Unit 2 The Atom What is a theory

















![Dot Diagrams for Ions Ca Cl +2 Ca [ Cl -1 ] Dot Diagrams for Ions Ca Cl +2 Ca [ Cl -1 ]](https://slidetodoc.com/presentation_image_h/cf6081fd6e602ac669bcbf84fad12d00/image-46.jpg)






- Slides: 58

Unit 2: The Atom

What is a theory? n a well-supported explanation of some aspect of the natural world n Organized and accepted with a lot of data to support it n "theories can incorporate facts and laws and tested hypotheses"

"If I have seen further it is by standing on the shoulders of Giants. " --Isaac Newton

Early Theories Ancient Greece Democritus: 4 B. C. : “atom” Believed there were 4 elements: Fire, Air, Water, Earth

Dalton: 1766 -1844 >All elements composed of tiny particles called atoms >Atoms of same element are identical; atoms of different elements are different >Atoms can physically mix together or chemically combine to form compounds >Chemical reactions cannot change atoms of one type of element to another

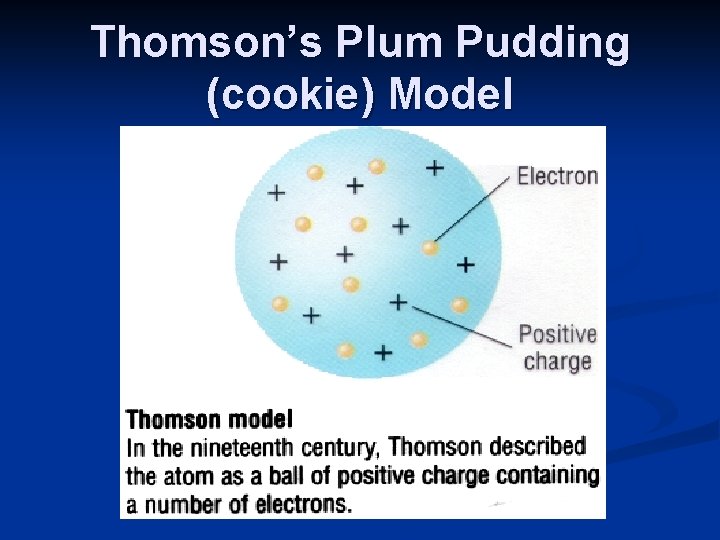
Thomson: 1856 -1940 >discovered electrons in 1897 >used a cathode ray tube >the ray produced was deflected by an electrical field (showed that atoms had particles with (-) charge)

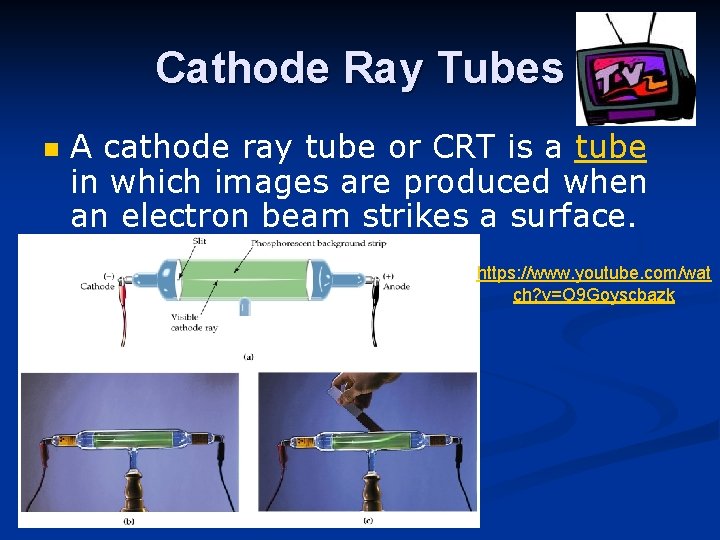
Cathode Ray Tubes n A cathode ray tube or CRT is a tube in which images are produced when an electron beam strikes a surface. https: //www. youtube. com/wat ch? v=O 9 Goyscbazk

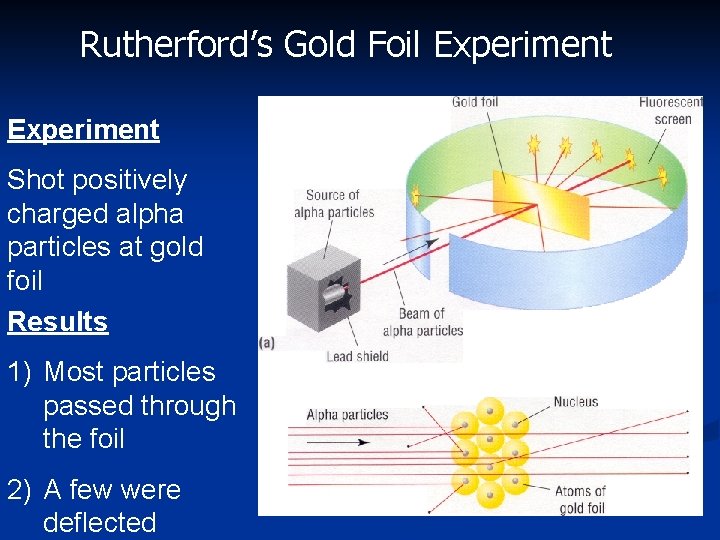
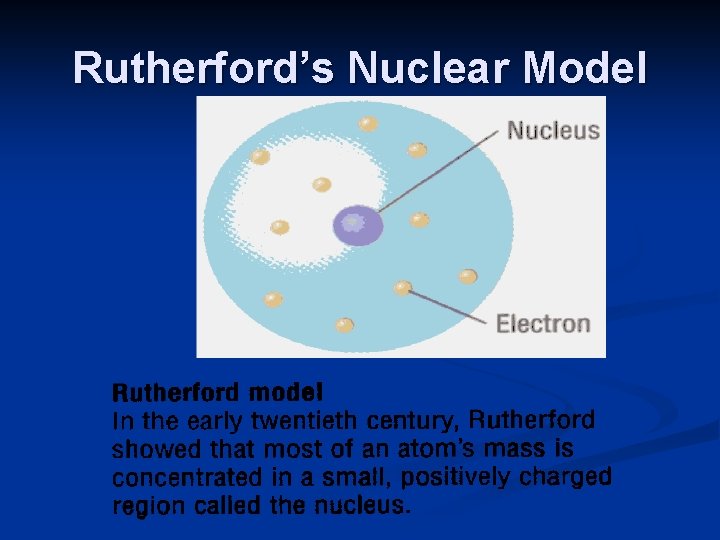
Rutherford: 1871 -1937 >Gold Foil Experiment >Discovered the nucleus

