Chapter 15 Water and Aqueous Systems 1 Water













- Slides: 36

Chapter 15 Water and Aqueous Systems 1

Water: The Molecular View • Water is most often thought of as a (l). (liquid at room temp) However, solid water (ice), and gaseous water (water vapor), also exist in large qty on Earth. Water is the only sub on Earth that exists in large qty in all 3 states. 2

Modeling Water: H-Bonding H-bonding: Ø a connection between the H atoms on one molecule and a highly electro -ve atom (O, N, F) on another. Ø one kind of intermolecular attraction Ø e. g. O in water molecule and H in another water molecule. 3

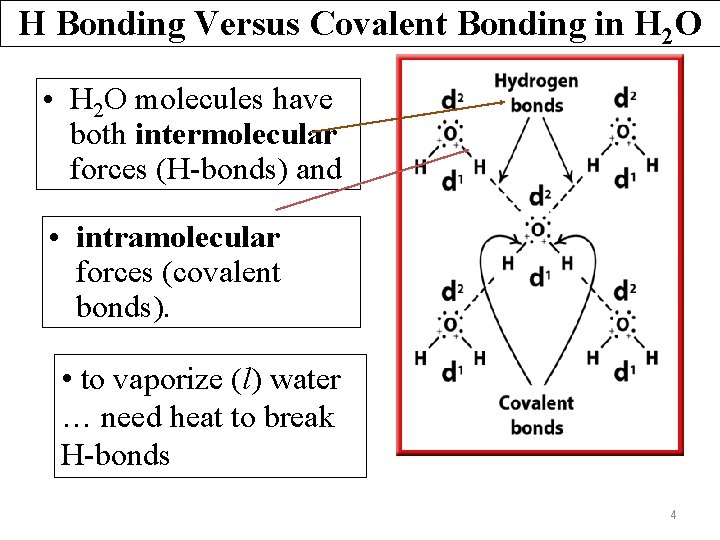
H Bonding Versus Covalent Bonding in H 2 O • H 2 O molecules have both intermolecular forces (H-bonds) and • intramolecular forces (covalent bonds). • to vaporize (l) water … need heat to break H-bonds 4

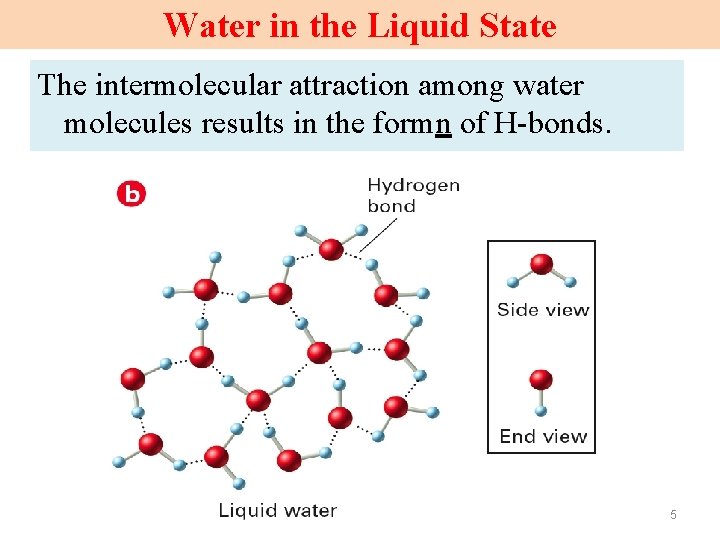
Water in the Liquid State The intermolecular attraction among water molecules results in the formn of H-bonds. 5

States of Water H 2 O occurs primarily in the (l) and (s) states on Earth, rather than as a (g). H-Bonds hold the H 2 O molecules together strongly enough that they cannot readily escape into the gaseous state at ordinary temperatures. 6

States of Water • That is why H 2 O has such a high b. p. (100° C) for such a small molecules • ethanol (C 2 H 5 OH) has a b. p. of 70°C 7

