15 2 Homogeneous Aqueous Systems Chapter 15 Water
























- Slides: 31

15. 2 Homogeneous Aqueous Systems > Chapter 15 Water and Aqueous Systems 15. 1 Water and Its Properties 15. 2 Homogeneous Aqueous Systems 15. 3 Heterogeneous Aqueous Systems 1 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > CHEMISTRY & YOU How can you make a pickle glow? Although it sounds absurd, an ordinary dill pickle from the deli can be a source of light when connected to an electric current! 2 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Solutions What types of substances dissolve most readily in water? • An aqueous solution is water that contains dissolved substances. 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Solutions Solvents and Solutes • In a solution, the dissolving medium is the solvent. • The dissolved particles in a solution are the solute. 4 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Solutions Solvents and Solutes • A solvent dissolves the solute. • The solute becomes dispersed in the solvent. • Solvents and solutes may be gases, liquids, or solids. 5 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Solutions Solvents and Solutes • Solutions are homogeneous mixtures. • Solute particles can be atoms, ions, or molecules. • If you filter a solution through filter paper, both the solute and solvent pass through the filter. 6 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Solutions Solvents and Solutes Substances that dissolve most readily in water include ionic compounds and polar covalent compounds. 7 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Solutions Solvents and Solutes Substances that dissolve most readily in water include ionic compounds and polar covalent compounds. • Nonpolar covalent compounds, such as methane, and compounds found in oil, grease, and gasoline, do not dissolve in water. • However, oil and grease will dissolve in gasoline. 8 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

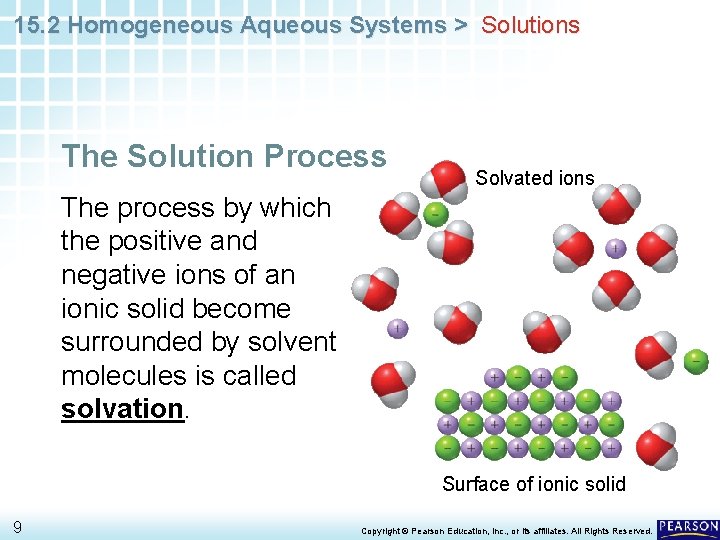
15. 2 Homogeneous Aqueous Systems > Solutions The Solution Process Solvated ions The process by which the positive and negative ions of an ionic solid become surrounded by solvent molecules is called solvation. Surface of ionic solid 9 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Solutions The Solution Process • Polar solvents such as water dissolve ionic compounds and polar compounds. • Nonpolar solvents such as gasoline dissolve nonpolar compounds. 10 • This relationship can be summed up in the expression “like dissolves like. ” Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Which of these compounds should not dissolve in water? A. HCl B. C 4 H 10 C. KI D. NH 3 11 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Which of these compounds should not dissolve in water? A. HCl B. C 4 H 10 C. KI D. NH 3 12 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Electrolytes and Nonelectrolytes Why are all ionic compounds electrolytes? • An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or in the molten state. 13 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Electrolytes and Nonelectrolytes All ionic compounds are electrolytes because they dissociate into ions. 14 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Electrolytes and Nonelectrolytes In order for the bulb to light, an electric current must flow between the two electrodes that are immersed in the solution. • Sodium chloride, a strong electrolyte, is nearly 100% dissociated into ions in water. To (+) electrode 15 To (–) electrode Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

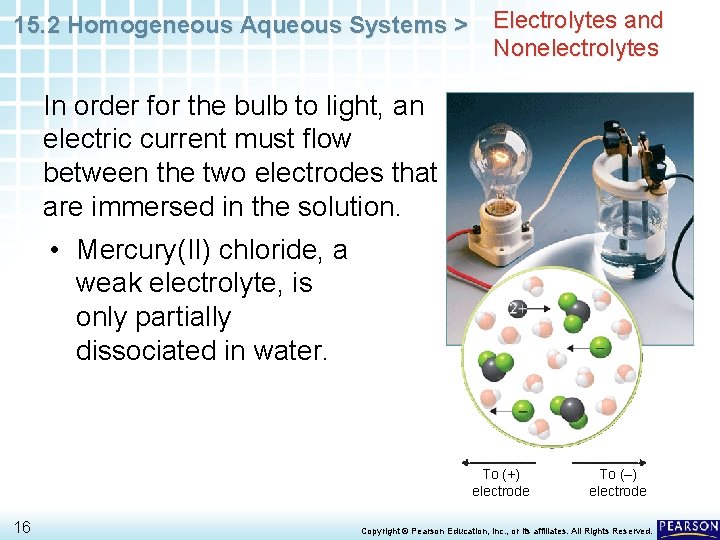
15. 2 Homogeneous Aqueous Systems > Electrolytes and Nonelectrolytes In order for the bulb to light, an electric current must flow between the two electrodes that are immersed in the solution. • Mercury(II) chloride, a weak electrolyte, is only partially dissociated in water. To (+) electrode 16 To (–) electrode Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