Rutherford’s Gold Foil Experiment Shot positively charged alpha particles at gold foil Results 1) Most particles passed through the foil 2) A few were deflected

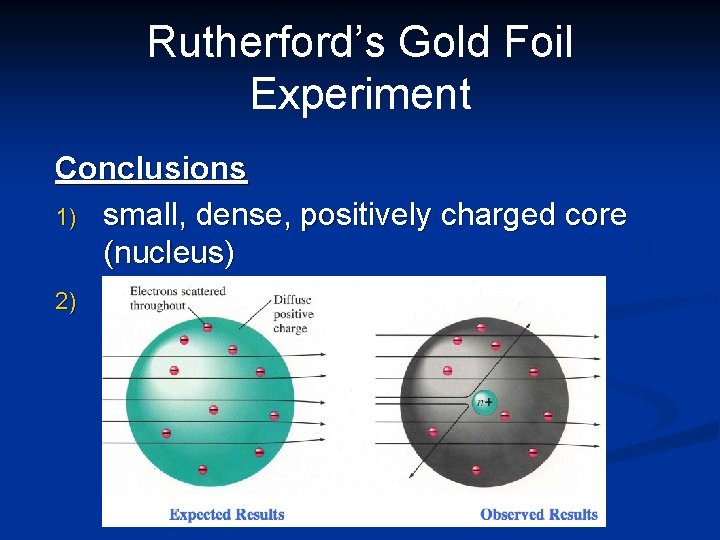
Rutherford’s Gold Foil Experiment Conclusions 1) small, dense, positively charged core (nucleus) 2) the rest of the atom is empty space

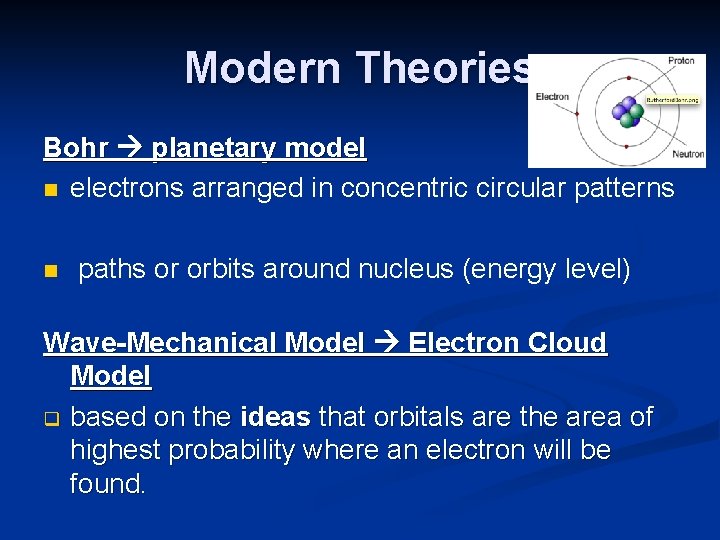
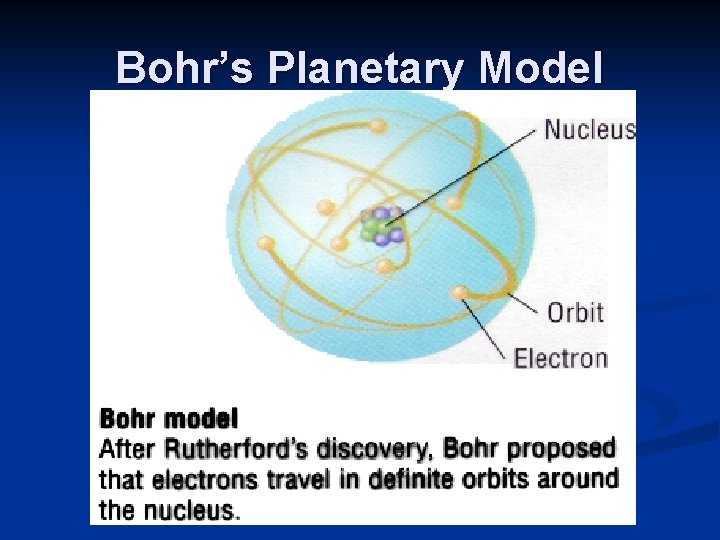
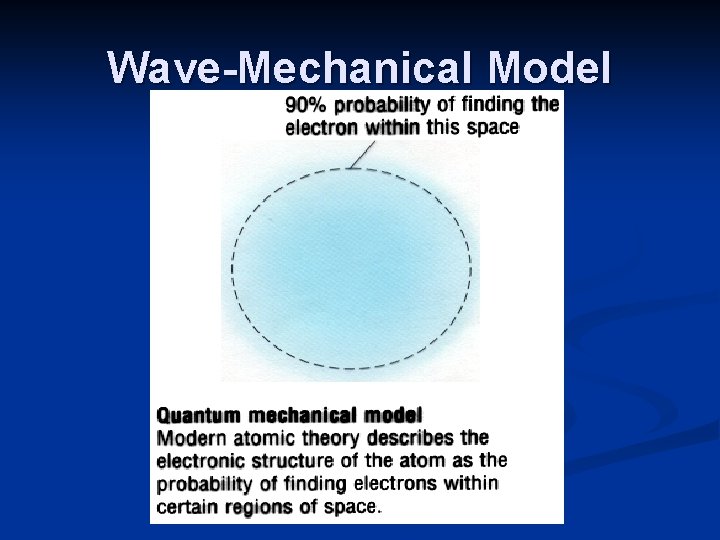
Modern Theories Bohr planetary model n electrons arranged in concentric circular patterns n paths or orbits around nucleus (energy level) Wave-Mechanical Model Electron Cloud Model q based on the ideas that orbitals are the area of highest probability where an electron will be found.

