The Story of Spontaneity and Energy Dispersal You
















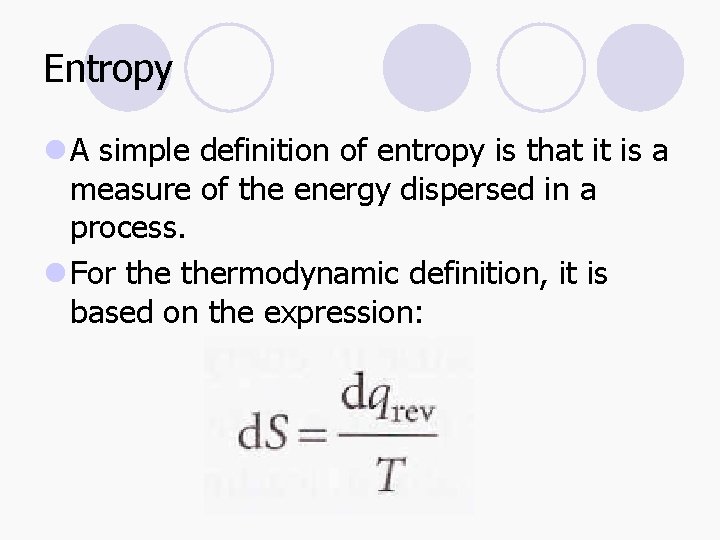




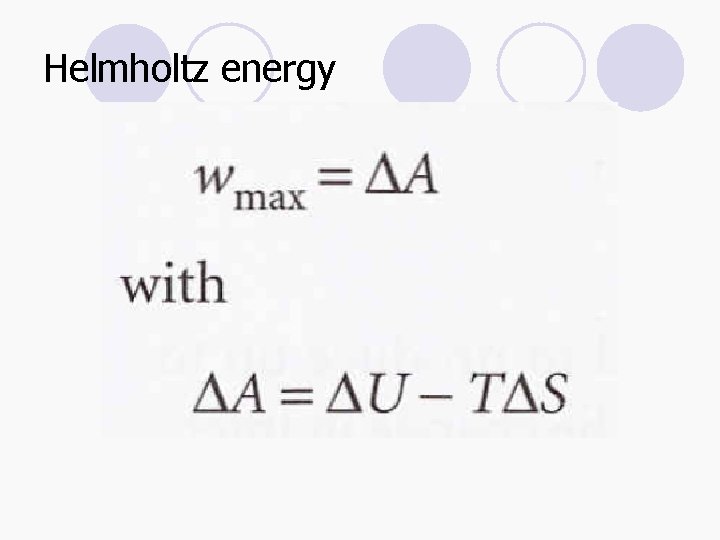







- Slides: 74

The Story of Spontaneity and Energy Dispersal You never get what you want: 100% return on investment

Spontaneity l Spontaneous process are those that occur naturally. ¡Hot body cools ¡A gas expands to fill the available volume l A spontaneous direction of change is where the direction of change does not require work to bring it about.

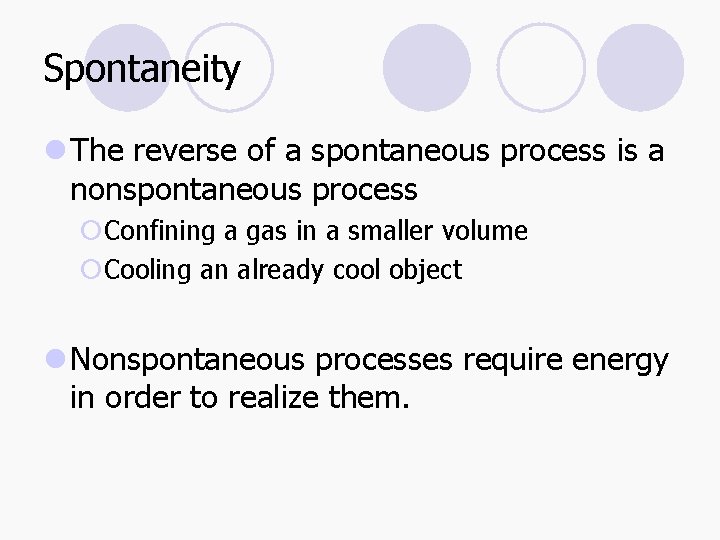
Spontaneity l The reverse of a spontaneous process is a nonspontaneous process ¡Confining a gas in a smaller volume ¡Cooling an already cool object l Nonspontaneous processes require energy in order to realize them.

Spontaneity l Note: ¡Spontaneity is often interpreted as a natural tendency of a process to take place, but it does not necessarily mean that it can be realized in practice. l Some spontaneous processes have rates sooo slow that the tendency is never realized in practice, while some are painfully obvious.

Spontaneity l The conversion of diamond to graphite is spontaneous, but it is joyfully slow. l The expansion of gas into a vacuum is spontaneous and also instantaneous.

2 ND LAW OF THERMODYNAMICS

Physical Statements of the 2 nd Law of Thermodynamics l Kelvin Statements l “No process is possible in which the sole result is the absorption of heat from a reservoir and its complete conversion into work”

l “It is impossible for a system to undergo a cyclic process whose sole effects are the flow of an amount of heat from the surroundings to the system and the performance of an equal amount of work on the surroundings. ” l “It is impossible for a system to undergo a cyclic process that turns heat completely into work done on the surroundings. ”

l Clausius statement l It is impossible for a process to occur that has the sole effect of removing a quantity of heat from an object at a lower temperature and transferring this quantity of heat to an object at a higher temperature. l Heat cannot flow spontaneously from a cooler to a hotter object if nothing else happens

The 2 nd Law of Thermodynamics l The 2 nd Law of Thermodynamics recognizes the two classes of processes, the spontaneous and nonspontaneous processes.

Implications of the 2 nd Law l No heat engine can have an efficiency as great as unity l No macroscopic process can decrease the entropy of the universe

Are you kidding me? Thermodynamic Cat

Hot Reservoir Heat Cold Reservoir Engine Heat Work I approve!


What determines the direction of spontaneous change? l The total internal energy of a system does NOT determine whether a process is spontaneous or not. l Per the First Law, energy is conserved in any process involving an isolated system.

What determines the direction of spontaneous change? l Instead, it is important to note that the direction of change is related to the distribution of energy. l Spontaneous changes are always accompanied by a dispersal of energy.

Energy Dispersal l Superheroes with energy blasts and similar powers as well as the Super Saiyans are impossible characters. l They seem to violate the Second Law of Thermodynamics!

Power Genki dama

Energy Dispersal l A ball on a warm floor can never be observed to spontaneously bounce as a result of the energy from the warm floor

Energy Dispersal l In order for this to happen, thermal energy represented by the random motion and vibrations of the floor atoms would have to be spontaneously diverted to accumulate into the ball.

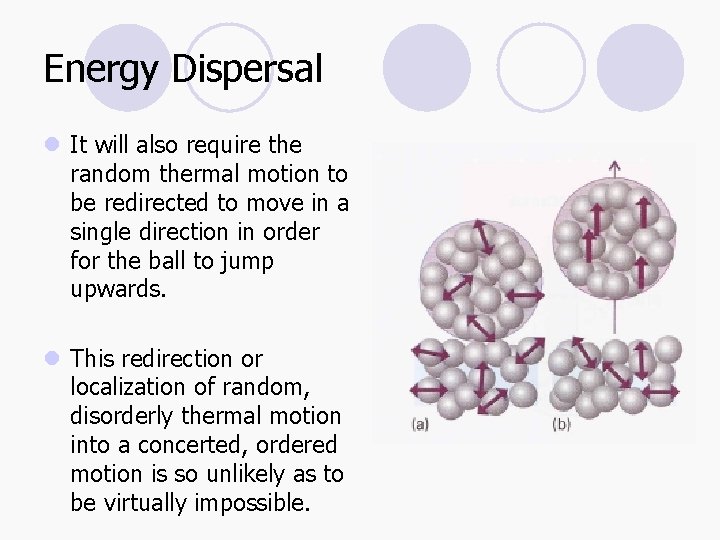
Energy Dispersal l It will also require the random thermal motion to be redirected to move in a single direction in order for the ball to jump upwards. l This redirection or localization of random, disorderly thermal motion into a concerted, ordered motion is so unlikely as to be virtually impossible.

