Florida Dept of Agriculture and Consumer Services Division













- Slides: 15

Florida Dept. of Agriculture and Consumer Services Division of Food Safety Labeling Guidance for Hemp Food Establishments

Hemp Resources • Section 581. 217, F. S. - State Hemp Program • Rule 5 K-4. 034, F. A. C - Hemp Extract in Food • https: //www. fdacs. gov/Cannabis. Hemp/Hemp-CBD-in-Florida • Hemp Food Establishment Guide

Definitions • “Hemp extract” means a substance or compound intended for ingestion, containing more than trace amounts of cannabinoid, or for inhalation which that is derived from or contains hemp and which that does not contain other controlled substances. ” • The term does not include synthetic CBD or seeds or seedderived ingredients that are generally recognized as safe by the United States Food and Drug Administration. • “Ingestion” means the process of taking Food into the body through the mouth and into the gastrointestinal tract through eating or drinking. • Florida Law now includes such products intended for inhalation and the Department is currently engaged in the rulemaking process.

Human Ingestion • The Division of Food Safety only regulates food products containing hemp extract if these products are intended for human ingestion. • The Division does not currently regulate pet food/treats, topical body care, tobacco, vaping, or alcohol related products containing hemp extract. • Pet food/treats marketers or growers should speak to Divisions of Agricultural Environmental Services or Plant Industry, respectively. • Sellers of “smokables” or “topicals” or hemp extract in restaurants should speak to Department of Business and Professional Regulation (DBPR).

Labeling Requirements for Prepackaged Food products q. Statement of Identity / Food product name and description q. Product weight / measurement in both metric and US measurement terms q. Manufacturer’s - or packer or distributor - details (name, address, contact) q. List of ingredients (including sub-ingredients) in order from most to least q. Allergen or prescribed warnings q. Nutrition Facts Panel or Supplements Facts Panel, unless exempt

Food Labeling The goal is truthful and factual information to inform consumers Refer to 21 CFR 101 for additional guidance (pertains to all food products). Food products containing hemp extract as defined in Florida Law must also comply with labeling requirements in Section 581. 217, F. S. Applies to foods produced in the U. S. and all imports All required information on the label must be legible and in English

Labeling for Food Products Containing Hemp Extract Food products containing Hemp Extract must be distributed and sold in a container with: 1. A scannable barcode or quick response code linked to the certificate of analysis of the Hemp Extract batch by an independent testing laboratory; 2. The batch number; 3. The internet address of a website where batch information may be obtained; 4. The expiration date; and 5. The number of milligrams of each marketed cannabinoid per serving. If cannabinoids are marketed, the milligrams per serving of the specific cannabinoid must be listed and the serving size shall be declared on the label.

Quick Response (QR) Code • The QR code on the label must either link directly to the Certificate of Analysis (COA) or to a website where the batch number may be entered to look up the COA. • The batch number on the COA must match the batch number on the product label.

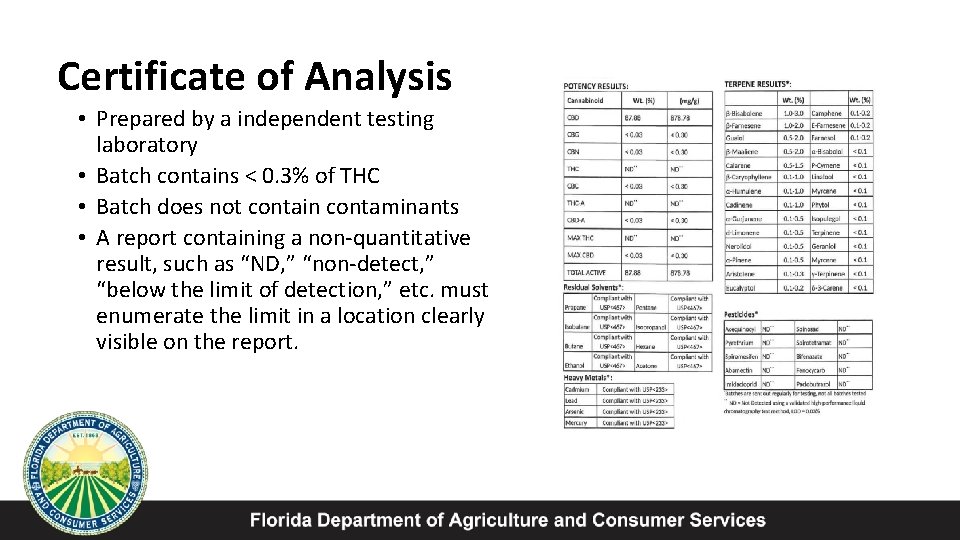
Certificate of Analysis • Prepared by a independent testing laboratory • Batch contains < 0. 3% of THC • Batch does not contain contaminants • A report containing a non-quantitative result, such as “ND, ” “non-detect, ” “below the limit of detection, ” etc. must enumerate the limit in a location clearly visible on the report.

