LECTURE NOTES ON MEDICAL HELMINTHOLOGY Dept of Parasitology










































































































- Slides: 156

LECTURE NOTES ON MEDICAL HELMINTHOLOGY Dept. of Parasitology FK Unpad

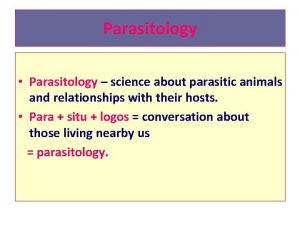
Introduction to Medical Helminthology ý Medical helminthology : the study of parasitic worms (helminthes/ vermes/cacing) affecting man, which : – Spend part or the entire life cycle in a human host or – Animal parasite causing disease in human

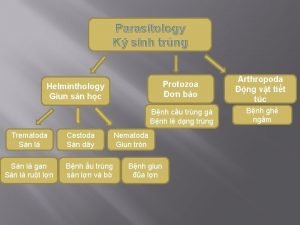
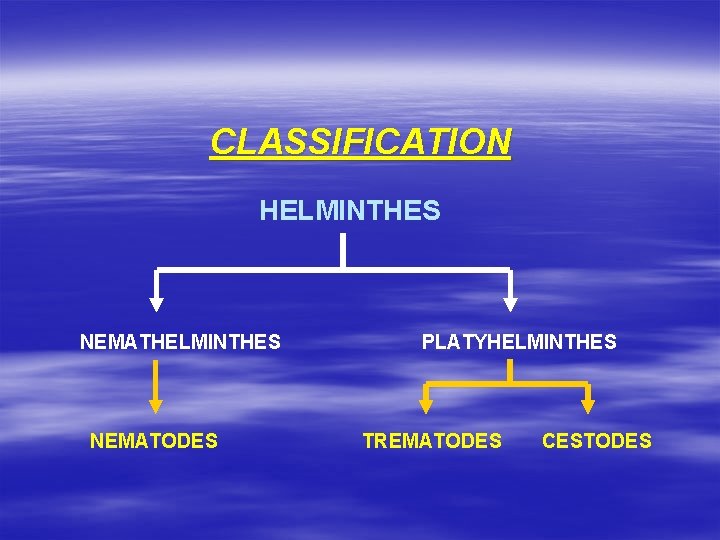
CLASSIFICATION HELMINTHES NEMATODES PLATYHELMINTHES TREMATODES CESTODES

General characteristics of Nematodes Ø The species parasitic in man range in length from 2 mm (Strongyloides stercoralis) to over a meter (Dracunculus medinensis) Ø The adult is an elongate cylindrical worm, bilaterally symmetrical like a thread; also known as roundworm Ø Unsegmented Ø Body covered by fine and smooth cuticle, sometimes striated Ø Has inner body cavity (pseudocoelom)

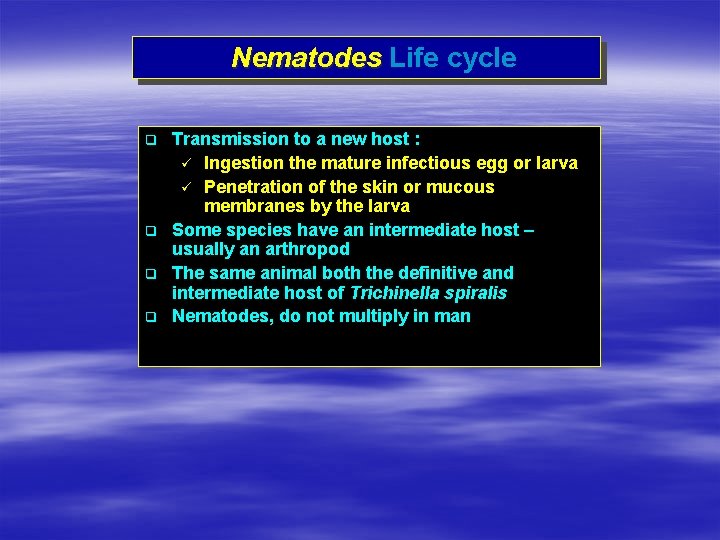
Nematodes Life cycle q q Transmission to a new host : ü Ingestion the mature infectious egg or larva ü Penetration of the skin or mucous membranes by the larva Some species have an intermediate host – usually an arthropod The same animal both the definitive and intermediate host of Trichinella spiralis Nematodes, do not multiply in man

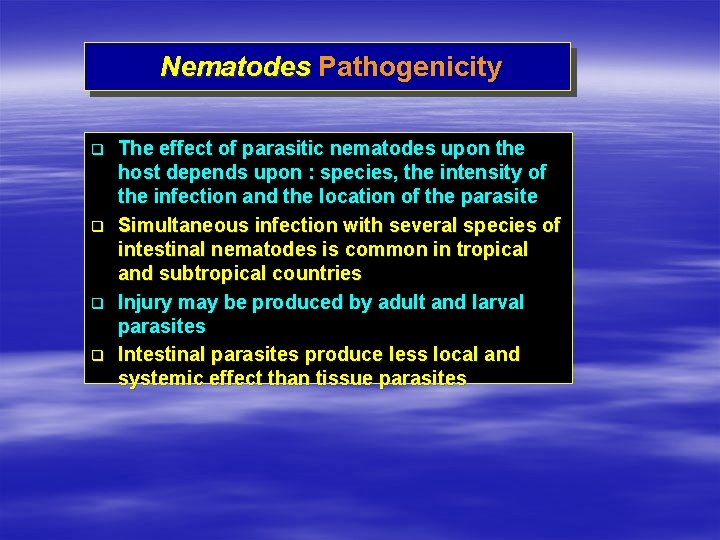
Nematodes Pathogenicity q q The effect of parasitic nematodes upon the host depends upon : species, the intensity of the infection and the location of the parasite Simultaneous infection with several species of intestinal nematodes is common in tropical and subtropical countries Injury may be produced by adult and larval parasites Intestinal parasites produce less local and systemic effect than tissue parasites

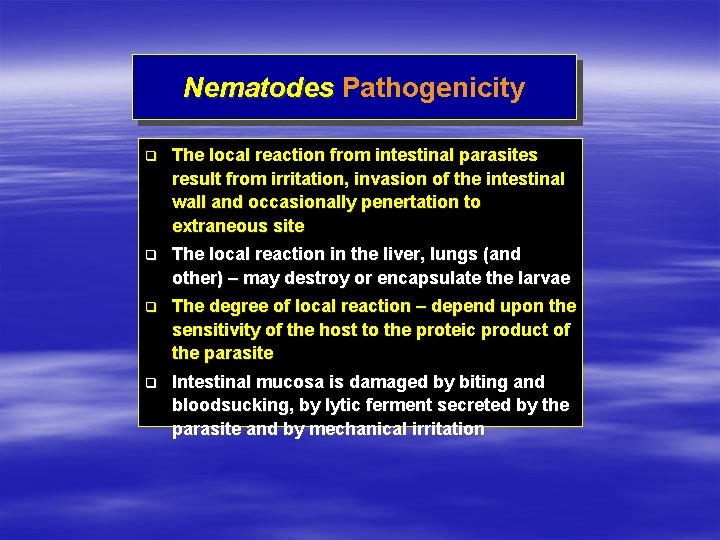
Nematodes Pathogenicity q The local reaction from intestinal parasites result from irritation, invasion of the intestinal wall and occasionally penertation to extraneous site q The local reaction in the liver, lungs (and other) – may destroy or encapsulate the larvae q The degree of local reaction – depend upon the sensitivity of the host to the proteic product of the parasite q Intestinal mucosa is damaged by biting and bloodsucking, by lytic ferment secreted by the parasite and by mechanical irritation

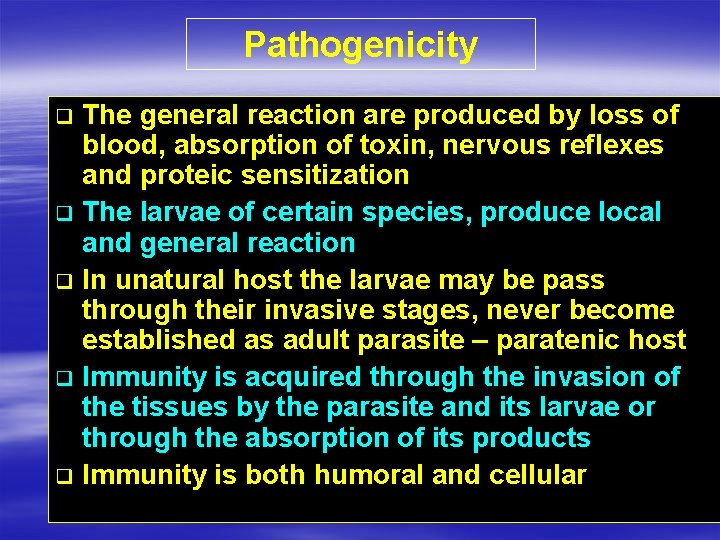
Pathogenicity The general reaction are produced by loss of blood, absorption of toxin, nervous reflexes and proteic sensitization q The larvae of certain species, produce local and general reaction q In unatural host the larvae may be pass through their invasive stages, never become established as adult parasite – paratenic host q Immunity is acquired through the invasion of the tissues by the parasite and its larvae or through the absorption of its products q Immunity is both humoral and cellular q

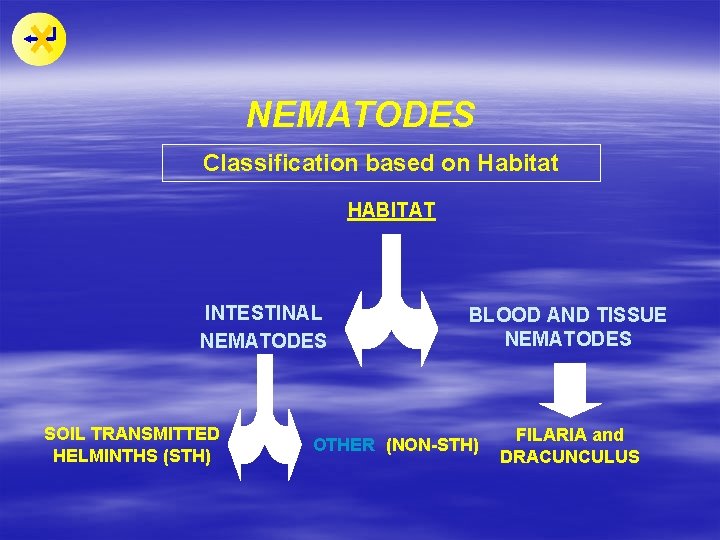
NEMATODES Classification based on Habitat HABITAT INTESTINAL NEMATODES SOIL TRANSMITTED HELMINTHS (STH) BLOOD AND TISSUE NEMATODES OTHER (NON-STH) FILARIA and DRACUNCULUS

NEMATODES q Soil Transmitted Helminthes ü Ascaris lumbricoides ü Trichuris trichiura ü Hookworm (Necator americanus, Ancylostoma duodenale) ü Strongyloides stercoralis q Non-Soil Transmitted Helminthes ü Enterobius vermicularis ü Trichinella spiralis q Filaria and Dracunculus ü Wuchereria bancrofti ü Brugia malayi ü Brugia timori Importan t

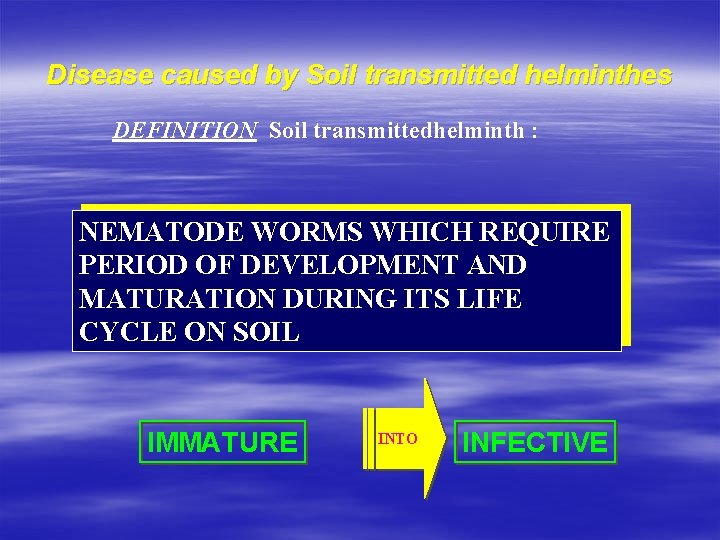
Disease caused by Soil transmitted helminthes DEFINITION Soil transmittedhelminth : NEMATODE WORMS WHICH REQUIRE PERIOD OF DEVELOPMENT AND MATURATION DURING ITS LIFE CYCLE ON SOIL IMMATURE INTO INFECTIVE