Energy Dispersal and Spontaneity l Spontaneous change can now be interpreted as the direction of change that leads to the dispersal of the total energy of an isolated system! INDEED!

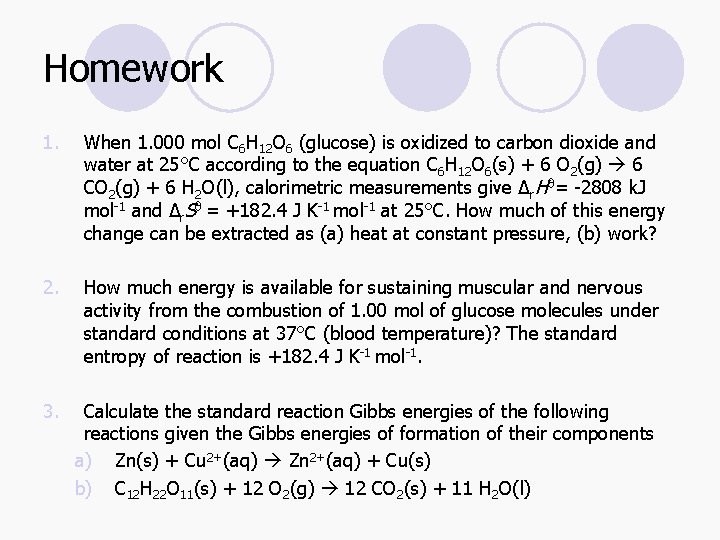
Entropy l A state function, denoted by S. l While the First Law can be associated with U, the Second Law may be expressed in terms of the S

Entropy and the Second Law l The Second Law can be expressed in terms of the entropy: The entropy of an isolated system increases over the course of a spontaneous change: ΔStot > 0 l Where Stot is the total entropy of the system and its surroundings.

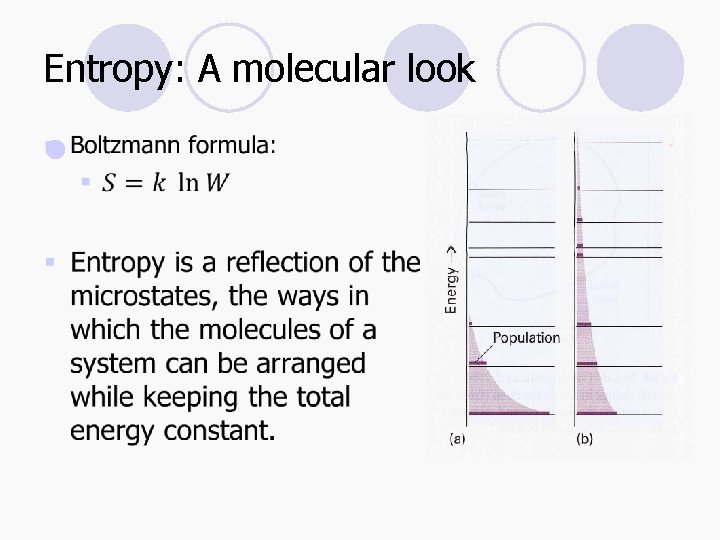
Entropy: A molecular look l
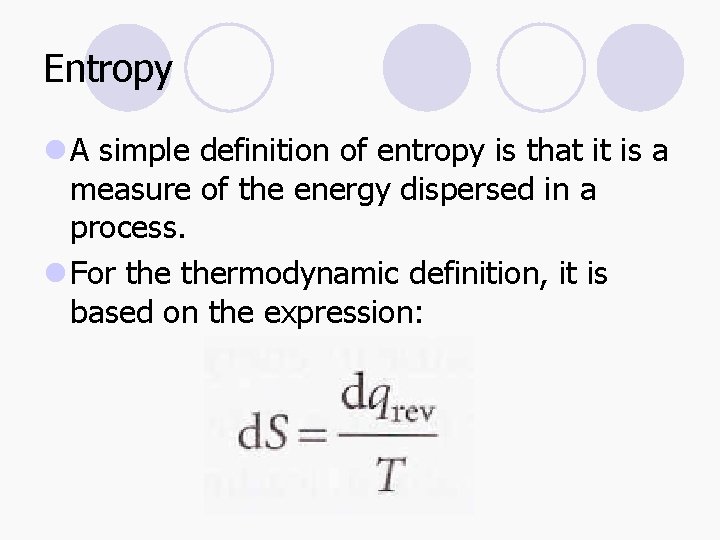
Entropy l A simple definition of entropy is that it is a measure of the energy dispersed in a process. l For thermodynamic definition, it is based on the expression:

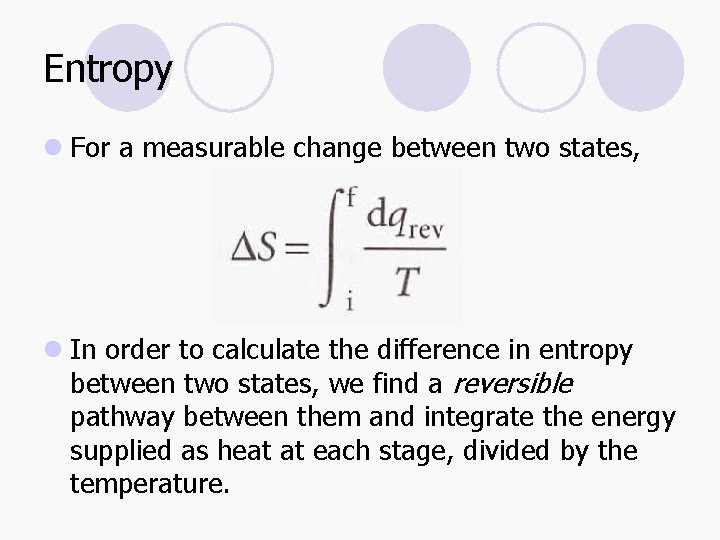
Entropy l For a measurable change between two states, l In order to calculate the difference in entropy between two states, we find a reversible pathway between them and integrate the energy supplied as heat at each stage, divided by the temperature.