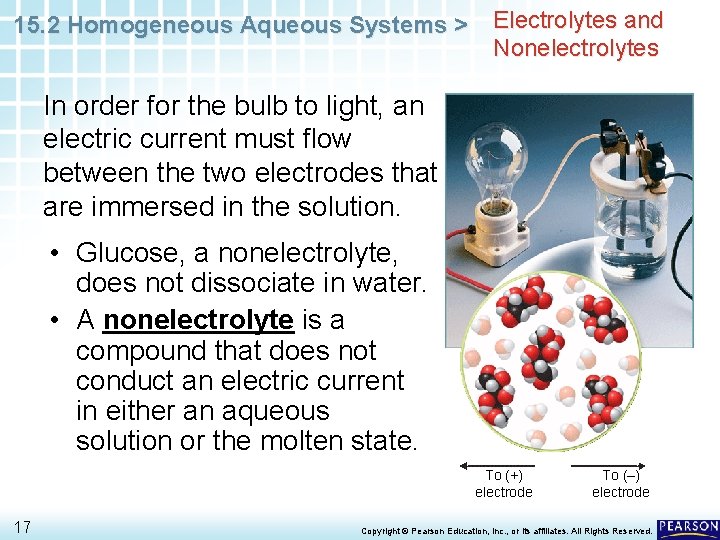
15. 2 Homogeneous Aqueous Systems > Electrolytes and Nonelectrolytes In order for the bulb to light, an electric current must flow between the two electrodes that are immersed in the solution. • Glucose, a nonelectrolyte, does not dissociate in water. • A nonelectrolyte is a compound that does not conduct an electric current in either an aqueous solution or the molten state. To (+) electrode 17 To (–) electrode Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Electrolytes and Nonelectrolytes Some polar molecular compounds are nonelectrolytes in the pure state but become electrolytes when they dissolve in water. • This change occurs because such compounds ionize in solution. 18 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Electrolytes and Nonelectrolytes Some polar molecular compounds are nonelectrolytes in the pure state but become electrolytes when they dissolve in water. • For example, ammonia (NH 3(g)) is not an electrolyte in the pure state. • Yet an aqueous solution of ammonia conducts an electric current because ammonium ions (NH 4+) and hydroxide ions (OH–) form when ammonia dissolves in water. NH 3(g) + H 2 O(l) NH 4+(aq) + OH–(aq) 19 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Electrolytes and Nonelectrolytes Not all electrolytes conduct electric current to the same degree. • In a solution that contains a strong electrolyte, all or nearly all of the solute exists as ions. • A weak electrolyte conducts an electric current poorly because only a fraction of the solute in the solution exists as ions. 20 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Electrolytes and Nonelectrolytes Your cells use electrolytes, such as sodium and potassium ions, to carry electrical impulses across themselves and to other cells. • An electrolyte imbalance can occur if you become dehydrated. • When you exercise, you can lose water and electrolytes from your body through perspiration. 21 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > CHEMISTRY & YOU Pickles contain table salt. Why can electric current flow through a pickle, causing it to glow? Electrolytes conduct an electric current when they are in an aqueous solution. Table salt, or Na. Cl, is a strong electrolyte. The water and salt in the pickle form a solution that conducts an electric current. The electric current causes the pickle to glow. 22 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Explain why you must be extremely careful when using electricity near a swimming pool. 23 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Explain why you must be extremely careful when using electricity near a swimming pool. The chlorinated water in a swimming pool is a solution that can conduct an electric current. If a current is introduced into the water, any swimmers could be shocked. 24 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Hydrates Hygroscopic Hydrates Calcium chloride monohydrate spontaneously absorbs a second molecule of water when exposed to moist air. • Calcium chloride is used as a desiccant in the laboratory. • A desiccant is a substance used to absorb moisture from the air and create a dry atmosphere. 25 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Key Concepts and Key Equation Substances that dissolve most readily in water include ionic compounds and polar covalent compounds. All ionic compounds are electrolytes because they dissociate into ions. The forces holding the water molecules in hydrates are not very strong, so the water is easily lost and regained. 26 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Glossary Terms • aqueous solution: water that contains dissolved substances • solvent: the dissolving medium in a solution • solute: dissolved particles in a solution • solvation: a process that occurs when an ionic solute dissolves; in solution, solvent molecules surround the positive and negative ions 27 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Glossary Terms • electrolyte: a compound that conducts an electric current when it is in an aqueous solution or in the molten state; all ionic compounds are electrolytes, but most covalent compounds are not • nonelectrolyte: a compound that does not conduct an electric current in aqueous solution or in the molten state • strong electrolyte: a solution in which a large portion of the solute exists as ions • weak electrolyte: a solution that conducts electricity poorly because only a fraction of the solute exists as ions 28 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > Glossary Terms • desiccant: a hygroscopic substance used as a drying agent 29 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > BIG IDEA Bonding and Interactions • Ionic compounds and polar covalent compounds dissolve most readily in water to form aqueous solutions. • Ionic compounds dissolve in water when the polar water molecules attract the ions of the solute, causing the individual solute ions to break away from the ionic crystal. 30 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 2 Homogeneous Aqueous Systems > END OF 15. 2 31 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.