacement Reaction 0 +1 Sr + 2 H 2 O 0 +1 +2 0 Sr(OH)2 + H 2 -1 Cl 2 + 2 KBr AC + B Hydrogen Displacement +2 0 2 Ag. NO 3 + Zn 0 A + BC 2 Ag + Zn(NO 3)2 Metal Displacement -1 0 2 KCl + Br 2 Halogen Displacement How do we know what can displace what? Activity Series Beautiful chemistry: Metal displacement; https: //vimeo. com/106807484

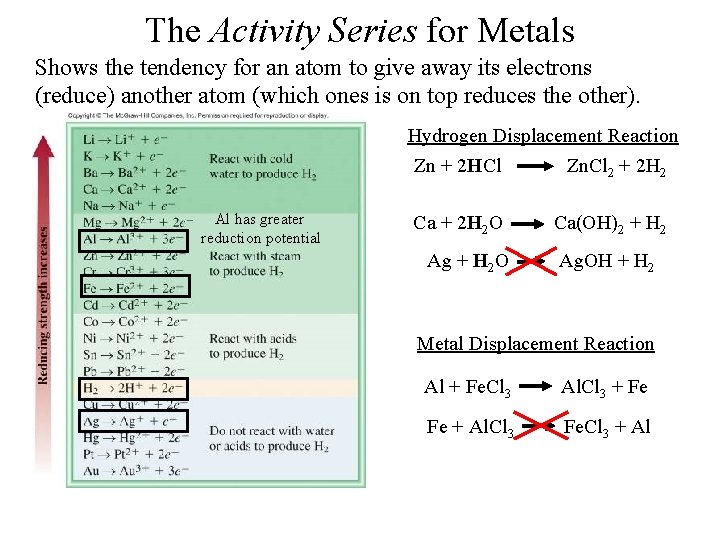
The Activity Series for Metals Shows the tendency for an atom to give away its electrons (reduce) another atom (which ones is on top reduces the other). Hydrogen Displacement Reaction Al has greater reduction potential Zn + 2 HCl Zn. Cl 2 + 2 H 2 Ca + 2 H 2 O Ca(OH)2 + H 2 Ag + H 2 O Ag. OH + H 2 Metal Displacement Reaction Al + Fe. Cl 3 Al. Cl 3 + Fe Fe + Al. Cl 3 Fe. Cl 3 + Al

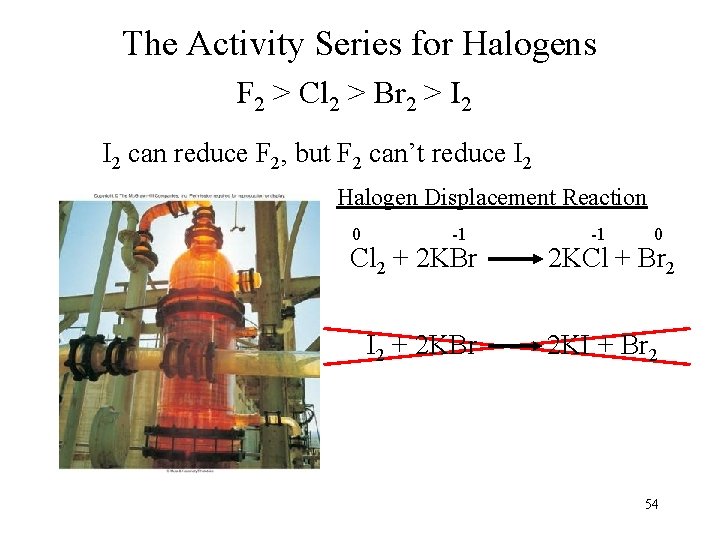
The Activity Series for Halogens F 2 > Cl 2 > Br 2 > I 2 can reduce F 2, but F 2 can’t reduce I 2 Halogen Displacement Reaction 0 -1 Cl 2 + 2 KBr I 2 + 2 KBr -1 0 2 KCl + Br 2 2 KI + Br 2 54

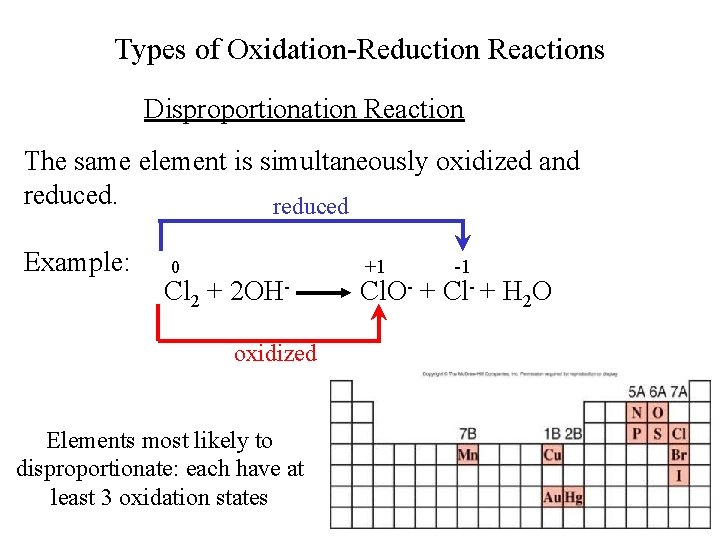
Types of Oxidation-Reduction Reactions Disproportionation Reaction The same element is simultaneously oxidized and reduced Example: 0 Cl 2 + 2 OHoxidized Elements most likely to disproportionate: each have at least 3 oxidation states +1 -1 Cl. O- + Cl- + H 2 O

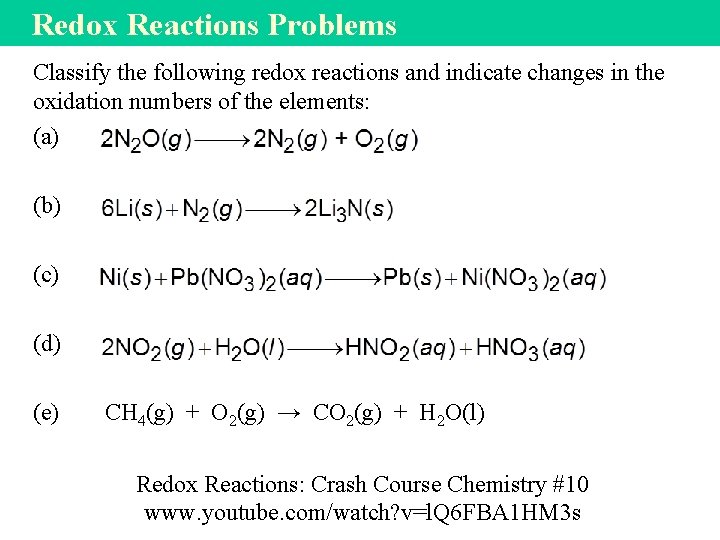
Redox Reactions Problems Classify the following redox reactions and indicate changes in the oxidation numbers of the elements: (a) (b) (c) (d) (e) CH 4(g) + O 2(g) → CO 2(g) + H 2 O(l) Redox Reactions: Crash Course Chemistry #10 www. youtube. com/watch? v=l. Q 6 FBA 1 HM 3 s