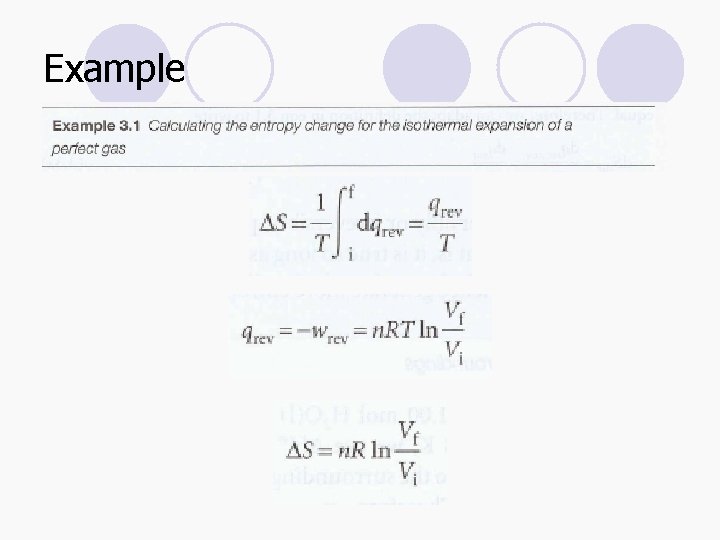
Example

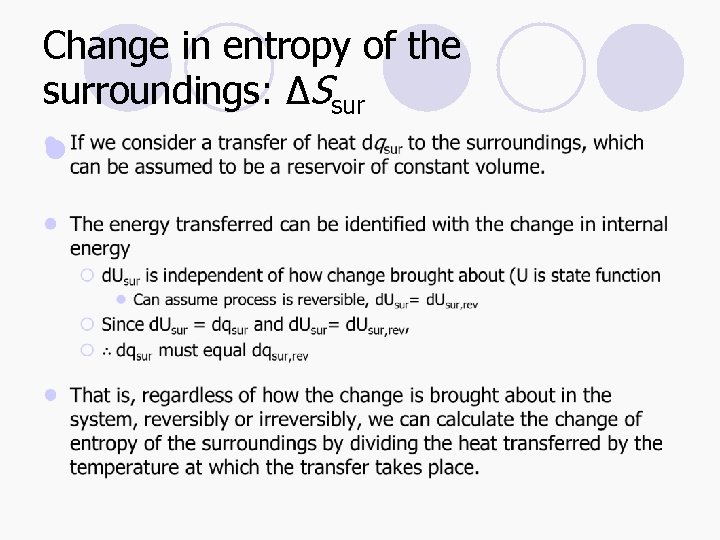
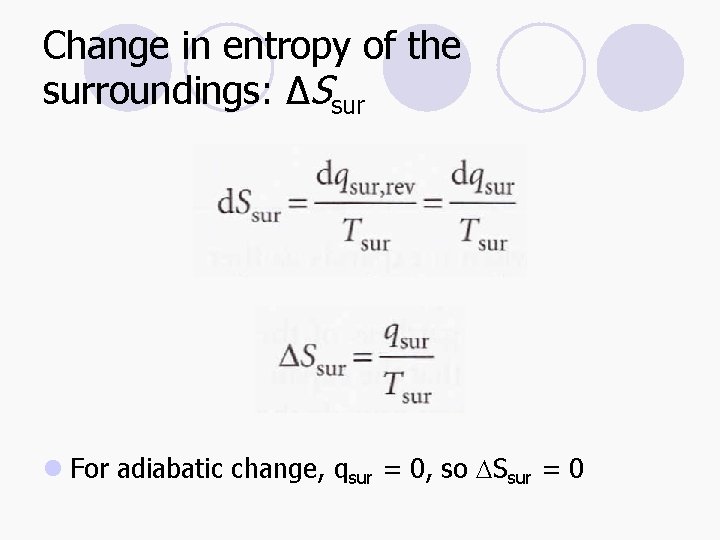
Change in entropy of the surroundings: ΔSsur l

Change in entropy of the surroundings: ΔSsur l For adiabatic change, qsur = 0, so DSsur = 0

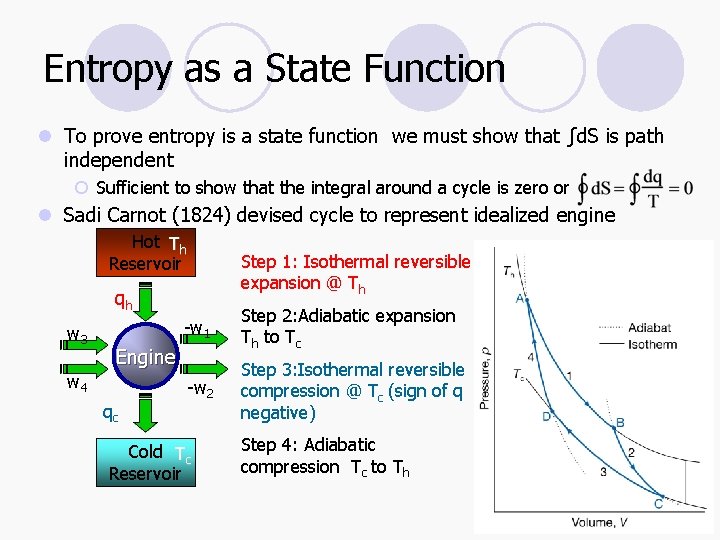
Entropy as a State Function l To prove entropy is a state function we must show that ∫d. S is path independent ¡ Sufficient to show that the integral around a cycle is zero or l Sadi Carnot (1824) devised cycle to represent idealized engine Hot Th Reservoir Step 1: Isothermal reversible expansion @ Th qh w 3 -w 1 Step 2: Adiabatic expansion Th to Tc -w 2 Step 3: Isothermal reversible compression @ Tc (sign of q negative) Engine w 4 qc Cold Tc Reservoir Step 4: Adiabatic compression Tc to Th

Carnot Engine How is that possible?

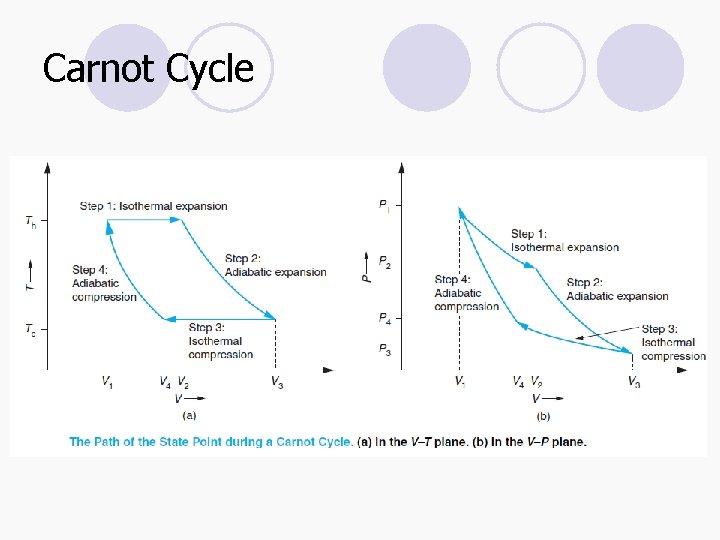
Carnot Cycle

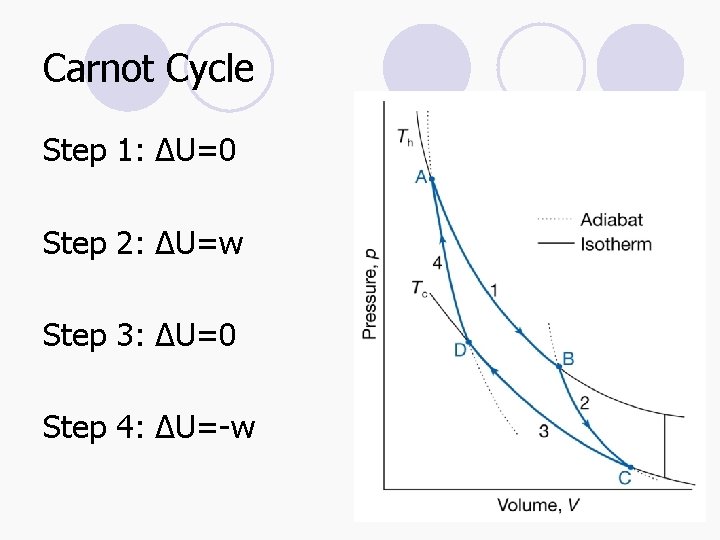
Carnot Cycle Step 1: ΔU=0 Step 2: ΔU=w Step 3: ΔU=0 Step 4: ΔU=-w