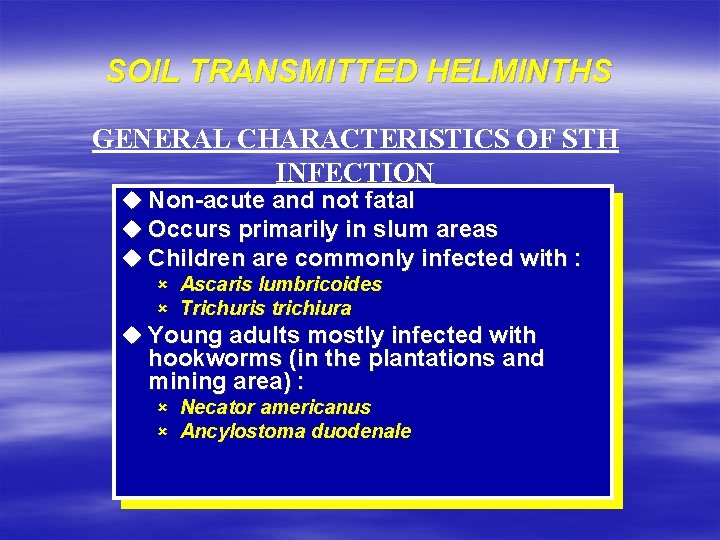
SOIL TRANSMITTED HELMINTHS GENERAL CHARACTERISTICS OF STH INFECTION u Non-acute and not fatal u Occurs primarily in slum areas u Children are commonly infected with : û Ascaris lumbricoides û Trichuris trichiura u Young adults mostly infected with hookworms (in the plantations and mining area) : û Necator americanus û Ancylostoma duodenale

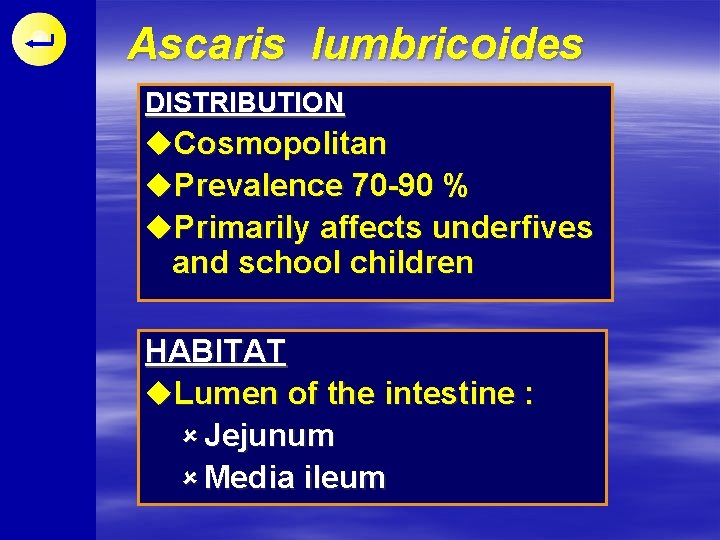
Ascaris lumbricoides DISTRIBUTION u. Cosmopolitan u. Prevalence 70 -90 % u. Primarily affects underfives and school children HABITAT u. Lumen of the intestine : û Jejunum û Media ileum

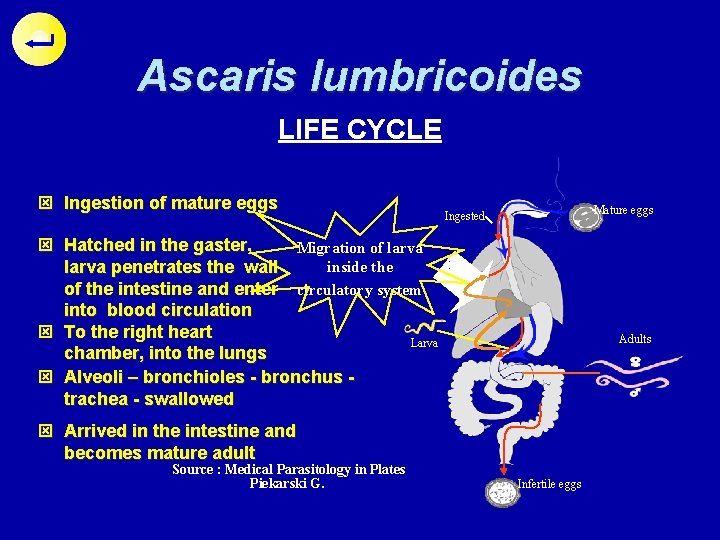
Ascaris lumbricoides LIFE CYCLE ý Ingestion of mature eggs Mature eggs Ingested ý Hatched in the gaster, Migration of larva inside the larva penetrates the wall of the intestine and enter circulatory system into blood circulation ý To the right heart Larva chamber, into the lungs ý Alveoli – bronchioles - bronchus trachea - swallowed Adults ý Arrived in the intestine and becomes mature adult Source : Medical Parasitology in Plates Piekarski G. Infertile eggs

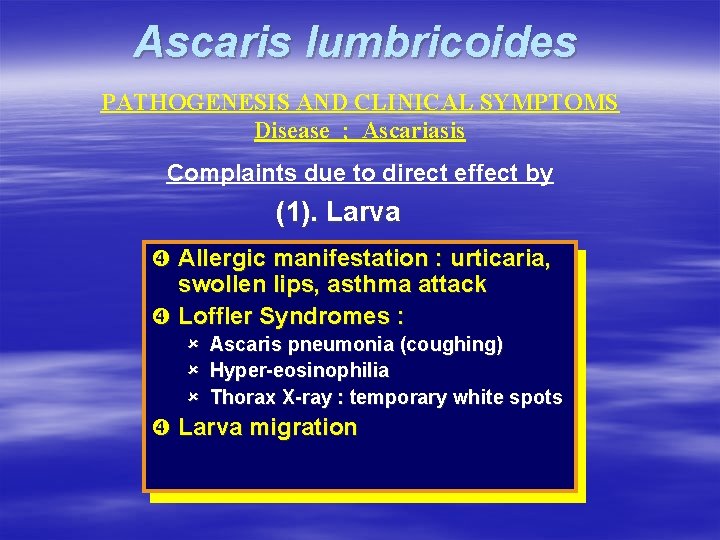
Ascaris lumbricoides PATHOGENESIS AND CLINICAL SYMPTOMS Disease ; Ascariasis Complaints due to direct effect by (1). Larva Allergic manifestation : urticaria, swollen lips, asthma attack Loffler Syndromes : û û û Ascaris pneumonia (coughing) Hyper-eosinophilia Thorax X-ray : temporary white spots Larva migration

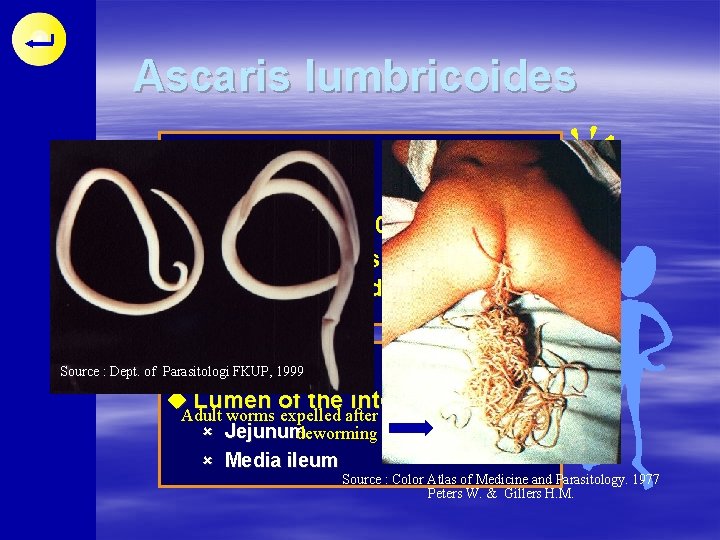
Ascaris lumbricoides DISTRIBUTION u Cosmopolitan u Prevalence 70 -90 % u Primarily affects underfives and school children HABITAT Source : Dept. of Parasitologi FKUP, 1999 u Lumen of the intestine : Adult worms expelled after û Jejunum deworming û Media ileum Source : Color Atlas of Medicine and Parasitology. 1977 Peters W. & Gillers H. M.

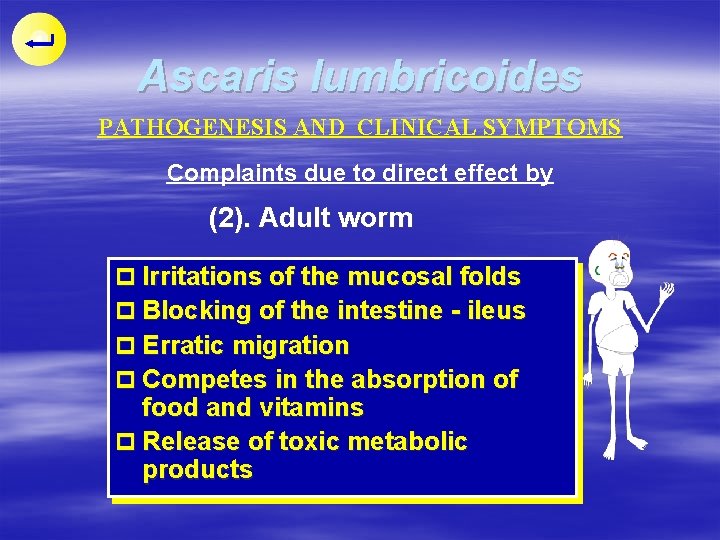
Ascaris lumbricoides PATHOGENESIS AND CLINICAL SYMPTOMS Complaints due to direct effect by (2). Adult worm p Irritations of the mucosal folds p Blocking of the intestine - ileus p Erratic migration p Competes in the absorption of food and vitamins p Release of toxic metabolic products

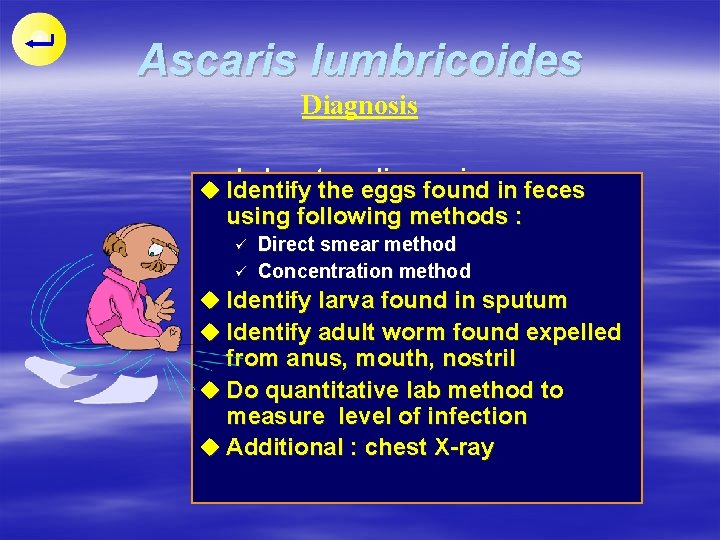
Ascaris lumbricoides Diagnosis Laboratory diagnosis u Identify the eggs found in feces using following methods : ü ü Direct smear method Concentration method u Identify larva found in sputum u Identify adult worm found expelled from anus, mouth, nostril u Do quantitative lab method to measure level of infection u Additional : chest X-ray

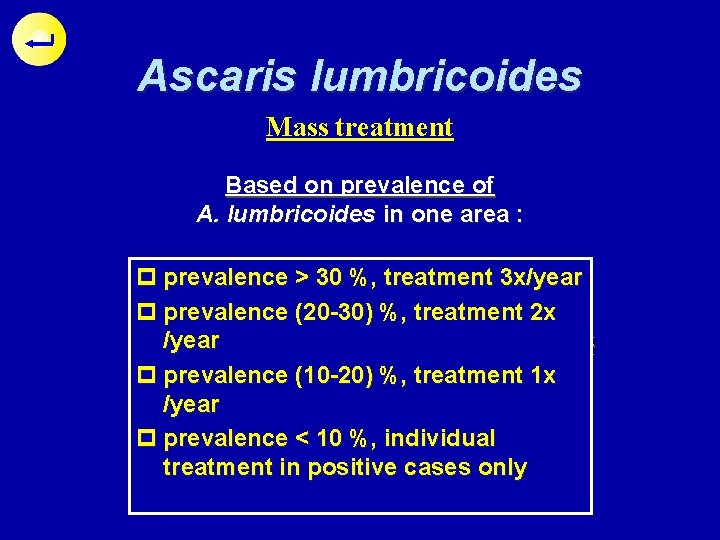
Ascaris lumbricoides Mass treatment Treatment Based on prevalence of Drug available A. lumbricoides in one area : o Pyrantel pamoate p prevalence > 30 %, treatment 3 x/year o Mebendazol ? p prevalence (20 -30) %, treatment 2 x /year o Oxantel pamoate o Piperazine p prevalence (10 -20) %, treatment 1 x /year o Albendazole p prevalence < 10 %, individual treatment in positive cases only