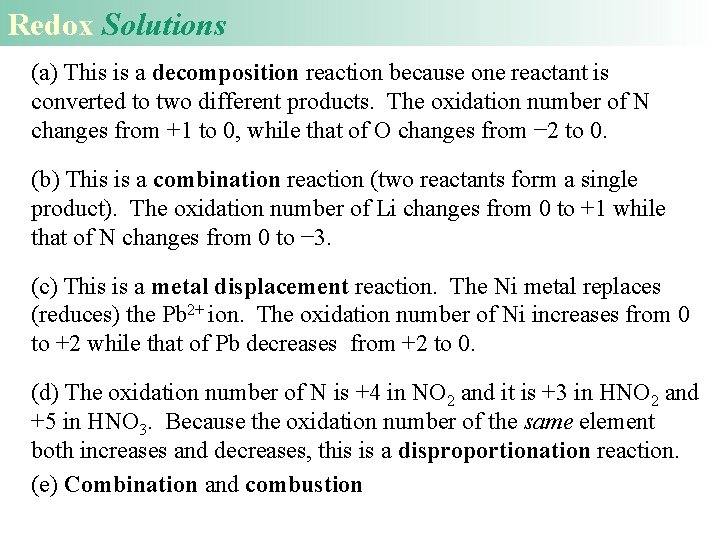
Redox Solutions (a) This is a decomposition reaction because one reactant is converted to two different products. The oxidation number of N changes from +1 to 0, while that of O changes from − 2 to 0. (b) This is a combination reaction (two reactants form a single product). The oxidation number of Li changes from 0 to +1 while that of N changes from 0 to − 3. (c) This is a metal displacement reaction. The Ni metal replaces (reduces) the Pb 2+ ion. The oxidation number of Ni increases from 0 to +2 while that of Pb decreases from +2 to 0. (d) The oxidation number of N is +4 in NO 2 and it is +3 in HNO 2 and +5 in HNO 3. Because the oxidation number of the same element both increases and decreases, this is a disproportionation reaction. (e) Combination and combustion

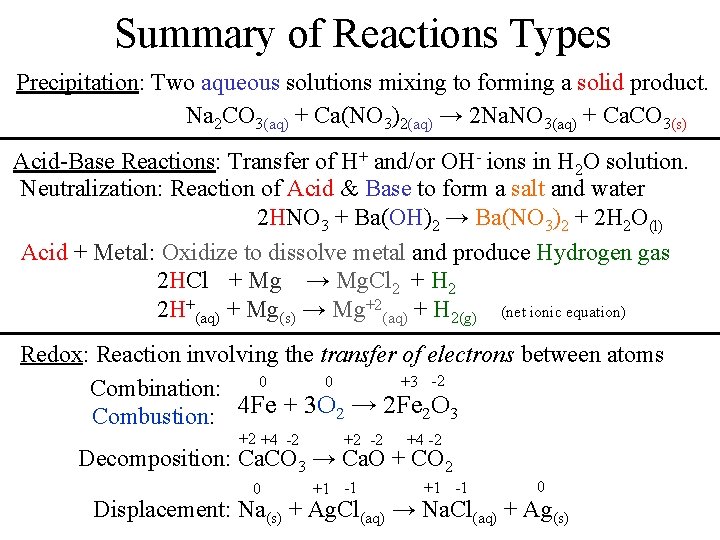
Summary of Reactions Types Precipitation: Two aqueous solutions mixing to forming a solid product. Na 2 CO 3(aq) + Ca(NO 3)2(aq) → 2 Na. NO 3(aq) + Ca. CO 3(s) Acid-Base Reactions: Transfer of H+ and/or OH- ions in H 2 O solution. Neutralization: Reaction of Acid & Base to form a salt and water 2 HNO 3 + Ba(OH)2 → Ba(NO 3)2 + 2 H 2 O(l) Acid + Metal: Oxidize to dissolve metal and produce Hydrogen gas 2 HCl + Mg → Mg. Cl 2 + H 2 2 H+(aq) + Mg(s) → Mg+2(aq) + H 2(g) (net ionic equation) Redox: Reaction involving the transfer of electrons between atoms 0 0 +3 -2 Combination: Combustion: 4 Fe + 3 O 2 → 2 Fe 2 O 3 +2 +4 -2 +2 -2 +4 -2 Decomposition: Ca. CO 3 → Ca. O + CO 2 0 +1 -1 0 Displacement: Na(s) + Ag. Cl(aq) → Na. Cl(aq) + Ag(s)

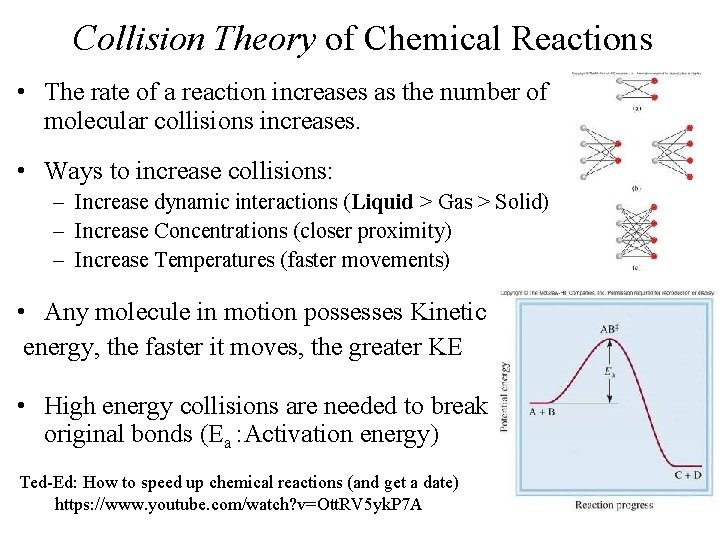
Collision Theory of Chemical Reactions • The rate of a reaction increases as the number of molecular collisions increases. • Ways to increase collisions: – Increase dynamic interactions (Liquid > Gas > Solid) – Increase Concentrations (closer proximity) – Increase Temperatures (faster movements) • Any molecule in motion possesses Kinetic energy, the faster it moves, the greater KE • High energy collisions are needed to break original bonds (Ea : Activation energy) Ted-Ed: How to speed up chemical reactions (and get a date) https: //www. youtube. com/watch? v=Ott. RV 5 yk. P 7 A

Disposing of excess WWII Sodium metal in 1947 2 Na + 2 H 2 O → 2 Na. OH + H 2(g) 60

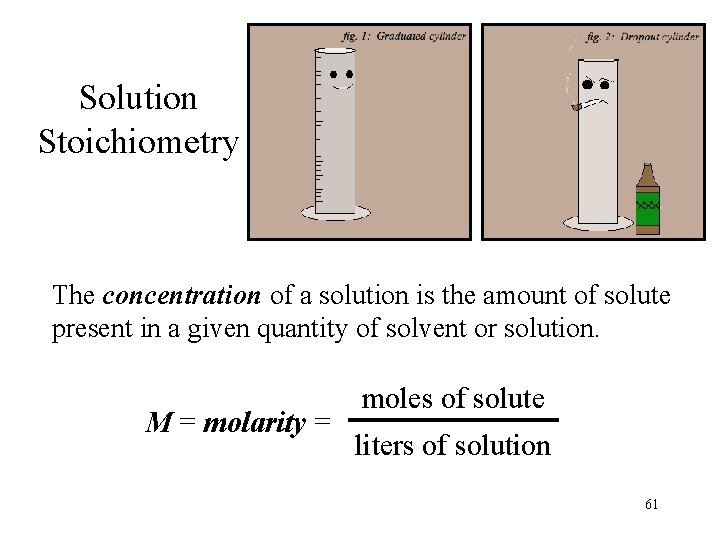
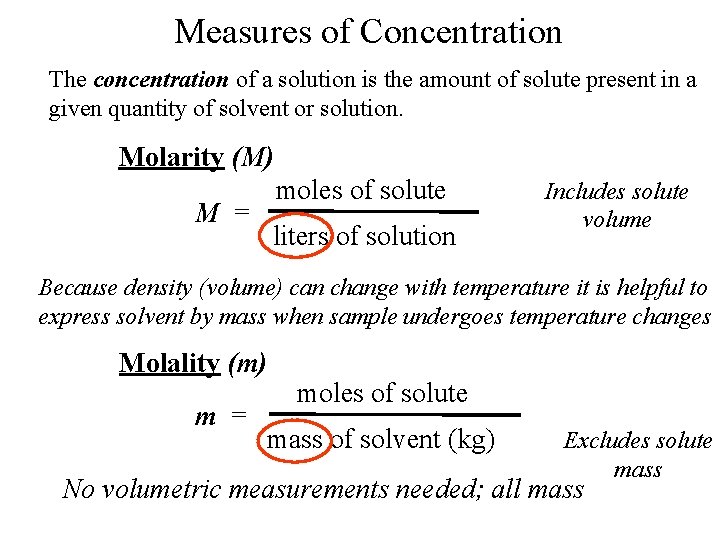
Solution Stoichiometry The concentration of a solution is the amount of solute present in a given quantity of solvent or solution. M = molarity = moles of solute liters of solution 61