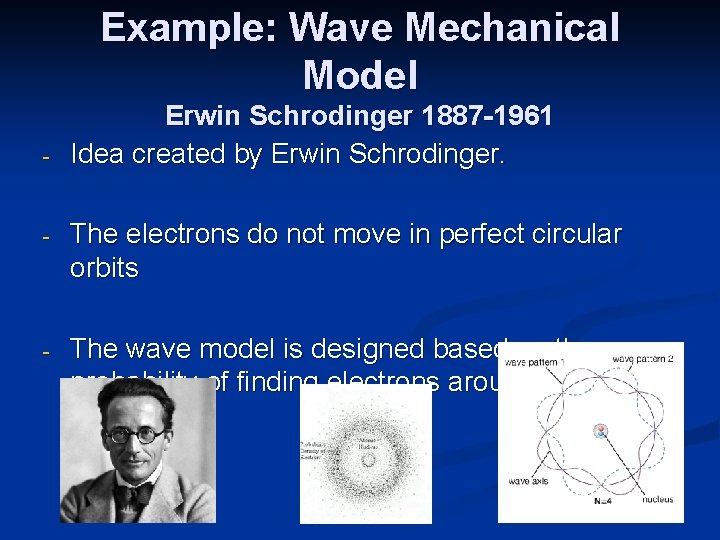
Example: Wave Mechanical Model - Erwin Schrodinger 1887 -1961 Idea created by Erwin Schrodinger. - The electrons do not move in perfect circular orbits - The wave model is designed based on the probability of finding electrons around a nucleus

Summary- Atomic Models Dalton’s Cannonball

Thomson’s Plum Pudding (cookie) Model

Rutherford’s Nuclear Model

Bohr’s Planetary Model

Wave-Mechanical Model

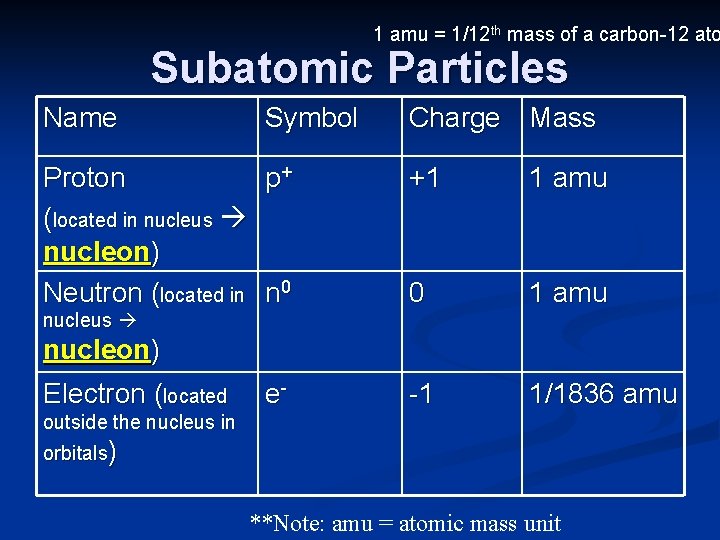
1 amu = 1/12 th mass of a carbon-12 ato Subatomic Particles Name Symbol Proton p+ (located in nucleus nucleon) Neutron (located in n 0 Charge Mass +1 1 amu 0 1 amu -1 1/1836 amu nucleus nucleon) Electron (located e- outside the nucleus in orbitals) **Note: amu = atomic mass unit

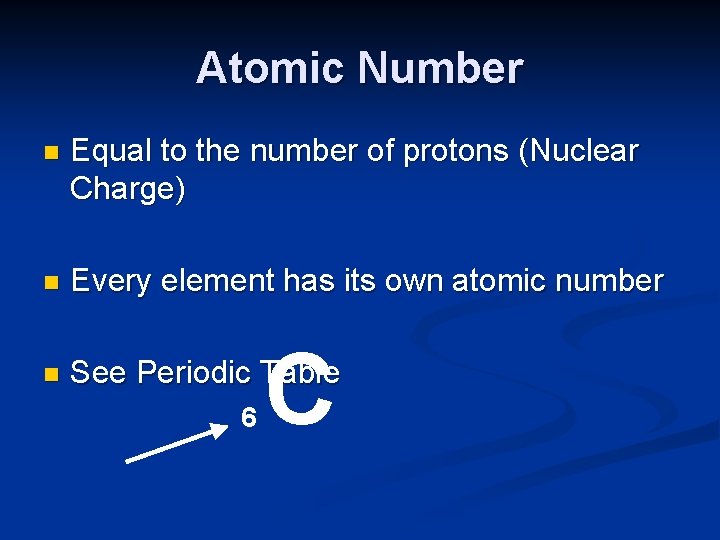
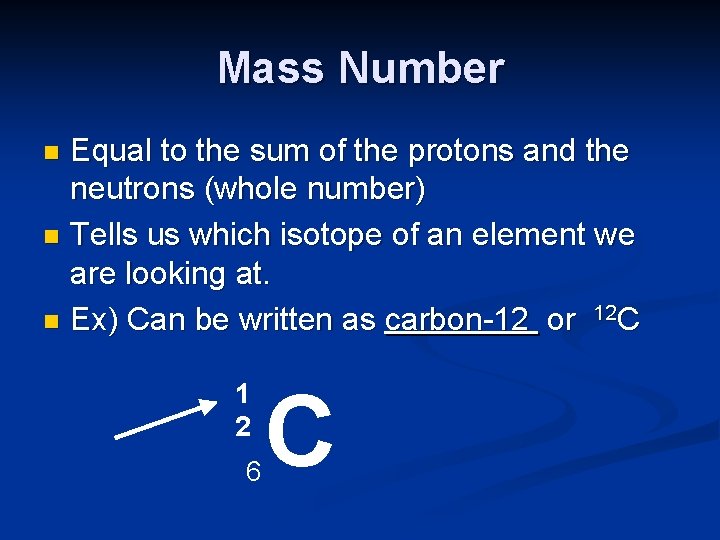
Atomic Number n Equal to the number of protons (Nuclear Charge) n Every element has its own atomic number n See Periodic Table 6 C

Mass Number Equal to the sum of the protons and the neutrons (whole number) n Tells us which isotope of an element we are looking at. n Ex) Can be written as carbon-12 or 12 C n 1 2 6 C

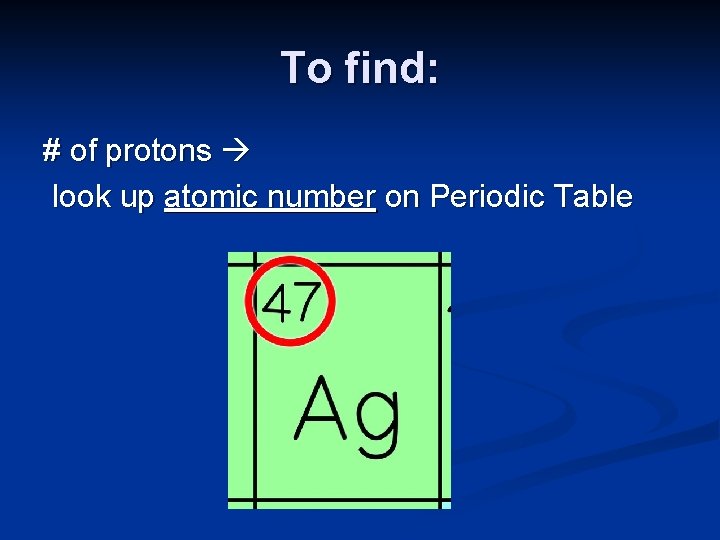
To find: # of protons look up atomic number on Periodic Table