Expiration Date • “Expiration Date” means month and year as determined by the manufacturer, packer, or distributor on the basis of tests or other information showing that the product, until that date, under the conditions of handling, storage, preparation, and use per label directions, will when consumed, contain not less than the quantity of each ingredient as set forth on its label. • The cannabinoid potency advertised on the label must be maintained throughout the shelf-life of the product, as determined by the expiration date on the label.

If cannabinoids are marketed on the label, the milligrams per serving of the cannabinoids must be listed and the serving size must be displayed on the label of the product • If the label only advertises generically “Hemp, ” “Hemp Oil, ” or “Hemp Extract, ” the amount of cannabinoid(s) per serving is not required to be stated. • If the label advertises a specific cannabinoid (most commonly CBD), the amount of the cannabinoid per serving and the serving size must be listed. • The serving size must be declared in a volumetric measurement. Statements like “one dropper, ” “one spoonful, ” ”one bite, ” etc. are not permitted for any food products.

Health Claims • The label may not contain claims indicating the product is intended for diagnosis, cure, mitigation, treatment, or prevention of disease rendering it a drug as defined in 21 U. S. C. 321(g)(1). • Unapproved health claims on the label are not acceptable and are considered “misbranding. ” • However, claims such as “helps, ” “supports, ” or “promotes” in relation to maintaining normal body functions are acceptable and allowed to be on the labeling.

Supplements • Hemp extract products intended for human ingestion that are labeled as supplements are regulated by the Division of Food Safety and must adhere to all supplement and hemp extract requirements, including hemp labeling requirements.

Hemp Labeling • The Division of Food Safety does not have labeling approval authority; however, labels will be reviewed for compliance during inspections. It is the responsibility of the Hemp Food Establishment to meet the requirements and properly develop product labels containing the necessary information and in accordance with 21 CFR Part 101 and Section 581. 217, F. S. • Food Establishments may utilize the Hemp Food Establishment Guide which contains amongst other things the labeling requirements pursuant to Section 581. 217, F. S.

Questions?
 Florida dept of agriculture and consumer services
Florida dept of agriculture and consumer services Florida dept of agriculture and consumer services
Florida dept of agriculture and consumer services Department of agriculture consumer services
Department of agriculture consumer services Interpret a food chain or food web
Interpret a food chain or food web Virginia department of agriculture and consumer services
Virginia department of agriculture and consumer services Oviposition
Oviposition Nebraska dept of agriculture
Nebraska dept of agriculture Ministry of agriculture veterinary services division
Ministry of agriculture veterinary services division Micah ennis
Micah ennis Albany county dept of social services
Albany county dept of social services Black box model consumer behaviour
Black box model consumer behaviour Consumer markets and consumer buyer behavior
Consumer markets and consumer buyer behavior Department of hotels and restaurants
Department of hotels and restaurants Greenfly carnivore
Greenfly carnivore Cengage
Cengage Consumer behaviour research process
Consumer behaviour research process



