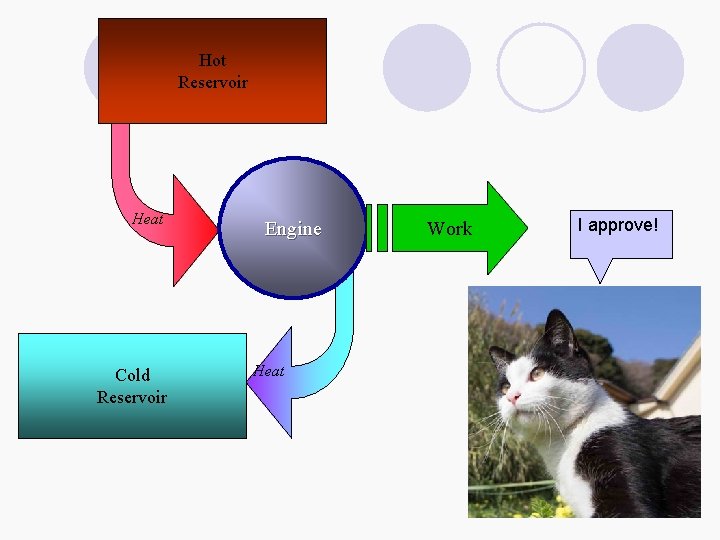
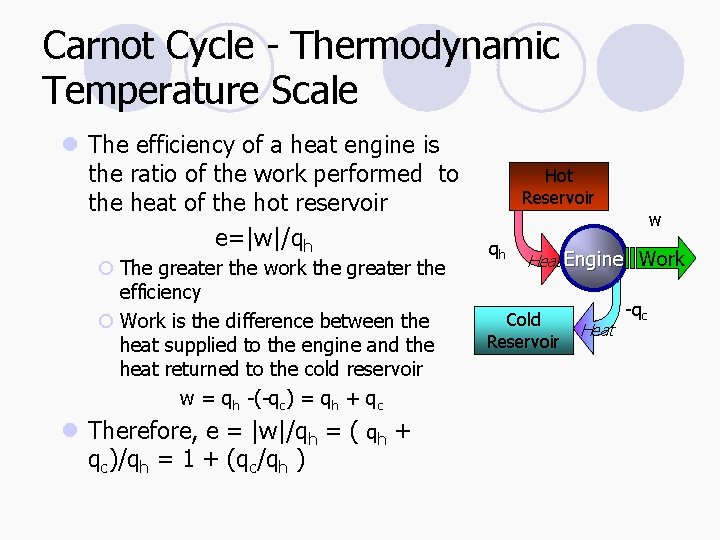
Carnot Cycle - Thermodynamic Temperature Scale l The efficiency of a heat engine is the ratio of the work performed to the heat of the hot reservoir e=|w|/qh ¡ The greater the work the greater the efficiency ¡ Work is the difference between the heat supplied to the engine and the heat returned to the cold reservoir w = qh -(-qc) = qh + qc l Therefore, e = |w|/qh = ( qh + qc)/qh = 1 + (qc/qh ) Hot Reservoir qh w Heat Engine Work Cold Reservoir Heat -qc

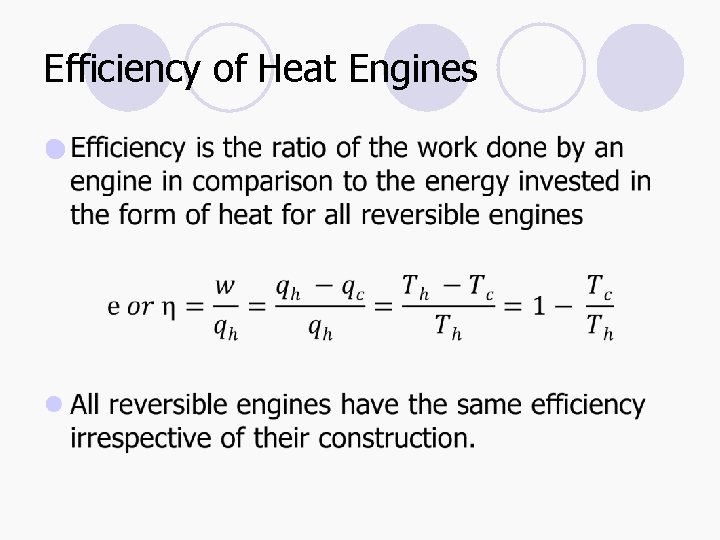
Efficiency of Heat Engines l

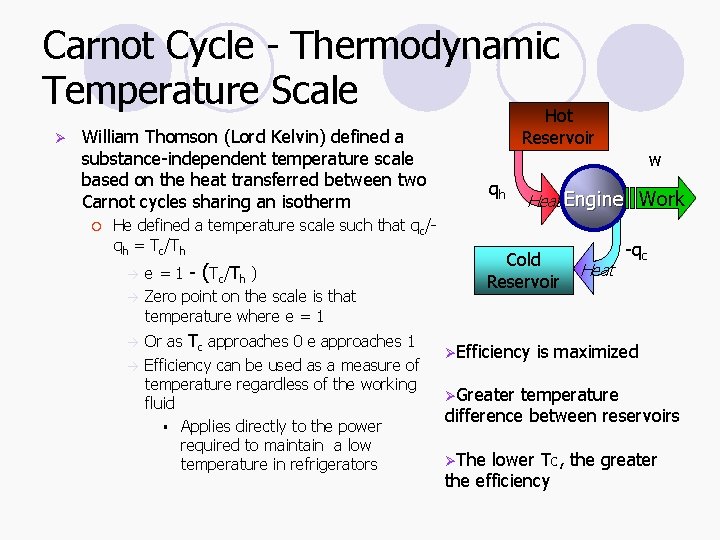
Carnot Cycle - Thermodynamic Temperature Scale Hot Ø William Thomson (Lord Kelvin) defined a substance-independent temperature scale based on the heat transferred between two Carnot cycles sharing an isotherm ¡ He defined a temperature scale such that qc/qh = Tc/Th e = 1 - (Tc/Th ) Zero point on the scale is that temperature where e = 1 Or as Tc approaches 0 e approaches 1 Efficiency can be used as a measure of temperature regardless of the working fluid § Applies directly to the power required to maintain a low temperature in refrigerators Reservoir qh w Heat Engine Work Cold Reservoir Heat -qc ØEfficiency is maximized ØGreater temperature difference between reservoirs ØThe lower Tc, the greater the efficiency

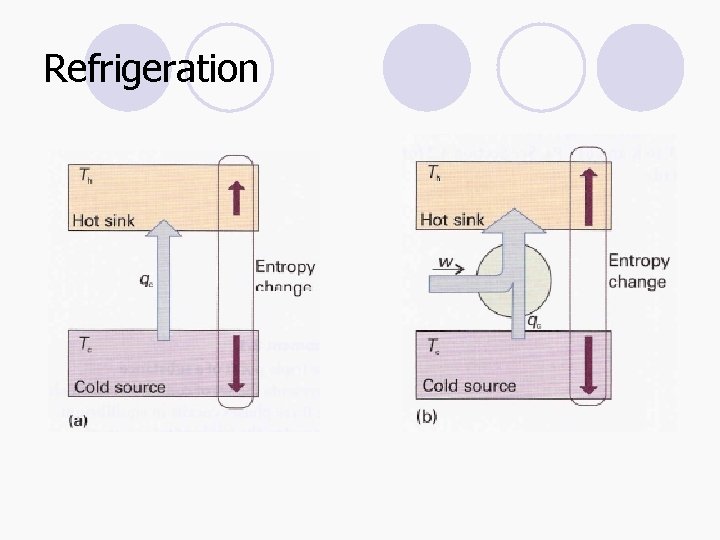
Refrigeration

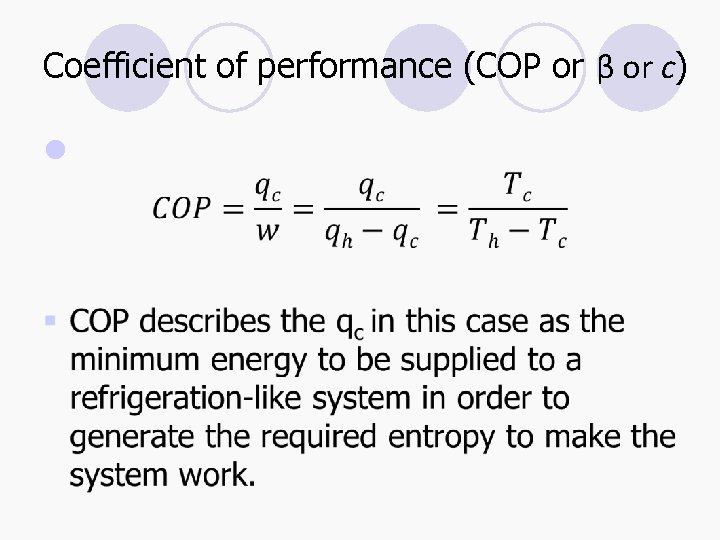
Coefficient of performance (COP or β or c) l

