Science 10 Review Part 2 Dissociation Equations Dissociation





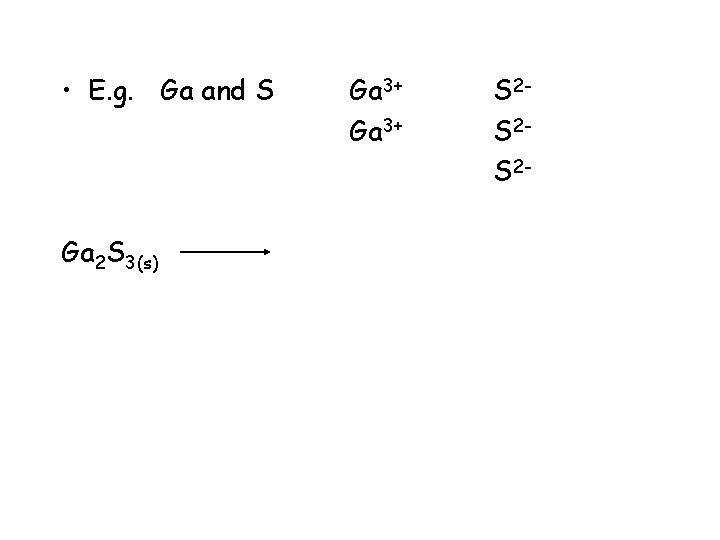
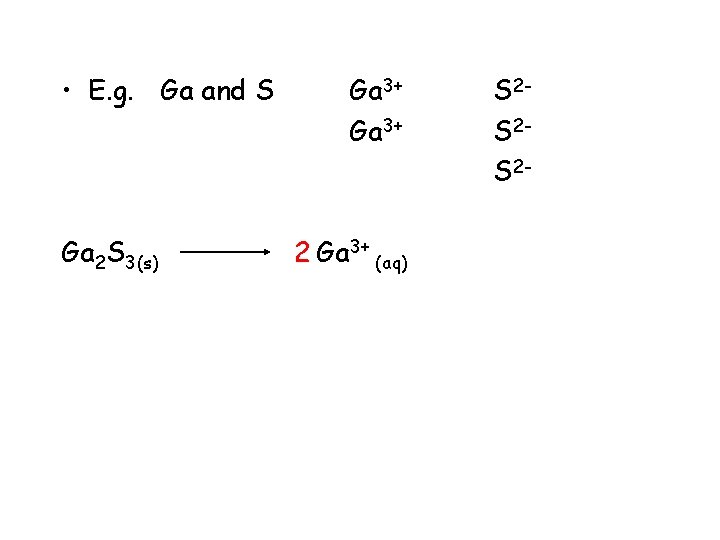
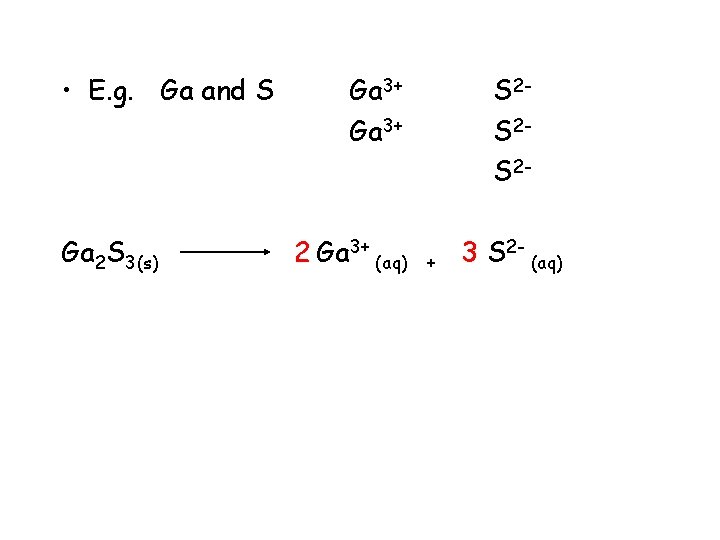
- Slides: 22