Measures of Concentration The concentration of a solution is the amount of solute present in a given quantity of solvent or solution. Molarity (M) moles of solute M = liters of solution Includes solute volume Because density (volume) can change with temperature it is helpful to express solvent by mass when sample undergoes temperature changes Molality (m) m = moles of solute mass of solvent (kg) Excludes solute mass No volumetric measurements needed; all mass

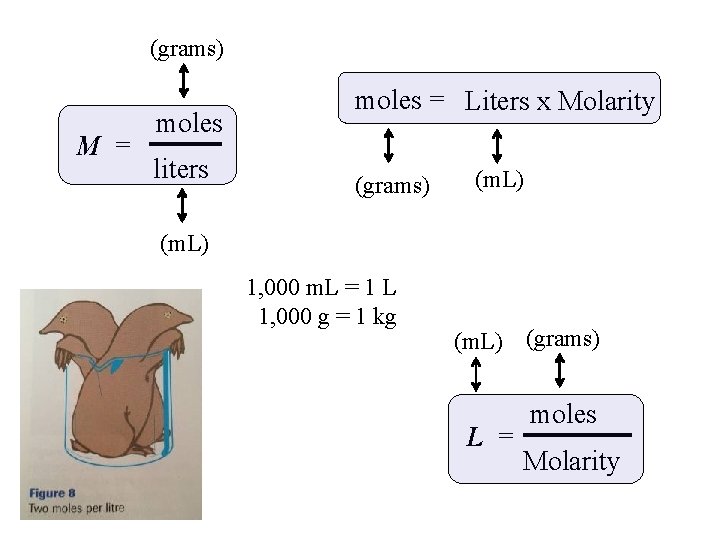
(grams) M = moles liters moles = Liters x Molarity (grams) (m. L) 1, 000 m. L = 1 L 1, 000 g = 1 kg (m. L) (grams) L = moles Molarity

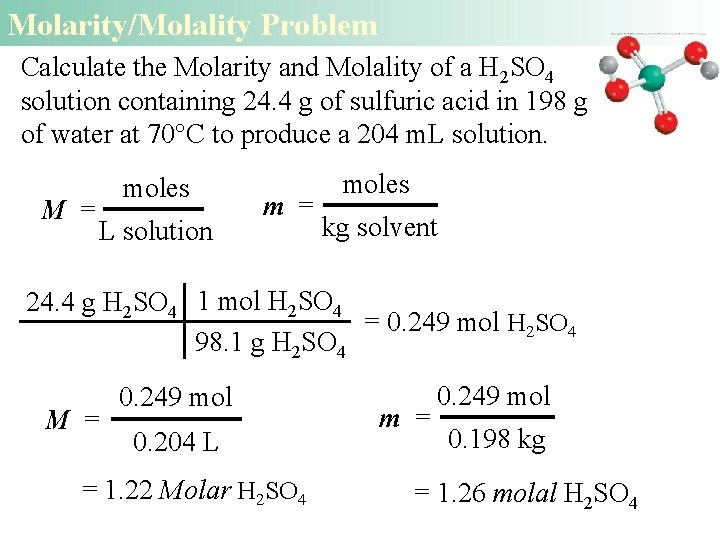
Molarity/Molality Problem Calculate the Molarity and Molality of a H 2 SO 4 solution containing 24. 4 g of sulfuric acid in 198 g of water at 70°C to produce a 204 m. L solution. M = moles L solution m = moles kg solvent 24. 4 g H 2 SO 4 1 mol H 2 SO 4 = 0. 249 mol H 2 SO 4 98. 1 g H 2 SO 4 M = 0. 249 mol 0. 204 L = 1. 22 Molar H 2 SO 4 m = 0. 249 mol 0. 198 kg = 1. 26 molal H 2 SO 4
![Ion Molarity Problem What is the Molar concentration of the Potassium ion K when Ion Molarity Problem What is the Molar concentration of the Potassium ion [K+] when](https://slidetodoc.com/presentation_image_h2/ba2eb3943b132c6d6c25d03ddd00ac93/image-65.jpg)
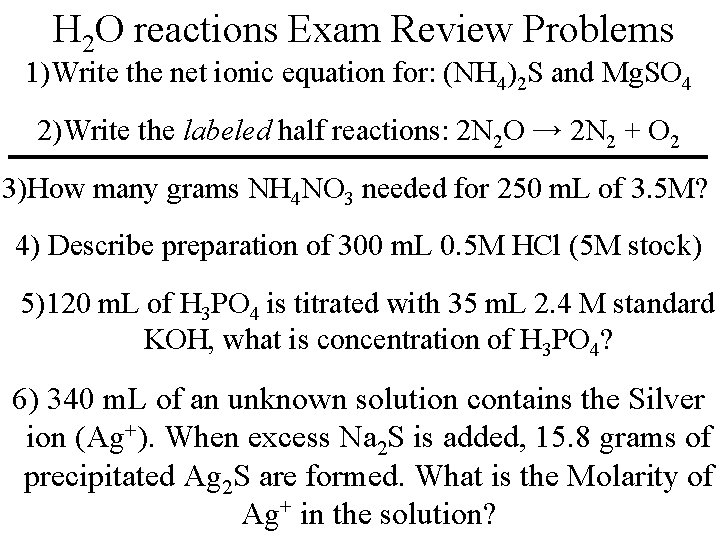
Ion Molarity Problem What is the Molar concentration of the Potassium ion [K+] when 9. 85 g of K 2 CO 3 are dissolved in a 250 m. L solution? 9. 85 g K 2 CO 3 1 mol K 2 CO 3 2 mol K+ 138. 2 g K 2 CO 3 1 mol K 2 CO 3(s) → 2 K+(aq) + CO 3 -2(aq) M = 0. 143 mol 0. 250 L = 0. 143 mol K+ = 0. 57 M K+ Practice: Molarity of [NO 3 -1] in 2. 5 grams Mg(NO 3)2 in 1. 4 L solution.