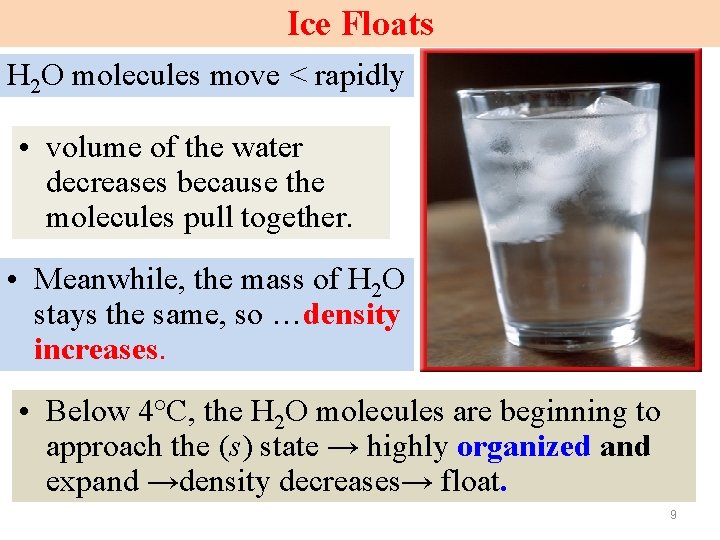
Ice Floats • Ice floats in H 2 O. • i. e. the density of the H 2 O(s) < that of (l) H 2 O. • As H cools from 60°C, its volume decreases and its density increases. 8

Ice Floats H 2 O molecules move < rapidly • volume of the water decreases because the molecules pull together. • Meanwhile, the mass of H 2 O stays the same, so …density increases. • Below 4°C, the H 2 O molecules are beginning to approach the (s) state → highly organized and expand →density decreases→ float. 9

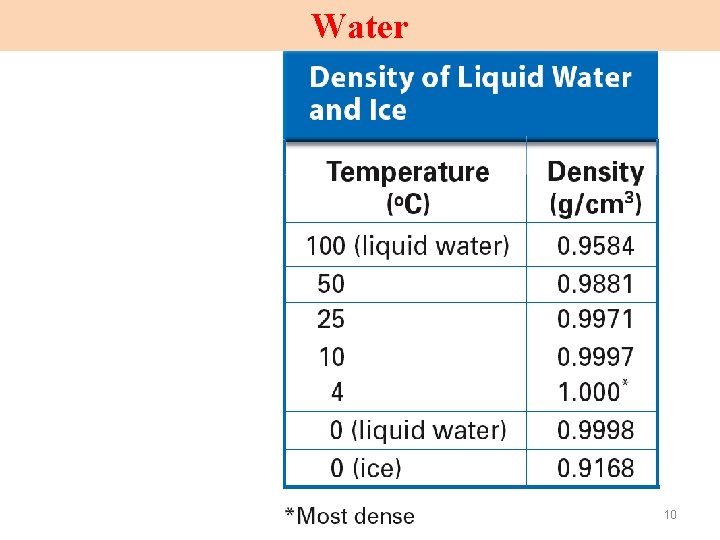
Water 10

Water Evaporation/Condensation Vaporization • change in state of (l) to (g) (vapor) • at or above b. p. (100°C for liquid water) or • at any temperatures → evaporation Evaporation • at surface of a (l). (molecules with enough k. e. jump …) • at any temperature (below b. p. ) 11

Evaporation/Condensation • (g) → (l) 12

Water: The Super Solvent Ø Most of the H 2 O on Earth is not pure (in solns). Ø because it is an excellent solvent for a variety of solutes. 13

Water: The Super Solvent • H 2 O is such a versatile solvent that it is sometimes called the universal solvent. 14

Solvents and Solutes aqueous solution • water that contains dissolved sub. solvent • the dissolving medium in a soln. • exist in larger amt than solute • the dissolved particles in a soln 15