Science 10 Review Part : 2 Dissociation Equations

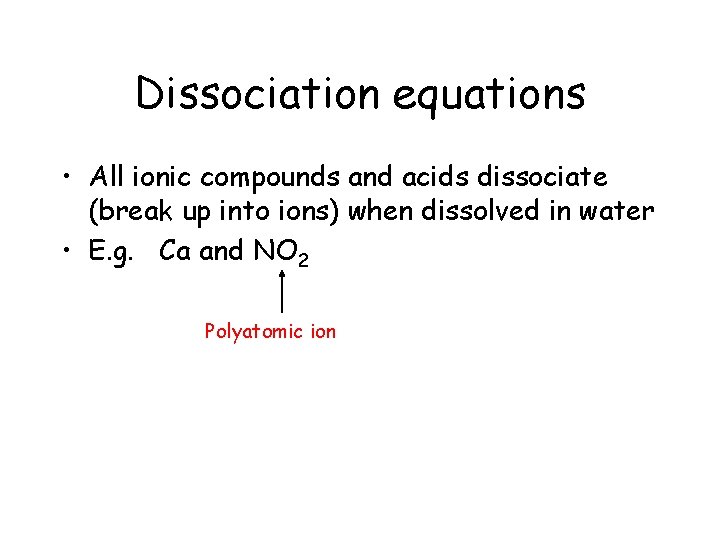
Dissociation equations

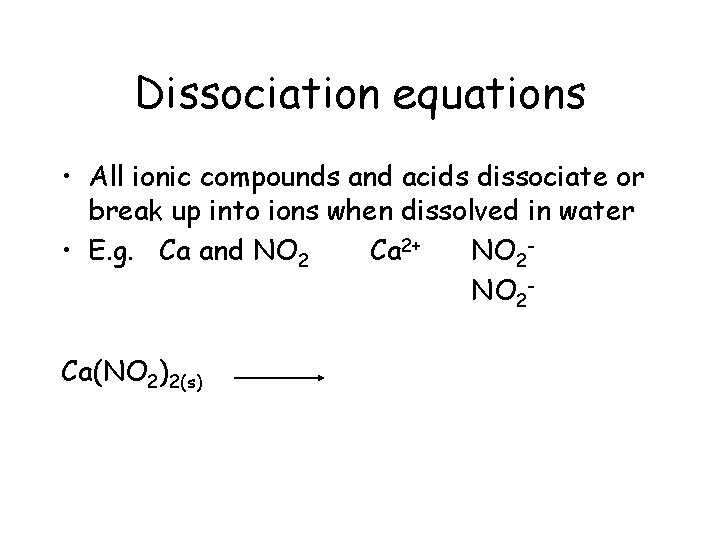
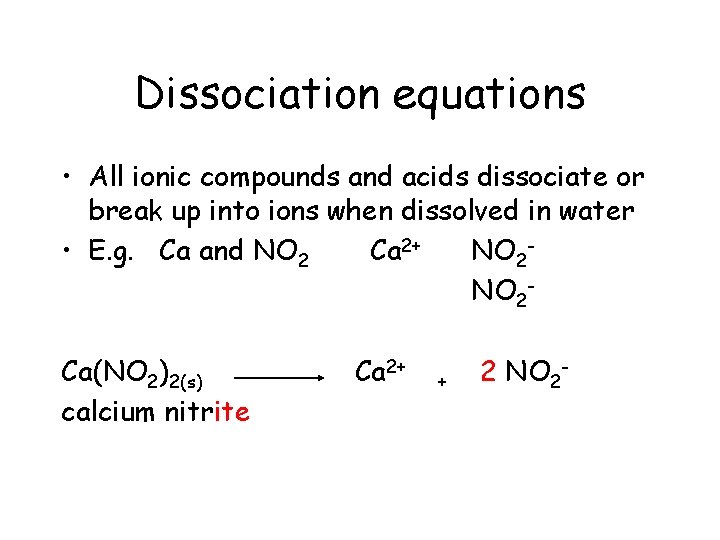
Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water