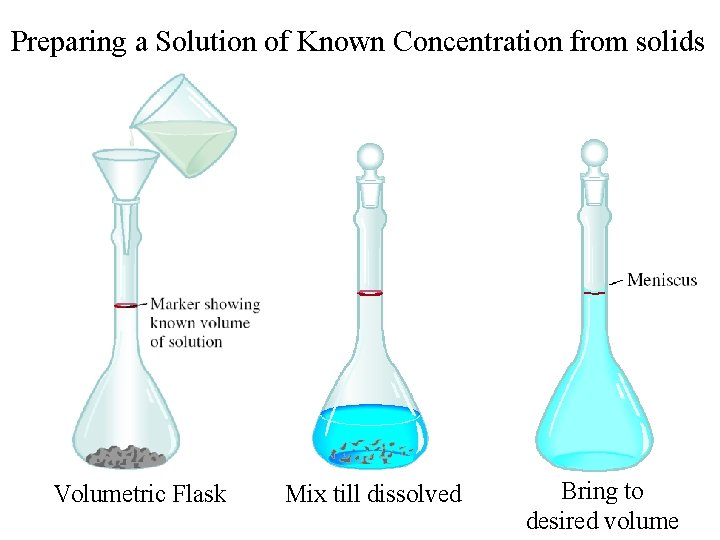
Preparing a Solution of Known Concentration from solids Volumetric Flask Mix till dissolved Bring to desired volume
![Molarity Problem What is the Molar concentration of the Sodium ion Na when 23 Molarity Problem What is the Molar concentration of the Sodium ion [Na+] when 23.](https://slidetodoc.com/presentation_image_h2/ba2eb3943b132c6d6c25d03ddd00ac93/image-67.jpg)
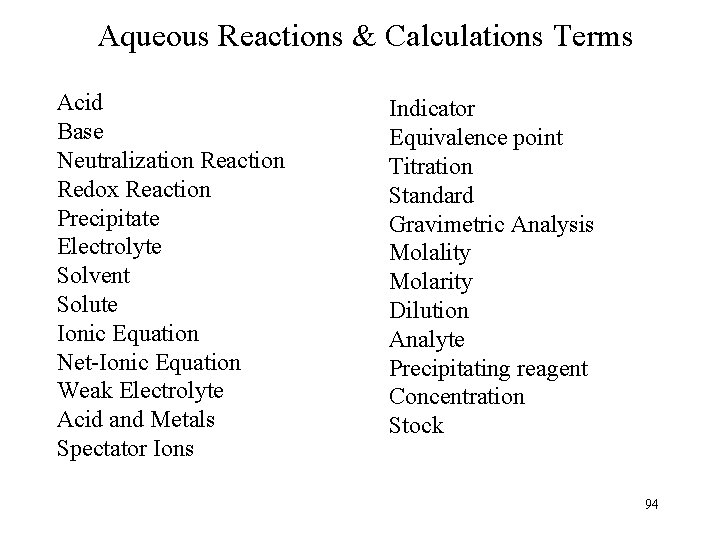
Molarity Problem What is the Molar concentration of the Sodium ion [Na+] when 23. 4 g of Na. Cl and 34. 1 g of Na 2 O are dissolved in 0. 60 L H 2 O? 23. 4 g Na. Cl 1 mol 58. 5 g Na. Cl 34. 1 g Na 2 O 1 mol Na 2 O = 0. 40 mol Na. Cl = 0. 40 mol Na+ 2 mol Na+ 62. 8 g Na 2 O 1 mol Na 2 O = 1. 08 mol Na+ (doubles from subscript) 0. 40 mol Na+ + 1. 08 mol Na+ = 1. 48 mol Na+ M = 1. 48 mol 0. 60 L = 2. 5 M Na+ 67

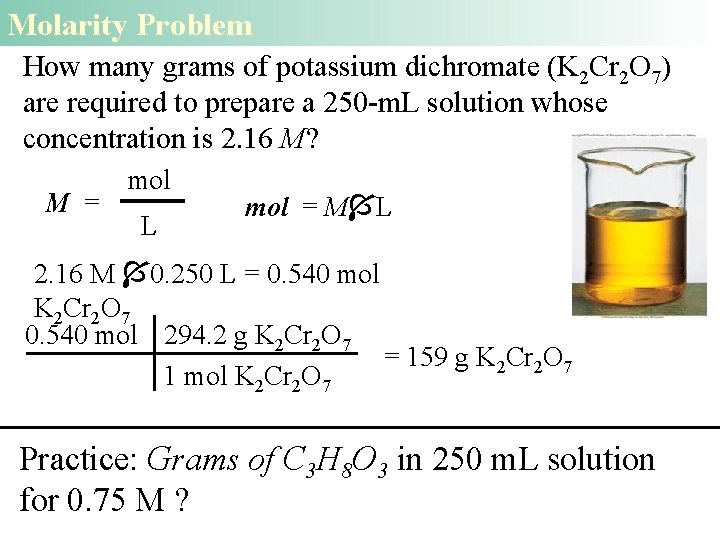
Molarity Problem How many grams of potassium dichromate (K 2 Cr 2 O 7) are required to prepare a 250 -m. L solution whose concentration is 2. 16 M? M = mol L mol = M L 2. 16 M 0. 250 L = 0. 540 mol K 2 Cr 2 O 7 0. 540 mol 294. 2 g K 2 Cr 2 O 7 = 159 g K 2 Cr 2 O 7 1 mol K 2 Cr 2 O 7 Practice: Grams of C 3 H 8 O 3 in 250 m. L solution for 0. 75 M ?

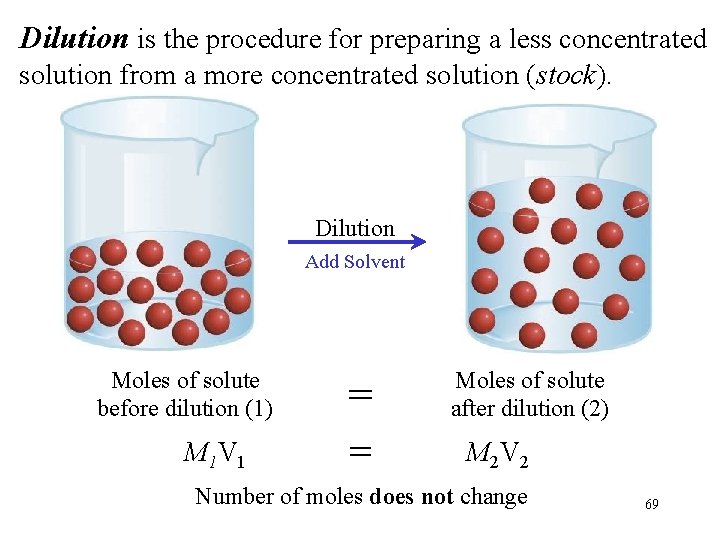
Dilution is the procedure for preparing a less concentrated solution from a more concentrated solution (stock). Dilution Add Solvent Moles of solute before dilution (1) M 1 V 1 = = Moles of solute after dilution (2) M 2 V 2 Number of moles does not change 69

Dilution Practice Describe how you would prepare 250 m. L of a 1. 75 M H 2 SO 4 solution, starting with an 8. 6 M stock solution of H 2 SO 4. Keep in mind that in dilution, the concentration of the solution decreases but the number of moles of the solute remains the same. Stock New soln M 1 V 1 = M 2 V 2 M 1 = 8. 6 V 1 = ? M 2 =1. 75 V 2 = 250 m. L (8. 6 M V 1) = (1. 75 M 250 m. L) V 1 = 50. 9 m. L Dilute 50. 9 m. L of the 8. 6 M H 2 SO 4 stock with water to give a final volume of 250 m. L. *A description required to answer the question

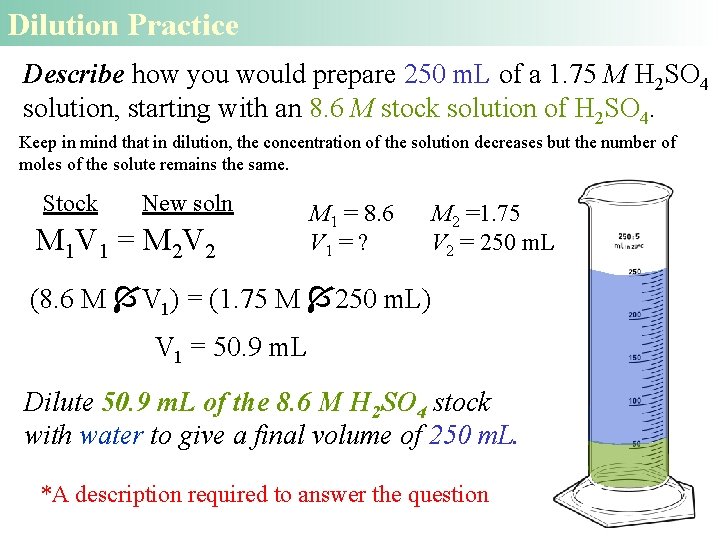
Dilution Problem Describe how you would prepare 300 m. L of a 0. 4 M C 6 H 12 O 6 solution, starting with an 2. 5 M stock solution of C 6 H 12 O 6. M 1 V 1 = M 2 V 2 (2. 5 M V 1) = (0. 4 M 300 m. L) V 1 = 48 m. L Thus, we must dilute 48 m. L of the 2. 5 M solution with water to give a final volume of 300 m. L

