Reactions in Solutions Dissociation Equations In aqueous solutions







- Slides: 11

Reactions in Solutions

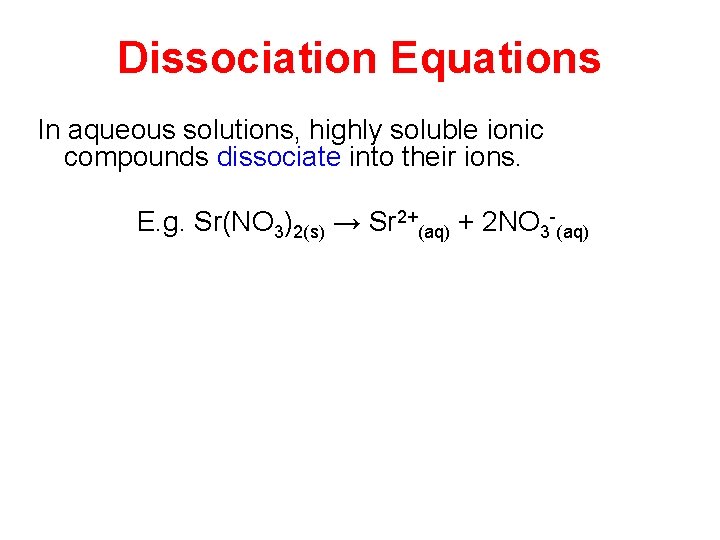
Dissociation Equations In aqueous solutions, highly soluble ionic compounds dissociate into their ions. E. g. Sr(NO 3)2(s) → Sr 2+(aq) + 2 NO 3 -(aq)

Reactions in Solution • When combining two aqueous ionic solutions there are two possible outcomes: 1. The solutions do not react and remain in solution 2. The solutions react (double displacement) forming either – A gas – A precipitate – Water

Reactions Producing a Gas Remember your gas tests? • Oxygen • Hydrogen • Carbon dioxide

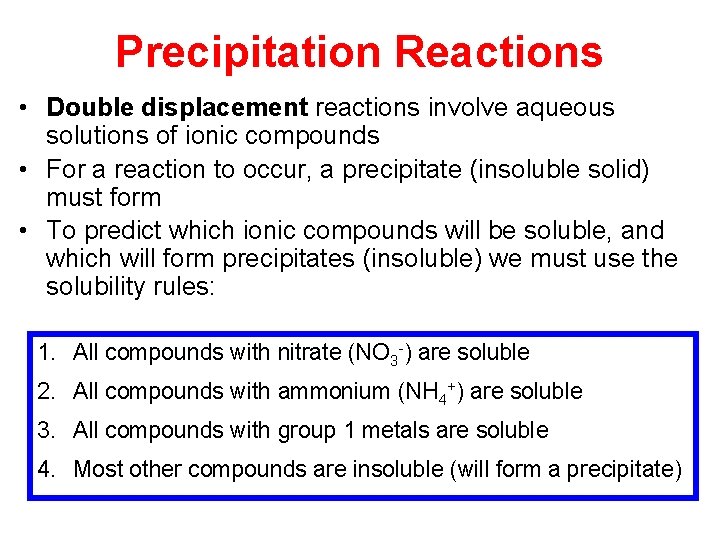
Precipitation Reactions • Double displacement reactions involve aqueous solutions of ionic compounds • For a reaction to occur, a precipitate (insoluble solid) must form • To predict which ionic compounds will be soluble, and which will form precipitates (insoluble) we must use the solubility rules: 1. All compounds with nitrate (NO 3 -) are soluble 2. All compounds with ammonium (NH 4+) are soluble 3. All compounds with group 1 metals are soluble 4. Most other compounds are insoluble (will form a precipitate)

