Compounds in Aqueous Solutions I Dissociation Separation of






- Slides: 9

Compounds in Aqueous Solutions

I. Dissociation • Separation of ions that occurs when an ionic compound dissolves in water • Balance chemical equation for charge as well as for atoms. • Ca. Cl 2(s) → Ca 2+(aq) + 2 Cl-(aq) • Assume 100% dissociation – 1 mol of calcium chloride produces one mol of calcium ions and 2 moles of chloride ions – 3 moles of ions total

II. Practice problems • Write the equation for the dissociation of each of the following in water, and then determine the # of moles of each ion produced as well as the total number of moles of ions produced. • A. 1 mol ammonium chloride • B. 1 mol sodium sulfide • C. 0. 5 mol barium nitrate

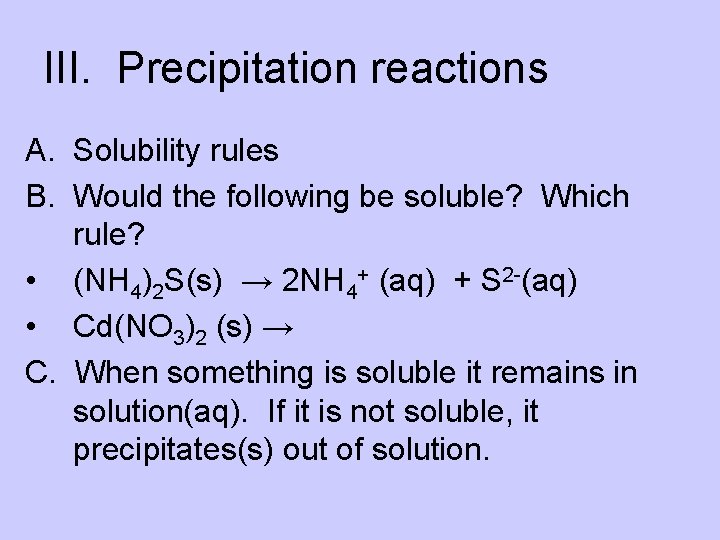
III. Precipitation reactions A. Solubility rules B. Would the following be soluble? Which rule? • (NH 4)2 S(s) → 2 NH 4+ (aq) + S 2 -(aq) • Cd(NO 3)2 (s) → C. When something is soluble it remains in solution(aq). If it is not soluble, it precipitates(s) out of solution.

IV. Net Ionic Equations • • Reactions of ions in aqueous solutions Only includes compounds and ions that undergo a chemical change in a reaction in an aqueous solution. 1. Write overall equation 2. Write formula equation (Is each product soluble or not) 3. Write overall ionic equation -Dissociate soluble compounds into ions. Precipitates are shown as solids. 4. Cancel out spectator ions. Do not undergo any chemical change and do not take part in the reaction. Found in solution before and after reaction. 5. Write net ionic equation.

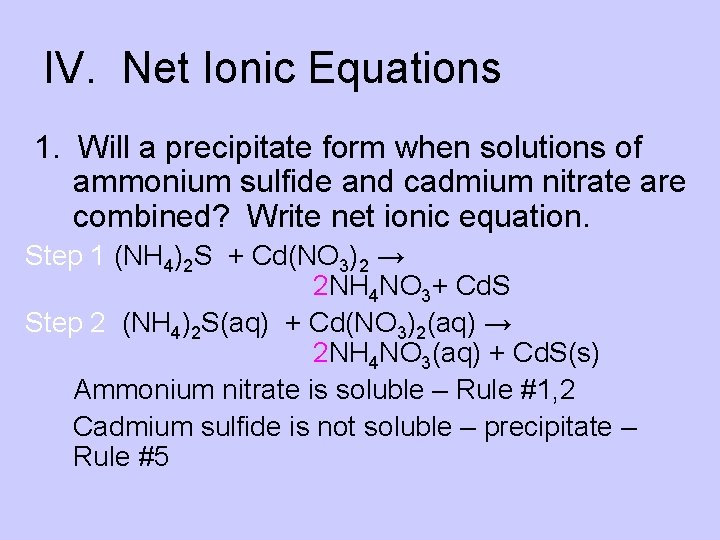
IV. Net Ionic Equations 1. Will a precipitate form when solutions of ammonium sulfide and cadmium nitrate are combined? Write net ionic equation. Step 1 (NH 4)2 S + Cd(NO 3)2 → 2 NH 4 NO 3+ Cd. S Step 2 (NH 4)2 S(aq) + Cd(NO 3)2(aq) → 2 NH 4 NO 3(aq) + Cd. S(s) Ammonium nitrate is soluble – Rule #1, 2 Cadmium sulfide is not soluble – precipitate – Rule #5

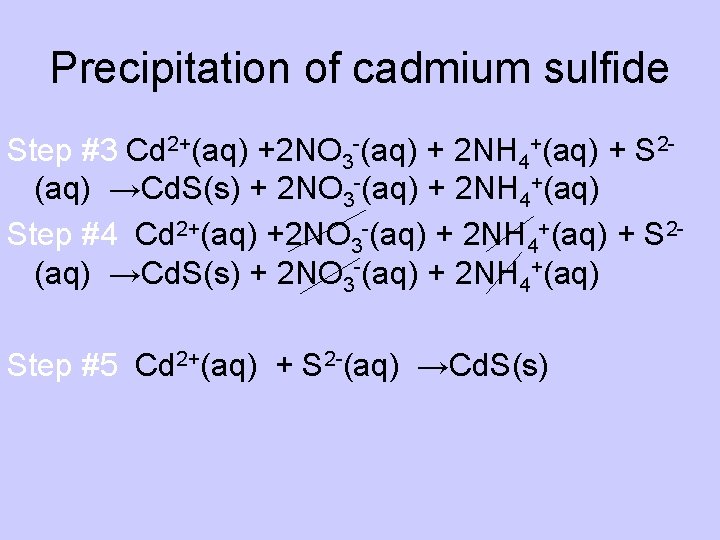
Precipitation of cadmium sulfide Step #3 Cd 2+(aq) +2 NO 3 -(aq) + 2 NH 4+(aq) + S 2(aq) →Cd. S(s) + 2 NO 3 -(aq) + 2 NH 4+(aq) Step #4 Cd 2+(aq) +2 NO 3 -(aq) + 2 NH 4+(aq) + S 2(aq) →Cd. S(s) + 2 NO 3 -(aq) + 2 NH 4+(aq) Step #5 Cd 2+(aq) + S 2 -(aq) →Cd. S(s)

V. Practice problems Problem #2 Will a precipitate form when aqueous solutions of zinc nitrate and ammonium sulfide are combined? 1. Equation for double replacement 2. Formula equation (includes state of products) 3. Overall ionic equation 4. Net ionic equation – cancel out spectator ions.

V. Practice problems 3. Will a precipitate form if solutions of potassium sulfate and barium nitrate are combined? If so, write the net ionic equation for the reaction. 4. Will a precipitate form if solutions of potassium nitrate and magnesium sulfate are combined? If so, write the net ionic equation for the reaction.