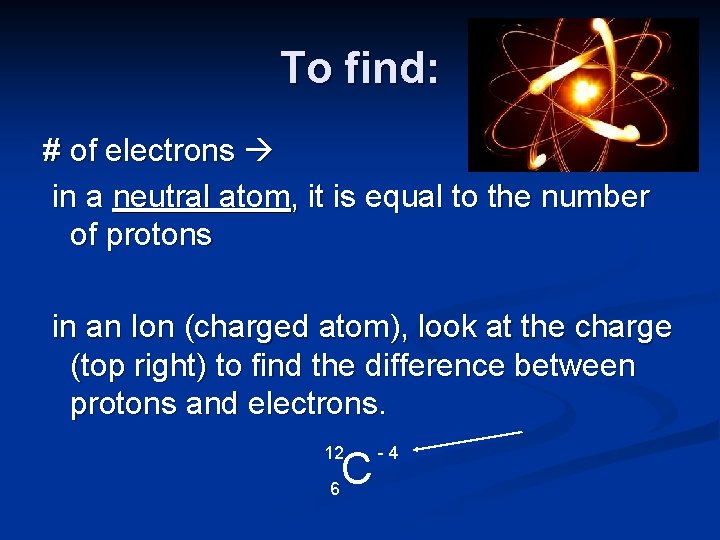
To find: # of electrons in a neutral atom, it is equal to the number of protons in an Ion (charged atom), look at the charge (top right) to find the difference between protons and electrons. 12 6 C -4

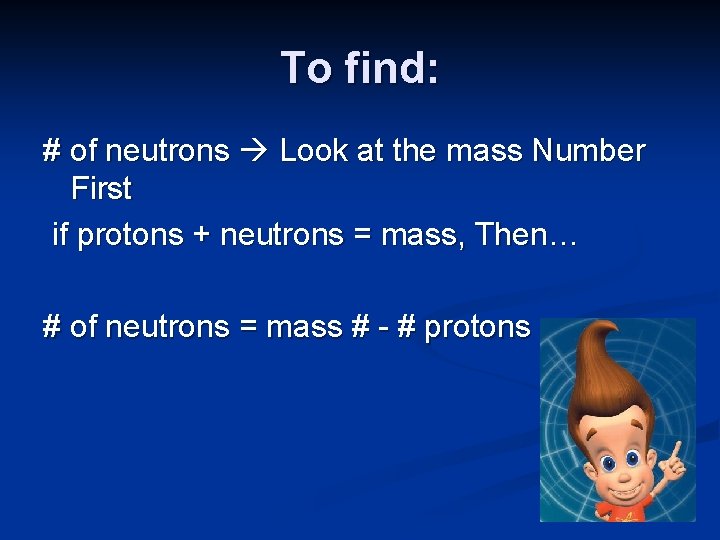
To find: # of neutrons Look at the mass Number First if protons + neutrons = mass, Then… # of neutrons = mass # - # protons

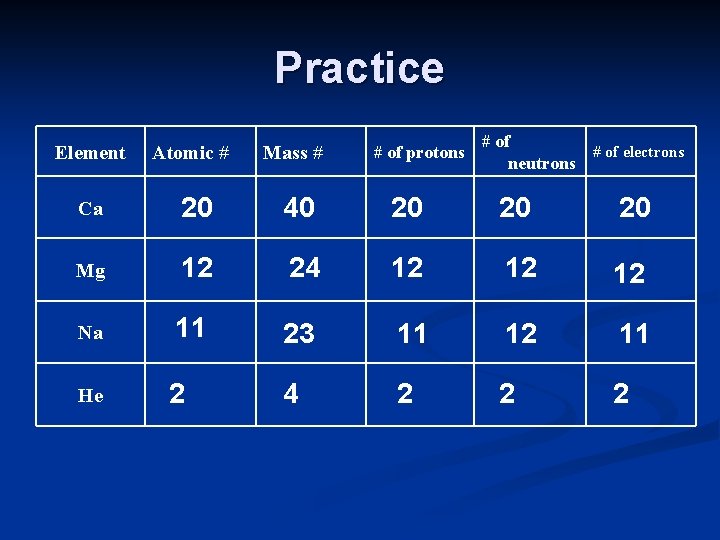
Practice Element Atomic # Mass # # of protons # of electrons neutrons Ca 20 40 20 20 20 Mg 12 24 12 12 12 Na 11 23 11 12 11 He 2 4 2 2 2

Do Now 1. What is the nuclear charge of a sulfur atom? 2. If an atom has 5 protons and 6 neutrons how many electrons does it have? What would the mass of this atom be? 3. What must all atoms of the same element have in common? 4. List the relative mass, charge and location of all three subatomic particles? 5. How many protons, neutrons and electrons does an atom of Lithium-7 have? 6. What conclusions about the atom were discovered as a result of the Gold Foil Experiment?

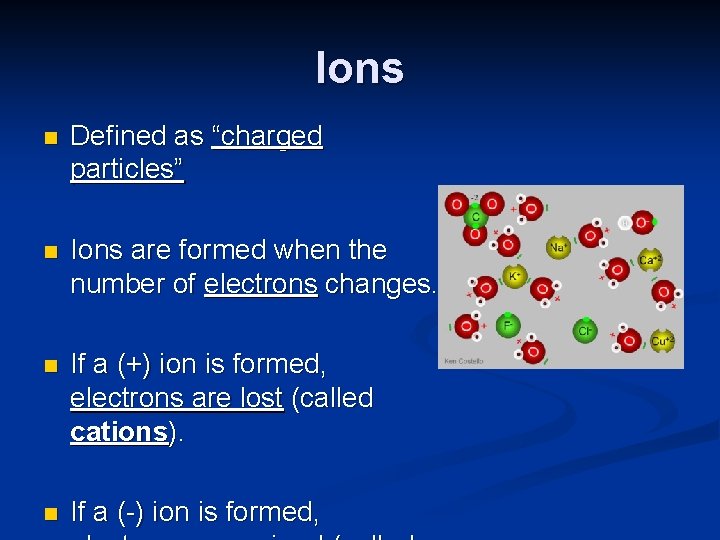
Ions n Defined as “charged particles” n Ions are formed when the number of electrons changes. n If a (+) ion is formed, electrons are lost (called cations). n If a (-) ion is formed,

Examples n Ca 2+ A Ca atom has 20 protons and 20 electrons. A Ca 2+ ion has lost two electrons to have 18.

