The Bohr Atom Evolution of Electron Configuration The

The Bohr Atom - Evolution of Electron Configuration The Bohr Atom Evolution of Electron Configuration This presentation is partially animated. Only use the control panel at the bottom of screen to review what you have seen. When using your mouse, make sure you click only when it is within the light blue frame that surrounds each slide.
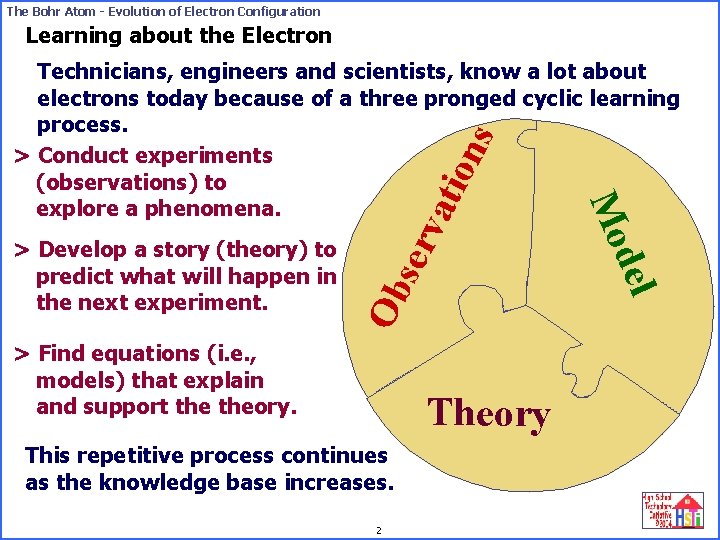
The Bohr Atom - Evolution of Electron Configuration Learning about the Electron ser v Ob > Find equations (i. e. , models) that explain and support theory. Theory This repetitive process continues as the knowledge base increases. 2 del > Develop a story (theory) to predict what will happen in the next experiment. Mo ati ons Technicians, engineers and scientists, know a lot about electrons today because of a three pronged cyclic learning process. > Conduct experiments (observations) to explore a phenomena.
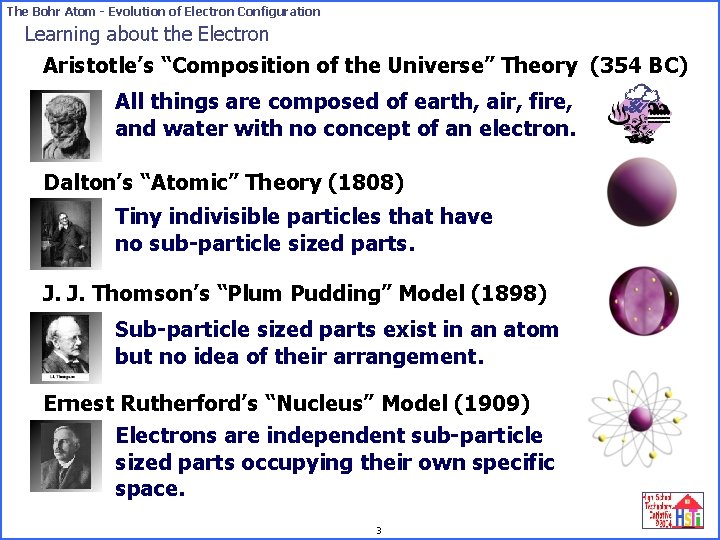
The Bohr Atom - Evolution of Electron Configuration Learning about the Electron Aristotle’s “Composition of the Universe” Theory (354 BC) All things are composed of earth, air, fire, and water with no concept of an electron. Dalton’s “Atomic” Theory (1808) Tiny indivisible particles that have no sub-particle sized parts. J. J. Thomson’s “Plum Pudding” Model (1898) Sub-particle sized parts exist in an atom but no idea of their arrangement. Ernest Rutherford’s “Nucleus” Model (1909) Electrons are independent sub-particle sized parts occupying their own specific space. 3
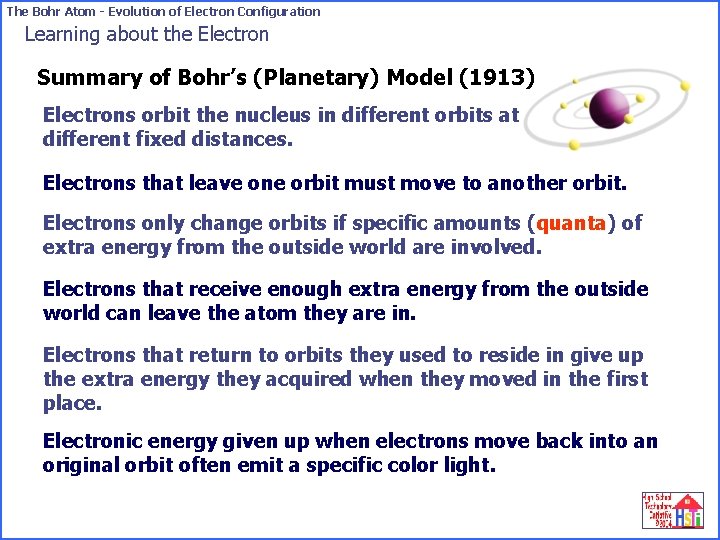
The Bohr Atom - Evolution of Electron Configuration Learning about the Electron Summary of Bohr’s (Planetary) Model (1913) Electrons orbit the nucleus in different orbits at different fixed distances. Electrons that leave one orbit must move to another orbit. Electrons only change orbits if specific amounts (quanta) of extra energy from the outside world are involved. Electrons that receive enough extra energy from the outside world can leave the atom they are in. Electrons that return to orbits they used to reside in give up the extra energy they acquired when they moved in the first place. Electronic energy given up when electrons move back into an original orbit often emit a specific color light.

on s The Bohr Atom - Evolution of Electron Configuration ati se rv Ob del Observations Mo The Puzzle Pieces Bohr had to Solve The most important observation Bohr needed to explain was the fact that when energy was added to atoms the atoms gave off (emitted) light. Theory The biggest problem with Rutherford’s story was why the electrons don’t eventually crash into the nucleus and destroy the atom. Model Up to this point in time (1913), nobody knew why Balmer’s equation predicted the color (frequency) of the light waves emitted by atoms. A difficult puzzle to solve, but Bohr made a major step in putting the pieces together. 5
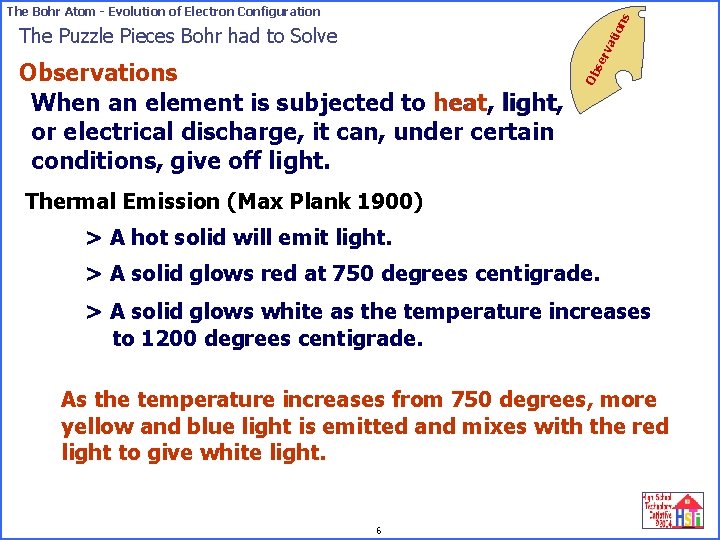
on s The Bohr Atom - Evolution of Electron Configuration Ob Observations When an element is subjected to heat, light, or electrical discharge, it can, under certain conditions, give off light. se rv ati The Puzzle Pieces Bohr had to Solve Thermal Emission (Max Plank 1900) > A hot solid will emit light. > A solid glows red at 750 degrees centigrade. > A solid glows white as the temperature increases to 1200 degrees centigrade. As the temperature increases from 750 degrees, more yellow and blue light is emitted and mixes with the red light to give white light. 6
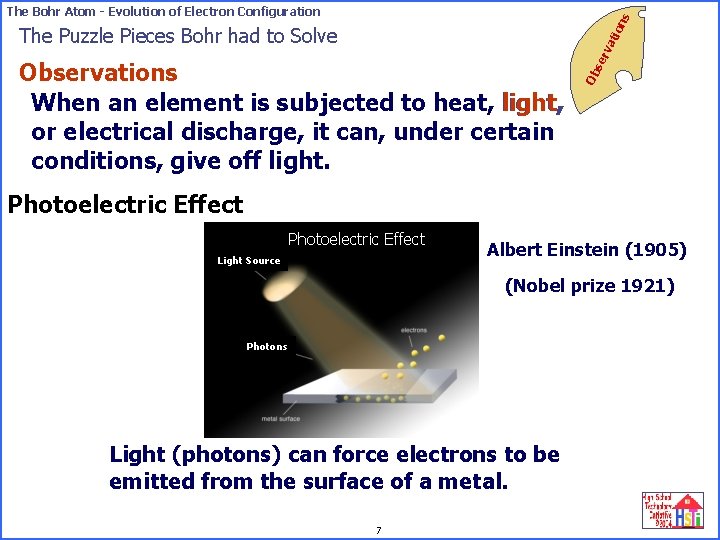
on s The Bohr Atom - Evolution of Electron Configuration Ob Observations When an element is subjected to heat, light, or electrical discharge, it can, under certain conditions, give off light. se rv ati The Puzzle Pieces Bohr had to Solve Photoelectric Effect Light Source Albert Einstein (1905) (Nobel prize 1921) Photons Light (photons) can force electrons to be emitted from the surface of a metal. 7

on s The Bohr Atom - Evolution of Electron Configuration Ob Observations When an element is subjected to heat, light, or electrical discharge, it can, under certain conditions, give off light. se rv ati The Puzzle Pieces Bohr had to Solve Corona Who, What, Where is it? Who St. Elmo’s Fire As described in “The Tempest” What/Where 8

on s The Bohr Atom - Evolution of Electron Configuration se rv ati The Puzzle Pieces Bohr had to Solve Ob Observations When an element is subjected to heat, light, or electrical discharge, it can, under certain conditions, give off light. Corona (1611) “I boarded the king’s ship; now in the beak, Now in the waist, the deck, in every cabin, I flamed amazement; sometimes I’d divide And burn in many places; on the topmast, The yards and bowsprit, would I flame distinctly, Then meet and join. ” A passage from “The Tempest” by William Shakespeare 9
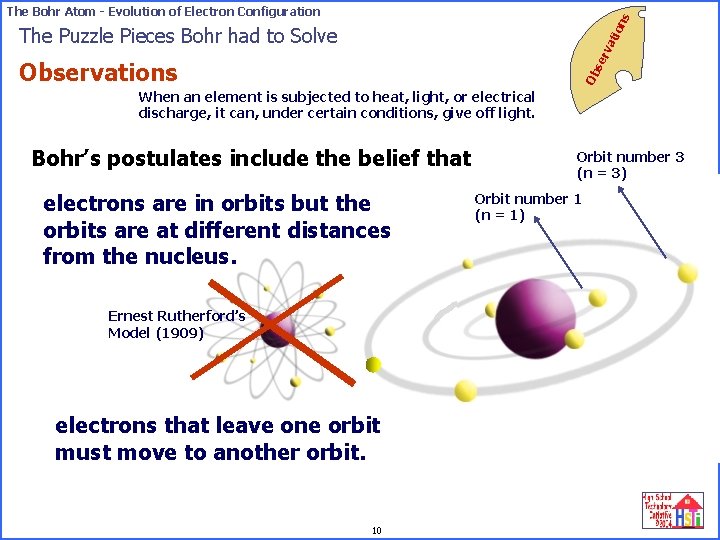
on s The Bohr Atom - Evolution of Electron Configuration se rv ati The Puzzle Pieces Bohr had to Solve Ob Observations When an element is subjected to heat, light, or electrical discharge, it can, under certain conditions, give off light. Bohr’s postulates include the belief that electrons are in orbits but the orbits are at different distances from the nucleus. Ernest Rutherford’s Model (1909) electrons that leave one orbit must move to another orbit. 10 Orbit number 3 (n = 3) Orbit number 1 (n = 1)