Dissociation equations • All ionic compounds and acids dissociate (break up into ions) when dissolved in water • E. g. Ca and NO 2 Polyatomic ion

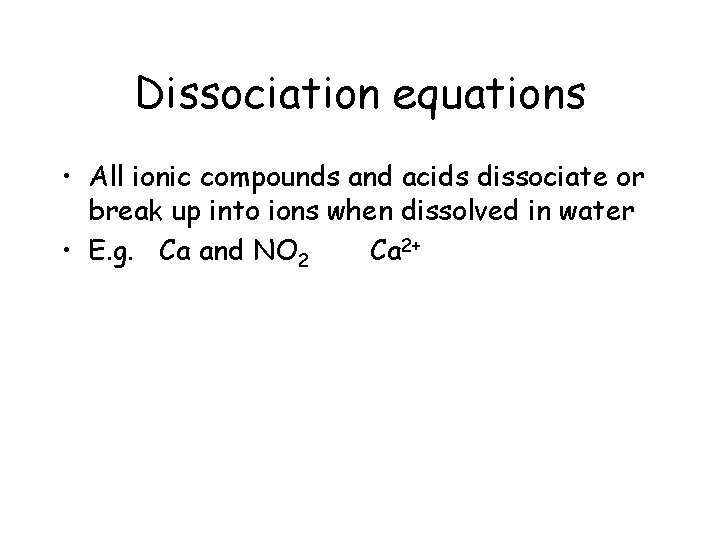
Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+

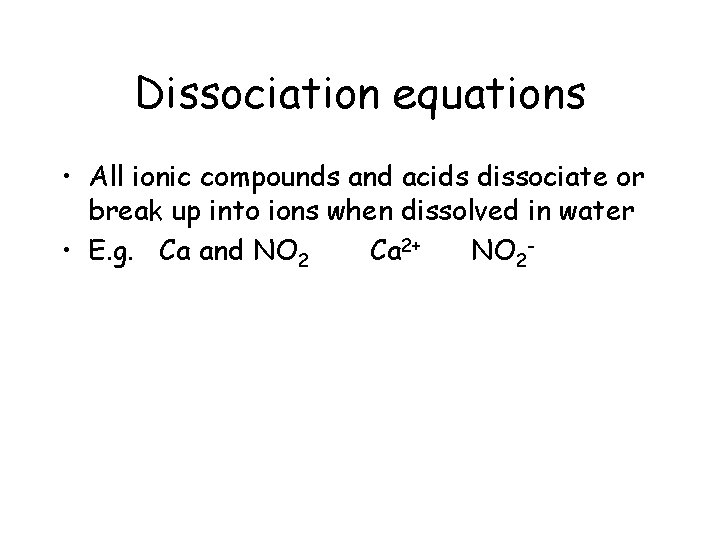
Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 -

Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 -

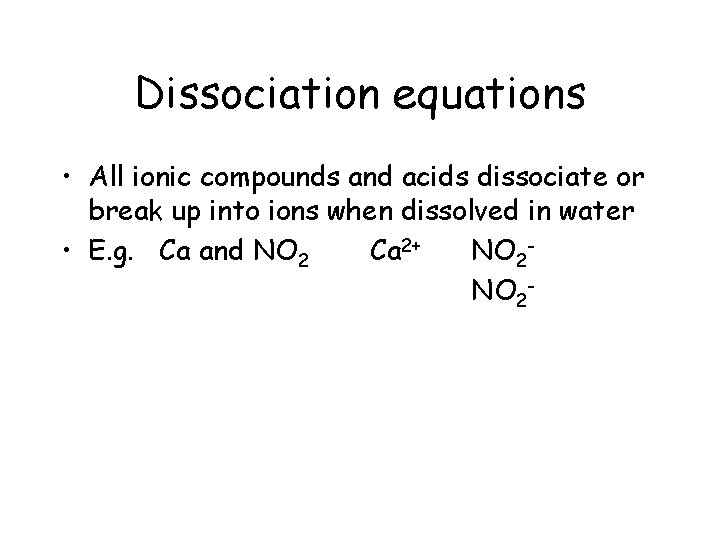
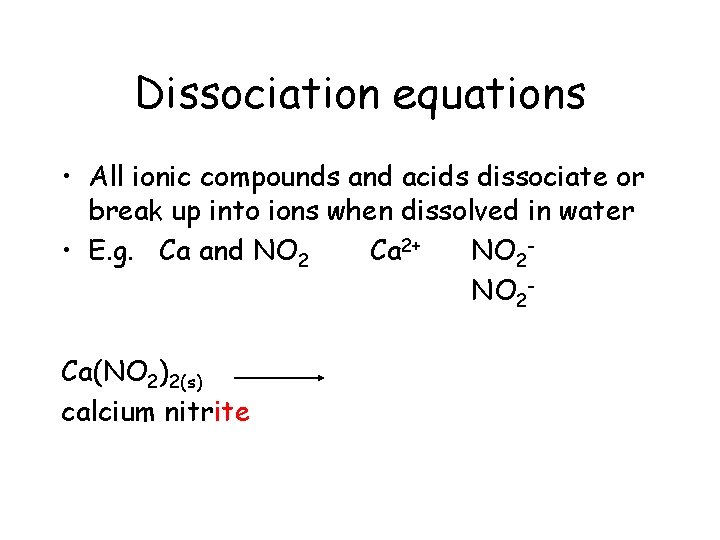
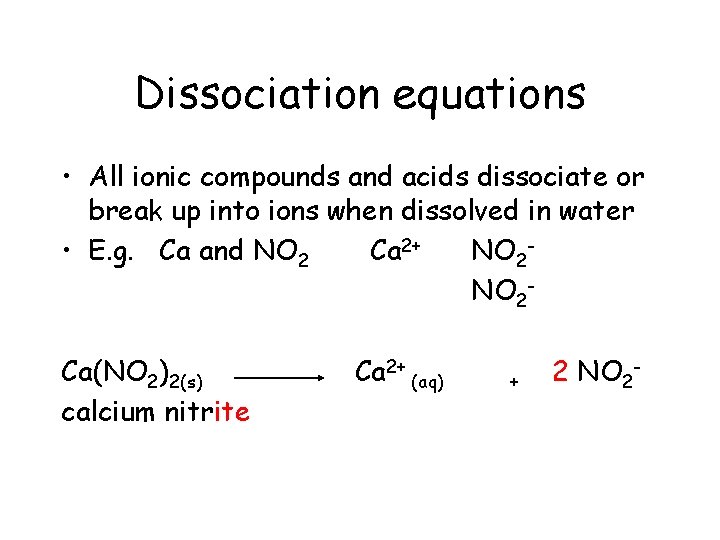
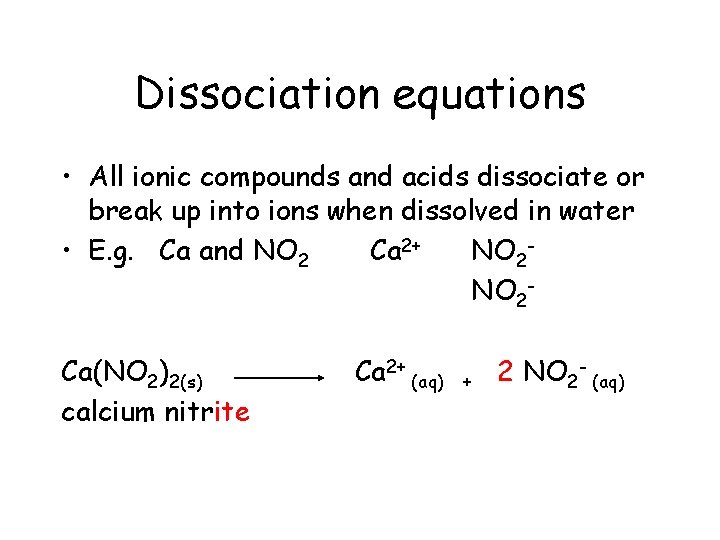
Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 Ca(NO 2)2(s)