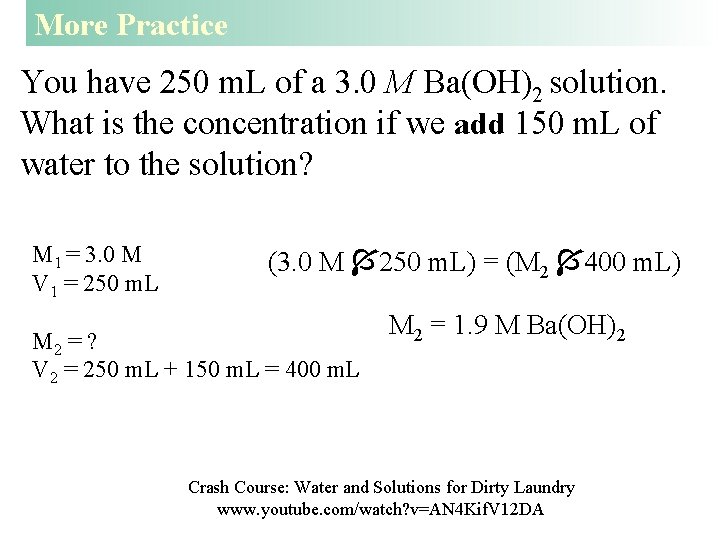
More Practice You have 250 m. L of a 3. 0 M Ba(OH)2 solution. What is the concentration if we add 150 m. L of water to the solution? M 1 = 3. 0 M V 1 = 250 m. L (3. 0 M 250 m. L) = (M 2 400 m. L) M 2 = ? V 2 = 250 m. L + 150 m. L = 400 m. L M 2 = 1. 9 M Ba(OH)2 Crash Course: Water and Solutions for Dirty Laundry www. youtube. com/watch? v=AN 4 Kif. V 12 DA

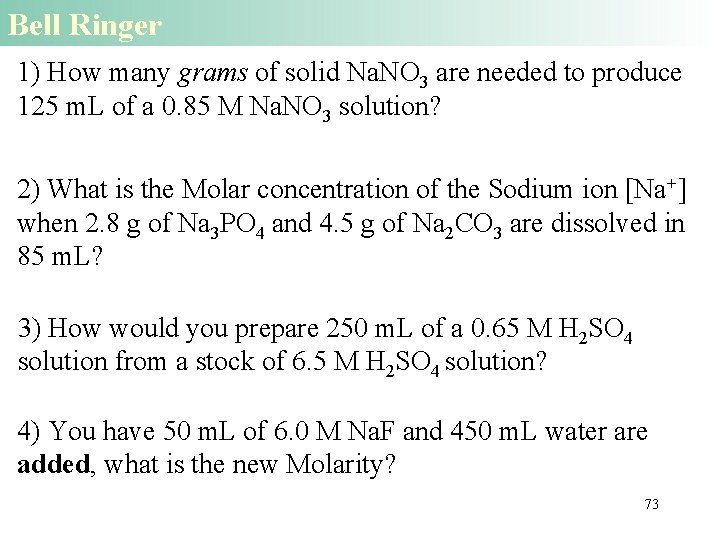
Bell Ringer 1) How many grams of solid Na. NO 3 are needed to produce 125 m. L of a 0. 85 M Na. NO 3 solution? 2) What is the Molar concentration of the Sodium ion [Na+] when 2. 8 g of Na 3 PO 4 and 4. 5 g of Na 2 CO 3 are dissolved in 85 m. L? 3) How would you prepare 250 m. L of a 0. 65 M H 2 SO 4 solution from a stock of 6. 5 M H 2 SO 4 solution? 4) You have 50 m. L of 6. 0 M Na. F and 450 m. L water are added, what is the new Molarity? 73

Titrations In a titration, a solution of accurately known concentration is added gradually to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Standard solution – solution with known concentration to be precisely added for comparison Equivalence (End) point – the point at which the reaction is complete example) 1 mol H 2 SO 4 2 mol Na. OH (obtained from balanced equation) 74

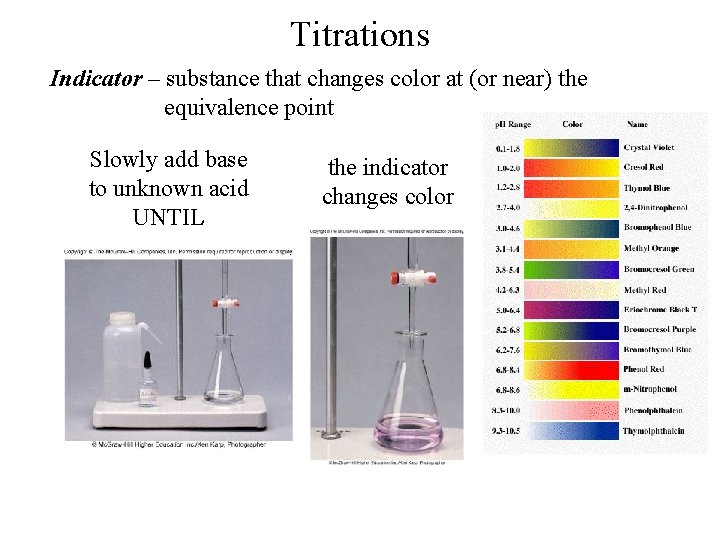
Titrations Indicator – substance that changes color at (or near) the equivalence point Slowly add base to unknown acid UNTIL the indicator changes color

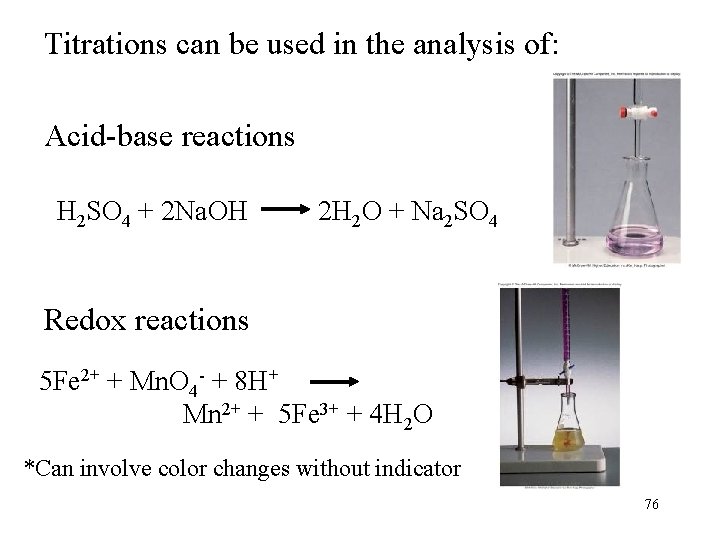
Titrations can be used in the analysis of: Acid-base reactions H 2 SO 4 + 2 Na. OH 2 H 2 O + Na 2 SO 4 Redox reactions 5 Fe 2+ + Mn. O 4 - + 8 H+ Mn 2+ + 5 Fe 3+ + 4 H 2 O *Can involve color changes without indicator 76

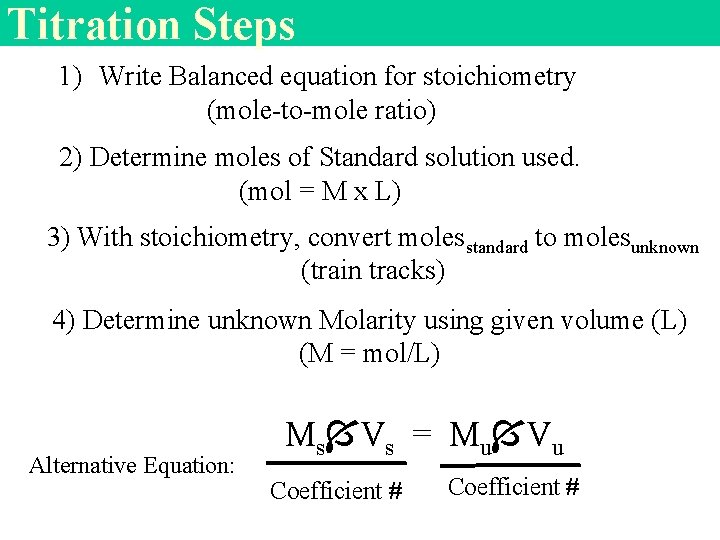
Titration Steps 1) Write Balanced equation for stoichiometry (mole-to-mole ratio) 2) Determine moles of Standard solution used. (mol = M x L) 3) With stoichiometry, convert molesstandard to molesunknown (train tracks) 4) Determine unknown Molarity using given volume (L) (M = mol/L) Alternative Equation: Ms Vs = Mu Vu Coefficient #