Solvents and Solutes both the solute and the solvent pass thru filter papers. 16

Solvents and Solutes • A solvent dissolves the solute. • solute disperses in the solvent. • Solvents and solutes may be (g), (l), or (s). • Solute particles can be atoms, ions, or molecules. 17

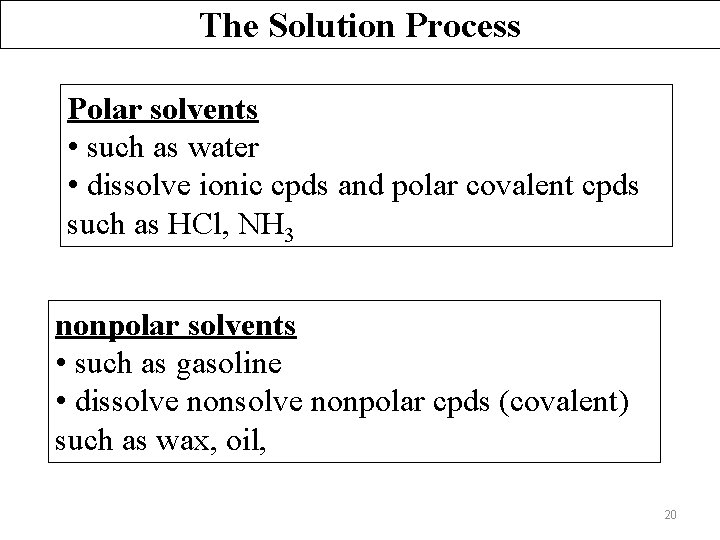
The Solution Process Polar solvents • e. g. water; • dissolve polar sub: most ionic cpds (e. g. Na. Cl), acids, bases, salts and some covalent cpds (polar covalent). Nonpolar solvents • e. g. gasoline, alcohol, kerosene, thinner, oil, acetone (organic solvents) • dissolve nonpolar sub (e. g. grease) Like dissolves like. 18

Electrolytes and Nonelectrolytes Electrolyte • a cpd that conducts an electric current when it is in an aq soln or in the molten state. (ionic cpds) • All ionic cpds (salts) are electrolytes → dissociate into ions. 19

The Solution Process Polar solvents • such as water • dissolve ionic cpds and polar covalent cpds such as HCl, NH 3 nonpolar solvents • such as gasoline • dissolve nonpolar cpds (covalent) such as wax, oil, 20

Concentrated vs Dilute • Don’t use strong and weak to soln concns. Use ‘concentrated’ and ‘dilute’ to describe concn. 21

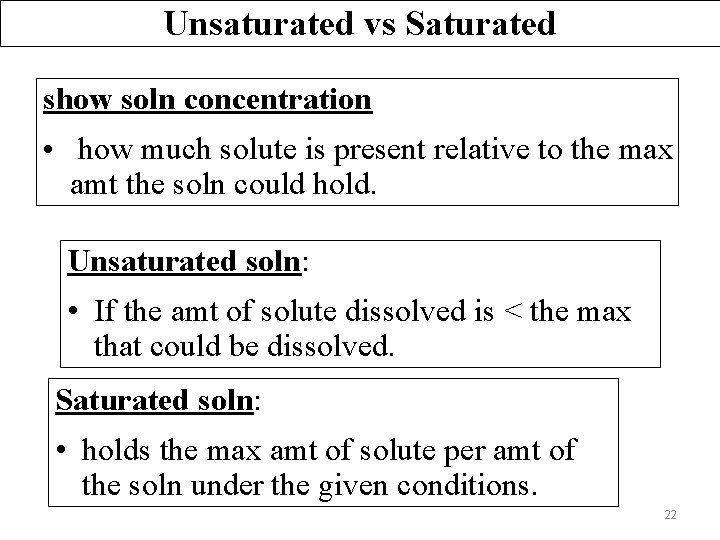
Unsaturated vs Saturated show soln concentration • how much solute is present relative to the max amt the soln could hold. Unsaturated soln: • If the amt of solute dissolved is < the max that could be dissolved. Saturated soln: • holds the max amt of solute per amt of the soln under the given conditions. 22