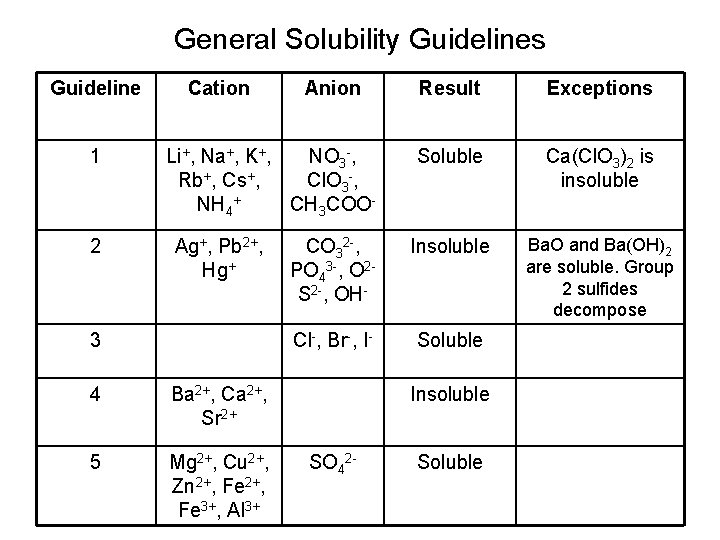
General Solubility Guidelines Guideline 1 2 Cation Anion Result Exceptions Soluble Ca(Cl. O 3)2 is insoluble CO 32 -, PO 43 -, O 2 S 2 -, OH- Insoluble Ba. O and Ba(OH)2 are soluble. Group 2 sulfides decompose Cl-, Br-, I- Soluble Li+, Na+, K+, NO 3 -, Rb+, Cs+, Cl. O 3 -, NH 4+ CH 3 COOAg+, Pb 2+, Hg+ 3 4 Ba 2+, Ca 2+, Sr 2+ 5 Mg 2+, Cu 2+, Zn 2+, Fe 3+, Al 3+ Insoluble SO 42 - Soluble

• Ba. Cl 2 is a white crystalline powder. Is it soluble or insoluble? • The Ba 2+ ion is insoluble. It is in guideline 4. • The Cl- ion is soluble. It is listed in guidelie 3. • The higher the guideline number takes precedence. • Ba. Cl 2 is soluble.

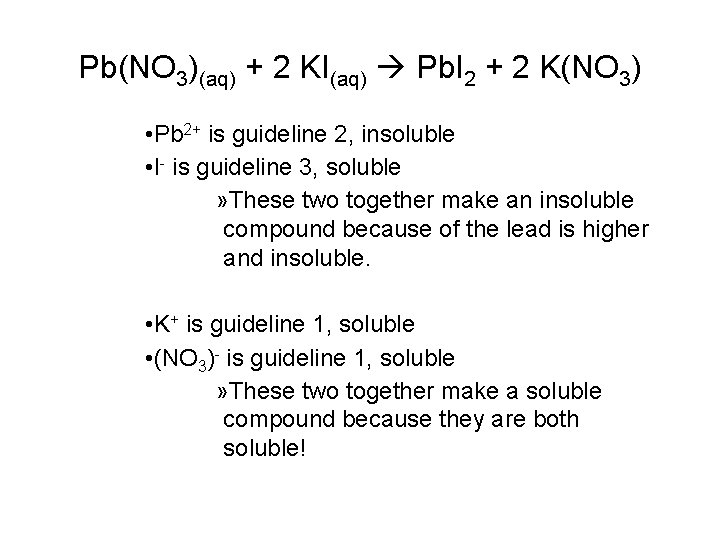
Pb(NO 3)(aq) + 2 KI(aq) Pb. I 2 + 2 K(NO 3) • Pb 2+ is guideline 2, insoluble • I- is guideline 3, soluble » These two together make an insoluble compound because of the lead is higher and insoluble. • K+ is guideline 1, soluble • (NO 3)- is guideline 1, soluble » These two together make a soluble compound because they are both soluble!

Practice Will the following solutions produce a precipitate? A. potassium carbonate and copper (I) sulfate B. ammonium chloride and sodium sulfate C. sodium carbonate and barium nitrate D. lithium hydroxide and ammonium chlorate

Reactions Producing Water • Neutralization reactions • Acid + base ➔ salt + water • p. H tests (litmus paper, phenolphthalein, bromothymol blue, universal indicator)

Practice • p. 332 #1, 2 • p. 138 -143 #1 -5, 7