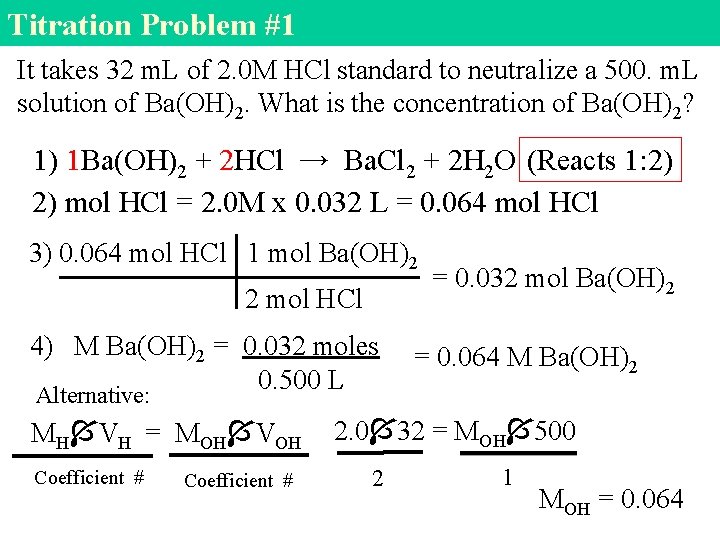
Titration Problem #1 It takes 32 m. L of 2. 0 M HCl standard to neutralize a 500. m. L solution of Ba(OH)2. What is the concentration of Ba(OH)2? 1) 1 Ba(OH)2 + 2 HCl → Ba. Cl 2 + 2 H 2 O (Reacts 1: 2) 2) mol HCl = 2. 0 M x 0. 032 L = 0. 064 mol HCl 3) 0. 064 mol HCl 1 mol Ba(OH)2 2 mol HCl 4) M Ba(OH)2 = 0. 032 moles 0. 500 L = 0. 032 mol Ba(OH)2 = 0. 064 M Ba(OH)2 Alternative: MH VH = MOH VOH Coefficient # 2. 0 32 = MOH 500 2 1 MOH = 0. 064

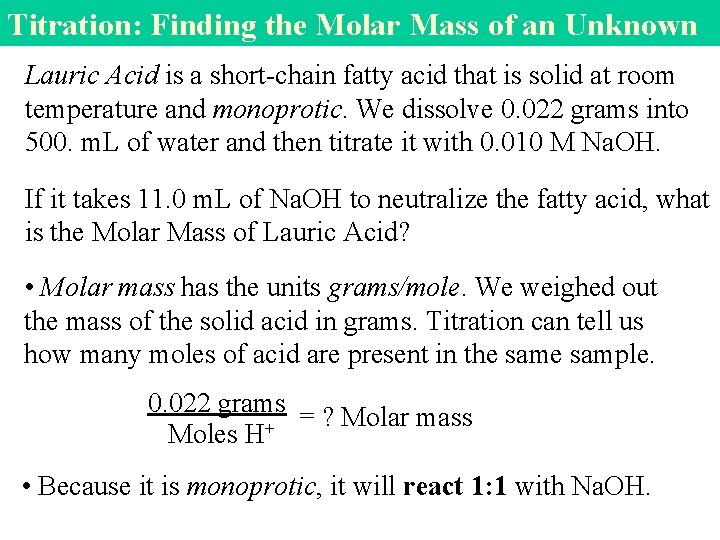
Titration: Finding the Molar Mass of an Unknown Lauric Acid is a short-chain fatty acid that is solid at room temperature and monoprotic. We dissolve 0. 022 grams into 500. m. L of water and then titrate it with 0. 010 M Na. OH. If it takes 11. 0 m. L of Na. OH to neutralize the fatty acid, what is the Molar Mass of Lauric Acid? • Molar mass has the units grams/mole. We weighed out the mass of the solid acid in grams. Titration can tell us how many moles of acid are present in the sample. 0. 022 grams = ? Molar mass + Moles H • Because it is monoprotic, it will react 1: 1 with Na. OH.

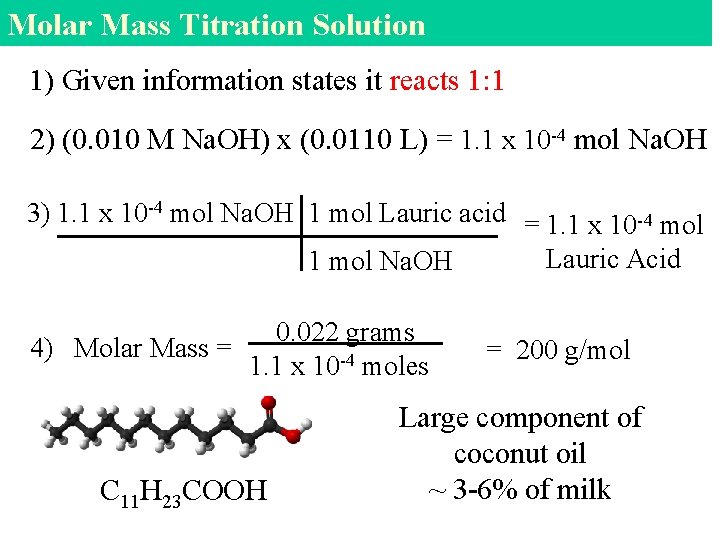
Molar Mass Titration Solution 1) Given information states it reacts 1: 1 2) (0. 010 M Na. OH) x (0. 0110 L) = 1. 1 x 10 -4 mol Na. OH 3) 1. 1 x 10 -4 mol Na. OH 1 mol Lauric acid = 1. 1 x 10 -4 mol Lauric Acid 1 mol Na. OH 0. 022 grams 4) Molar Mass = 1. 1 x 10 -4 moles C 11 H 23 COOH = 200 g/mol Large component of coconut oil ~ 3 -6% of milk

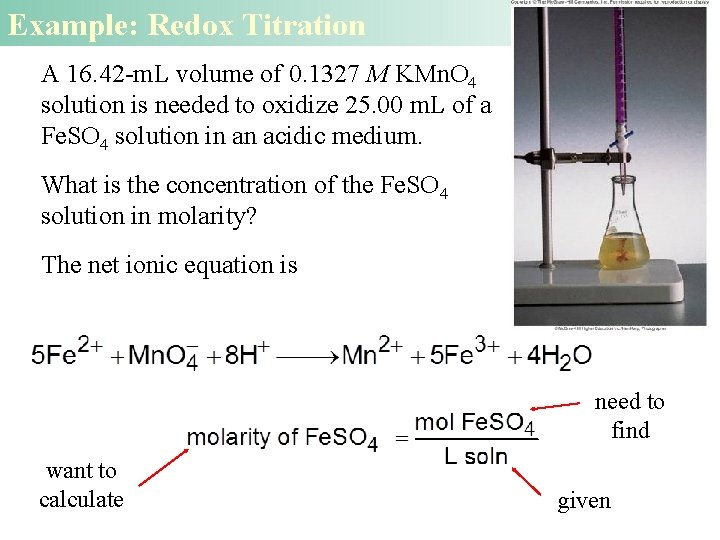
Example: Redox Titration A 16. 42 -m. L volume of 0. 1327 M KMn. O 4 solution is needed to oxidize 25. 00 m. L of a Fe. SO 4 solution in an acidic medium. What is the concentration of the Fe. SO 4 solution in molarity? The net ionic equation is need to find want to calculate given