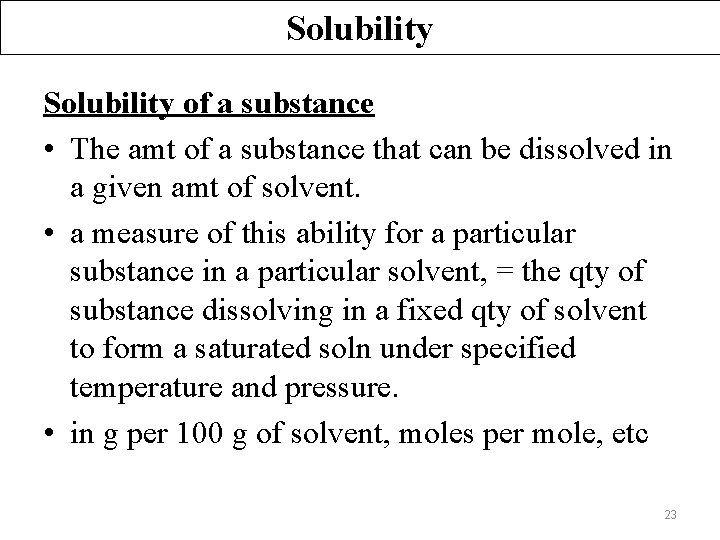
Solubility of a substance • The amt of a substance that can be dissolved in a given amt of solvent. • a measure of this ability for a particular substance in a particular solvent, = the qty of substance dissolving in a fixed qty of solvent to form a saturated soln under specified temperature and pressure. • in g per 100 g of solvent, moles per mole, etc 23

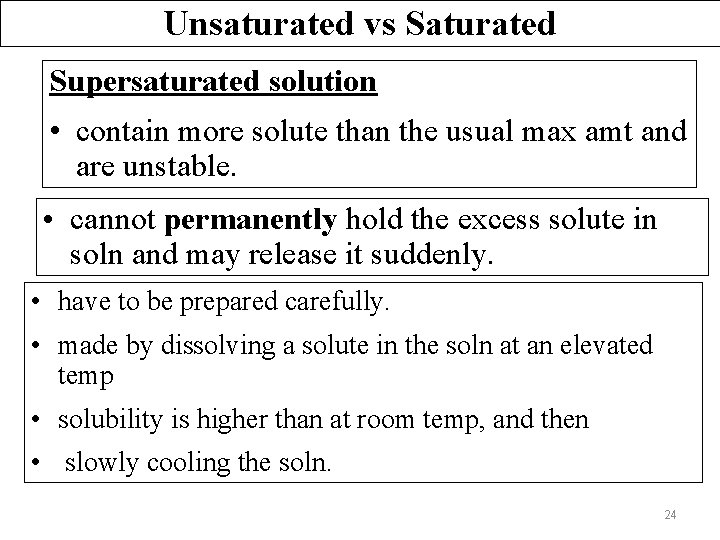
Unsaturated vs Saturated Supersaturated solution • contain more solute than the usual max amt and are unstable. • cannot permanently hold the excess solute in soln and may release it suddenly. • have to be prepared carefully. • made by dissolving a solute in the soln at an elevated temp • solubility is higher than at room temp, and then • slowly cooling the soln. 24

Factors Affecting Solubiity 25

Suspensions A suspension • a mixture from which particles settle out upon standing. e. g. sand mixes with water • particles are much larger and denser--- do not stay suspended indefinitely. 26