Ascaris lumbricoides PREVENTION p treatment of individual case p Provision of sanitary public bath, wash and toilet facilities p Media information and health education p Routine health check up of children

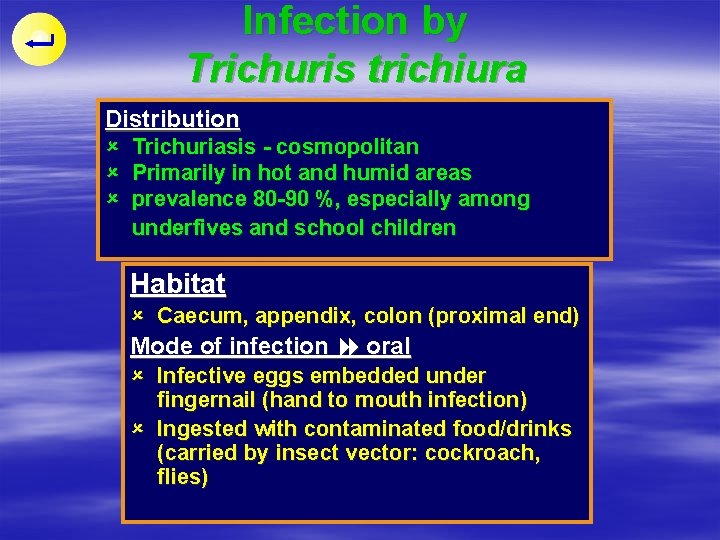
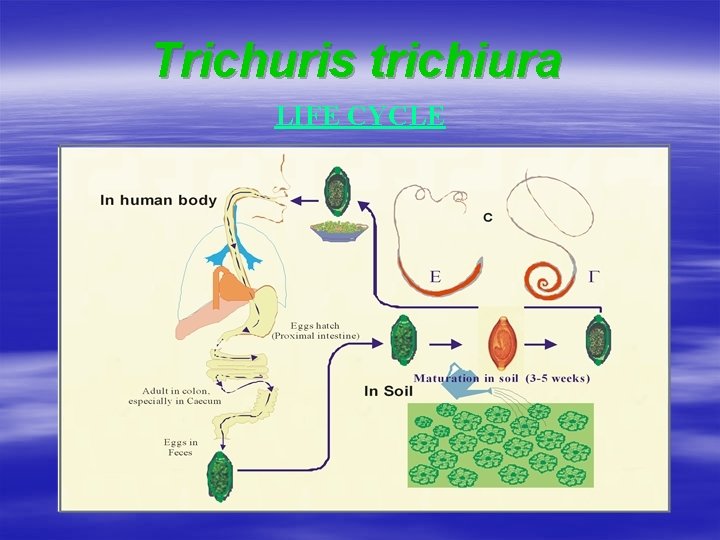
Infection by Trichuris trichiura Distribution û û û Trichuriasis - cosmopolitan Primarily in hot and humid areas prevalence 80 -90 %, especially among underfives and school children Habitat û Caecum, appendix, colon (proximal end) Mode of infection oral û Infective eggs embedded under fingernail (hand to mouth infection) û Ingested with contaminated food/drinks (carried by insect vector: cockroach, flies)

Trichuris trichiura LIFE CYCLE

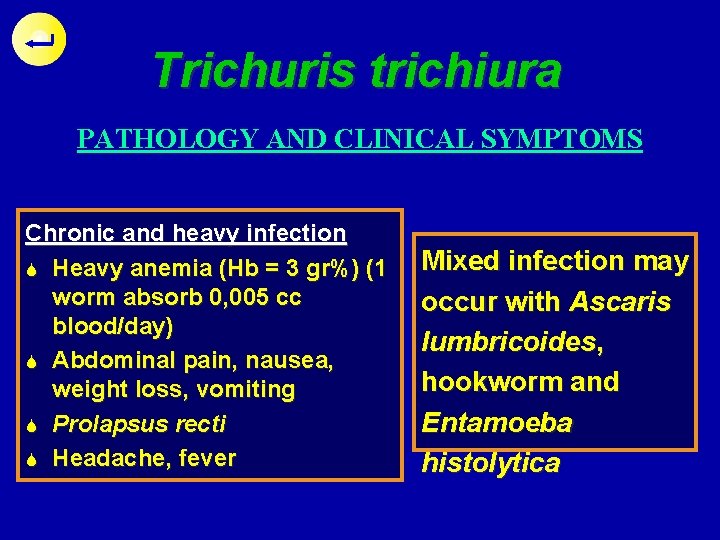
Trichuris trichiura PATHOLOGY AND CLINICAL SYMPTOMS Disease : Trichuriasis Heavy infection worm migrate to colon, rectum S Prolapsus recti, worm found in mucosal lining (due to frequent defecation)

Trichuris trichiura PATHOLOGY AND CLINICAL SYMPTOMS Chronic and heavy infection S Heavy anemia (Hb = 3 gr%) (1 worm absorb 0, 005 cc blood/day) S Abdominal pain, nausea, weight loss, vomiting S Prolapsus recti S Headache, fever Mixed infection may occur with Ascaris lumbricoides, hookworm and Entamoeba histolytica

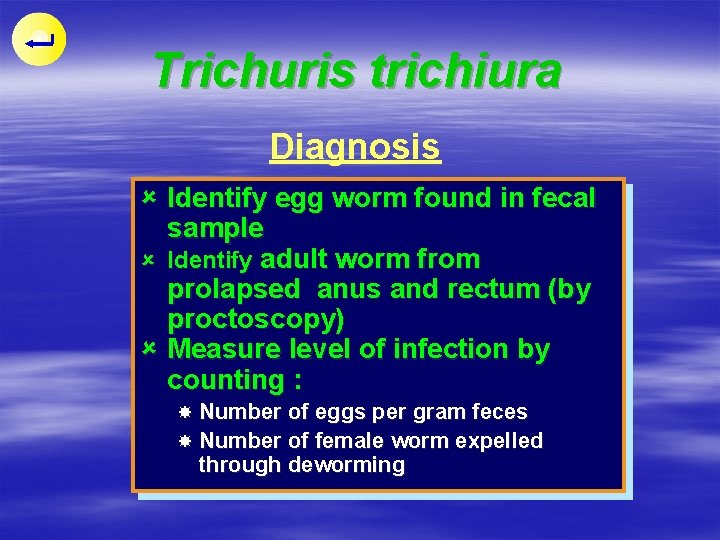
Trichuris trichiura Diagnosis û Identify egg worm found in fecal sample û Identify adult worm from prolapsed anus and rectum (by proctoscopy) û Measure level of infection by counting : Number of eggs per gram feces Number of female worm expelled through deworming

Trichuris trichiura PREVENTION Treatment û available Elimination Drugs : of source of infection û Improved personal hygiene (hand û Oxantel pamoate û Mebendazol (drug of training) choice) washing, toilet û Through washing of sold vegetables û Health education û Provision of sanitary public toilet

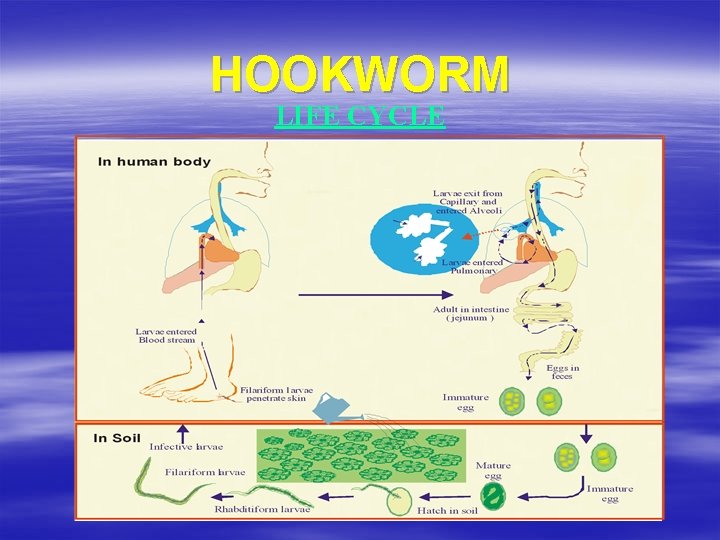
Infection by Hookworm PREFERENTIAL HABITAT û Small intestine (jejunum) û In heavy infection : duodenum, colon GEOGRAPHIC DISTRIBUTION Cosmopolitan, especially : – Tropical equator – Coal/tin mines, coffee/rubber plantations û Ideal soil for egg development : – – – Sandy soil Clay soil Muddy soil hindered from excessive dryness or wetness

HOOKWORM LIFE CYCLE

HOOKWORM PATHOLOGY AND CLINICAL SYMPTOMS û û û Disease: Ancylostomiasis Synonym: Uncinariasis, necatoriasis infection by A. duodenale are more serious than N. americanus û Chronic infection rarely produce acute manifestation û Tissue damage and symptoms are caused by : – Larva stage – Adult worm

HOOKWORM PATHOLOGY CAUSED BY LARVA STAGE û Larva penetrates the skin maculopapules - erythema - heavy itching : ground itch/dew itch û In sensitive patient, larva carried in the circulation, may cause: – Bronchitis / Pneumonitis

HOOKWORM PATHOLOGY CAUSED BY ADULT WORM û Hooked to the intestinal mucosal wall : abdominal pain, nausea, diarrhea û Absorbing 0, 2 -0, 3 ml of blood/day/worm : progressive anemia, hypo chrome, microcytic type of Fe deficiency anemia û Heavy anemia (Hb may reach 2 gr %) : – Dyspnea, physical weakness, headache – Rapid pulse beat, cardiac weakness – Children : physical growth retardation, mental

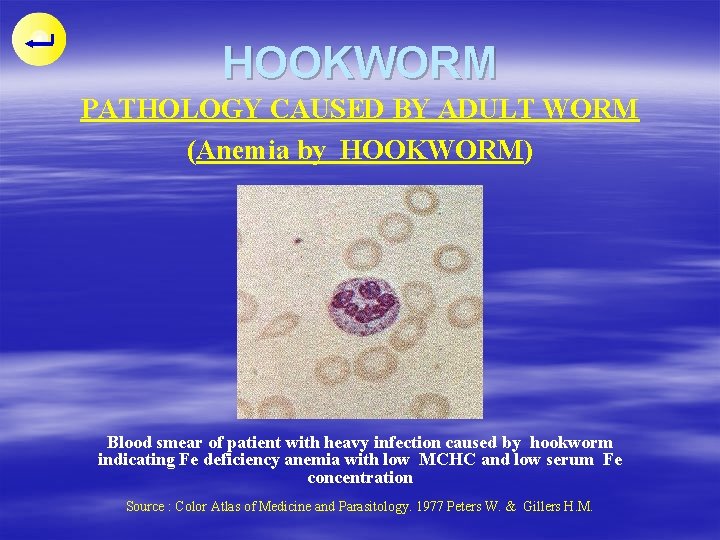
HOOKWORM PATHOLOGY CAUSED BY ADULT WORM (Anemia by HOOKWORM) Blood smear of patient with heavy infection caused by hookworm indicating Fe deficiency anemia with low MCHC and low serum Fe concentration Source : Color Atlas of Medicine and Parasitology. 1977 Peters W. & Gillers H. M.

HOOKWORM PATHOLOGY CAUSED BY ADULT WORM (Anemia by hookworm) û Atrophic glossitis found with hypo chromic microcytic anemia, caused by heavy infection of hookworm û Tongue surface become smooth and lacking of papillae

HOOKWORM PATHOLOGY CAUSED BY ADULT WORM (Anemia by hookworm) û Glositis atrofik pada with atrophic glossitis û Patient anemi hipokrom also show fingernail deformity mikrositer yang (koilonichia) disebabkan infection û Fingernail becomes thin and berat HOOKWORM concave û Tampak lidah halus with elevated ridge dan kurang papila source : Atlas Parasitologi Kedokteran, Zaman P. Alih Bahasa : Anwar C. ; Mursal Y.