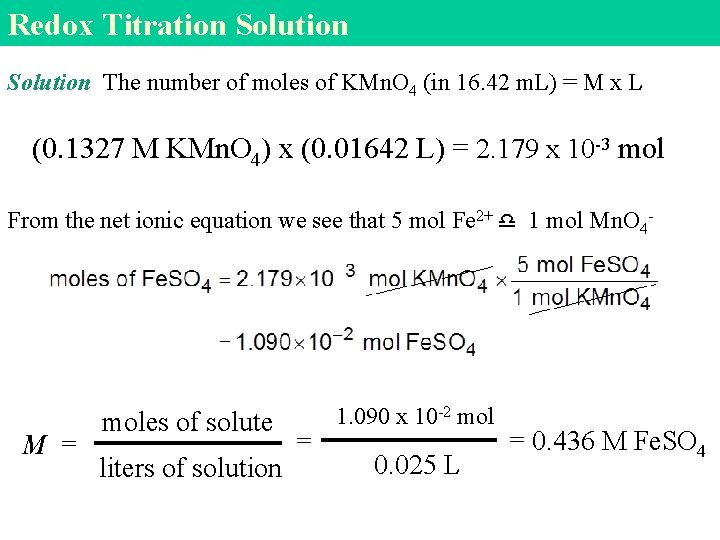
Redox Titration Solution The number of moles of KMn. O 4 (in 16. 42 m. L) = M x L (0. 1327 M KMn. O 4) x (0. 01642 L) = 2. 179 x 10 -3 mol From the net ionic equation we see that 5 mol Fe 2+ 1 mol Mn. O 4 - M = moles of solute liters of solution = 1. 090 x 10 -2 mol 0. 025 L = 0. 436 M Fe. SO 4

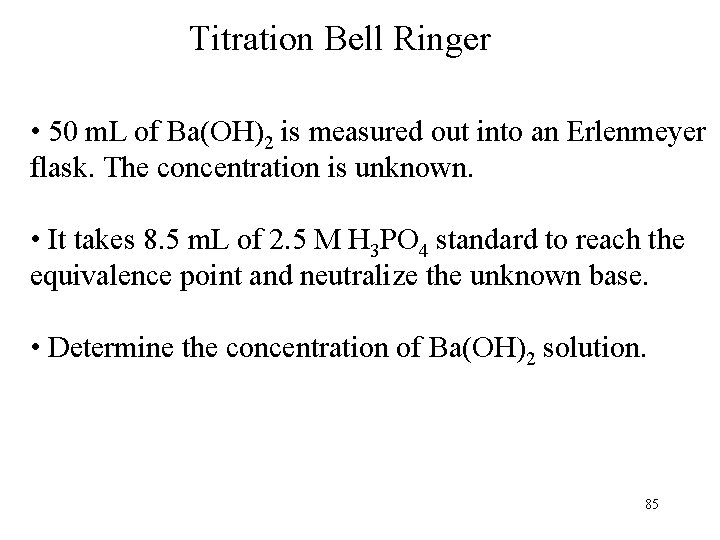
Titration Bell Ringer • 50 m. L of Ba(OH)2 is measured out into an Erlenmeyer flask. The concentration is unknown. • It takes 8. 5 m. L of 2. 5 M H 3 PO 4 standard to reach the equivalence point and neutralize the unknown base. • Determine the concentration of Ba(OH)2 solution. 85

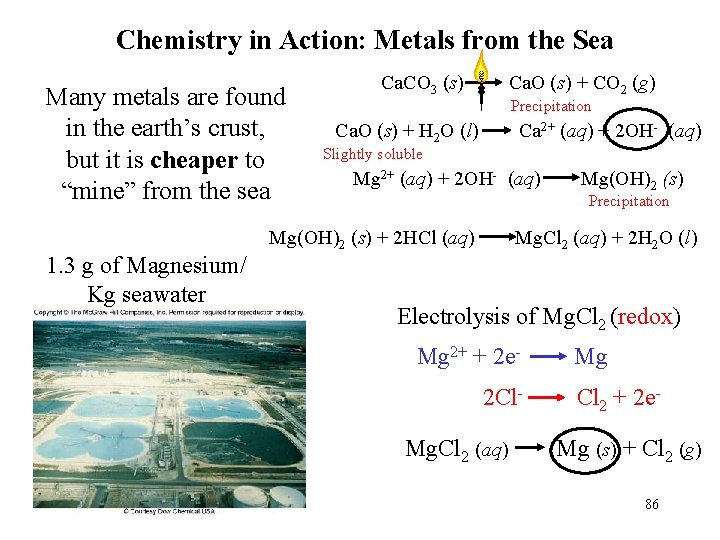
Chemistry in Action: Metals from the Sea Many metals are found in the earth’s crust, but it is cheaper to “mine” from the sea Ca. CO 3 (s) Ca. O (s) + CO 2 (g) Precipitation Ca. O (s) + H 2 O (l) Ca 2+ (aq) + 2 OH- (aq) Slightly soluble Mg 2+ (aq) + 2 OH- (aq) Precipitation Mg(OH)2 (s) + 2 HCl (aq) 1. 3 g of Magnesium/ Kg seawater Mg(OH)2 (s) Mg. Cl 2 (aq) + 2 H 2 O (l) Electrolysis of Mg. Cl 2 (redox) Mg 2+ + 2 e 2 Cl. Mg. Cl 2 (aq) Mg Cl 2 + 2 e. Mg (s) + Cl 2 (g) 86

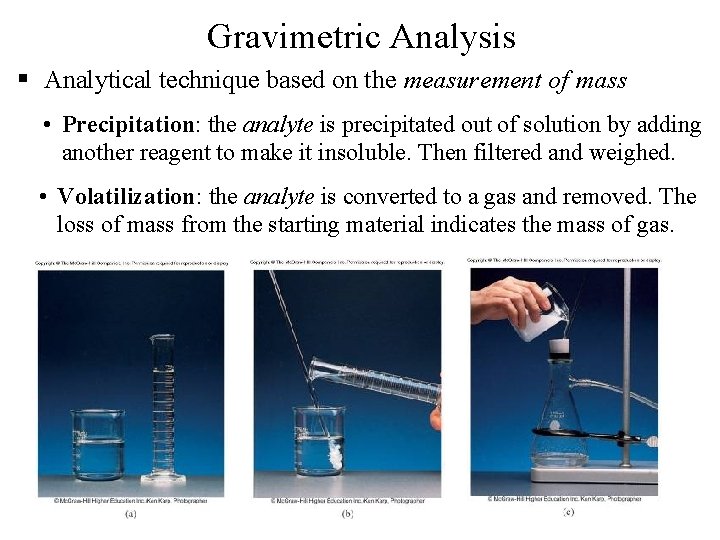
Gravimetric Analysis § Analytical technique based on the measurement of mass • Precipitation: the analyte is precipitated out of solution by adding another reagent to make it insoluble. Then filtered and weighed. • Volatilization: the analyte is converted to a gas and removed. The loss of mass from the starting material indicates the mass of gas. 87

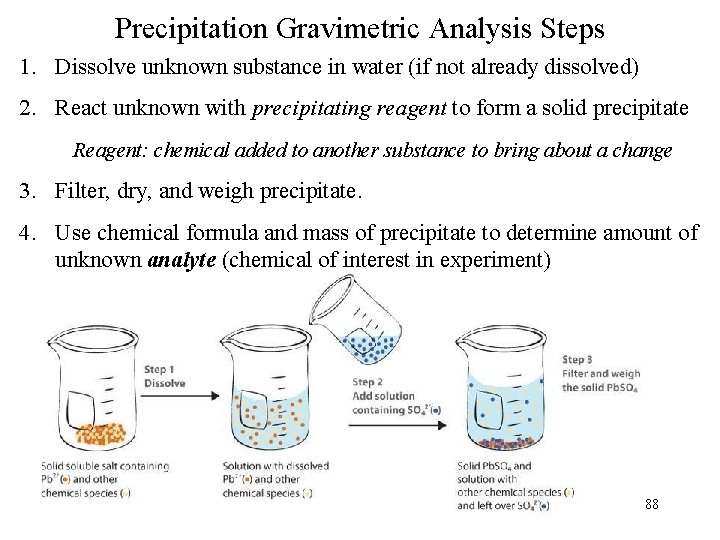
Precipitation Gravimetric Analysis Steps 1. Dissolve unknown substance in water (if not already dissolved) 2. React unknown with precipitating reagent to form a solid precipitate Reagent: chemical added to another substance to bring about a change 3. Filter, dry, and weigh precipitate. 4. Use chemical formula and mass of precipitate to determine amount of unknown analyte (chemical of interest in experiment) 88