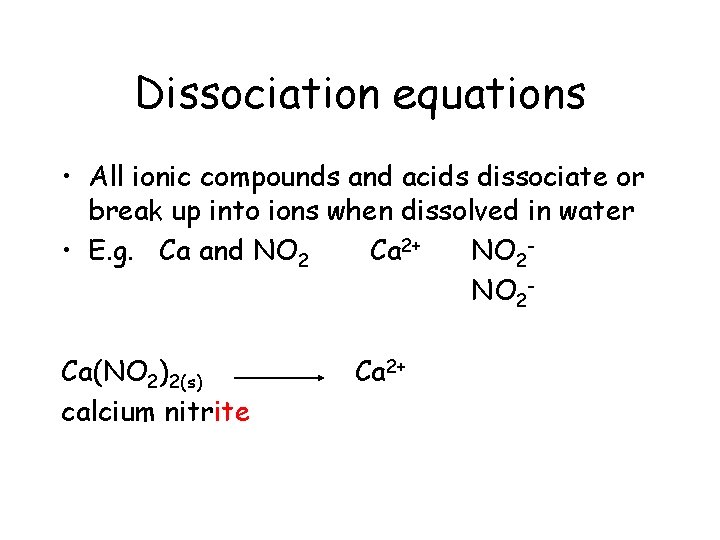
Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 Ca(NO 2)2(s) calcium nitrite

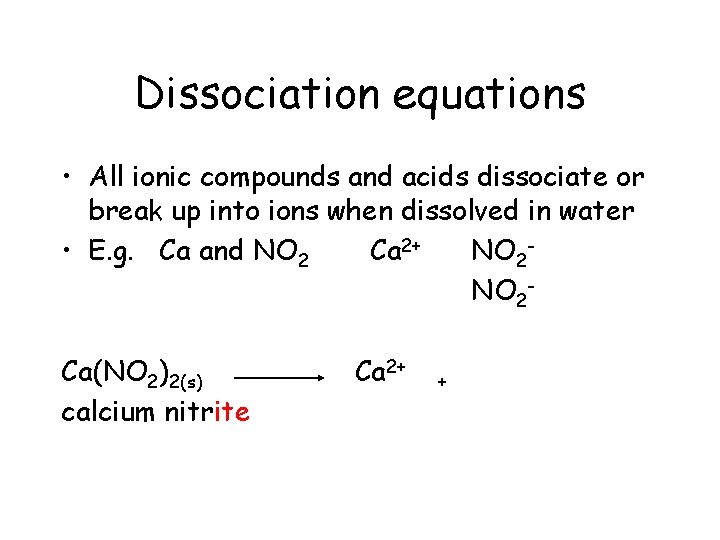
Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 Ca(NO 2)2(s) calcium nitrite Ca 2+

Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 Ca(NO 2)2(s) calcium nitrite Ca 2+ +

Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 Ca(NO 2)2(s) calcium nitrite Ca 2+ + 2 NO 2 -

Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 Ca(NO 2)2(s) calcium nitrite Ca 2+ (aq) + 2 NO 2 -

Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 Ca(NO 2)2(s) calcium nitrite Ca 2+ (aq) + 2 NO 2 - (aq)