Examples Cl A Cl atom has 17 protons and 17 electrons. A Cl- ion has gained one electron to have 18. n

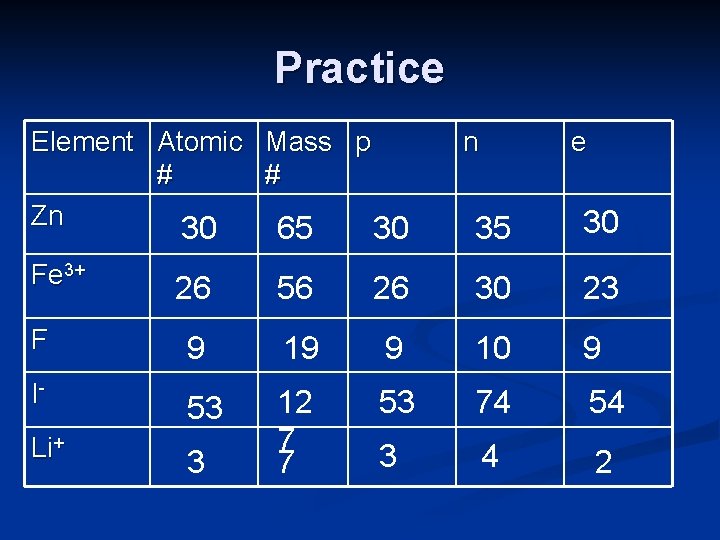
Practice Element Atomic Mass p # # Zn 30 65 30 Fe 3+ n e 35 30 26 56 26 30 23 F 9 19 9 10 9 I- 53 53 74 54 Li+ 3 12 7 7 3 4 2

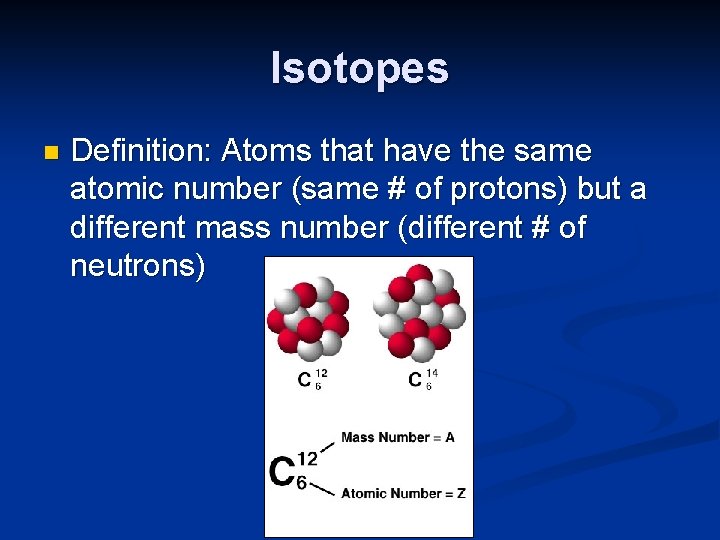
Isotopes n Definition: Atoms that have the same atomic number (same # of protons) but a different mass number (different # of neutrons)

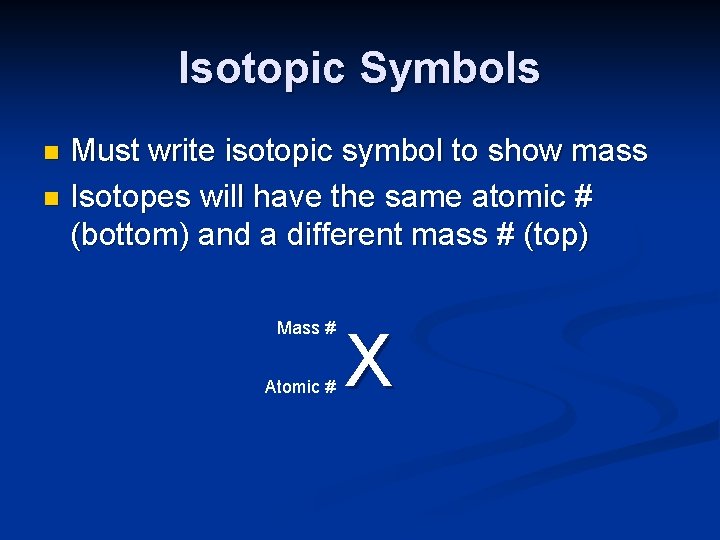
Isotopic Symbols Must write isotopic symbol to show mass n Isotopes will have the same atomic # (bottom) and a different mass # (top) n Mass # Atomic # X

Write the isotopic symbol for: n Carbon-14 (write a symbol for a different isotope of carbon) 14 6 C

Write the isotopic symbol for: n Oxygen-17 (write a symbol for a different isotope of carbon) 17 8 O

Write the isotopic symbol for: n Chlorine-37 (write a symbol for a different isotope of carbon) 37 17 Cl

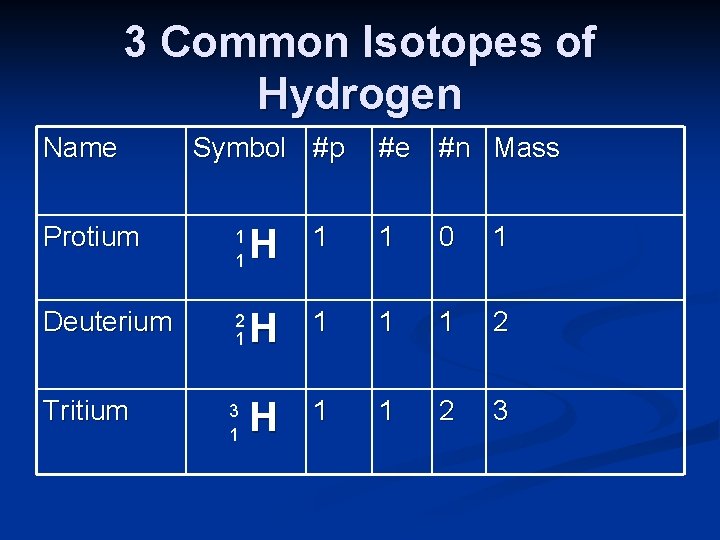
3 Common Isotopes of Hydrogen Name Symbol #p #e #n Mass Protium 1 1 H 1 1 0 1 Deuterium 2 1 H 1 1 1 2 H 1 1 2 3 Tritium 3 1