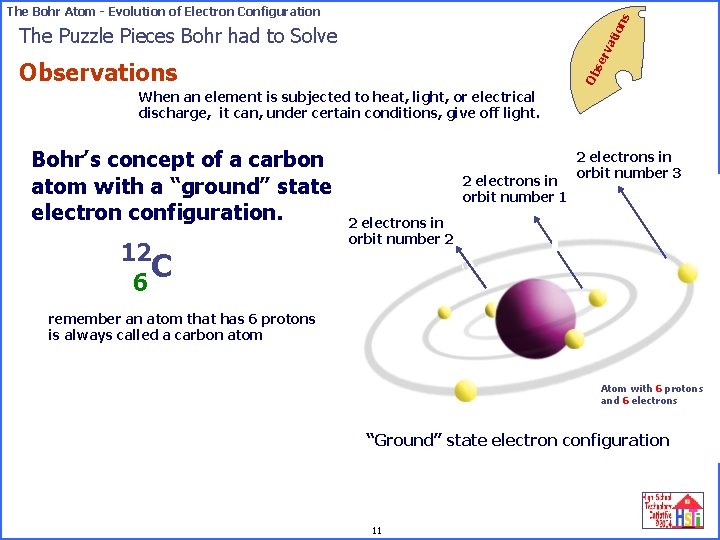
on s The Bohr Atom - Evolution of Electron Configuration se rv ati The Puzzle Pieces Bohr had to Solve Ob Observations When an element is subjected to heat, light, or electrical discharge, it can, under certain conditions, give off light. Bohr’s concept of a carbon atom with a “ground” state electron configuration. 12 C 6 2 electrons in orbit number 1 2 electrons in orbit number 3 2 electrons in orbit number 2 remember an atom that has 6 protons is always called a carbon atom Atom with 6 protons and 6 electrons “Ground” state electron configuration 11

on s The Bohr Atom - Evolution of Electron Configuration se rv ati The Puzzle Pieces Bohr had to Solve Ob Observations When an element is subjected to heat, light, or electrical discharge, it can, under certain conditions, give off light. The electron configuration of an atom changes when energy (heat, light, or electricity) is added to the atom. electrons only change orbits if specific amounts (quanta) of extra energy from the outside world are involved. 12 C 6 Atom with 6 protons and 6 electrons “Ground” state electron configuration “Excited” 12
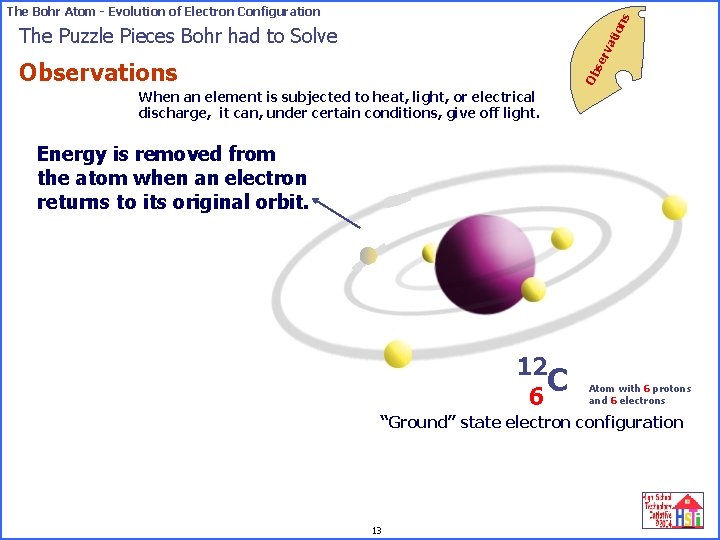
on s The Bohr Atom - Evolution of Electron Configuration se rv ati The Puzzle Pieces Bohr had to Solve Ob Observations When an element is subjected to heat, light, or electrical discharge, it can, under certain conditions, give off light. Energy is removed from the atom when an electron returns to its original orbit. 12 C 6 Atom with 6 protons and 6 electrons “Excited” state electron configuration “Ground” 13
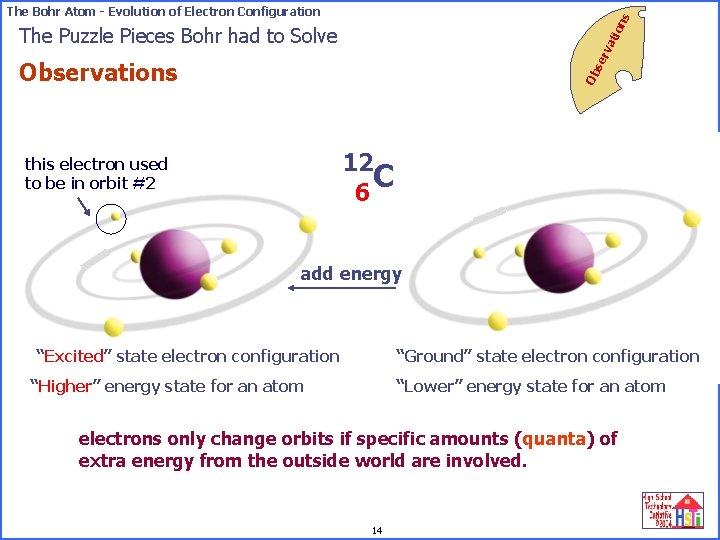
on s The Bohr Atom - Evolution of Electron Configuration se rv ati The Puzzle Pieces Bohr had to Solve Ob Observations 12 C 6 this electron used to be in orbit #2 add energy “Excited” state electron configuration “Ground” state electron configuration “Higher” energy state for an atom “Lower” energy state for an atom electrons only change orbits if specific amounts (quanta) of extra energy from the outside world are involved. 14
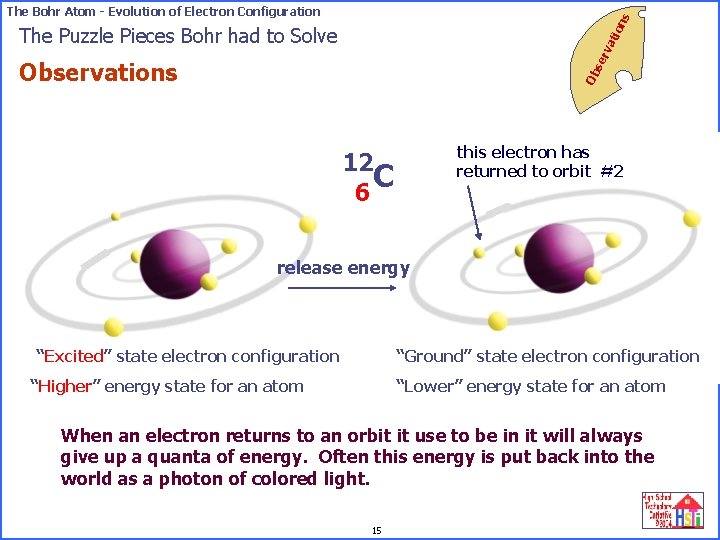
on s The Bohr Atom - Evolution of Electron Configuration se rv ati The Puzzle Pieces Bohr had to Solve Ob Observations this electron has returned to orbit #2 12 C 6 release energy “Excited” state electron configuration “Ground” state electron configuration “Higher” energy state for an atom “Lower” energy state for an atom When an electron returns to aniforbit it use to be in(quanta) it will always electrons only change orbits specific amounts of give up energy a quanta of energy. Often this are energy is put back into the extra from the outside world involved. world as a photon of colored light. 15