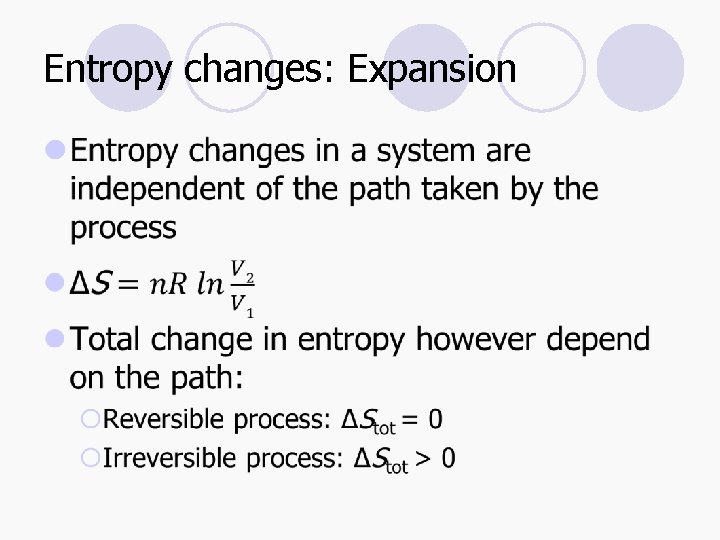
Entropy changes: Expansion l

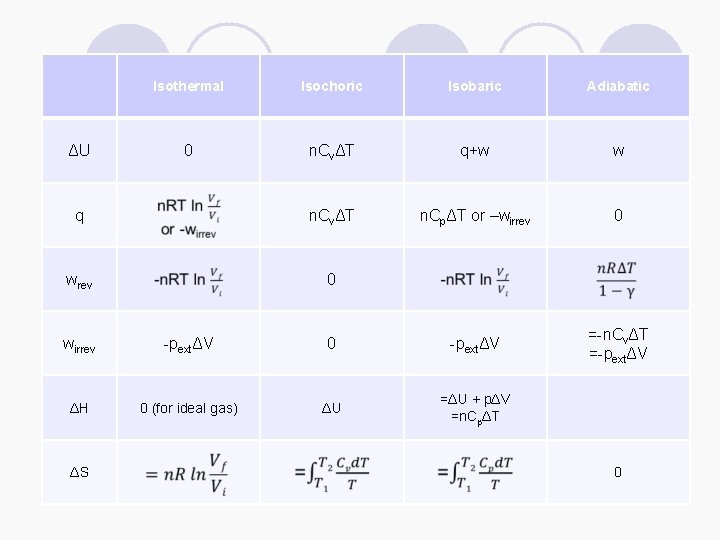
Isothermal Isochoric Isobaric Adiabatic 0 n. CvΔT q+w w q n. CvΔT n. CpΔT or –wirrev 0 wrev 0 =-n. CvΔT =-pextΔV ΔU wirrev -pextΔV 0 -pextΔV ΔH 0 (for ideal gas) ΔU =ΔU + pΔV =n. CpΔT ΔS 0

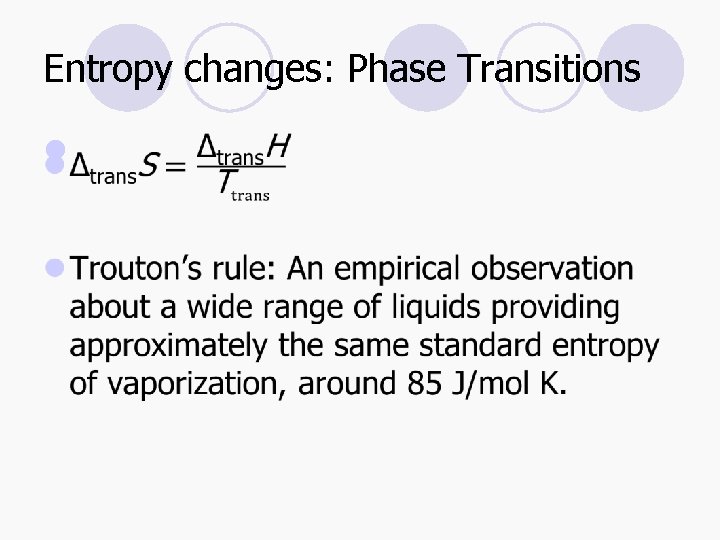
Entropy changes: Phase Transitions l

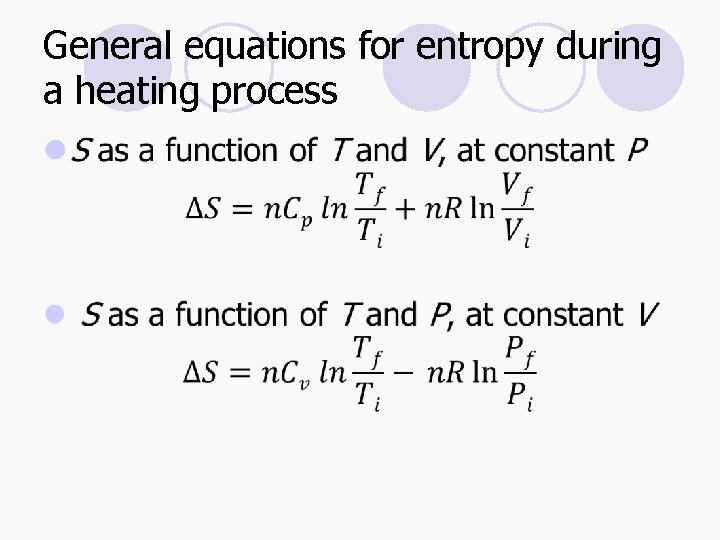
General equations for entropy during a heating process l

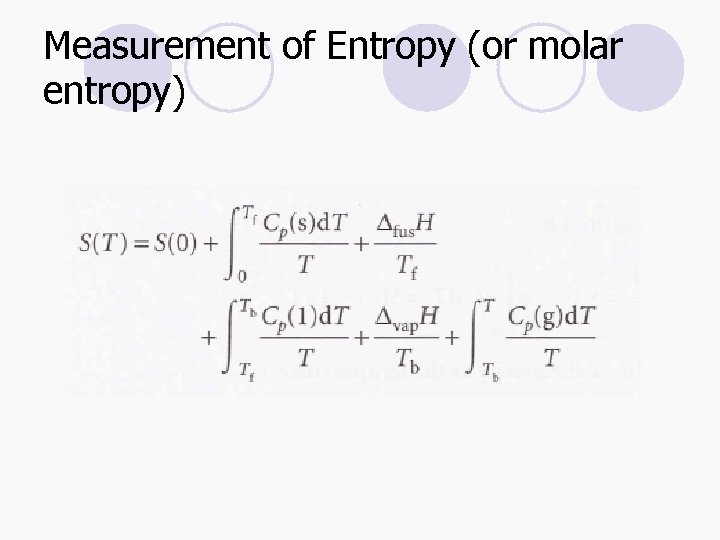
Measurement of Entropy (or molar entropy)

Measurement of Entropy (or molar entropy) l The terms in the previous equation can be calculated or determined experimentally l The difficult part is assessing heat capacities near T = 0. l Such heat capacities can be evaluated via the Debye extrapolation