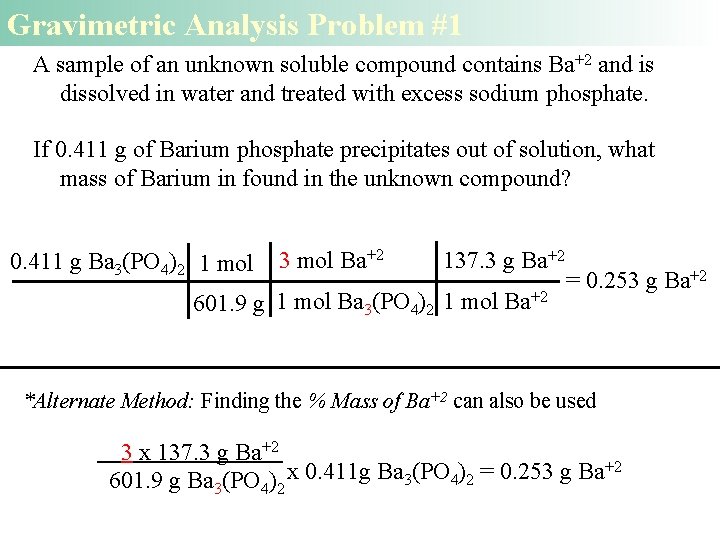
Gravimetric Analysis Problem #1 A sample of an unknown soluble compound contains Ba+2 and is dissolved in water and treated with excess sodium phosphate. If 0. 411 g of Barium phosphate precipitates out of solution, what mass of Barium in found in the unknown compound? 0. 411 g Ba 3(PO 4)2 1 mol 3 mol Ba+2 137. 3 g Ba+2 601. 9 g 1 mol Ba 3(PO 4)2 1 mol Ba+2 = 0. 253 g Ba+2 *Alternate Method: Finding the % Mass of Ba+2 can also be used 3 x 137. 3 g Ba+2 +2 x 0. 411 g Ba (PO ) = 0. 253 g Ba 3 4 2 601. 9 g Ba 3(PO 4)2

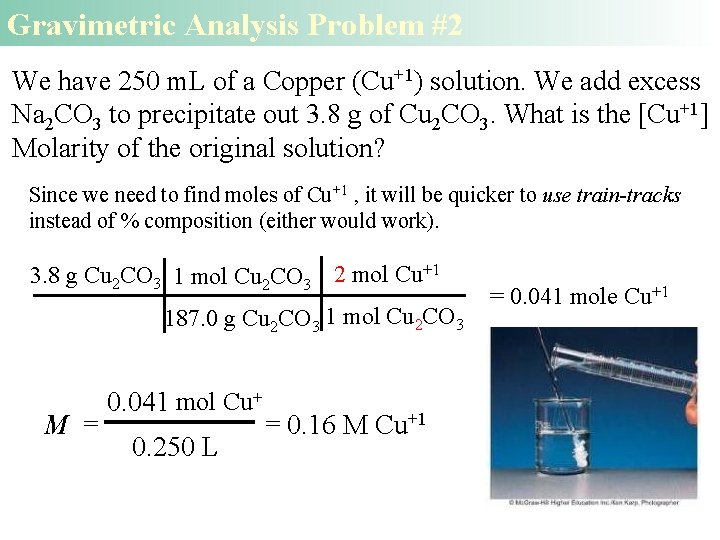
Gravimetric Analysis Problem #2 We have 250 m. L of a Copper (Cu+1) solution. We add excess Na 2 CO 3 to precipitate out 3. 8 g of Cu 2 CO 3. What is the [Cu+1] Molarity of the original solution? Since we need to find moles of Cu+1 , it will be quicker to use train-tracks instead of % composition (either would work). 3. 8 g Cu 2 CO 3 1 mol Cu 2 CO 3 2 mol Cu+1 187. 0 g Cu 2 CO 3 1 mol Cu 2 CO 3 M = 0. 041 mol Cu+ 0. 250 L = 0. 16 M Cu+1 = 0. 041 mole Cu+1

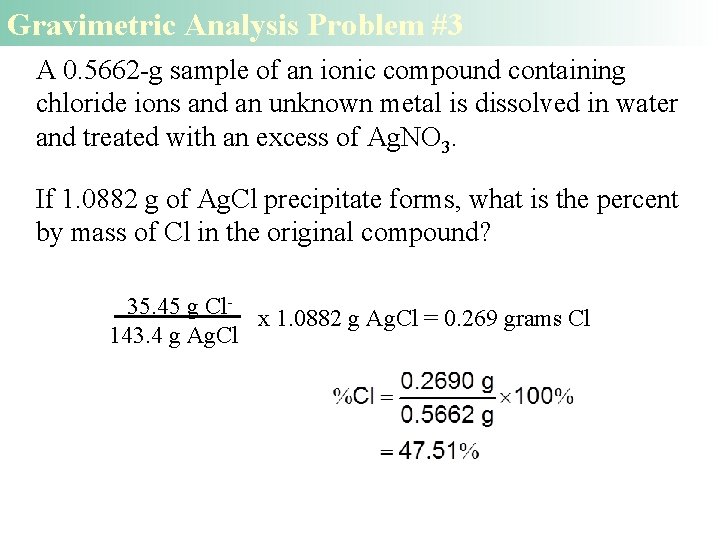
Gravimetric Analysis Problem #3 A 0. 5662 -g sample of an ionic compound containing chloride ions and an unknown metal is dissolved in water and treated with an excess of Ag. NO 3. If 1. 0882 g of Ag. Cl precipitate forms, what is the percent by mass of Cl in the original compound? 35. 45 g Clx 1. 0882 g Ag. Cl = 0. 269 grams Cl 143. 4 g Ag. Cl

More Gravimetric Review Problems 1) An unknown ionic compound contains Carbonate (CO 3 -2). To precipitate the carbonate, we add excess Ca. Cl 2 and collect 25. 3 grams of Ca. CO 3 precipitate. Calculate mass of Carbonate present in the original compound. 2) 50 m. L of a solution contains an unknown amount of Ni+ ions. We add excess Na 3 PO 4 to precipitate out 5. 67 grams of Ni 3 PO 4. What is the Molarity of Ni+? 3) 340 m. L of an unknown solution contains the Silver ion (Ag+). When excess Na 2 S is added, 15. 8 grams of precipitated Ag 2 S are formed. What is the Molarity of Ag+ in the solution?

H 2 O reactions Exam Review Problems 1)Write the net ionic equation for: (NH 4)2 S and Mg. SO 4 2)Write the labeled half reactions: 2 N 2 O → 2 N 2 + O 2 3)How many grams NH 4 NO 3 needed for 250 m. L of 3. 5 M? 4) Describe preparation of 300 m. L 0. 5 M HCl (5 M stock) 5)120 m. L of H 3 PO 4 is titrated with 35 m. L 2. 4 M standard KOH, what is concentration of H 3 PO 4? 6) 340 m. L of an unknown solution contains the Silver ion (Ag+). When excess Na 2 S is added, 15. 8 grams of precipitated Ag 2 S are formed. What is the Molarity of Ag+ in the solution?

Aqueous Reactions & Calculations Terms Acid Base Neutralization Reaction Redox Reaction Precipitate Electrolyte Solvent Solute Ionic Equation Net-Ionic Equation Weak Electrolyte Acid and Metals Spectator Ions Indicator Equivalence point Titration Standard Gravimetric Analysis Molality Molarity Dilution Analyte Precipitating reagent Concentration Stock 94