HOOKWORM Diagnosis û Identify eggs from feces sample û Identify larva from : – Fecal culture – Old feces sample

HOOKWORM TIHELMINTHICS trachlorethylen ebendazole rantel pamoate oskanate phenium hidroxynaphtoate PREVENTION û Same as with Ascariasis but with the addition of : wearing shoes during

Infection by Strongyloides stercoralis HOST, HABITAT AND DISTRIBUTION û Man is the definitive Host û Habitat of female worm, mucosal lining of : l Duodenum l Jejunum (proximal end) û Found very cosmopolitan, especially in the tropical and subtropical region

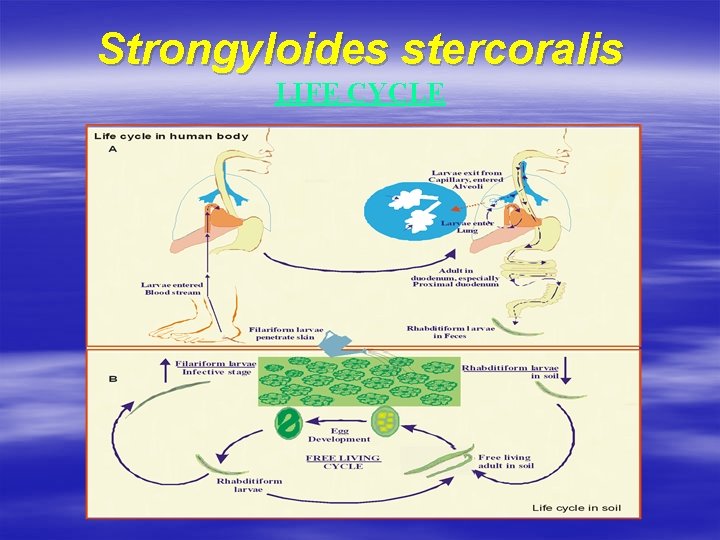
Strongyloides stercoralis LIFE CYCLE

Strongyloides stercoralis CLINICAL FEATURES û Disease : Strongyloidiasis, Strongyloidosis, Cochin China diarrhea û Level of infection : l Mild - asymptomatic l Moderate l Heavy and chronic

In moderate infection û Female worm embedded in the mucosal wall of duodenum û Burning sensation and stinging pain in the epigastrium û Nausea, vomiting, diarrhea and constipation

In heavy and chronic infection û Loss of body weight; Anemia û Dysentery (chronic); Slight fever û May be accompanied by secondary bacterial infection where worm inhabits the entire intestinal epithelium up to the distal colon)

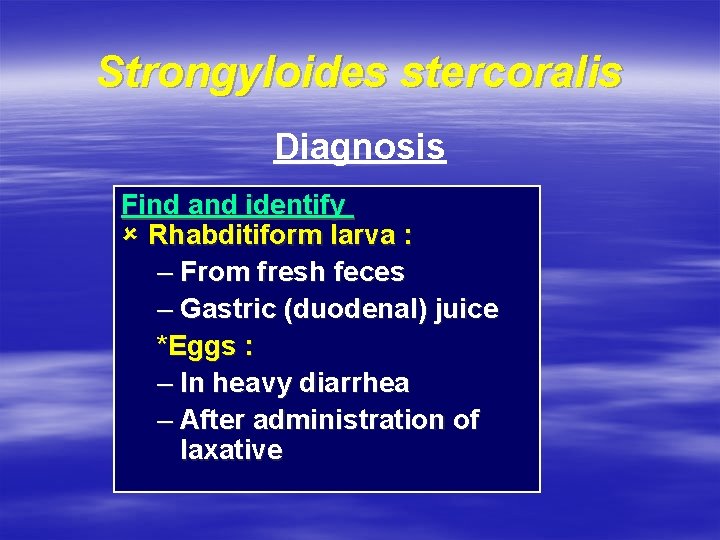
Strongyloides stercoralis Diagnosis Find and identify û Rhabditiform larva : – From fresh feces – Gastric (duodenal) juice *Eggs : – In heavy diarrhea – After administration of laxative

Strongyloides stercoralis TREATMENT AND PREVENTION Drugs given û û û Thiabendazole Mebendazole Pyrvinium pamoate

PREVENTION r Similar to the prevention of hookworm r Autoinfection is prevented by means of : – Avoid constipation – Anal hygiene

NON-SOIL TRANSMITTED HELMINTHS = Members of intestinal nematode that have transmissions are not via soil. = It happens because egg or larva of non-soil transmitted helminths don’t do maturation process (to become infectious) in the soil.

NON-SOIL TRANSMITTED HELMINTHS 1. Enterobius vermicularis 2. Trichinella spiralis

Enterobius vermicularis A. MORPHOLOGY > Enterobius vermicularis = Oxyuris vermicularis = pinworm. > In its life, this worm (ovipar) develops from: egg larva worm. > Its egg is oval, assymetry, that contains embrio. > This worm has lateral ala cephalic in anterior tip. > One female worm can produce 11. 000 eggs in one day. > The female worm will die after producing eggs. > The male worm will die after copulation.

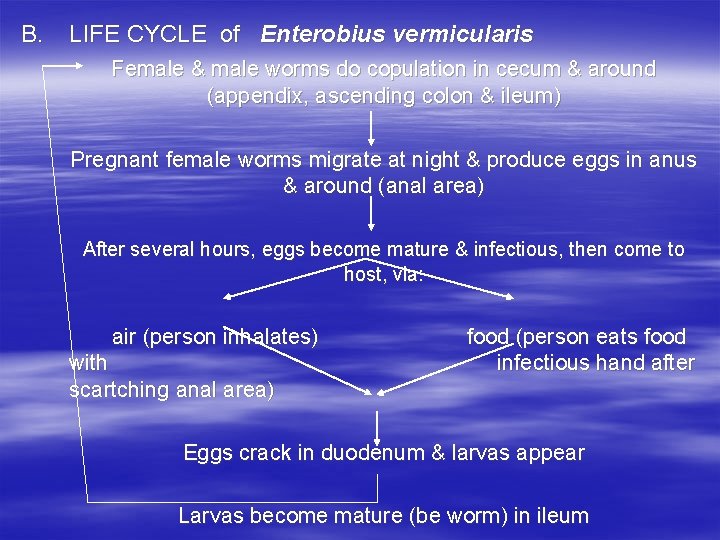
B. LIFE CYCLE of Enterobius vermicularis Female & male worms do copulation in cecum & around (appendix, ascending colon & ileum) Pregnant female worms migrate at night & produce eggs in anus & around (anal area) After several hours, eggs become mature & infectious, then come to host, via: air (person inhalates) with scartching anal area) food (person eats food infectious hand after Eggs crack in duodenum & larvas appear Larvas become mature (be worm) in ileum

Enterobius vermicularis C. SPREADING > Cosmopolite (spread around the world), especially in children. D. PATHOLOGY & CLINIC > The worm causes disease, called enterobiasis. > This disease has clinical symptoms: - Ithcing in anal area (pruritus ani) at night. - In girl, this disease can make inflammation in fallopian tube (salpingitis). - Intestine is seldom disturbed, etc.

Enterobius vermicularis E. DIAGNOSIS > Scotch adhesive tape swab method, is done before taking a bath or defecating. F. TREATMENT > Mebendazole, thiabendazole, etc. G. PREVENTION > Washing hand before eating > Cutting long fingernail, etc.

Trichinella spiralis A. MORPHOLOGY > Trichinella spiralis = porkworm. > In its life, this worn (vivipar) develops from larva worm. > Its larva can become cyst (circular larva which is covered by hyaline capsule). > This worm has stylet mouth to invade intestine or muscle tissue. > One female worm can produce about 1. 350 -2. 000 larvas. > The male worm will die after copulation.

B. LIFE CYCLE of Trichinella spiralis Female & male worms do copulation in mouse/pig/person duodenum to cecum Pregnant female worms enter to intestinal villi and then lymphatic sinus Pregnant female worms bear larvas in lymphatic sinus Larva is brought by lymphatic flow, to thorachic duct, right heart, lung, left heart, and then to around body Host (person/pig/etc) can die Larva enter to mouse/pig/person muscle tissue & make cysts (larva can live until 30 years in muscle) Health person eats meat (pig muscle) before cooked perfectly Cyst wall rupture & larva release & larva become mature (be worm) in health person duodenum The worm can also pass placenta & mammary

Trichinella spiralis C. SPREADING > Cosmopolite (spread around the world). D. PATHOLOGY & CLINIC > The worm causes disease, called trichinosis. > There are 3 clinical stadium: - Intestinal invasion that is done by worm (1 -2 days after eating infectious muscle). - Larva migration (7 -28 days after eating infectious muscle). It results cerebral disturbing, cardiac distrubing, fever, even die. -Forming cyst & healing process (90 days after eating infectious muscle).

BLOOD AND TISSUE NEMATODE THERE ARE THREE GROUPS : —Filaria dan Dracunculus —Larva Migrans (TROPICAL MEDICINE) —Rarely found nematode (LESS IMPORTANT !)

FILARIA LIFE CYCLE þ Insect as a vector : Anopheles, Aedes, Mansoni, Culex juga Simulium, Chrysops atau Culicoides þ Habitat : blood, lymph, muscle, conective tissue, serous cavity

BLOOD AND TISSUES NEMATODE Filaria – caused Filariasis TOPICS (As problem of Public Health in Indonesia) Ø Wuchereria bancrofti Ø Brugia malayi Ø Brugia timori All species above – nocturnal periodicity

LIFE CYCLE OF FILARIA þ In the human body, found stages : – Adult filaria – lymph node and vessel, difficult to find – not important – Microfilariae – pralarvae stage – in the peripheral blood – important for diagnostic þ Microfilariae, must be attention about : – Morphology for identified species of filaria – Periodicity for chose right time for take blood þ Periodicity – when microfilariae found in most number : – Nocturnal Periodicity – only in the night – Subperiodic nocturnal – in the night more number than in the daytime – Diurnal Periodicity – only in the daytime – Subperiodic diurnal – in the daytime more number than in the night – Nonperiodic – same number in the daytime and in the night

MORPHOLOGY OF MICROFILARIAE q Appearance graceful or stiff q Sheathed, its clear in part of the head or the tail (the three of them are sheathed) q “Nuclei” in the body : spread in average or in groups, show in the part of : Ø Head – without nuclei, named cephalic space, compare its length with its wide Ø Tail, contain the nuclei or not

PRIMARY CAUSE § Wuchereria bancrofti § Brugia malayi § Brugia timori

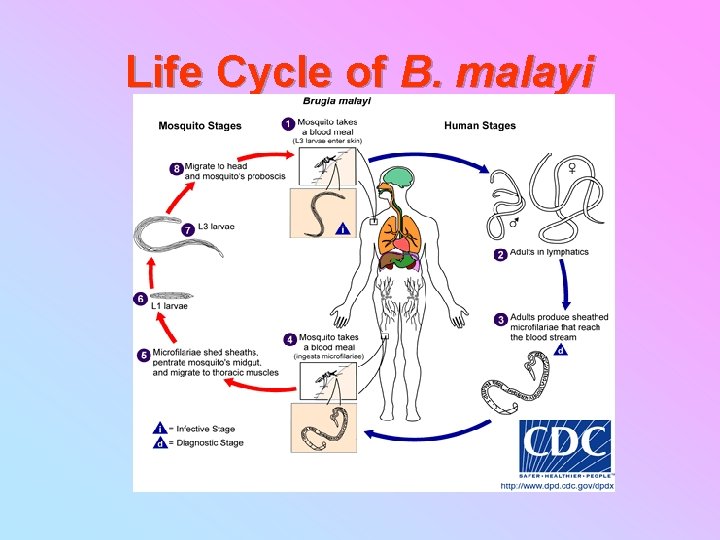
Life Cycle of B. malayi

Mansonia, Anopheles, Culex, Aedes

MAIN MOSQUITO VECTORS § W. bancrofti Anopheles sp § B. malayi Mansonia sp § B. timori An. barbirostris

Wuchereria bancrofti Habitat and Distribution HABITAT ÞVessel and lymph node (bellow the diaphragm) ÞCan live 10 -18 years ÞMicrofilaria in blood, penetrate placenta Þ 3 times metamorfosa

DISTRIBUTION : § Urban bancroftian filariasis, vektor Culex fatigans § Rural bancroftian filariasis, vektor Aedes, Anopheles dan Mansoni

Wuchereria bancrofti Periodicity PERIODICITY (WHO, 1967) v Commonly nocturna, also in Indonesia v In Polynesia, subperiodic diurna, vector Aedes polynisiensis

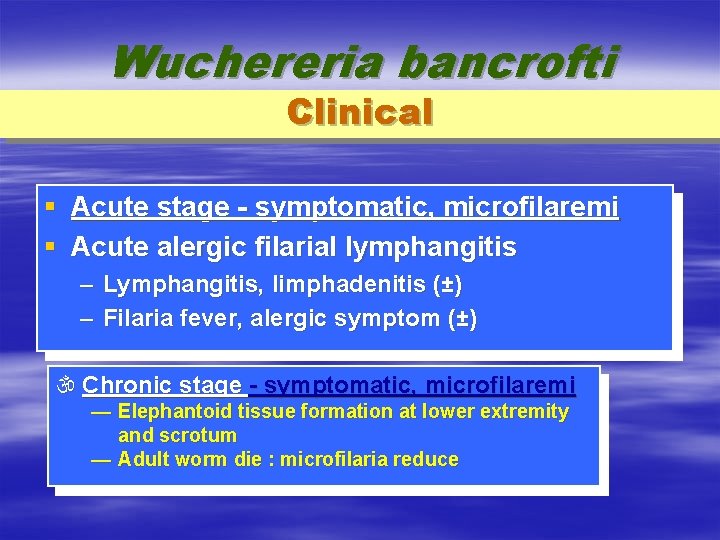
Wuchereria bancrofti Clinical DIVIDED IN : Biologic incubation periode Asymptomatic periode Acute stage Chronic stage Biologic incubation periode asymptomatic, amicrofilaremi larva - microfilaremi (± 1 year) § Asymptomatic Periode asymptomatic, microfilaremi § symptom (-), microfilaria (+), especially in endemic area

Wuchereria bancrofti Clinical § Acute stage - symptomatic, microfilaremi § Acute alergic filarial lymphangitis – Lymphangitis, limphadenitis (±) – Filaria fever, alergic symptom (±) Chronic stage - symptomatic, microfilaremi — Elephantoid tissue formation at lower extremity and scrotum — Adult worm die : microfilaria reduce

PERIODICITY § § § Nocturnal Periodicity Sub periodic nocturnal Diurnal Periodicity Sub periodic diurnal Non periodic All 3 species have nocturnal periodicity

Clinical manifestation § § Acute filariasis Chronic filariasis Atypical presentation Asymptomatic carrier

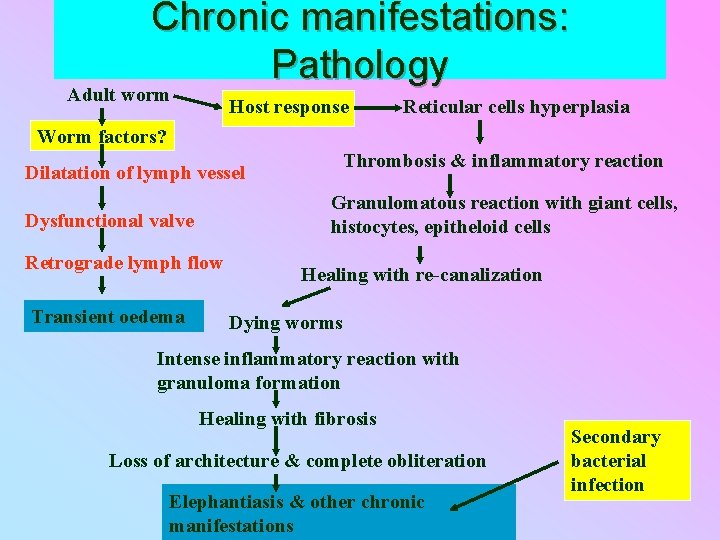
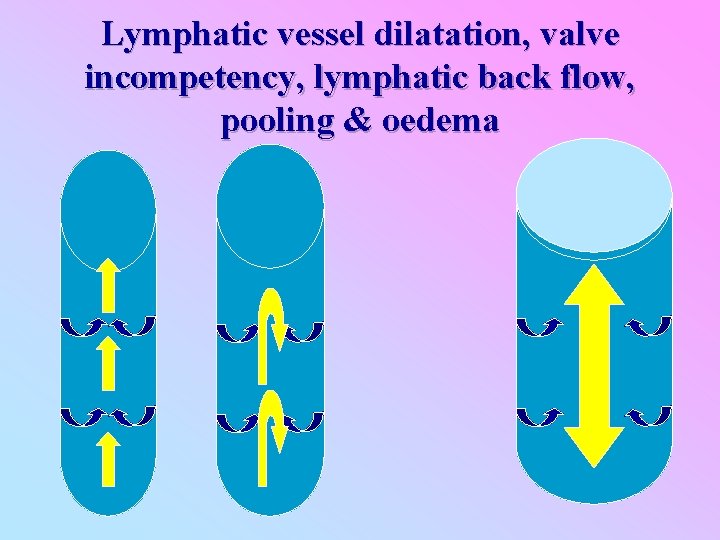
Chronic manifestations: Pathology Adult worm Host response Reticular cells hyperplasia Worm factors? Thrombosis & inflammatory reaction Dilatation of lymph vessel Granulomatous reaction with giant cells, histocytes, epitheloid cells Dysfunctional valve Retrograde lymph flow Transient oedema Healing with re-canalization Dying worms Intense inflammatory reaction with granuloma formation Healing with fibrosis Loss of architecture & complete obliteration Elephantiasis & other chronic manifestations Secondary bacterial infection

Lymphatic vessel dilatation, valve incompetency, lymphatic back flow, pooling & oedema

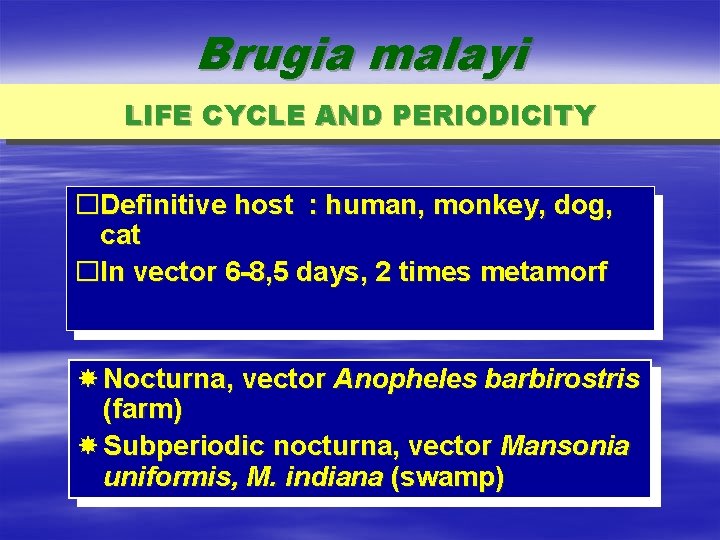
Brugia malayi LIFE CYCLE AND PERIODICITY �Definitive host : human, monkey, dog, cat �In vector 6 -8, 5 days, 2 times metamorf Nocturna, vector Anopheles barbirostris (farm) Subperiodic nocturna, vector Mansonia uniformis, M. indiana (swamp)

Brugia malayi CLINICAL, THERAPY AND PREVENTION CLINICAL: - Main symptom : fever, limphangytis, limphadenitis - Elephantiasis : lower extremity bellow knee, elbow, inguinal, rarely scrotum THERAPY : ý Hetrazan, po 0, 1 gr, 3 -4 x/day, as long as 10 days PREVENTION : ý Pentachlorophenol (dowicide G), kill water plant Pistia stratioides, Eichornia, Salvinia

Example from Thailand ELEPHANTIASIS by B. malayi Not involving external genitalia Courtesy Prof. Radomyos P. Mahidol U.

Example of filariasis from Malaysia

Filariasis Case at Pusat Pencegah Penyakit Untut – Kepala Batas

Filariasis research - IMR

Blood Trematoda (Schistosoma sp. )

There are four species of schistosome which are infective to humans: § Schistosoma mansoni, found in Africa, Brazil, Venezuela, Suriname , Puerto Rico, and the Dominican Republic. § Freshwater snails of the Biomphalaria genus are an important host for this trematode.

§ S. japonicum whose common name is simply blood fluke is found widely spread in Eastern Asia and the southwestern Pacific region. In Taiwan this species only affects animals, not humans. Freshwater snails of the Oncomelania genus are an important host for S. japonicum.

§ S. mekongi is related to S. japonicum and affects both superior and inferior mesenteric veins. S. mekongi differs in that it has smaller eggs, a different intermediate host, and longer prepatent period in the mammalian host.

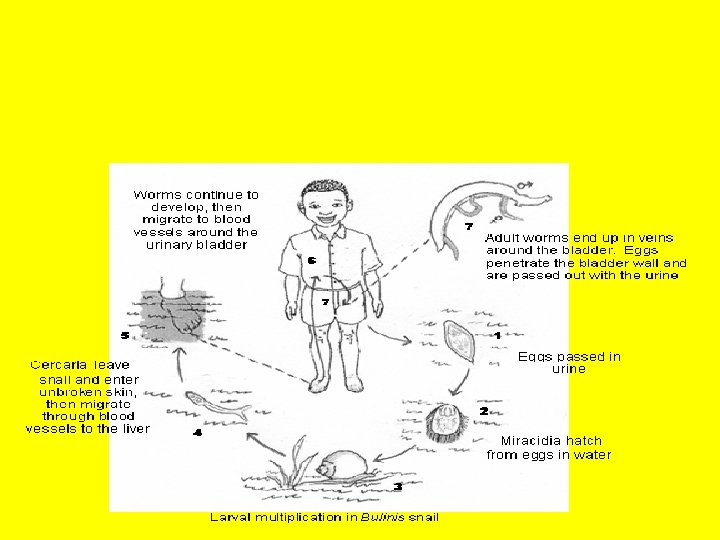
§ S. haematobium, commonly referred to as the bladder fluke, originally found in Africa, the Near East, and the Mediterranean basin, was introduced into India during World War II. Freshwater snails of the Bulinus genus are an important host for this parasite

§ Schistosoma mansoni causes intestinal schistosomiasis § Schistosoma haematobium causes urinary schistosomiasis § Schistosoma japonicum and Schistosoma mekongi cause Asian intestinal schistosomiasis

HABITAT § S. Haematobium Venous vessel (Vv) of urinary bladder, colon; Hepar § S. Japonicum Vv. of intestine; Hepar § S. Mekongi Vv. of intestine; Hepar § S. Mansoni Vv. of Colon, Rectum; Hepar

EPIDEMIOLOGY Disease of schistomiasis or bilharzasis in Indonesia only schistomiasis japonica found in middle sulawesi, relate to agriculture getting water from irrigation. Group of age hit by between 5 -50 year. Mass treatment every 6 month.

S. japonicum § The S. japonicum worms are yellow or yellow-brown. The males of this species are slightly larger than the other Schistosomes and they measure ~ 1. 2 cm by 0. 5 mm. The females measure 2 cm by 0. 4 mm. The adult worms are longer and narrower than the related S. mansoni worms.

S. japonicum § This Parasite at human being cause oriental of schistosomiasis, schistosomiasis japonica, katayama fever or Snail Fever disease

Katayama Fever § Acute schistosomiasis (Katayama's fever) may occur weeks after the initial infection, especially by S. mansoni and S. japonicum. Manifestations include: § Abdominal pain § Cough § Diarrhea § Eosinophilia § Fever § Fatigue § Hepatosplenomegaly

§ Faeces with egg of s. JAponicum have contact with water § Those egg will hatch and release free-swimming larva called Miracidia § Miracidia will infect Oncomelaria hupensis by penetrating their skin § Inside the snail they will undergo asexual reproduction, and will become sporocysts § Further reproduction will produce large number of Cercaria, which is freeswimming larva § Cercaria can infect us by penetrating our skin § After penetration, they will loose their tail and become Schistosomule § Enter circulation, and end at the mesenteric veins § There, they will undergo sexual reproduction and produce egg § Egg will penetrate the tissue and are passed with the faeces

S. mansoni § The male S. mansoni is approximately 1 cm long (0. 6 to 1. 4 cm) and is 0. 11 cm wide. It is white and it has a funnelshaped oral sucker at its anterior end § The female has a cilindric body, longer and thinner than the male (1. 2 to 1. 6 cm long by 0. 016 cm wide). The female parasite is darker and it looks gray. The darker color is due to the presence of a pigment (hemozoin) in its digestive tube

CLINICAL SYMPTOM AND PATHOLOGY Similar with S. japonicum, but it is lighter

MORPHOLOGY AND LIFE CYCLE Adult Worm of fairish male about 1, 3 cm and female about 2, 0 cm. its life in small vena flank. Egg found in urine, and rectum genitals


Pathology § It can break the wall of the urinary bladder § The most characteristic symptom is haematuria. § Dysuria also can occur. § Inflammatory reaction to eggs in the bladder wall and later fibrosis and calcification often leads to mucosal hyperplasia and papilloma formation § In Egypt vesical schistosomiasis has been considered a common cause of malignancy of the bladder in male agricultural workers

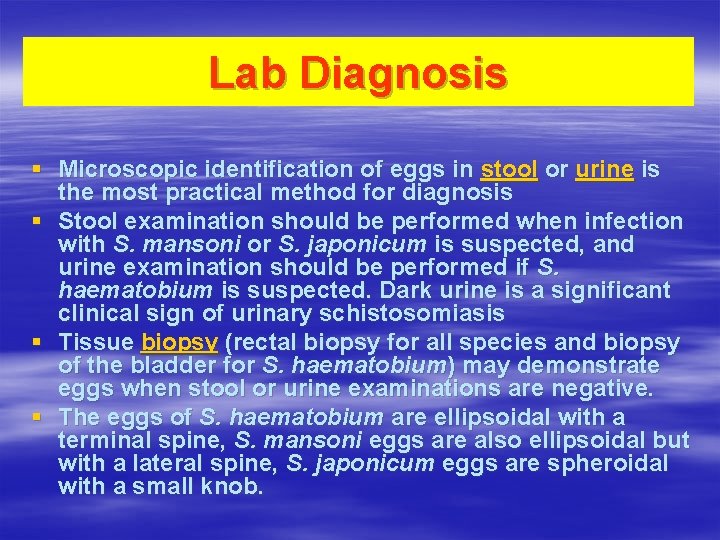
Lab Diagnosis § Microscopic identification of eggs in stool or urine is the most practical method for diagnosis § Stool examination should be performed when infection with S. mansoni or S. japonicum is suspected, and urine examination should be performed if S. haematobium is suspected. Dark urine is a significant clinical sign of urinary schistosomiasis § Tissue biopsy (rectal biopsy for all species and biopsy of the bladder for S. haematobium) may demonstrate eggs when stool or urine examinations are negative. § The eggs of S. haematobium are ellipsoidal with a terminal spine, S. mansoni eggs are also ellipsoidal but with a lateral spine, S. japonicum eggs are spheroidal with a small knob.

Treatment § Schistosomiasis is readily treated using a single oral dose of the drug Praziquantel

Prevention § The main focus of prevention is eliminating the water-borne snails which are natural reservoirs for the disease. This is usually done by identifying bodies of water, such as lakes, ponds, etc. , which are infested, forbidding or warning against swimming and adding niclosamide, acrolein, copper sulfate, etc. , to the water in order to kill the snails. § Individuals can guard against schistosomiasis infection by avoiding bodies of water known or likely to harbor the carrier snails

Liver Trematodes v Clonorchis sinensis v Dicrocoelium dendriticum v Opistorchis felineus v Opistorchis viverini v Fasciola hepatica

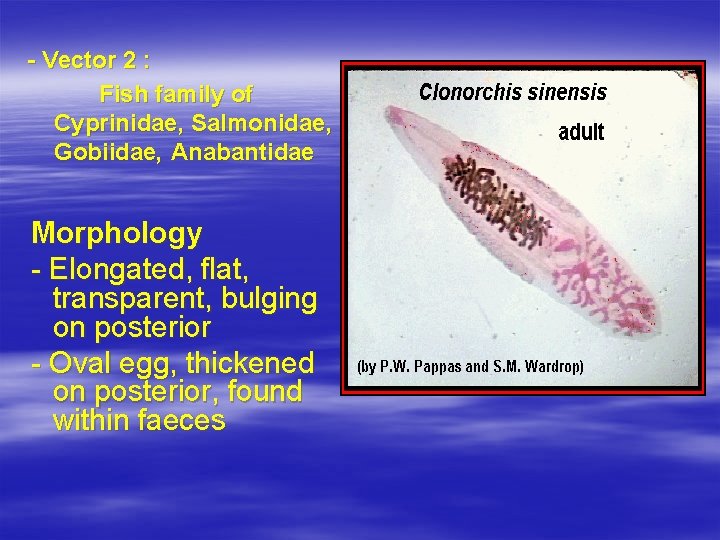
Clonorchis sinensis Epidemiologiy - China, Japan, Korea, Taiwan, Vietnam Habitat - Biliary tract, pancreatic tract Host - definitive : human, cat, dog - vector 1 : Water snail genus of Bulimus, Thiara, or species Melanoides tuberculatus

- Vector 2 : Fish family of Cyprinidae, Salmonidae, Gobiidae, Anabantidae Morphology - Elongated, flat, transparent, bulging on posterior - Oval egg, thickened on posterior, found within faeces

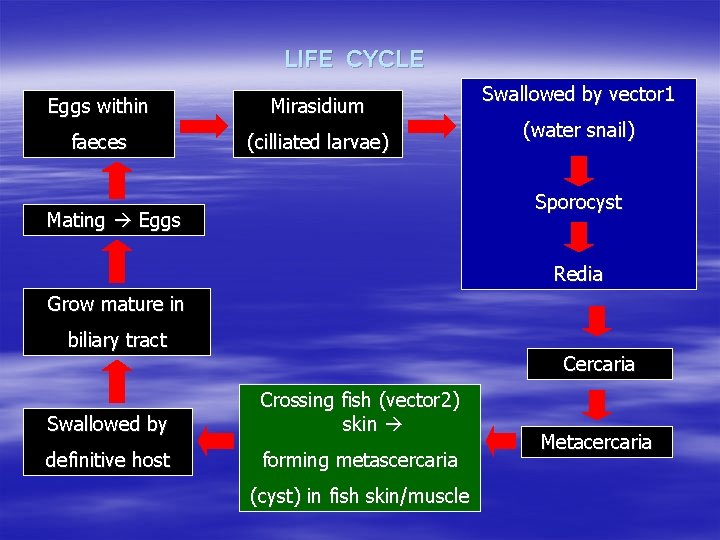
LIFE CYCLE Eggs within Mirasidium faeces (cilliated larvae) Swallowed by vector 1 (water snail) Sporocyst Mating Eggs Redia Grow mature in biliary tract Cercaria Swallowed by Crossing fish (vector 2) skin definitive host forming metascercaria (cyst) in fish skin/muscle Metacercaria

Diagnosis - Eggs within faeces - Immunology diagnosis Prevention - Avoid eating raw fish must be completely cooked

Dicrocoelium dendriticum Epidemiology - Cosmopolite for lamb & other herbivores in Asia, Africa, Europe & America Morphology - Mature form: flat, slender - Eggs: dark brown, thick wall, contain completelygrown mirasidium Host - Definitive: lamb - Vector 1: snail genus of Abida, Zebrina - Vector 2: ant, species Formica fusca Life Cycle : comparable to C. sinensis

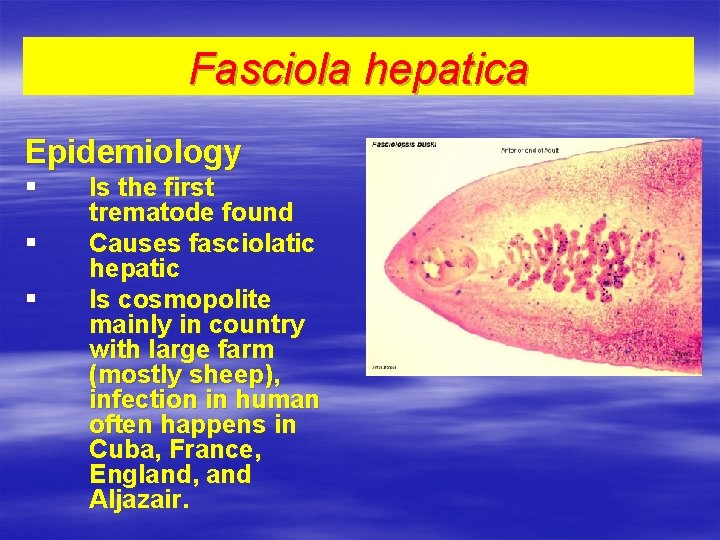
Fasciola hepatica Epidemiology § § § Is the first trematode found Causes fasciolatic hepatic Is cosmopolite mainly in country with large farm (mostly sheep), infection in human often happens in Cuba, France, England, and Aljazair.

Habitat and host § § Habitat is mainly in billiary duct, may penetrate hepatic tissue causing abses. Definitive hosts are human, sheep and other cattle. Intermediate host I is family Lymnaeidae, especially genus Lymnaea. Intermediate host II is water plants especially Nasturtium officinale.

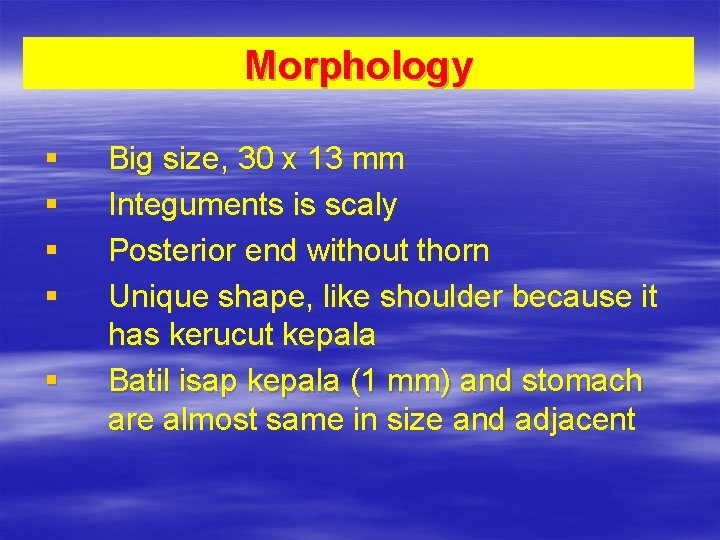
Morphology § § § Big size, 30 x 13 mm Integuments is scaly Posterior end without thorn Unique shape, like shoulder because it has kerucut kepala Batil isap kepala (1 mm) and stomach are almost same in size and adjacent

§ Caecum is hyper branching until posterior end § Hyper branching testis is lied between 2/4 – ¾ posterior body, one is behind the other § Ovary branches, anterior to testis and smaller § Uterus is short, curved, located between ootype and porus genital. § Egg is 130 -150 x 63 -90 µ in size and has operculum. Immature eggs are lied in billiary duct and exit with feces.

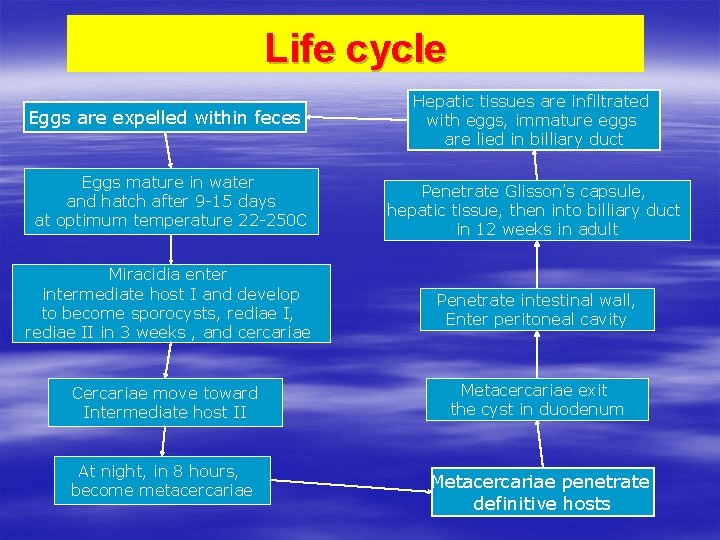
Life cycle Eggs are expelled within feces Eggs mature in water and hatch after 9 -15 days at optimum temperature 22 -250 C Hepatic tissues are infiltrated with eggs, immature eggs are lied in billiary duct Penetrate Glisson’s capsule, hepatic tissue, then into billiary duct in 12 weeks in adult Miracidia enter intermediate host I and develop to become sporocysts, rediae II in 3 weeks , and cercariae Penetrate intestinal wall, Enter peritoneal cavity Cercariae move toward Intermediate host II Metacercariae exit the cyst in duodenum At night, in 8 hours, become metacercariae Metacercariae penetrate definitive hosts

Clinical Symptom § Until metacercaria penetrate Glisson’s capsule, these is no complain. But trauma and necrotic lesion rise during migration through hepatic tissues. § In heavy infection, epithelia is scraped and young worm will return to hepatic parenchyma, form abscess pouch, and hepatic tissues are infiltrated with eggs. § When larvae migrates to peritoneal cavity, focus ectopic may happen in blood vessel, lungs, subcutaneous tissue, brain ventricles, and eyes where abscess will be formed. § Symptoms : colic, ikterus obstructiva, cough and vomiting, abdomen rigidity, acute epigastria pain, leukocytosist and eosinophily until above 60%

Diagnosis § Eggs are found in feces, duodenal fluid, or bile. Patient, who will be examined, do not eat liver. § Need early diagnosis to prevent fatal liver damage. § Serodiagnostic test is very helpful, although it is not an routine diagnostic tool.

Prevention § Treating patient and cattle that act as reservoir. § Incinerating water snails. § Cooking vegetables that will be eaten.

Lung Trematode § Paragonimus westermani § Disease : Paragonimiasis , Pulmonary distomiasis

Paragonimus westermani Ø Epidemiology – It is cosmopolite in human, mainly east in Japan, Philippine, Korea, China, Muangthai, Taiwan, Africa, etc. – In Indonesia, it is autochthon infection in animal. In human, as import case.

Host § Definitive hosts are human and mammalians such as dog and cat § Intermediate hosts I are Semisulcaspira libertina (Japan), Brotia asperata (Philipina), B. costula episcpalis (Malaysia), Syncera sp. , and Melania. § Intermediate hosts II are freshwater crabs genus Potamon, Eriocheir, Sesarma sp. , and crayfish.

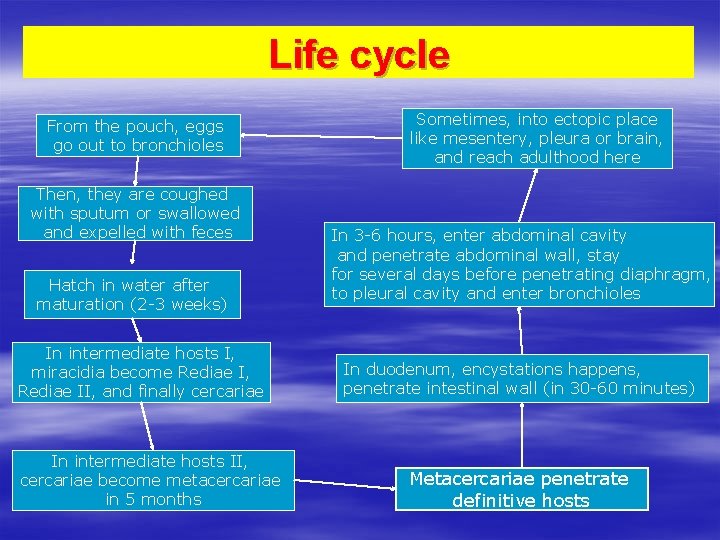
Life cycle From the pouch, eggs go out to bronchioles Then, they are coughed with sputum or swallowed and expelled with feces Hatch in water after maturation (2 -3 weeks) In intermediate hosts I, miracidia become Rediae I, Rediae II, and finally cercariae In intermediate hosts II, cercariae become metacercariae in 5 months Sometimes, into ectopic place like mesentery, pleura or brain, and reach adulthood here In 3 -6 hours, enter abdominal cavity and penetrate abdominal wall, stay for several days before penetrating diaphragm, to pleural cavity and enter bronchioles In duodenum, encystations happens, penetrate intestinal wall (in 30 -60 minutes) Metacercariae penetrate definitive hosts

Clinical signs and pathology § Around parasite, leukocyte infiltration happens and is followed by forming fibrosis capsule and form cyst containing 2 worms, purulent blood, and eggs. § Lungs : (1) nonsuppurative, (2) tubercle-like, (3) suppurative, (4) ulcerative. § First fever, shaking chills, dry cough, then hemaptoe with sticky and rush-colored sputum mainly when wake up in the morning. § Physical examination reveals a bronchopneumonia or bronchiectasi with pleural effusion.

Diagnosis § Finding eggs in sputum, aspiration pleural fluid, and feces § Serology test with intradermal test, complement fixation test.

Treatment and Prevention TREATMENT - Praziquantel PREVENTION § Treating patients § Cooking crab before being eaten


§ Cestodes are members of phylum Platyhelminthes, also known as tapeworms. General morphology § Scolex : organ of attachment, usually bearing suckers or hooks § Neck : part which it grows § Strobilla : the boby, consist of proglotids (segments)

§ Proglotids Immature : segment that is attached to the neck Mature : has a reproductive structure Gravid (pregnant): filled with eggs. §Tegument: covers adult cestode, allows materials to enter and waste to be excreted from the body § Hermaphrodite, each proglotid has a male and female reproductive structure. § Mature embryo has six hooks and also known


• Is not yet found in Indonesia • Resides in ileum of human, dogs, and cats Morphology • Adult worm 3 – 10 m, can be as long as 60 m, with 3000 -4000 proglotids. • In the place of sucker or hooks, are two shallow sucking grooves called bothria. • Operculated eggs

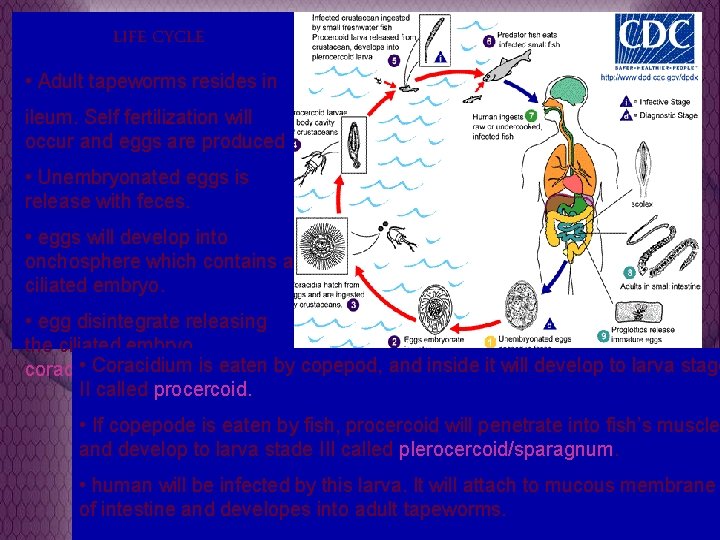
LIFE CYCLE • Adult tapeworms resides in ileum. Self fertilization will occur and eggs are produced • Unembryonated eggs is release with feces. • eggs will develop into onchosphere which contains a ciliated embryo. • egg disintegrate releasing the ciliated embryo , • Coracidium is eaten coracidium, larva stage I. by copepod, and inside it will develop to larva stage II called procercoid. • If copepode is eaten by fish, procercoid will penetrate into fish’s muscle and develop to larva stade III called plerocercoid/sparagnum. • human will be infected by this larva. It will attach to mucous membrane of intestine and developes into adult tapeworms.

Pathogenesis Disease: diphyllobothriasis Usually asymptomatic, can cause abdominal pain, weight loss, diarrhea, vomiting, deficiency of vit B 12. Diagnosis Eggs or proglotids found in feces. Treatment Niclosamide, praziquantel, bithionol, atabrin Prevention Avoid ingestion of undercooked fish


• Also called porkworm and resides in jejenum of human. • Larva is known as cycticercus celullosae 10 x 5 mm. usually inhabit the muscle. Morphology • Adult worm is 2 – 4 m • Has a rostellum with two rows of hooks plus four suckers. • Genital pores located lateral to proglotids. • Eggs doesn’t have opperculum, but contains onchosphere.

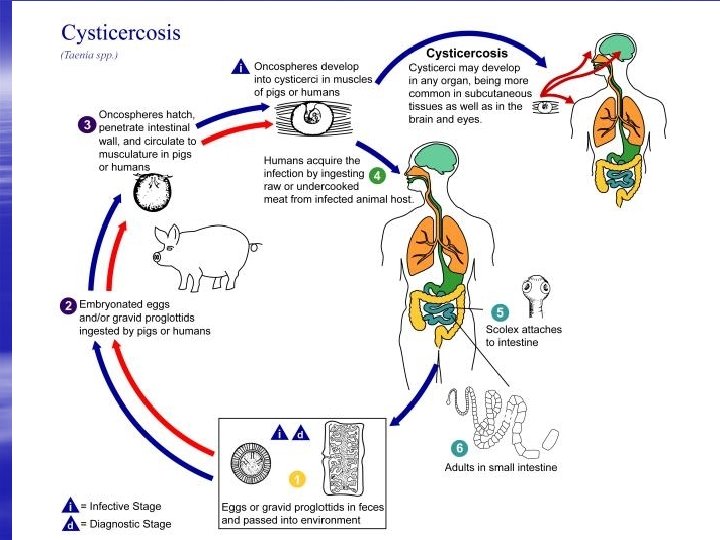
L i f e cycle § Eggs are digested out in the stomach of the pig. § Using its hook and lysis substrate, the oncosphere will then penetrate intestinal wall into blood stream and reside in muscle forming cysticercus celullose. § Human will be infected by digestion of undercooked pork. § The larva will attach inside the small intestine, and develop into adult tapeworms that can produce eggs. § These eggs will then pass through feces


Pathogenesis • Disease: cysticercosis • Most cases are asymptomatic. Diarrhea, abdominal pain may occur. • If cysticercus migrate to brain, can cause neurocysticercosis. Headaches, seizures, even death can occur. Diagnosis Gravid proglotids or egg in feces, CT scan, MRI. Treatment Praziquantel and niclosamide, also surgical removal.


• Also known as beef worm. • Larva is called cysticercus bovis, Morphology • Adult beefworm is 5 m, but can grow as far as 25 m. • Has four suckers, but no hooks.


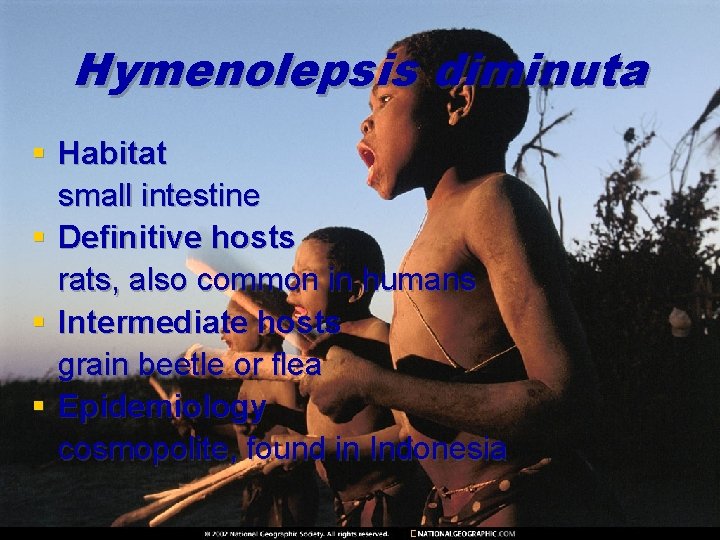
Hymenolepsis diminuta § Habitat small intestine § Definitive hosts rats, also common in humans § Intermediate hosts grain beetle or flea § Epidemiology cosmopolite, found in Indonesia

Hymenolepsis diminuta § Morphology Adult worms 10 -60 mm long, 3 -5 mm wide, have 800 -1000 proglotids. Rounded scolex bears a small rostellum with four suckers, proglotid 0, 8 mm long and 2, 5 mm wide. Eggs without the polar filaments, sized 6080μm.

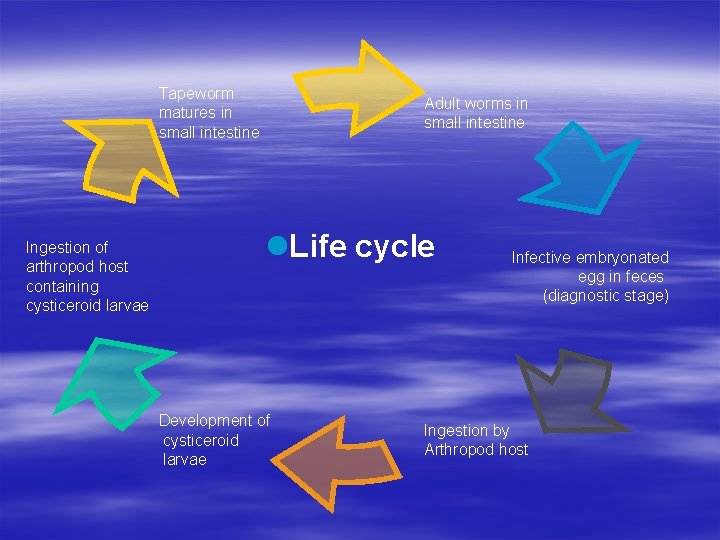
Tapeworm matures in small intestine Ingestion of arthropod host containing cysticeroid larvae Adult worms in small intestine l. Life cycle Development of cysticeroid larvae Infective embryonated egg in feces (diagnostic stage) Ingestion by Arthropod host

Hymenolepsis diminuta § Transmission or infection occurs by ingestion of infected beetles or other arthropods, usually in grains and cereals § Pathogenesis Symptoms, if any, are mild, and usually include diarrhea, nausea, and slight abdominal pain § Diagnosis Recovery of characteristic eggs in feces

Hymenolepsis diminuta § Treatment Niclosamide, Praziquantel § Prevention Limit exposure of grains and cereals to rats and insects.


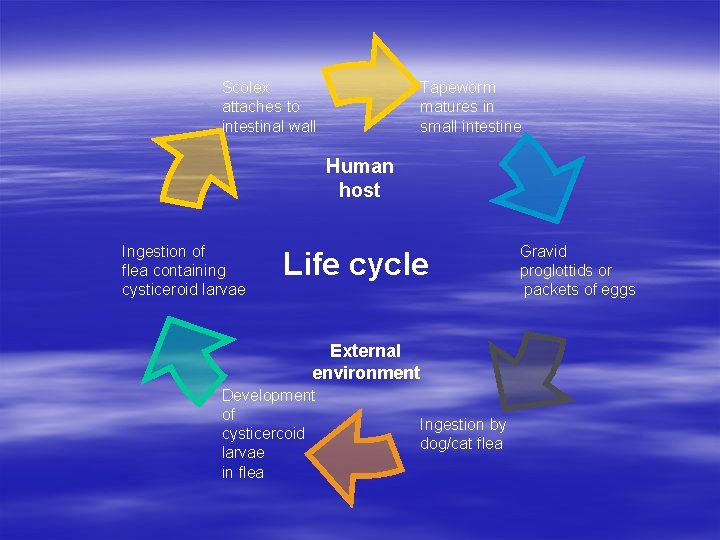
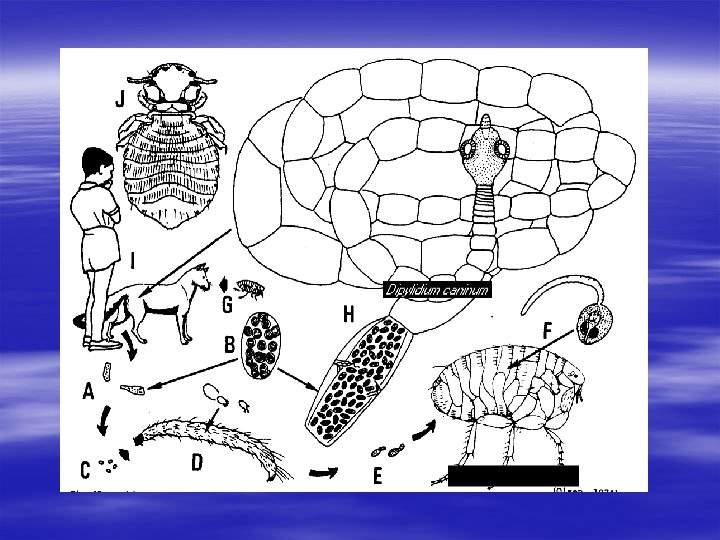
Dyphilidium caninum § Synonym Double-pore dog tapeworm § Definitive hosts Cats and dogs, , , also found in humans’ small intestine. § Intermediate hosts Dog/cat flea; Ctenocephalides canis and C. felis.