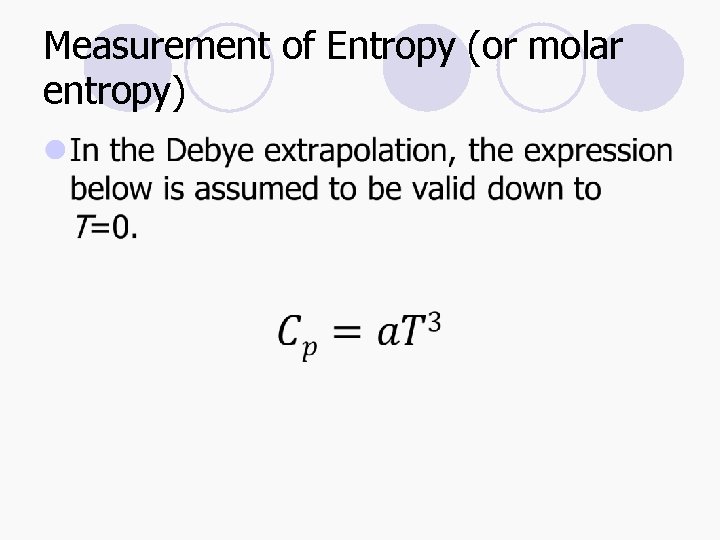
Measurement of Entropy (or molar entropy) l

Third Law of Thermodynamics l At T = 0, all energy of thermal motion has been quenched, and in a perfect crystal all the atoms or ions are in a regular, uniform array. l The localization of matter and the absence of thermal motion suggest that such materials also have zero entropy. l This conclusion is consistent with the molecular interpretation of entropy, because S = 0 if there is only one way of arranging the molecules and only one microstate is accessible (the ground state).

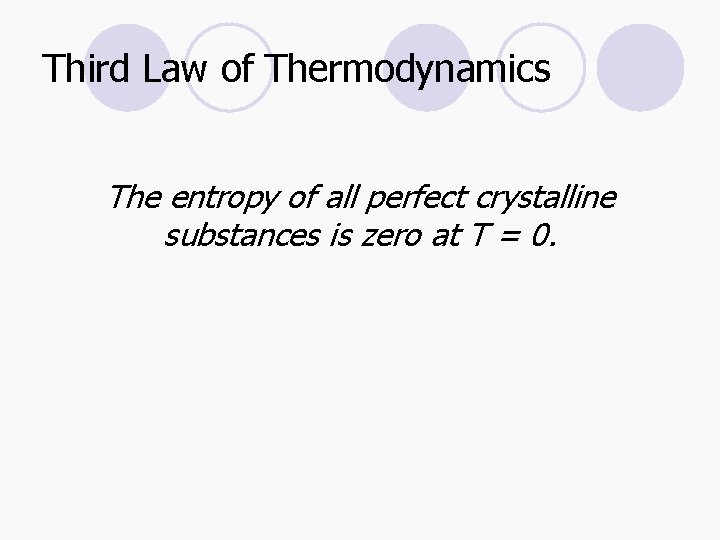
Third Law of Thermodynamics The entropy of all perfect crystalline substances is zero at T = 0.

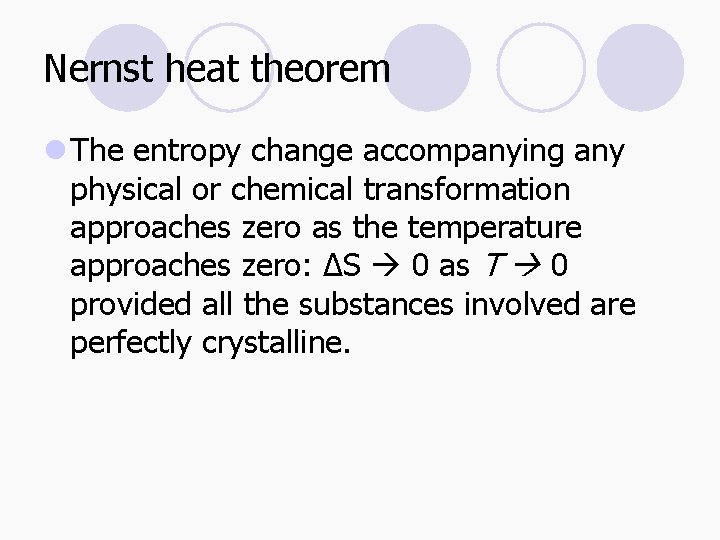
Nernst heat theorem l The entropy change accompanying any physical or chemical transformation approaches zero as the temperature approaches zero: ΔS 0 as T 0 provided all the substances involved are perfectly crystalline.

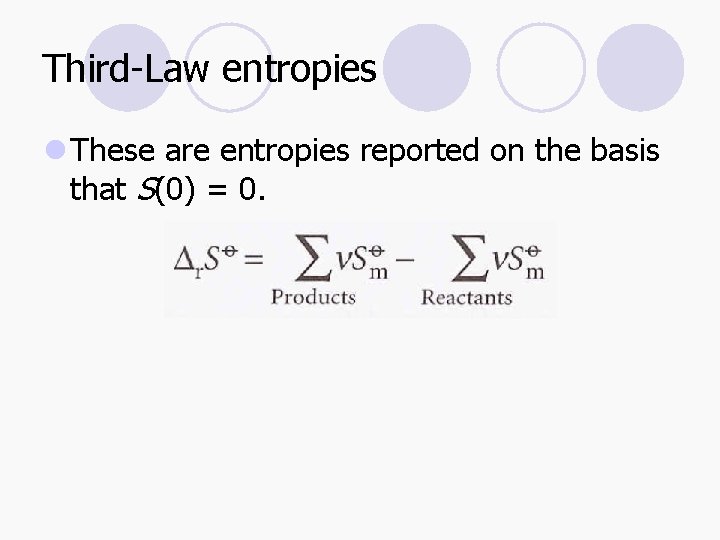
Third-Law entropies l These are entropies reported on the basis that S(0) = 0.