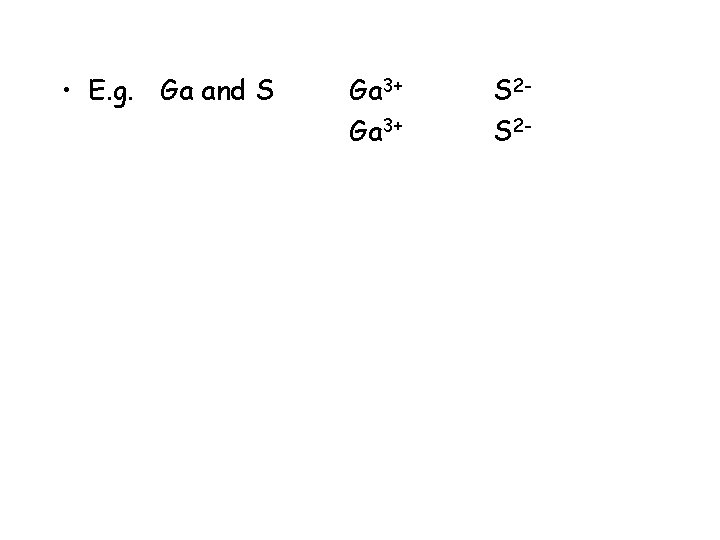
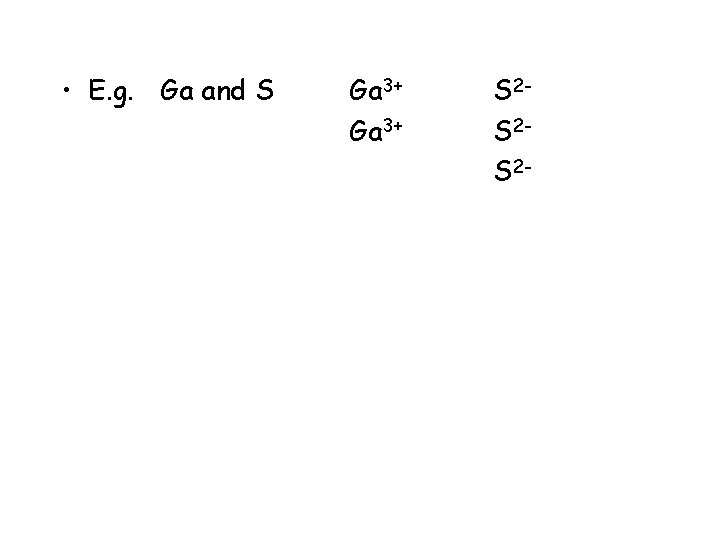
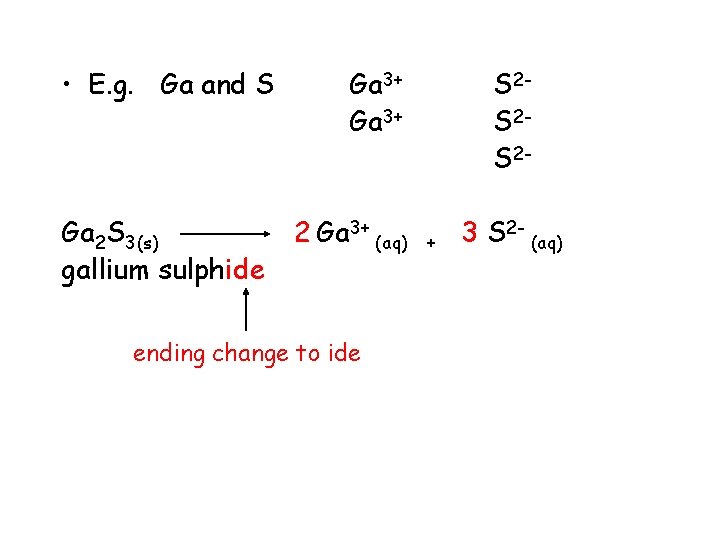
• E. g. Ga and S Ga 3+ S 2 -

• E. g. Ga and S Ga 3+ S 2 S 2 -

• E. g. Ga and S Ga 3+ S 2 S 2 -

• E. g. Ga and S Ga 3+ S 2 S 2 S 2 -
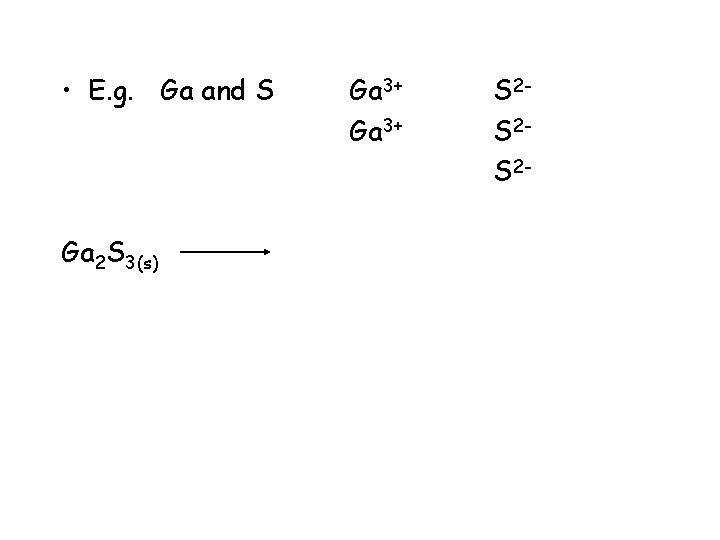
• E. g. Ga and S Ga 2 S 3(s) Ga 3+ S 2 S 2 S 2 -
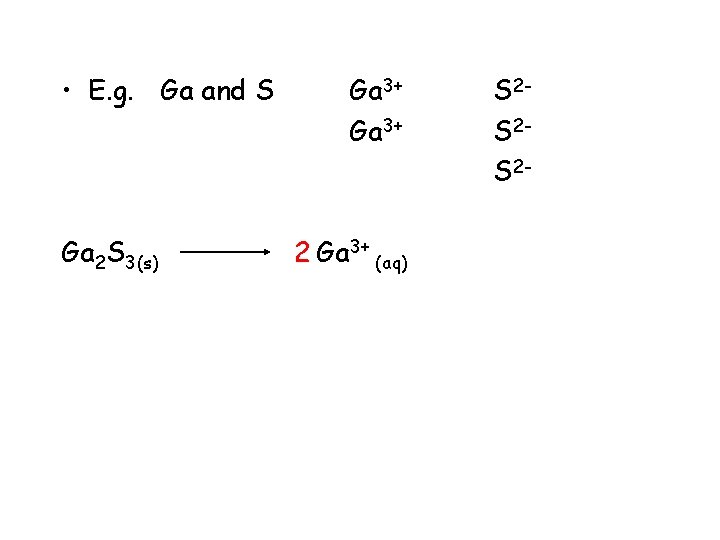
• E. g. Ga and S Ga 2 S 3(s) Ga 3+ 2 Ga 3+ (aq) S 2 S 2 S 2 -
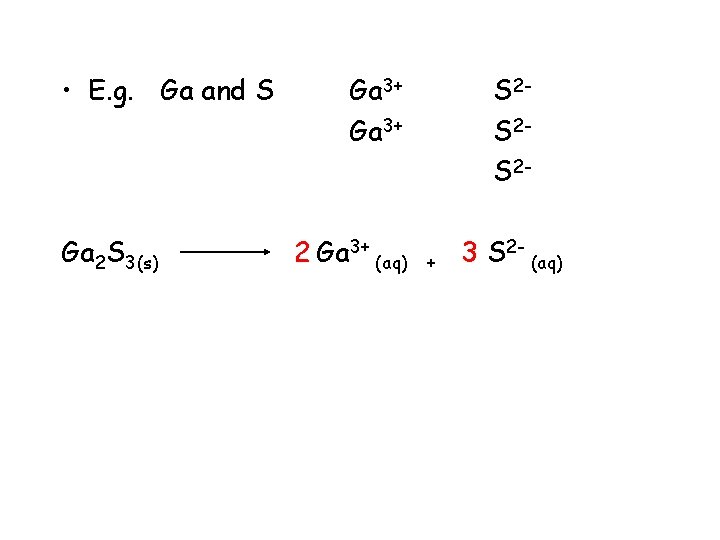
• E. g. Ga and S Ga 2 S 3(s) Ga 3+ 2 Ga 3+ (aq) S 2 S 2 S 2+ 3 S 2 - (aq)

• E. g. Ga and S Ga 2 S 3(s) gallium sulphide Ga 3+ 2 Ga 3+ (aq) ending change to ide S 2 S 2 S 2+ 3 S 2 - (aq)