Suspensions are heterogenous as at least 2 sub can be clearly identified. 27

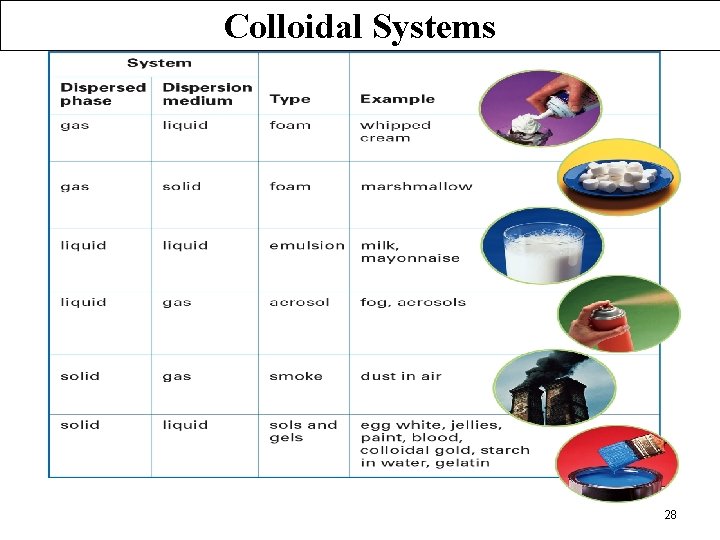
Colloidal Systems 28

Colloids colloid • a heterogeneous mixture • spread throughout the dispersion medium. • Particles smaller than those in suspensions and larger than those in solns. 29

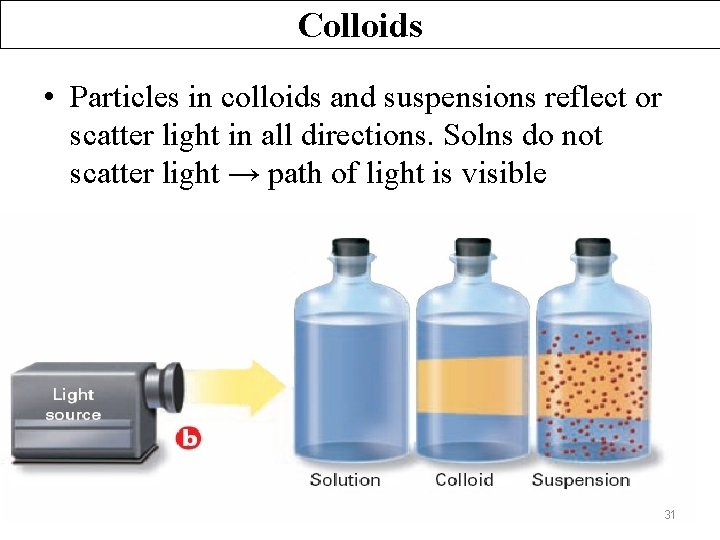
Colloids The Tyndall Effect Ø The scattering of visible light by colloidal and suspension particles. 30

Colloids • Particles in colloids and suspensions reflect or scatter light in all directions. Solns do not scatter light → path of light is visible 31

Colloids Emulsions Ø a colloidal dispersion of a (l) in a (l). ØAn emulsifying agent is essential for the formn of an emulsion and for maintaining the emulsion’s stability. 32

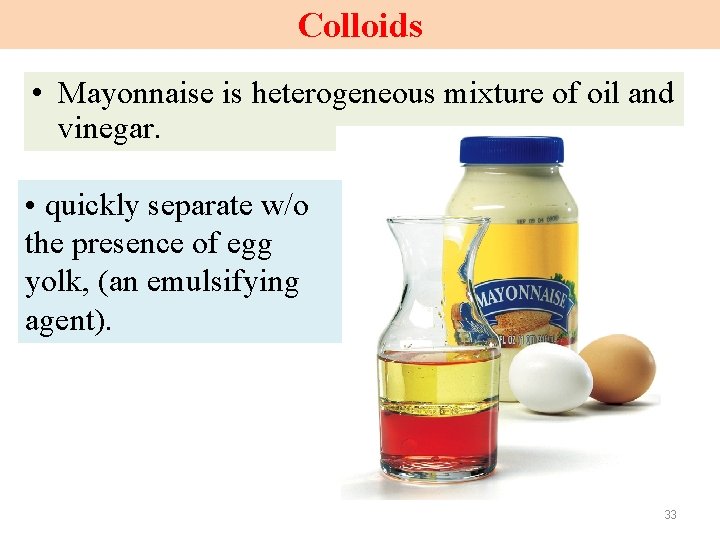
Colloids • Mayonnaise is heterogeneous mixture of oil and vinegar. • quickly separate w/o the presence of egg yolk, (an emulsifying agent). 33

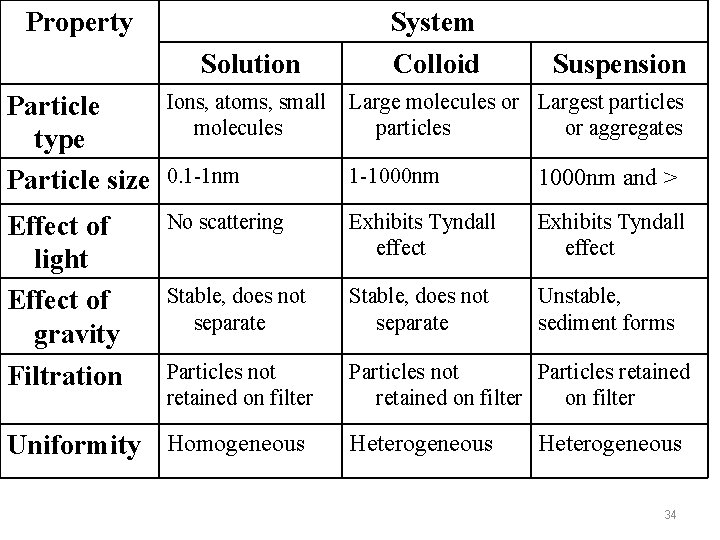
Property Solution System Colloid Suspension Particle type Particle size Ions, atoms, small molecules Large molecules or Largest particles or aggregates 0. 1 -1 nm 1 -1000 nm and > Effect of light Effect of gravity Filtration No scattering Exhibits Tyndall effect Stable, does not separate Unstable, sediment forms Particles not retained on filter Particles not Particles retained on filter Uniformity Homogeneous Heterogeneous 34

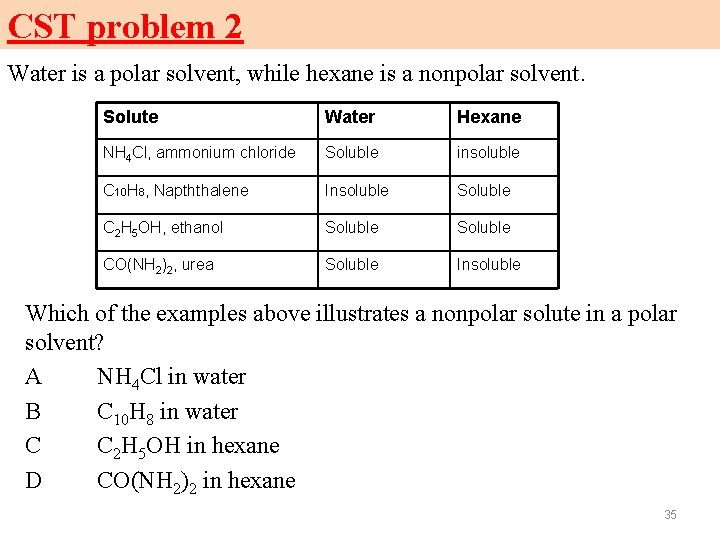
CST problem 2 Water is a polar solvent, while hexane is a nonpolar solvent. Solute Water Hexane NH 4 Cl, ammonium chloride Soluble insoluble C 10 H 8, Napththalene Insoluble Soluble C 2 H 5 OH, ethanol Soluble CO(NH 2)2, urea Soluble Insoluble Which of the examples above illustrates a nonpolar solute in a polar solvent? A NH 4 Cl in water B C 10 H 8 in water C C 2 H 5 OH in hexane D CO(NH 2)2 in hexane 35

The End 36