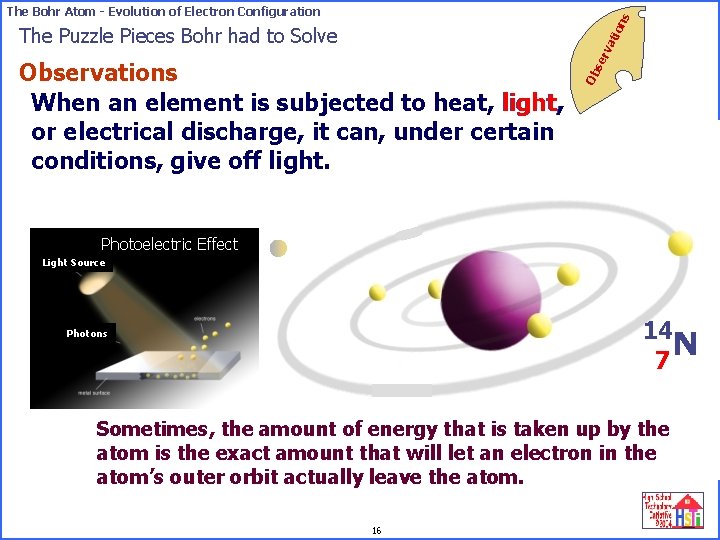
on s The Bohr Atom - Evolution of Electron Configuration Ob Observations When an element is subjected to heat, light, or electrical discharge, it can, under certain conditions, give off light. se rv ati The Puzzle Pieces Bohr had to Solve “Ground” state electron configuration Photoelectric Effect Light Source 14 N 7 Photons Sometimes, the amount of energy that is taken up by the atom is the exact amount that will let an electron in the atom’s outer orbit actually leave the atom. 16
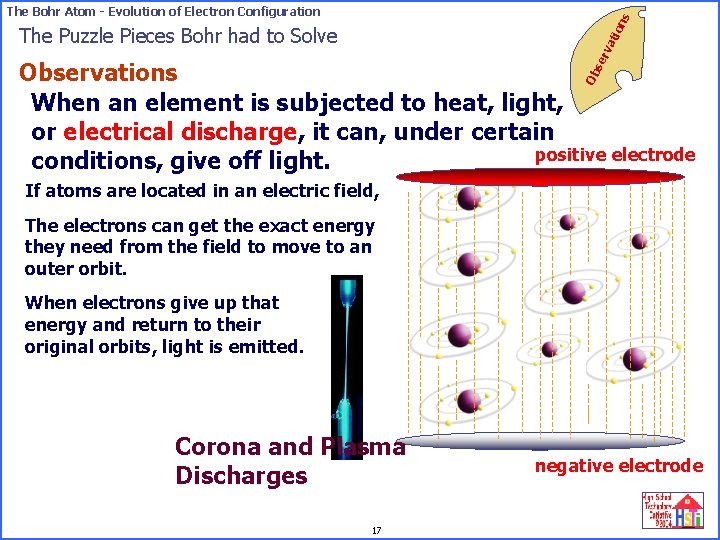
on s The Bohr Atom - Evolution of Electron Configuration se rv ati The Puzzle Pieces Bohr had to Solve Ob Observations When an element is subjected to heat, light, or electrical discharge, it can, under certain positive electrode conditions, give off light. If atoms are located in an electric field, The electrons can get the exact energy they need from the field to move to an outer orbit. When electrons give up that energy and return to their original orbits, light is emitted. Corona and Plasma Discharges 17 negative electrode
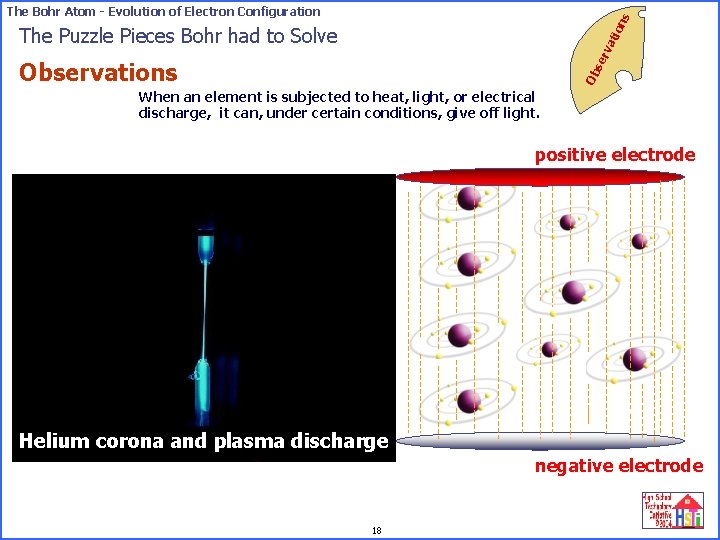
on s The Bohr Atom - Evolution of Electron Configuration se rv ati The Puzzle Pieces Bohr had to Solve Ob Observations When an element is subjected to heat, light, or electrical discharge, it can, under certain conditions, give off light. positive electrode Helium corona and plasma discharge negative electrode 18
on s The Bohr Atom - Evolution of Electron Configuration ati se rv Ob del Theory Mo The Puzzle Pieces Bohr had to Solve The biggest problem with Rutherford’s story was why the electrons do not eventually crash into the nucleus and destroy the atom. Bohr’s postulates include the belief that Electrons can only have a specific energy value to be in a specific orbit of an atom. Electrons remain in a specific orbit unless they obtain additional energy to move into orbits that are further from the nucleus. Therefore, electrons are not satellites (Rutherford’s theory) like man made satellites in continuous decaying orbits that gradually spiral toward the center. 19 Theory
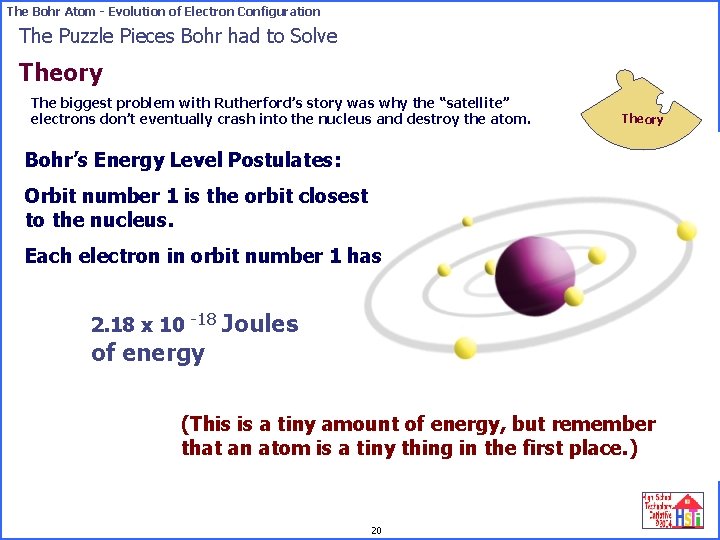
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Theory The biggest problem with Rutherford’s story was why the “satellite” electrons don’t eventually crash into the nucleus and destroy the atom. Theory Bohr’s Energy Level Postulates: Orbit number 1 is the orbit closest to the nucleus. Each electron in orbit number 1 has 2. 18 x 10 -18 Joules of energy (This is a tiny amount of energy, but remember that an atom is a tiny thing in the first place. ) 20
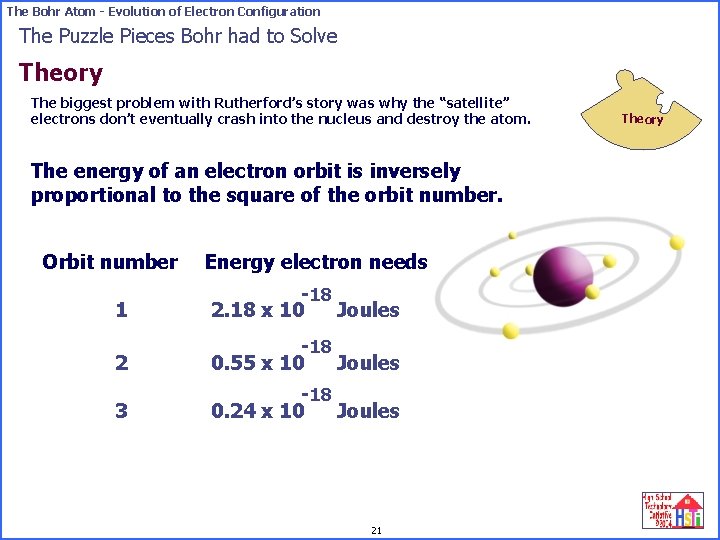
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Theory The biggest problem with Rutherford’s story was why the “satellite” electrons don’t eventually crash into the nucleus and destroy the atom. The energy of an electron orbit is inversely proportional to the square of the orbit number. Orbit number Energy electron needs 1 -18 2. 18 x 10 Joules 2 -18 0. 55 x 10 Joules 3 -18 0. 24 x 10 Joules 21 Theory
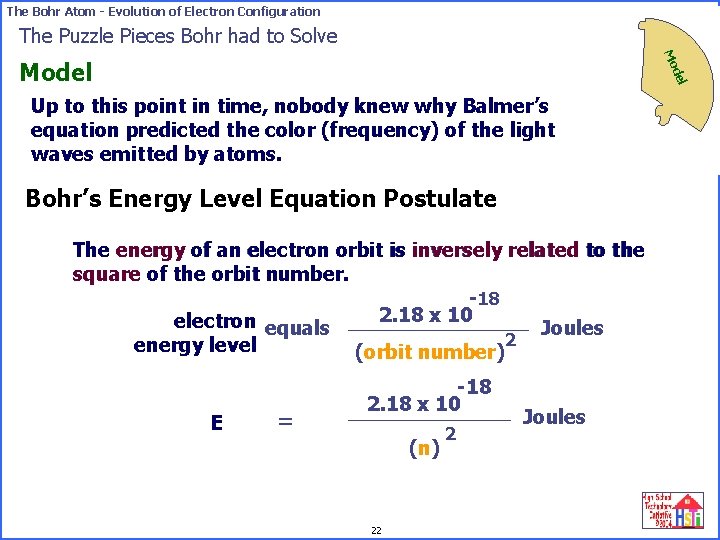
The Bohr Atom - Evolution of Electron Configuration Up to this point in time, nobody knew why Balmer’s equation predicted the color (frequency) of the light waves emitted by atoms. Ob se rv Model Theory Bohr’s Energy Level Equation Postulate The energy of an electron orbit is inversely related to the square of the orbit number. -18 2. 18 x 10 electron equals Joules 2 energy level (orbit number) E = -18 2. 18 x 10 (n) 22 2 Joules ddeell M Moo ati on s The Puzzle Pieces Bohr had to Solve
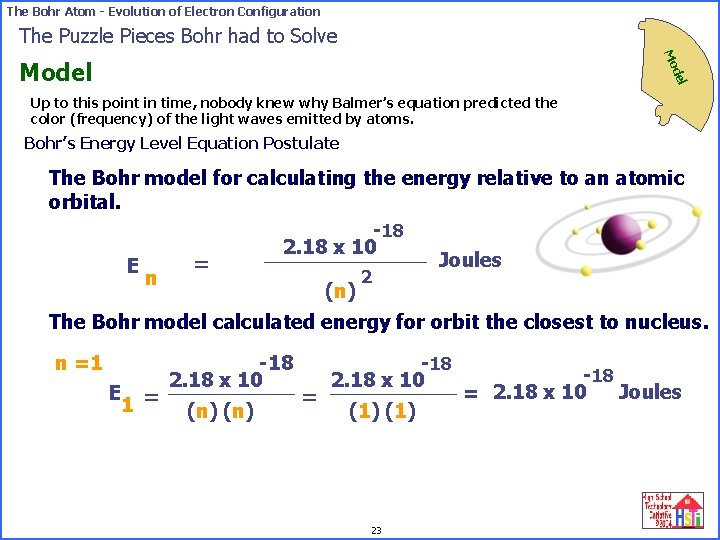
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo del Model Up to this point in time, nobody knew why Balmer’s equation predicted the color (frequency) of the light waves emitted by atoms. Bohr’s Energy Level Equation Postulate The Bohr model for calculating the energy relative to an atomic orbital. -18 2. 18 x 10 Joules = E 2 n (n) The Bohr model calculated energy for orbit the closest to nucleus. n =1 E 1 = -18 2. 18 x 10 (n) = -18 2. 18 x 10 (1) 23 -18 Joules = 2. 18 x 10
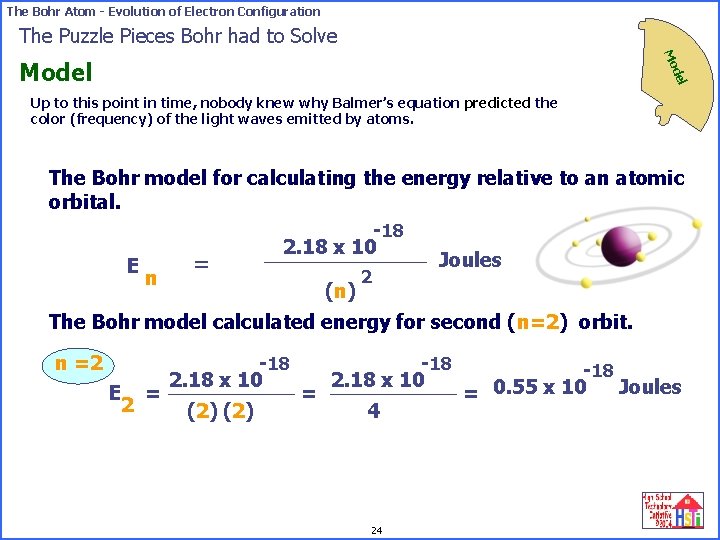
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo del Model Up to this point in time, nobody knew why Balmer’s equation predicted the color (frequency) of the light waves emitted by atoms. The Bohr model for calculating the energy relative to an atomic orbital. -18 2. 18 x 10 Joules = E 2 n (n) The Bohr model calculated energy for second (n=2) orbit. n =2 E = 2 -18 2. 18 x 10 (2) = -18 2. 18 x 10 4 24 -18 Joules = 0. 55 x 10
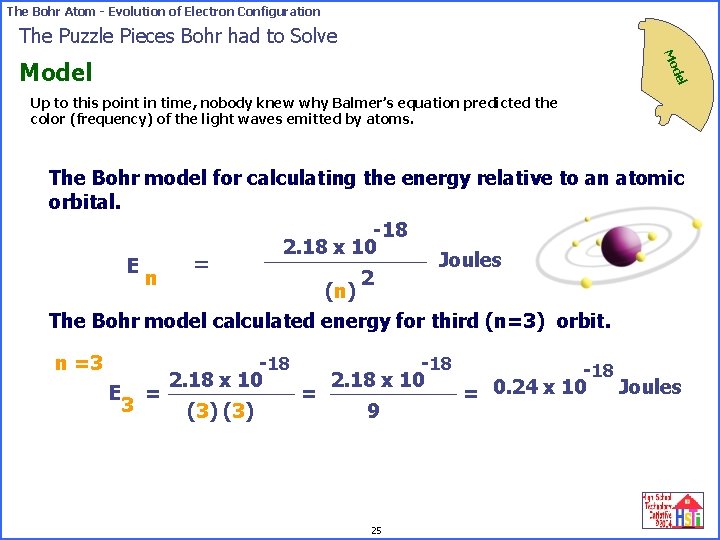
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo del Model Up to this point in time, nobody knew why Balmer’s equation predicted the color (frequency) of the light waves emitted by atoms. The Bohr model for calculating the energy relative to an atomic orbital. -18 2. 18 x 10 Joules = E n 2 (n) The Bohr model calculated energy for third (n=3) orbit. n =3 E = 3 -18 2. 18 x 10 (3) = -18 2. 18 x 10 9 25 -18 Joules = 0. 24 x 10
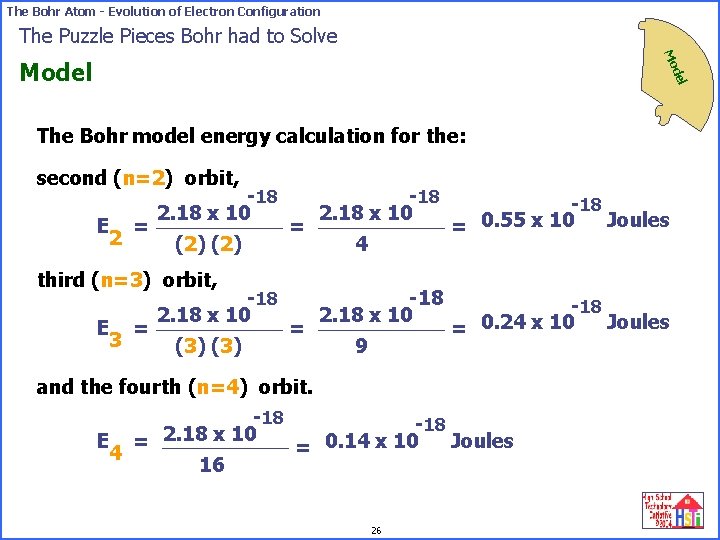
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo del Model The Bohr model energy calculation for the: second (n=2) orbit, E = 2 -18 2. 18 x 10 (2) = third (n=3) orbit, E = 3 -18 2. 18 x 10 (3) = -18 2. 18 x 10 4 -18 2. 18 x 10 9 -18 Joules = 0. 55 x 10 -18 Joules = 0. 24 x 10 and the fourth (n=4) orbit. -18 2. 18 x 10 E = Joules = 0. 14 x 10 4 16 26
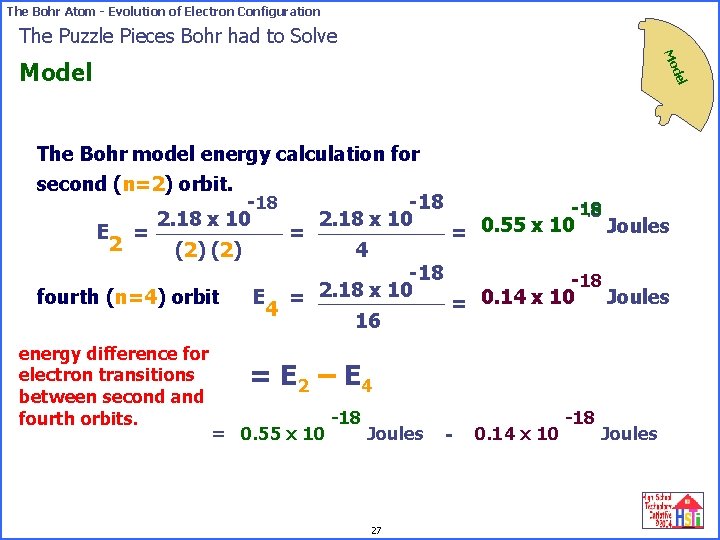
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo del Model The Bohr model energy calculation for second (n=2) orbit. -18 -18 2. 18 x 10 Joules = 0. 55 x 10 E = = 2 4 (2) -18 2. 18 x 10 E = Joules fourth (n=4) orbit = 0. 14 x 10 4 16 energy difference for electron transitions between second and fourth orbits. = E 2 – E 4 = 0. 55 x 10 -18 Joules 27 - 0. 14 x 10 -18 Joules
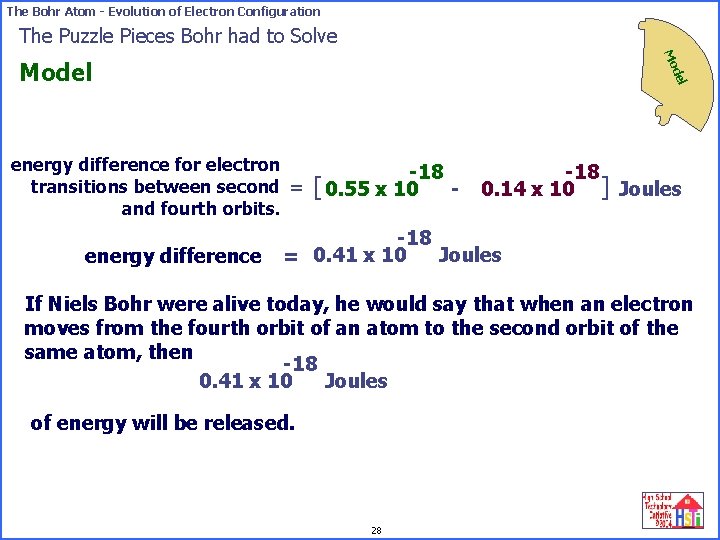
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo del Model energy difference for electron transitions between second = and fourth orbits. energy difference -18 [ 0. 55 x 10 - -18 0. 14 x 10 ] Joules -18 Joules = 0. 41 x 10 If Niels Bohr were alive today, he would say that when an electron moves from the fourth orbit of an atom to the second orbit of the same atom, then -18 Joules 0. 41 x 10 of energy will be released. 28

The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo del Model -18 Niels Bohr believed that 0. 41 x 10 Joules of energy would be released when an electron went from orbit 4 to orbit 2 in an atom. Now he just had to convince the rest of the world that what he believed was what really happened. How do you think he did that? 29
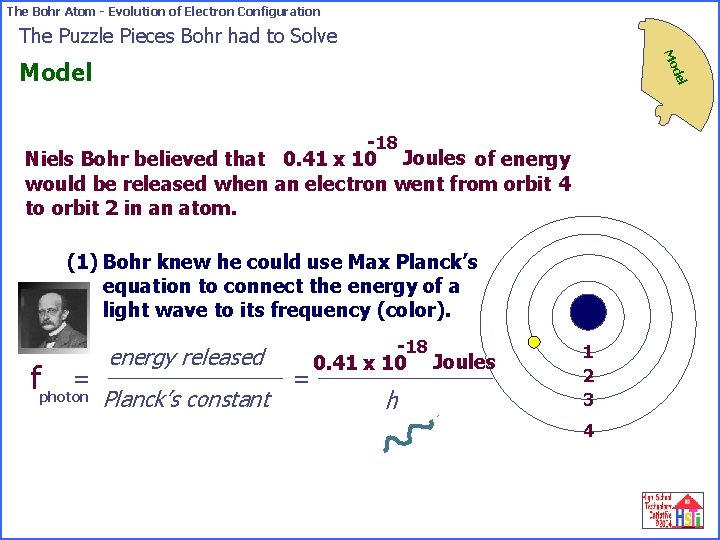
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo del Model -18 Niels Bohr believed that 0. 41 x 10 Joules of energy would be released when an electron went from orbit 4 to orbit 2 in an atom. (1) Bohr knew he could use Max Planck’s equation to connect the energy of a light wave to its frequency (color). = fphoton energy released Planck’s constant = -18 0. 41 x 10 Joules h 1 2 3 4
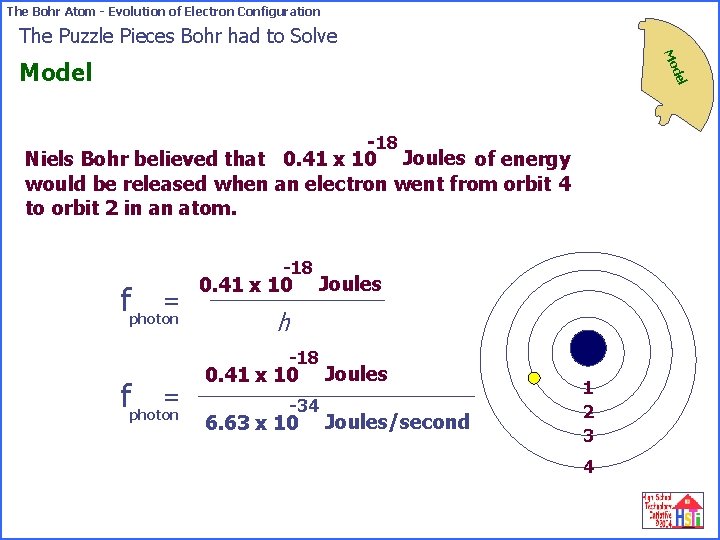
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo del Model -18 Niels Bohr believed that 0. 41 x 10 Joules of energy would be released when an electron went from orbit 4 to orbit 2 in an atom. -18 = fphoton 0. 41 x 10 Joules h -18 = fphoton 0. 41 x 10 -34 6. 63 x 10 Joules/second 1 2 3 4
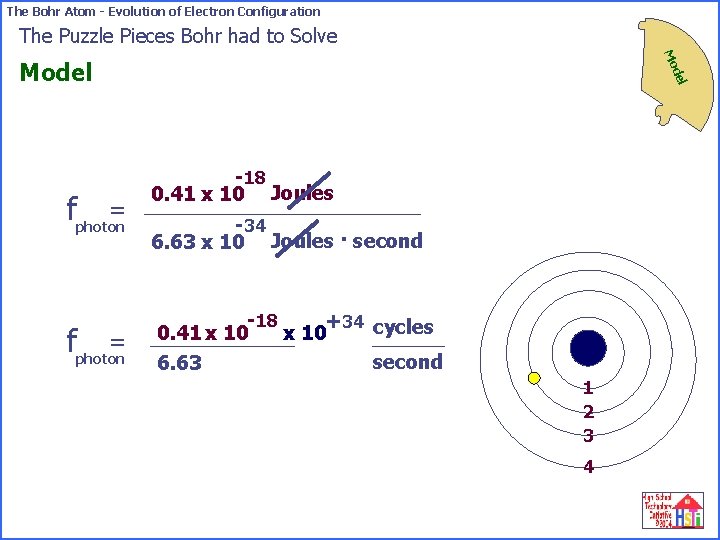
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo = fphoton del Model -18 0. 41 x 10 Joules -34 6. 63 x 10 Joules · second -18 +34 cycles 0. 41 x 10 second 6. 63 1 2 3 4
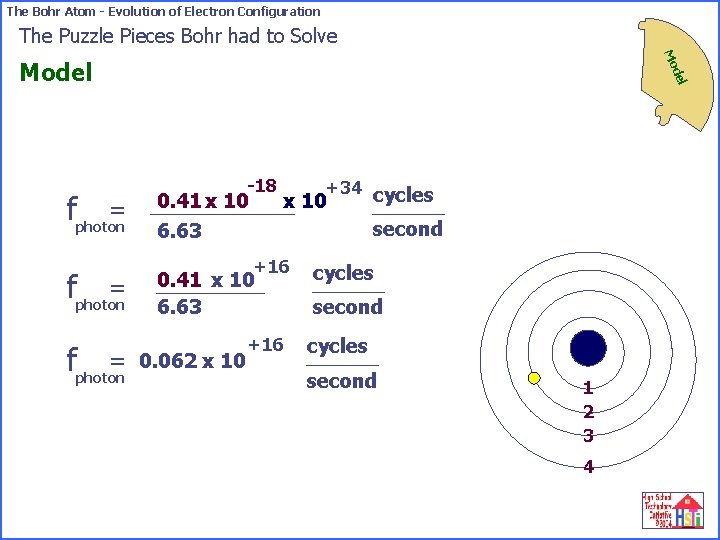
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo del Model -18 = fphoton 0. 41 x 10 6. 63 = fphoton second +16 0. 062 x 10 +34 cycles x 10 +16 cycles second 1 2 3 4
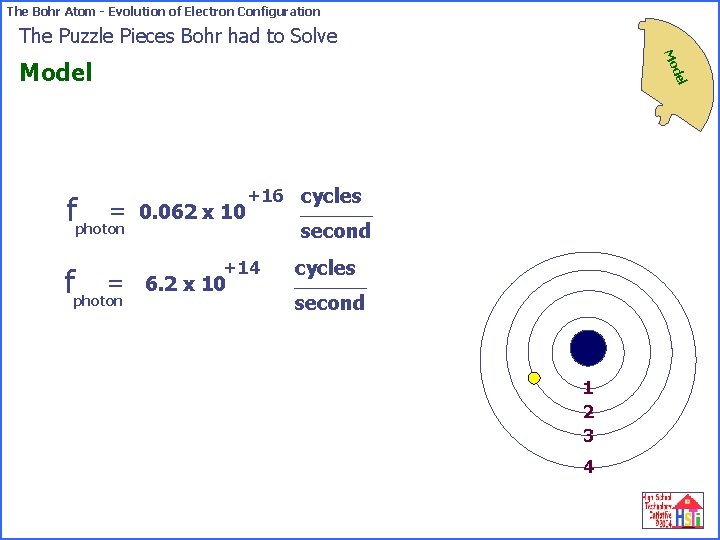
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo = fphoton del Model 0. 062 x 10 +16 cycles +14 6. 2 x 10 second cycles second 1 2 3 4
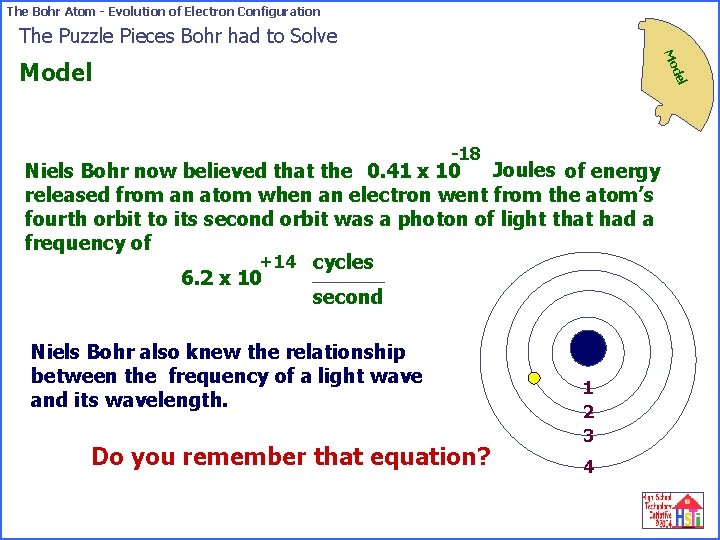
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo del Model -18 Joules of energy Niels Bohr now believed that the 0. 41 x 10 released from an atom when an electron went from the atom’s fourth orbit to its second orbit was a photon of light that had a frequency of +14 cycles 6. 2 x 10 second Niels Bohr also knew the relationship between the frequency of a light wave and its wavelength. Do you remember that equation? 1 2 3 4
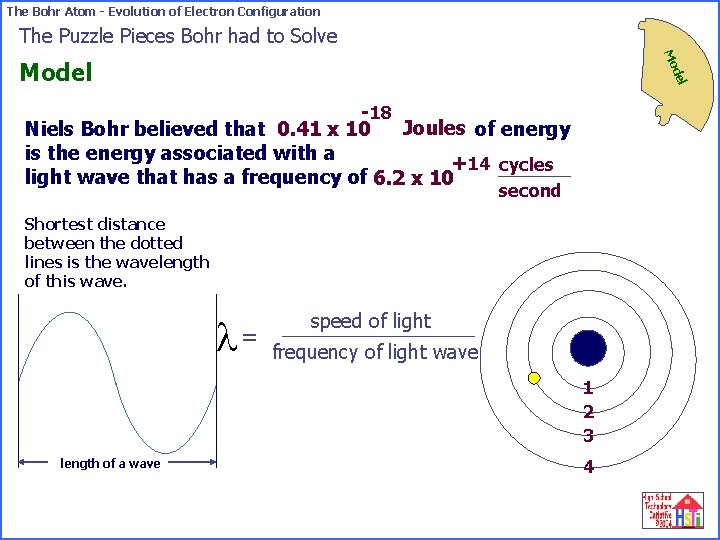
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo del Model -18 Joules of energy Niels Bohr believed that 0. 41 x 10 is the energy associated with a +14 cycles light wave that has a frequency of 6. 2 x 10 second Shortest distance between the dotted lines is the wavelength of this wave. l= speed of light frequency of light wave 1 2 3 length of a wave 4
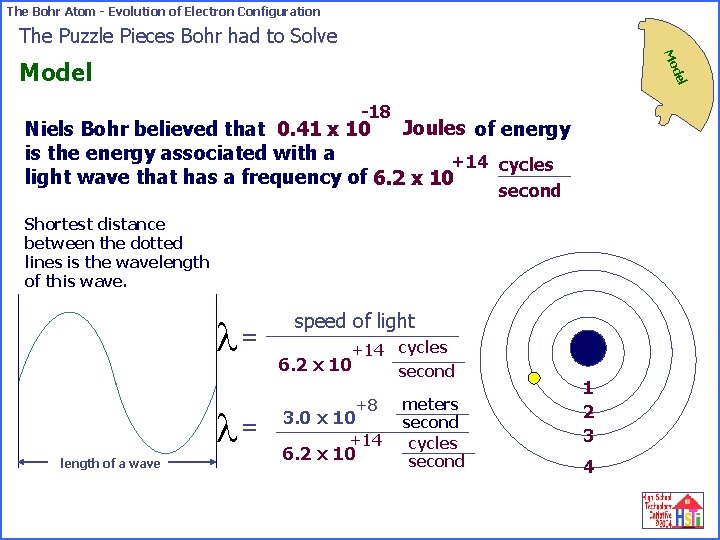
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo del Model -18 Joules of energy Niels Bohr believed that 0. 41 x 10 is the energy associated with a +14 cycles light wave that has a frequency of 6. 2 x 10 second Shortest distance between the dotted lines is the wavelength of this wave. l= l= length of a wave speed of light +14 cycles 6. 2 x 10 second +8 3. 0 x 10 +14 6. 2 x 10 meters second cycles second 1 2 3 4
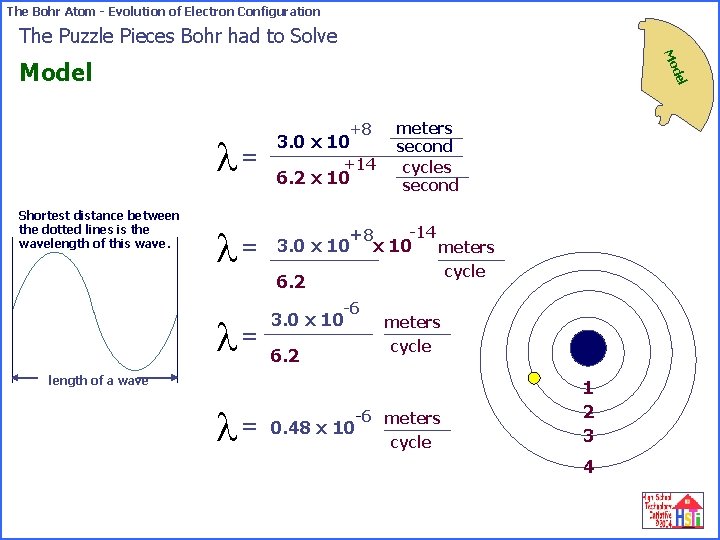
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo Shortest distance between the dotted lines is the wavelength of this wave. del Model +8 meters second cycles second l= 3. 0 x 10 l= -14 +8 3. 0 x 10 meters l= +14 6. 2 x 10 cycle 6. 2 -6 3. 0 x 10 6. 2 meters cycle length of a wave l = -6 meters cycle 0. 48 x 10 1 2 3 4
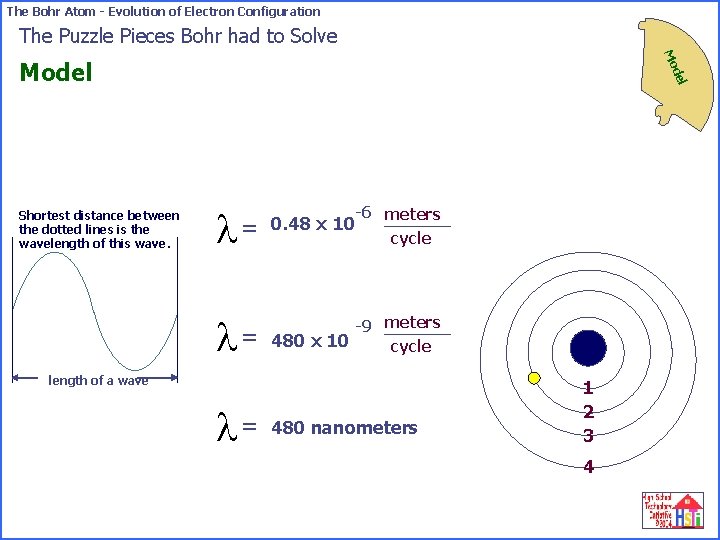
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo Shortest distance between the dotted lines is the wavelength of this wave. del Model -6 meters l= 0. 48 x 10 l= -9 meters 480 x 10 cycle length of a wave l= 480 nanometers 1 2 3 4
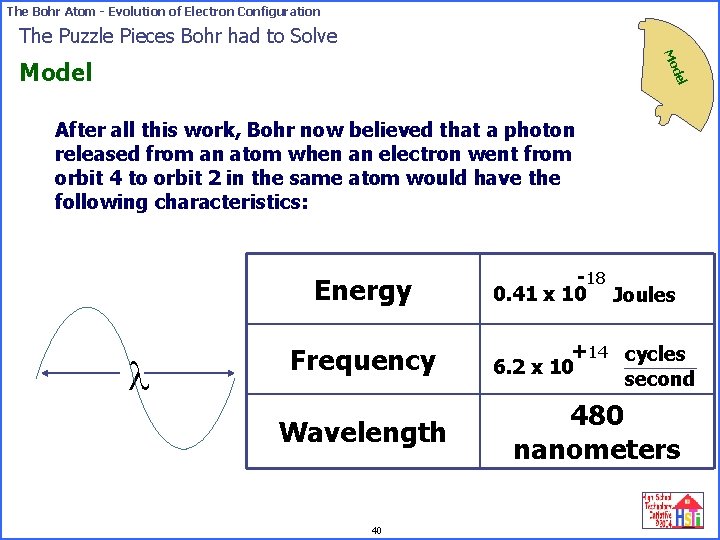
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo del Model After all this work, Bohr now believed that a photon released from an atom when an electron went from orbit 4 to orbit 2 in the same atom would have the following characteristics: Energy l -18 0. 41 x 10 Joules Frequency +14 cycles 6. 2 x 10 second Wavelength 480 nanometers 40
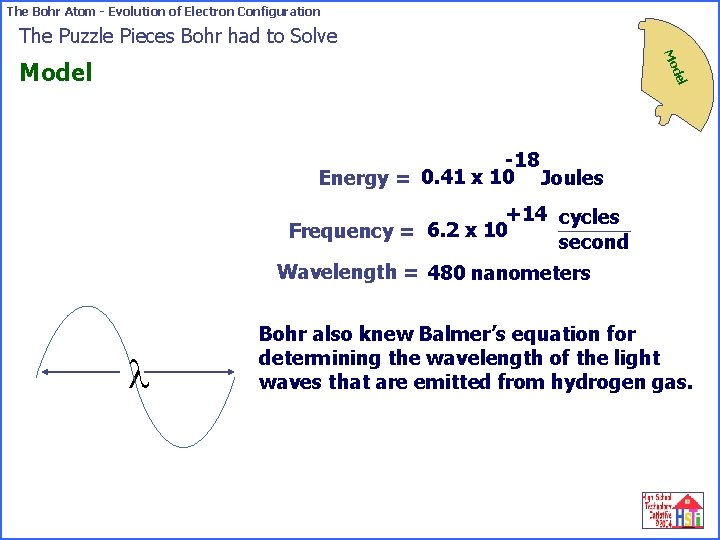
The Bohr Atom - Evolution of Electron Configuration The Puzzle Pieces Bohr had to Solve Mo del Model -18 Energy = 0. 41 x 10 Joules +14 cycles Frequency = 6. 2 x 10 second Wavelength = 480 nanometers l Bohr also knew Balmer’s equation for determining the wavelength of the light waves that are emitted from hydrogen gas.
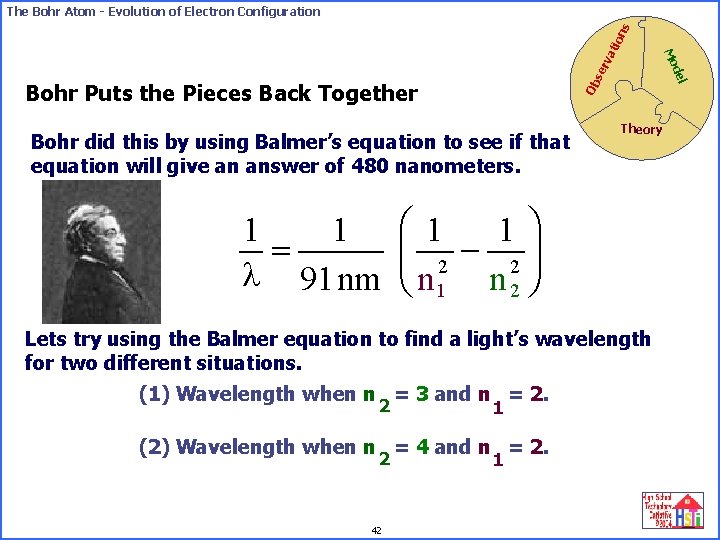
se rv Ob Bohr did this by using Balmer’s equation to see if that equation will give an answer of 480 nanometers. 1 1 = l 91 nm del Bohr Puts the Pieces Back Together Theory æ 1 ö 1 çç 2 - 2 ÷÷ è n 1 n 2 ø Lets try using the Balmer equation to find a light’s wavelength for two different situations. (1) Wavelength when n = 3 and n = 2. 2 1 (2) Wavelength when n = 4 and n = 2. 2 42 Mo ati on s The Bohr Atom - Evolution of Electron Configuration 1
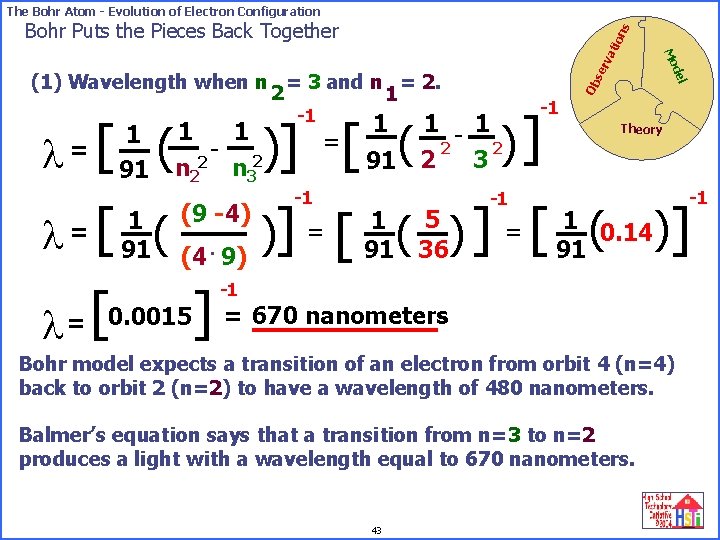
The Bohr Atom - Evolution of Electron Configuration l [ ( = 1 91 -1 se rv Ob -1 )] 1 - 1 2 2 2 3 91 )] =[ ( 1 1 - 2 2 n 3 del (1) Wavelength when n = 3 and n = 2. 2 1 Mo ati on s Bohr Puts the Pieces Back Together -1 Theory -1 [ ( (4 9) )] [ ( )] 0. 0015] = 670 nanometers [ = l l =? 1 91 (9 -4) = 5 1 91 36 = 1 0. 14 91 -1 Bohr model expects a transition of an electron from orbit 4 (n=4) back to orbit 2 (n=2) to have a wavelength of 480 nanometers. Balmer’s equation says that a transition from n=3 to n=2 produces a light with a wavelength equal to 670 nanometers. 43
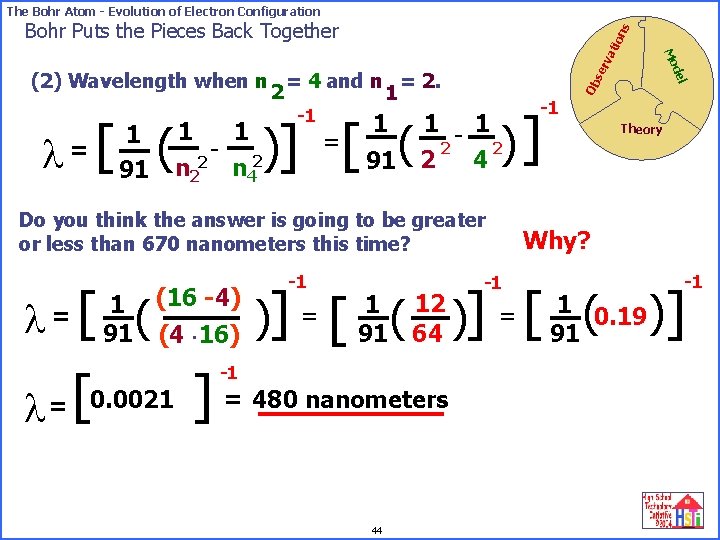
The Bohr Atom - Evolution of Electron Configuration l [ ( = 1 91 -1 se rv Ob 1 - 1 2 2 2 4 91 Do you think the answer is going to be greater or less than 670 nanometers this time? l l [ ( (16 -4) 1 =? 91 (4 16) [ = 0. 0021 -1 )] =[ ( 1 1 - 2 2 n 4 del (2) Wavelength when n = 4 and n = 2. 2 1 Mo ati on s Bohr Puts the Pieces Back Together Theory Why? -1 -1 [ ( )] 1 12 91 64 ] = 480 nanometers -1 44 = 1 0. 19 91
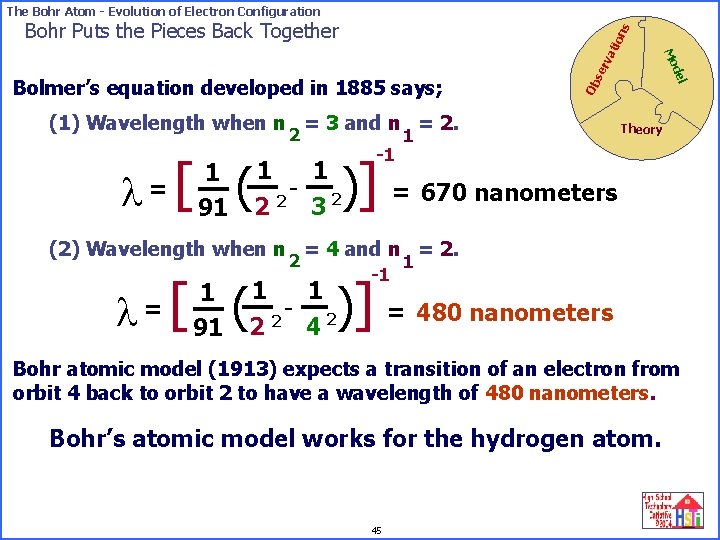
The Bohr Atom - Evolution of Electron Configuration se rv Ob (1) Wavelength when n = 3 and n = 2. 2 l= [ 1 91 ( -1 del Bolmer’s equation developed in 1885 says; Mo ati on s Bohr Puts the Pieces Back Together 1 Theory )] = 670 nanometers 1 1 - 2 2 2 3 (2) Wavelength when n = 4 and n = 2. l= [ 2 1 91 ( -1 1 )] = 480 nanometers 1 1 - 2 2 2 4 Bohr atomic model (1913) expects a transition of an electron from orbit 4 back to orbit 2 to have a wavelength of 480 nanometers. Bohr’s atomic model works for the hydrogen atom. 45
The Bohr Atom - Evolution of Electron Configuration Summary of Bohr’s (Planetary) Model (1913) Electrons orbit the nucleus in different orbits at different fixed distances. Electrons that leave one orbit must move to another orbit. Electrons only change orbits if specific amounts (quanta) of extra energy from the outside world are involved. Electrons that receive enough extra energy from the outside world can leave the atom they are in. Electrons that return to orbits they used to reside in give up the extra energy they acquired when they moved in the first place. Electronic energy given up when electrons move back into an original orbit often show up as a specific color light.
The Bohr Atom - Evolution of Electron Configuration Good news! Bohr’s model explains the light waves that are emitted by hydrogen! Bad News! Bohr’s model does not explains the light waves that are emitted by any other atom! Good news! The expanded quantum theory does explain light waves that are emitted by other atoms! 47

The Bohr Atom - Evolution of Electron Configuration 48
- Slides: 48