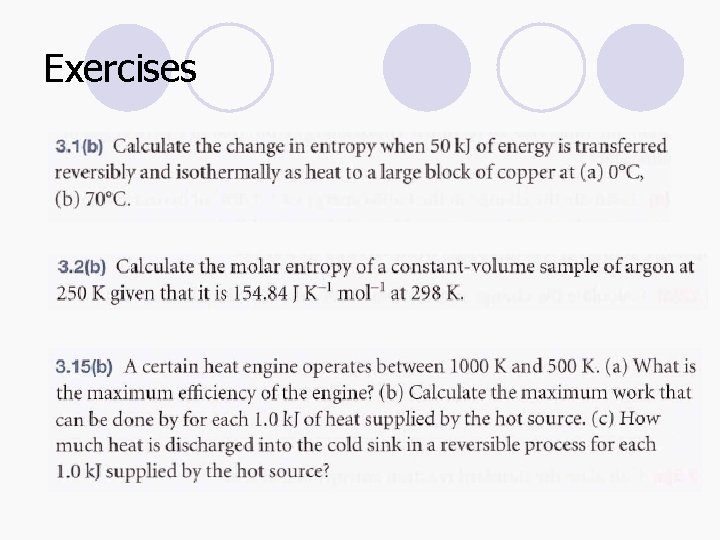
Exercises

HELMHOLTZ AND GIBBS ENERGIES

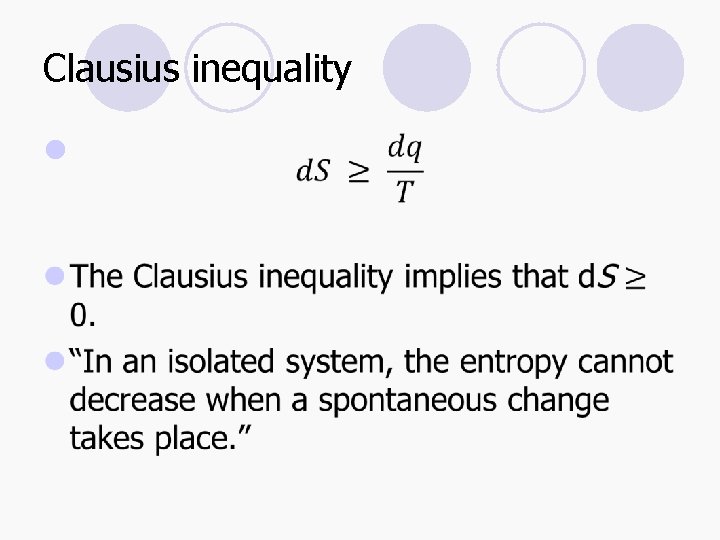
Clausius inequality l

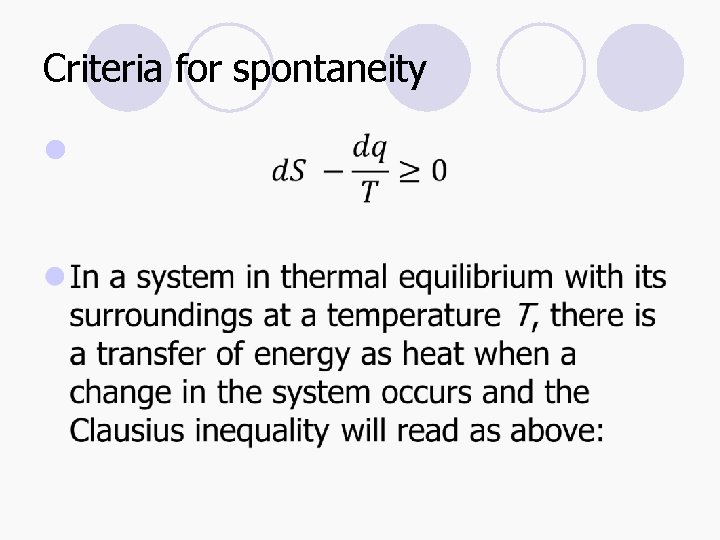
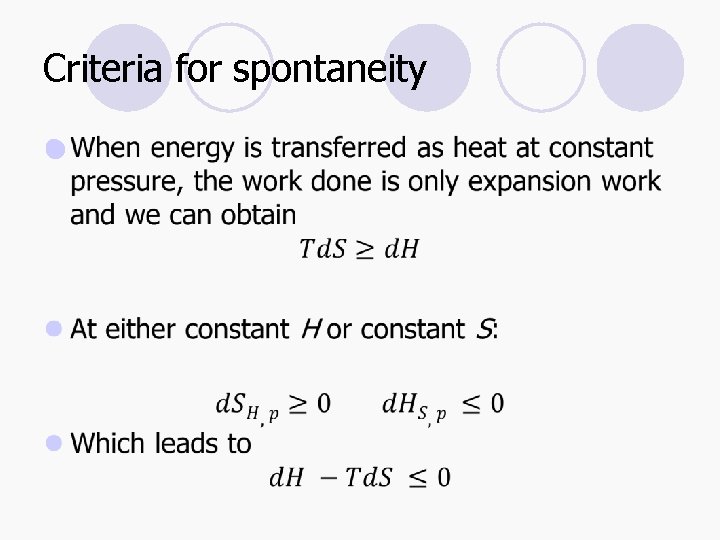
Criteria for spontaneity l

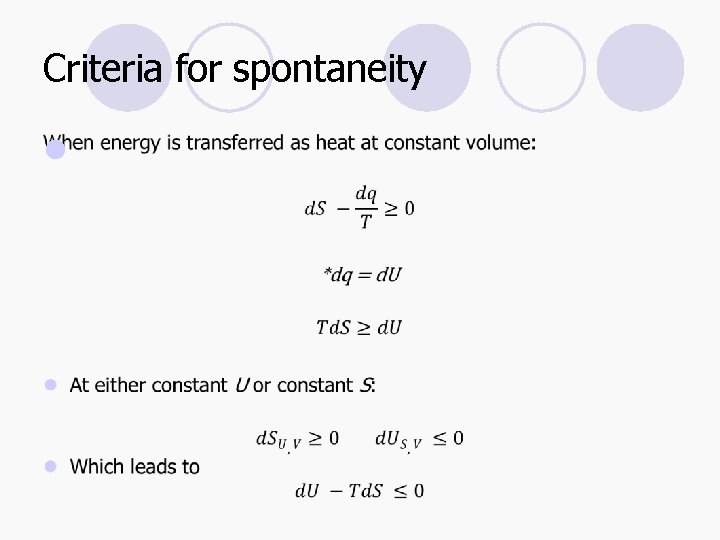
Criteria for spontaneity l

Criteria for spontaneity l

Criteria for spontaneity l

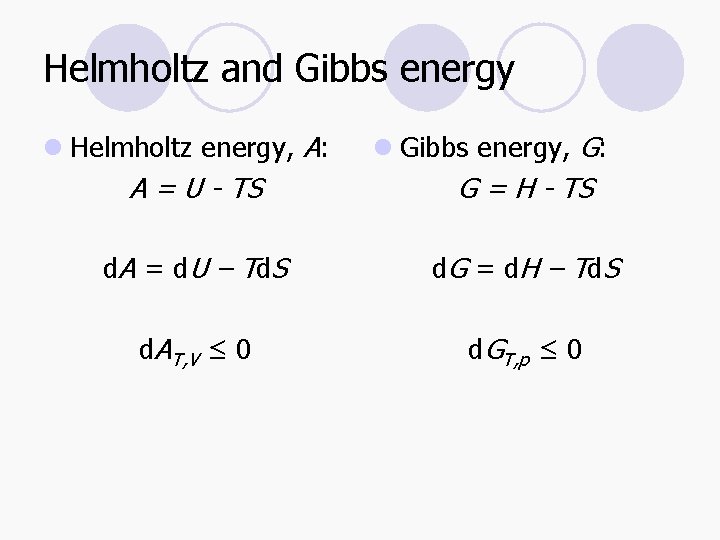
Helmholtz and Gibbs energy l Helmholtz energy, A: A = U - TS l Gibbs energy, G: G = H - TS d. A = d. U – Td. S d. G = d. H – Td. S d. AT, V ≤ 0 d. GT, p ≤ 0

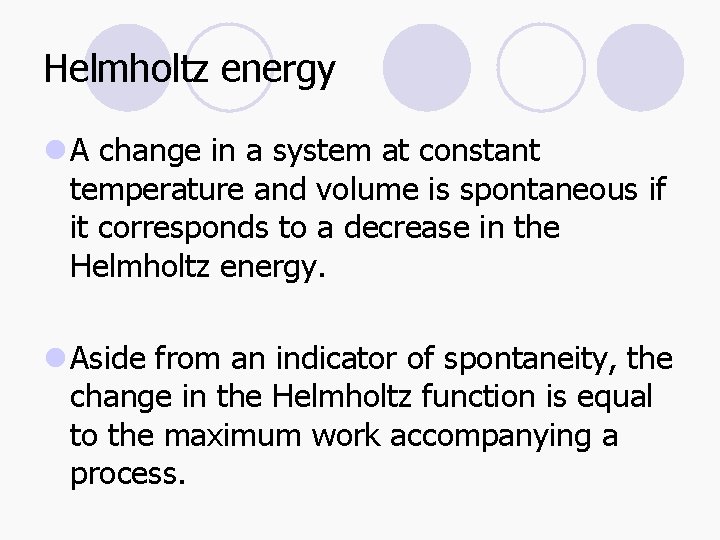
Helmholtz energy l A change in a system at constant temperature and volume is spontaneous if it corresponds to a decrease in the Helmholtz energy. l Aside from an indicator of spontaneity, the change in the Helmholtz function is equal to the maximum work accompanying a process.
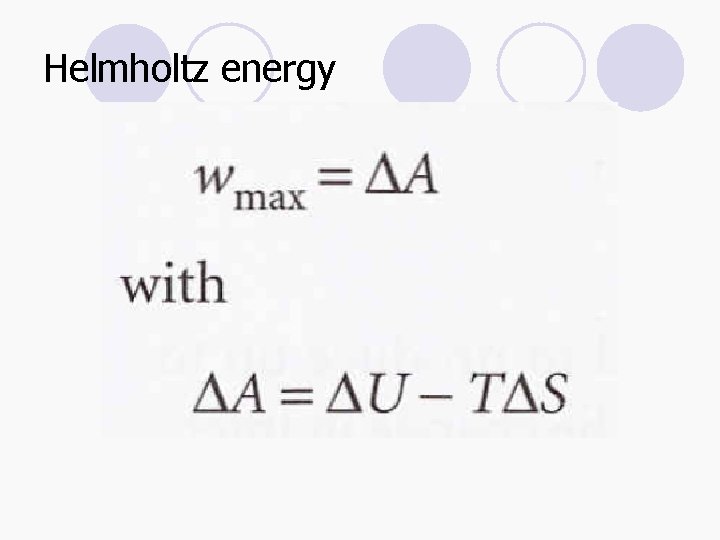
Helmholtz energy