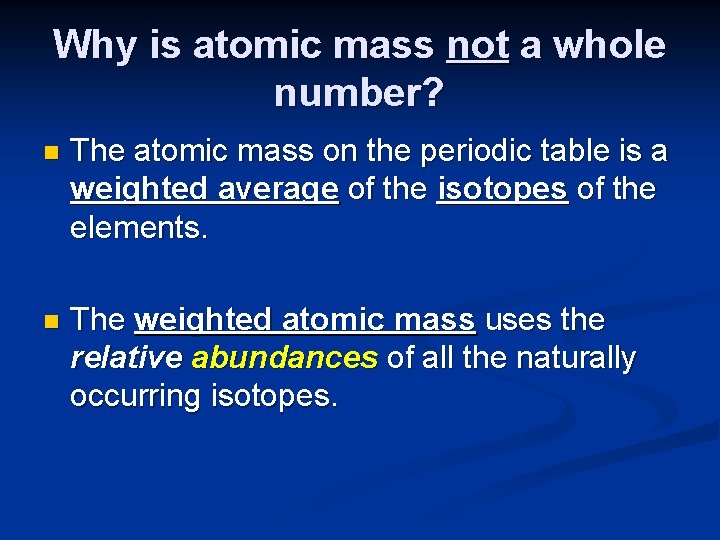
Why is atomic mass not a whole number? n The atomic mass on the periodic table is a weighted average of the isotopes of the elements. n The weighted atomic mass uses the relative abundances of all the naturally occurring isotopes.

How do you calculate a weighted average? n First move the decimal for the abundance percentages two spaces to the left. n Multiply these decimals by the mass for each isotope. n Add them all up.

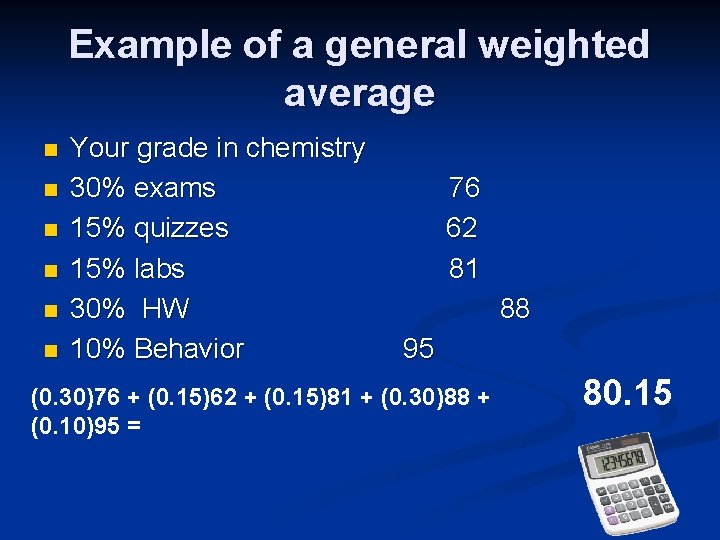
Example of a general weighted average n n n Your grade in chemistry 30% exams 15% quizzes 15% labs 30% HW 10% Behavior 76 62 81 88 95 (0. 30)76 + (0. 15)62 + (0. 15)81 + (0. 30)88 + (0. 10)95 = 80. 15

Example 1: n n n Determine weighted atomic mass Boron-10 19. 78% 10. 013 amu Boron-11 80. 22% 11. 009 amu (0. 1978) 10. 013 + (. 8022) 11. 009 = 10. 812 amu

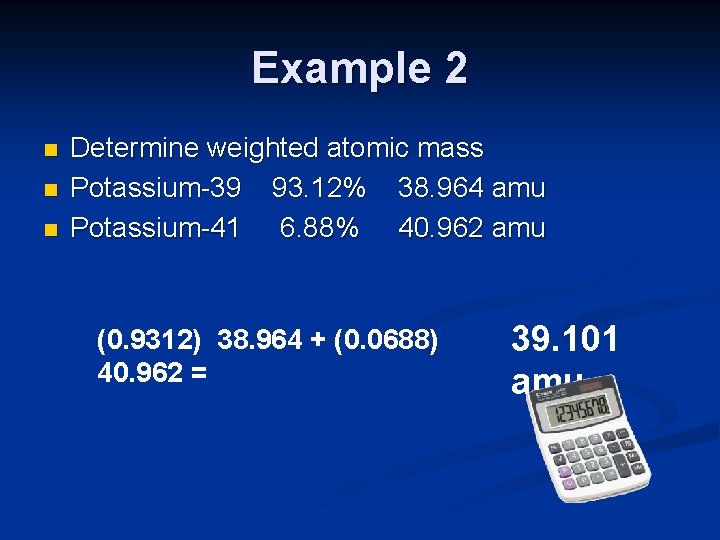
Example 2 n n n Determine weighted atomic mass Potassium-39 93. 12% 38. 964 amu Potassium-41 6. 88% 40. 962 amu (0. 9312) 38. 964 + (0. 0688) 40. 962 = 39. 101 amu

Do Now 1. How many total electrons does an Al+3 ion have? 2. If a neutral atom has 10 neutrons and 8 electrons how many protons does it have? 3. How does an atom of Lithium-7 differ from an atom of Beryllium-9 4. Compare a Na+1 ion to a Na atom? 14 15 16

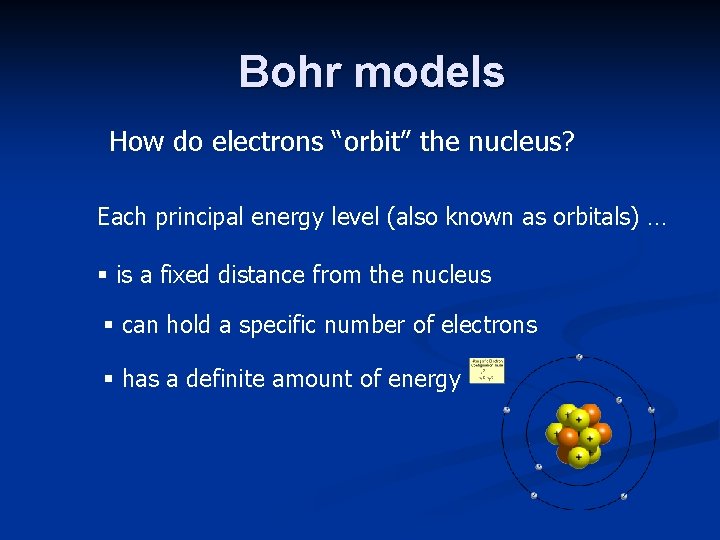
Bohr models How do electrons “orbit” the nucleus? Each principal energy level (also known as orbitals) … § is a fixed distance from the nucleus § can hold a specific number of electrons § has a definite amount of energy

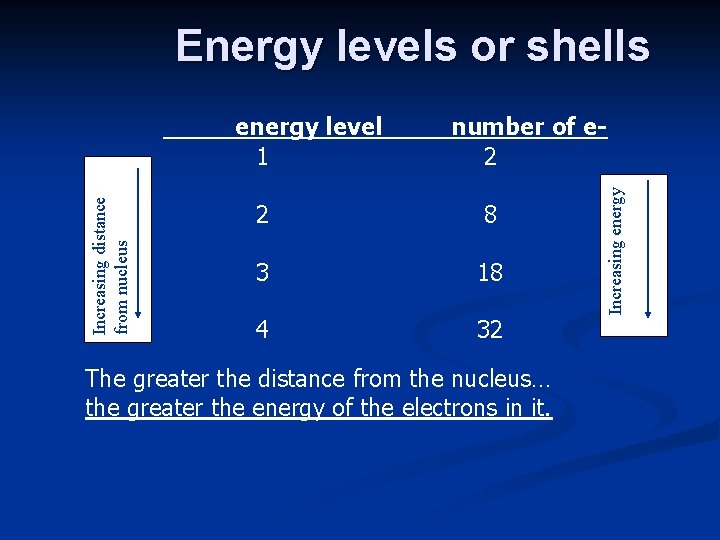
Energy levels or shells number of e 2 2 8 3 18 4 32 The greater the distance from the nucleus… the greater the energy of the electrons in it. Increasing energy Increasing distance from nucleus energy level 1

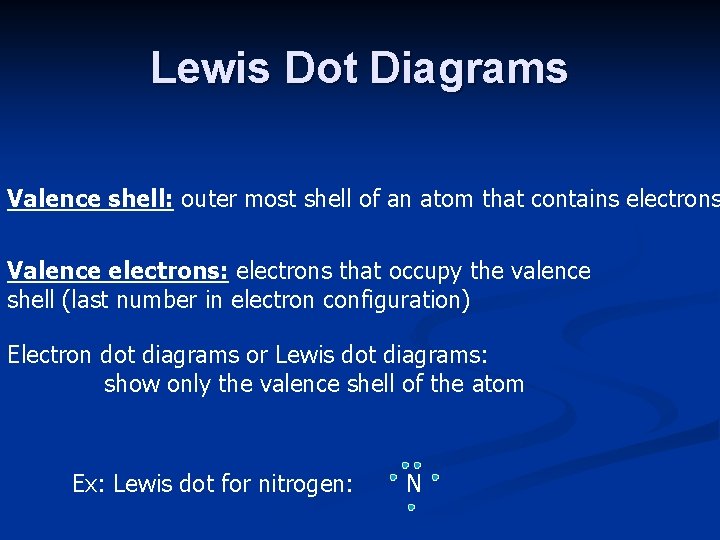
Lewis Dot Diagrams Valence shell: outer most shell of an atom that contains electrons Valence electrons: electrons that occupy the valence shell (last number in electron configuration) Electron dot diagrams or Lewis dot diagrams: show only the valence shell of the atom Ex: Lewis dot for nitrogen: N

TRY THESE O F C Ne I K
![Dot Diagrams for Ions Ca Cl 2 Ca Cl 1 Dot Diagrams for Ions Ca Cl +2 Ca [ Cl -1 ]](https://slidetodoc.com/presentation_image_h/cf6081fd6e602ac669bcbf84fad12d00/image-46.jpg)
Dot Diagrams for Ions Ca Cl +2 Ca [ Cl -1 ]

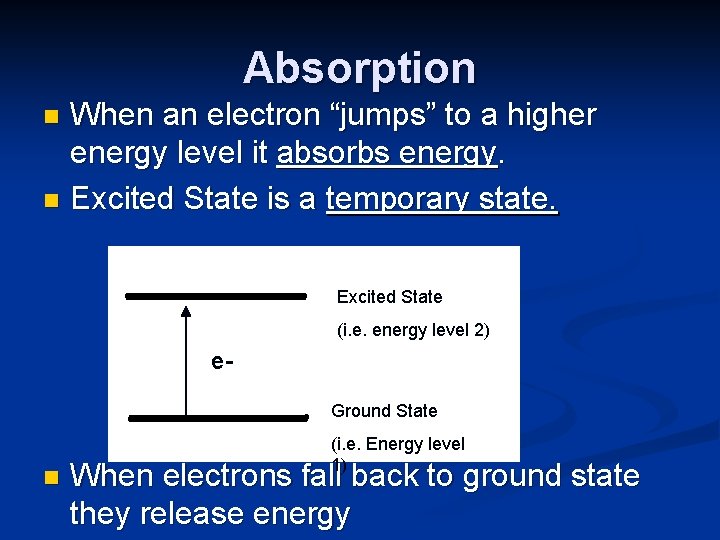
Ground State vs. Excited State n When electrons in an atom fill the lowest orbitals, it is said to be in the ground state. n When electrons absorb energy, they have the ability to jump to higher energy levels. n Excited state is when electrons no longer occupy the lowest available energy levels. (this is temporary)

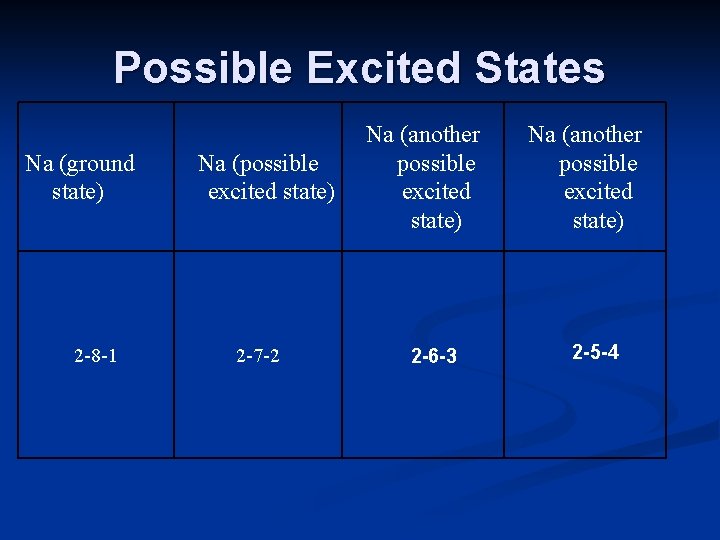
Possible Excited States Na (ground state) 2 -8 -1 Na (possible excited state) 2 -7 -2 Na (another possible excited state) 2 -6 -3 Na (another possible excited state) 2 -5 -4

Absorption When an electron “jumps” to a higher energy level it absorbs energy. n Excited State is a temporary state. n Excited State (i. e. energy level 2) e. Ground State n (i. e. Energy level 1) When electrons fall back to ground state they release energy

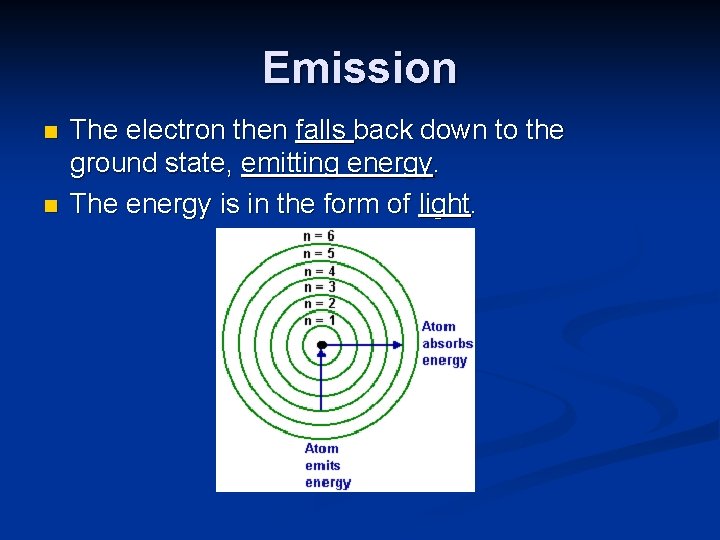
Emission n n The electron then falls back down to the ground state, emitting energy. The energy is in the form of light.

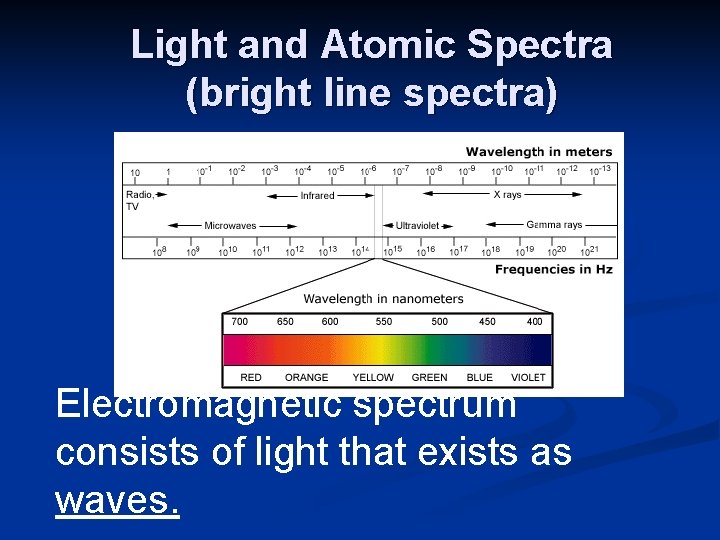
Light and Atomic Spectra (bright line spectra) Electromagnetic spectrum consists of light that exists as waves.

Sunlight and prisms n Sunlight produces a continuous range of wavelengths and frequencies that can be separated into all the colors of the rainbow. n ROY G B I V.

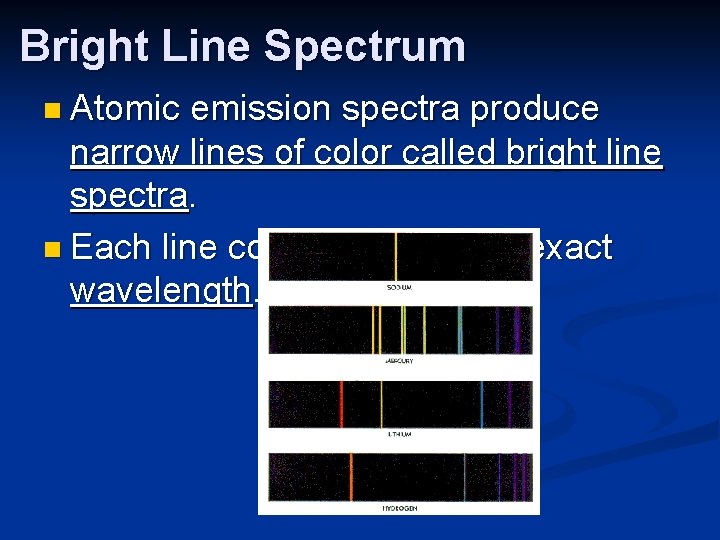
Bright Line Spectrum n Atomic emission spectra produce narrow lines of color called bright line spectra. n Each line corresponds to an exact wavelength.

Experiments – Flame Tests n Flame Tests – demonstrates the emission spectrum of a substance n By heating elements to high temperatures they enter excited state n Characteristic color will be emitted as excited electrons return to ground state.

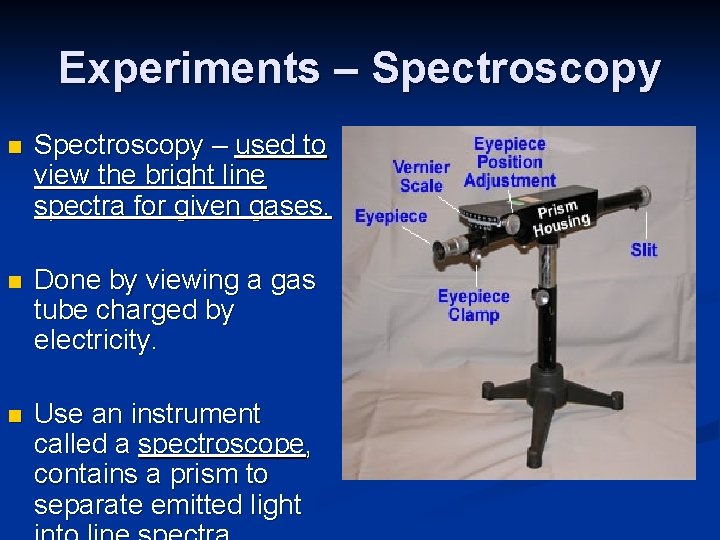
Experiments – Spectroscopy n Spectroscopy – used to view the bright line spectra for given gases. n Done by viewing a gas tube charged by electricity. n Use an instrument called a spectroscope, contains a prism to separate emitted light