Dyphilidium caninum § Morphology Adult tapeworm is 10 to 50 cm in length. Several rows of tiny hooklike spines are present on the coneshaped rostellum. The uterus in the gravid proglottid contains numerous packets of eggs, each packet containing 5 to 20 eggs. Colorless eggs ~ 30 -60μm in diameter, with a six hooked oncosphere present in each egg. The eggs are enclosed in membrane-bound packets.

Dyphilidium caninum § Transmission Infection after ingestion of cysticeroid larvae. § Pathogenesis Most of infected individuals are asymptomatic, although some with a heavy worm burden may experience mild gastrointestinal symptoms, such as nausea, diarrhea, indigestion, and slight abdominal pain.

Scolex attaches to intestinal wall Tapeworm matures in small intestine Human host Ingestion of flea containing cysticeroid larvae Life cycle External environment Development of cysticercoid larvae in flea Ingestion by dog/cat flea Gravid proglottids or packets of eggs


Dyphilidium caninum § Diagnosis Recovery of characteristic gravid proglottids and egg packets (following rupture of proglottids) in human feces. § Treatment Niclosamide, Praziquantel. § Prevention Good veterinary care of cats and dogs, keeping animal freely from parasites. Good personal hygiene.


Hymenolopsis nana § Morphology: § smallest tapeworm, 25 -40 mm, found in mice and other rodents, doesn’t need intermediate host § the scolex bears a short rostellum with one row of hooks, along with four suckers § proglottids 2 mm wide and 1 mm long § Sac-like gravid uterus is usually full of eggs § Egg: round to oval thin-shelled, 30 -45 micrometer, contains an inner envelope with two polar thickenings, each having four to eight filaments, which extend into the space within the shell

Hymenolopsis nana § Transmission: Infection is usually transmitted by ingestion of infective eggs. Although most cases are asymptomatic, mild gastrointestinal symptoms, such as diarrhea, abdominal pain, and weight may occur

Hymenolopsis nana § Laboratory Diagnosis: recovery of characteristic eggs in human feces. Adult worms and proglottids are rarely seen in the stool

Hymenolopsis nana § Treatment and Prevention: Praziquantel is the treatment of choice for infection with H. nana. Niclosamide is also effective. Good sanitary practices are essential in the prevention of infection


Echinococcus granulosus § Morphology: hydatid tapeworm, 4 mm in length, scolex bearing four suckers, numerous hooks, and three proglottids § Eggs: although not found, but it is identical to those of Taenia species

Echinococcus granulosus § Transmission and Pathogenesis Ingestion of eggs, usually by hand-to-mouth contact with infected dog feces. Symptoms vary, depending on the location of the cyst in tissue. Although cysts may form in many areas of the body, the lung and the liver are most commonly affected. Pulmonary symptoms, such as cough and chest pain, may develop with lung infection. One serious complication of hydatid cyst disease is the risk of anaphylactic shock, following rupture of the cyst.

Echinococcus granulosus § Laboratory Diagnosis: The eggs are not found in human feces. The diagnosis of hydatid cyst disease may be made by radiographic and serologic studies. A combination of assays, including enzyme immunoassay and immunoblot techniques, has been suggested ti diagnose hydatid cyst disease. The demonstration of hydatid sand is also diagnostic.

Echinococcus granulosus § Treatment and prevention Although surgical removal of hydatid cyst has long been considered the treatment of choice, several antihelminthic agents are now available. These include praziquantel and mebendazole. Infection is also prevented by good personal hygiene to prevent hand-to-mouth transmission of eggs from dogs to humans, avoidance of ingestion of sheep viscera by dogs, and antihelminthic treatment of dogs, as necessary

 Wormhost chest worm locations
Wormhost chest worm locations General parasitology lecture notes
General parasitology lecture notes 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Helminthology
Helminthology Parasitology classification
Parasitology classification Definition of metazoa
Definition of metazoa Essentials of medical parasitology
Essentials of medical parasitology Quadrant streak
Quadrant streak Father of veterinary parasitology
Father of veterinary parasitology Cryptosporidium parvum
Cryptosporidium parvum Parasitology
Parasitology Hymenolepis nana
Hymenolepis nana Parasitology
Parasitology Taenia saginata egg
Taenia saginata egg Toxoplaxmosis
Toxoplaxmosis Bilhariziasis
Bilhariziasis Parasitology
Parasitology Medical
Medical Emaplex
Emaplex Formal ether concentration technique
Formal ether concentration technique Project procurement management lecture notes
Project procurement management lecture notes Theology proper lecture notes
Theology proper lecture notes Advantages of government accounting
Advantages of government accounting 4 p's of management spectrum
4 p's of management spectrum Magnetism
Magnetism Classical mechanics
Classical mechanics Physical science lecture notes
Physical science lecture notes Power system dynamics and stability lecture notes
Power system dynamics and stability lecture notes Microbial physiology and metabolism lecture notes
Microbial physiology and metabolism lecture notes Mechatronics notes
Mechatronics notes Limits fits and tolerances lecture notes
Limits fits and tolerances lecture notes Financial engineering notes
Financial engineering notes Bipolar junction transistor lecture notes
Bipolar junction transistor lecture notes Software engineering lecture notes
Software engineering lecture notes Introduction to ofdm
Introduction to ofdm Land use planning lecture notes
Land use planning lecture notes Project management lecture notes doc
Project management lecture notes doc Lecture notes on homiletics
Lecture notes on homiletics Foundation engineering lecture notes
Foundation engineering lecture notes Image processing lecture notes
Image processing lecture notes Intermediate microeconomics lecture notes
Intermediate microeconomics lecture notes Parallel and distributed computing lecture notes
Parallel and distributed computing lecture notes Bayesian decision theory lecture notes
Bayesian decision theory lecture notes Nonlinear regression lecture notes
Nonlinear regression lecture notes Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes Fundamental deviation table
Fundamental deviation table Global stiffness matrix
Global stiffness matrix Reservoir routing and channel routing
Reservoir routing and channel routing Unit root test lecture notes
Unit root test lecture notes Shape memory alloys lecture notes
Shape memory alloys lecture notes Notes on research methods
Notes on research methods Financial markets and institutions - ppt
Financial markets and institutions - ppt Physics 101 lecture
Physics 101 lecture Operations management lecture notes doc
Operations management lecture notes doc Nlp lecture notes
Nlp lecture notes Linux lecture notes
Linux lecture notes Introduction to biochemistry lecture notes
Introduction to biochemistry lecture notes Stern-gerlach experiment lecture notes
Stern-gerlach experiment lecture notes Land use planning lecture notes
Land use planning lecture notes Exploratory data analysis lecture notes
Exploratory data analysis lecture notes Slidetodoc. com
Slidetodoc. com Elements of interior design ppt
Elements of interior design ppt Bayesian classification in data mining lecture notes
Bayesian classification in data mining lecture notes Data mining lecture notes
Data mining lecture notes Computer architecture notes
Computer architecture notes Franck-condon principle lecture notes
Franck-condon principle lecture notes Biopotential electrodes lecture notes
Biopotential electrodes lecture notes Catalysis lecture notes
Catalysis lecture notes Sargur srihari
Sargur srihari Gujarati basic econometrics lecture notes ppt
Gujarati basic econometrics lecture notes ppt Anderson localization lecture notes
Anderson localization lecture notes Operating system lecture notes
Operating system lecture notes Microwave remote sensing lecture notes
Microwave remote sensing lecture notes Lecture note tiu
Lecture note tiu Natural language processing lecture notes
Natural language processing lecture notes Sensitivity analysis lecture notes
Sensitivity analysis lecture notes Embryotomy ppt
Embryotomy ppt Translation lecture notes
Translation lecture notes Optical amplifiers lecture notes
Optical amplifiers lecture notes Land use planning '' lecture notes
Land use planning '' lecture notes Environmental sociology lecture notes
Environmental sociology lecture notes Factor analysis lecture notes
Factor analysis lecture notes Physics 101 lecture notes pdf
Physics 101 lecture notes pdf Lecture notes tiu
Lecture notes tiu Er diagram ppt
Er diagram ppt Analysis of algorithms lecture notes
Analysis of algorithms lecture notes Project cost management lecture notes
Project cost management lecture notes Magnetically coupled circuits lecture notes
Magnetically coupled circuits lecture notes Atomic emission spectroscopy lecture notes
Atomic emission spectroscopy lecture notes Contemporary management practice
Contemporary management practice Engineering ethics lecture notes
Engineering ethics lecture notes Nlp lecture notes
Nlp lecture notes Power system analysis lecture notes
Power system analysis lecture notes Ethem alpaydin
Ethem alpaydin Machine learning lecture notes
Machine learning lecture notes Flame hardening mild steel
Flame hardening mild steel Computer aided drug design lecture notes
Computer aided drug design lecture notes Introduction to algorithms lecture notes
Introduction to algorithms lecture notes Power semiconductor devices lecture notes
Power semiconductor devices lecture notes Be8255 ppt
Be8255 ppt Chemistry of carbohydrates notes
Chemistry of carbohydrates notes Pdf
Pdf Health management information system lecture notes
Health management information system lecture notes Natural language processing lecture notes
Natural language processing lecture notes Switch mode power supply lecture notes
Switch mode power supply lecture notes Ic fabrication technology
Ic fabrication technology Spalart-allmaras model
Spalart-allmaras model Atmospheric physics lecture notes
Atmospheric physics lecture notes Lightning elves
Lightning elves Natural language processing lecture notes
Natural language processing lecture notes Natural language processing lecture notes
Natural language processing lecture notes Matlab lecture notes
Matlab lecture notes Micropaleontology definition
Micropaleontology definition Cmos vlsi design lecture notes
Cmos vlsi design lecture notes Health economics lecture notes
Health economics lecture notes Power system dynamics and stability lecture notes
Power system dynamics and stability lecture notes Data mining lecture notes
Data mining lecture notes Data mining lecture notes
Data mining lecture notes Project planning and management lecture notes ppt
Project planning and management lecture notes ppt Network management principles
Network management principles Medical genetics lecture
Medical genetics lecture Medical ethics lecture
Medical ethics lecture Medical statistics lecture
Medical statistics lecture Definition of health psychology
Definition of health psychology Dept nmr spectroscopy
Dept nmr spectroscopy Department of agriculture consumer services
Department of agriculture consumer services Finance departments
Finance departments Inspectors in worcester
Inspectors in worcester Dept. name of organization (of affiliation)
Dept. name of organization (of affiliation) Mn dept of education
Mn dept of education Dept of finance and administration
Dept of finance and administration Dept. name of organization
Dept. name of organization Ohio dept of dd
Ohio dept of dd Affiliation poster presentation
Affiliation poster presentation Vaginal dept
Vaginal dept Gome dept
Gome dept Gome dept
Gome dept Horizontal
Horizontal Gome dept
Gome dept Hoe dept
Hoe dept Battalion chief interview questions
Battalion chief interview questions Oviposition
Oviposition Dept of education
Dept of education Florida dept of agriculture and consumer services
Florida dept of agriculture and consumer services Florida dept of agriculture and consumer services
Florida dept of agriculture and consumer services Dept a
Dept a Central islip fire department
Central islip fire department Rowan county medicaid
Rowan county medicaid Dept of education
Dept of education Tabella nmr
Tabella nmr Pt dept logistik
Pt dept logistik Nys department of homeland security
Nys department of homeland security La dept of revenue
La dept of revenue La revenue dept
La revenue dept Rewley house continuing education library
Rewley house continuing education library Nebraska dept of agriculture
Nebraska dept of agriculture Iit
Iit