, useful


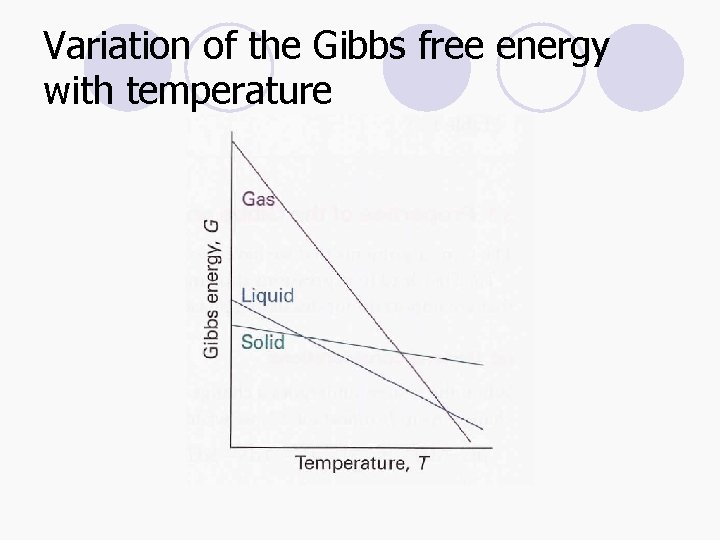
Variation of the Gibbs free energy with temperature

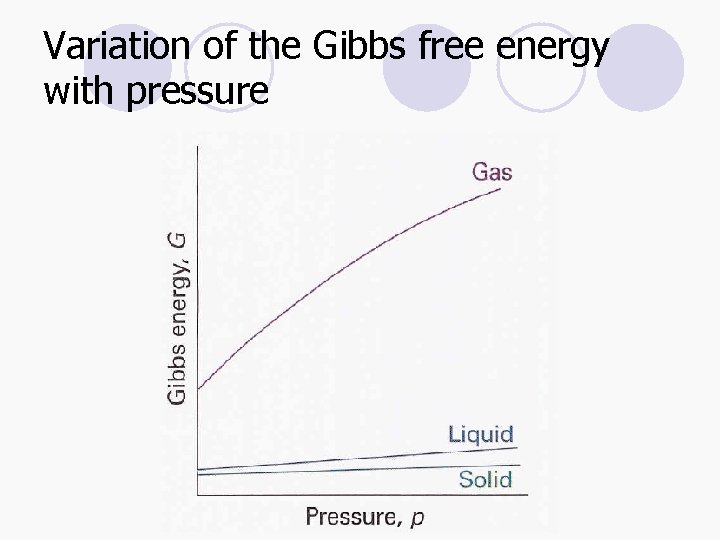
Variation of the Gibbs free energy with pressure

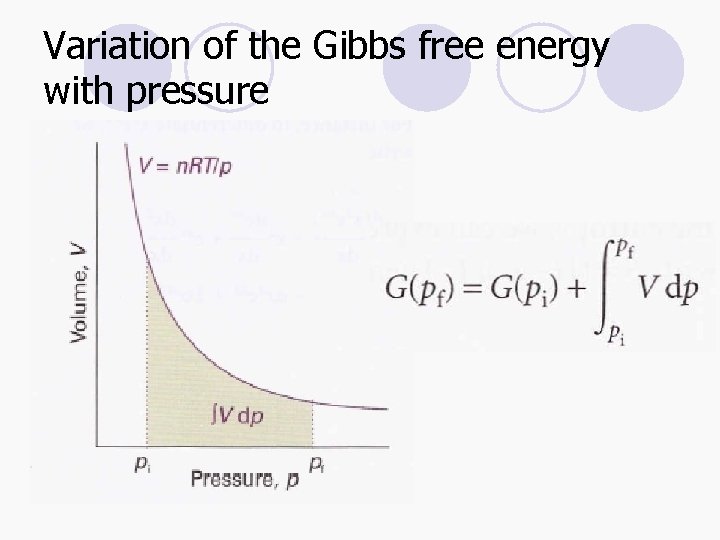
Variation of the Gibbs free energy with pressure

Homework 1. When 1. 000 mol C 6 H 12 O 6 (glucose) is oxidized to carbon dioxide and water at 25°C according to the equation C 6 H 12 O 6(s) + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(l), calorimetric measurements give Δr. Hθ= -2808 k. J mol-1 and Δr. Sθ = +182. 4 J K-1 mol-1 at 25°C. How much of this energy change can be extracted as (a) heat at constant pressure, (b) work? 2. How much energy is available for sustaining muscular and nervous activity from the combustion of 1. 00 mol of glucose molecules under standard conditions at 37°C (blood temperature)? The standard entropy of reaction is +182. 4 J K-1 mol-1. 3. Calculate the standard reaction Gibbs energies of the following reactions given the Gibbs energies of formation of their components a) Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s) b) C 12 H 22 O 11(s) + 12 O 2(g) 12 CO 2(s) + 11 H 2 O(l)




One for the road l Life requires the assembly of a large number of simple molecules into more complex but very ordered macromolecules. Does life violate the Second Law of Thermodynamics? Why or why not?
 Chemistry microstates
Chemistry microstates Bpch21
Bpch21 How to calculate gibbs free energy
How to calculate gibbs free energy Ap chemistry spontaneity entropy and free energy
Ap chemistry spontaneity entropy and free energy Thermodynamics ppt
Thermodynamics ppt Predicting spontaneity
Predicting spontaneity Balancing redox reactions in acidic solution
Balancing redox reactions in acidic solution Predicting spontaneity
Predicting spontaneity Complete the following table on reaction spontaneity.
Complete the following table on reaction spontaneity. Is the redox spontaneity rule empirical
Is the redox spontaneity rule empirical Sycamore dispersal method
Sycamore dispersal method Aeromicroflora and dispersal of microbes
Aeromicroflora and dispersal of microbes Lily seed dispersal
Lily seed dispersal Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Acorn seed dispersal
Acorn seed dispersal Seed dispersal by animals worksheet
Seed dispersal by animals worksheet Definition of avian
Definition of avian How do acorns disperse their seeds
How do acorns disperse their seeds Mango dispersal
Mango dispersal Ap human geography chapter 5 frq
Ap human geography chapter 5 frq Multiculturalism definition ap human geography
Multiculturalism definition ap human geography Pollination fertilisation seed dispersal germination
Pollination fertilisation seed dispersal germination How does seed dispersal happen
How does seed dispersal happen Dispersal by animals
Dispersal by animals Agent of dispersal
Agent of dispersal Dispersal by animals
Dispersal by animals Banana seed dispersal
Banana seed dispersal Mustard seed dispersal
Mustard seed dispersal Radiological dispersal device
Radiological dispersal device Female part of a flower
Female part of a flower Seed dispersal flow chart
Seed dispersal flow chart Where are you going where have you been true story
Where are you going where have you been true story Tell me what you eat and i shall tell you what you are
Tell me what you eat and i shall tell you what you are Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton
Tư thế worm breton Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Môn thể thao bắt đầu bằng chữ f
Môn thể thao bắt đầu bằng chữ f Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên
Thế nào là giọng cùng tên Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Bảng số nguyên tố
Bảng số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu