Do Now Sit SILENTLY and answer the following





















































































- Slides: 124

Do Now • Sit SILENTLY and answer the following in your notes. You do not have to copy the questions, though I am confident some of you will still ask…le sigh • What are the 2 atoms that make up a water molecule? • Balance the following… – ___H 2 + ____O 2 ____H 2 O • If water changes from a solid to a liquid to a gas then it is undergoing what kind of change? How do you know? • What are some ways that we as humans pollute water systems?

What makes water so special?

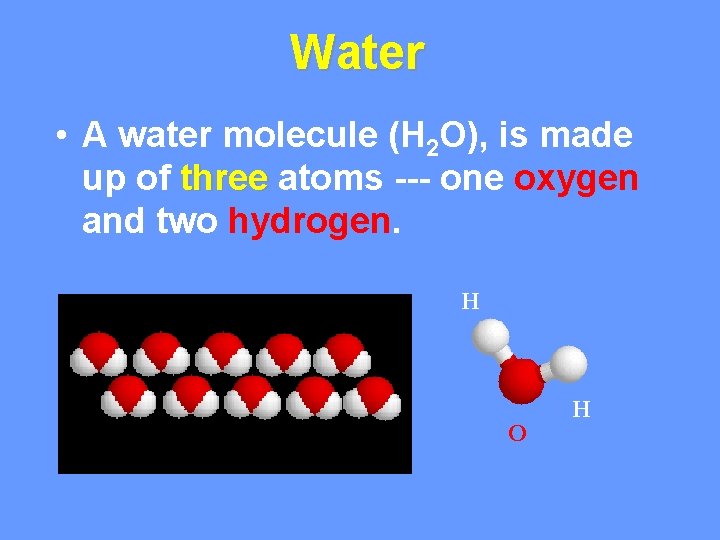
Water • A water molecule (H 2 O), is made up of three atoms --- one oxygen and two hydrogen. H O H

Special Properties • Water is the only substance on Earth that occurs naturally as a solid, a liquid, and a gas. • It is often referred to as ‘the universal solvent’ because so many other substances dissolve in it. • This characteristic is one reason that the water encountered on Earth is rarely pure.

So…what makes water so special?

Water is Polar!!!! • The oxygen end “acts” negative • The hydrogen end “acts” positive • Causes the water to be POLAR, like a magnet.

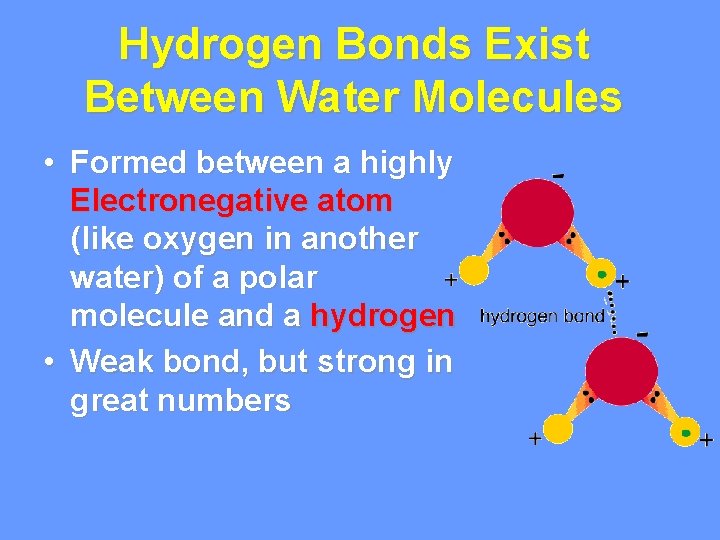
Hydrogen Bonds Exist Between Water Molecules • Formed between a highly Electronegative atom (like oxygen in another water) of a polar molecule and a hydrogen • Weak bond, but strong in great numbers

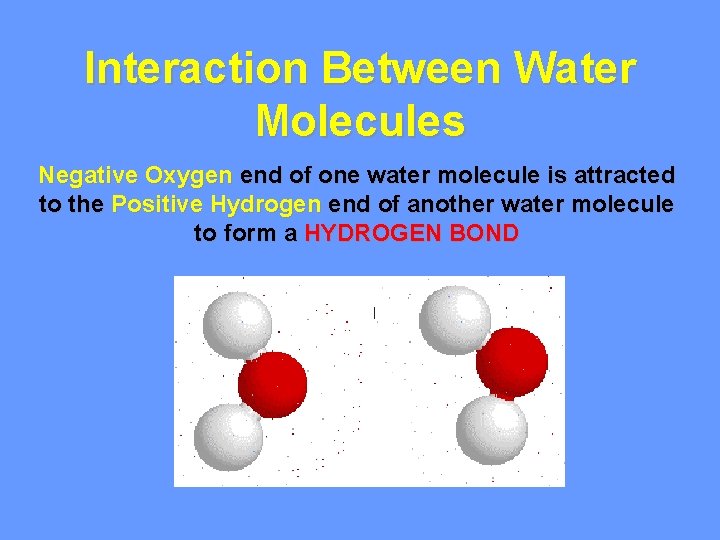
Interaction Between Water Molecules Negative Oxygen end of one water molecule is attracted to the Positive Hydrogen end of another water molecule to form a HYDROGEN BOND

What are the Properties of Water?

Properties of Water • Cohesion

Properties of Water • Cohesion • Adhesion

Properties of Water • Cohesion • Adhesion • High Specific Heat

Properties of Water • Cohesion • Adhesion • High Specific Heat • High Heat of Vaporization

Properties of Water • Cohesion • Adhesion • High Specific Heat • High Heat of Vaporization • Less Dense as a Solid

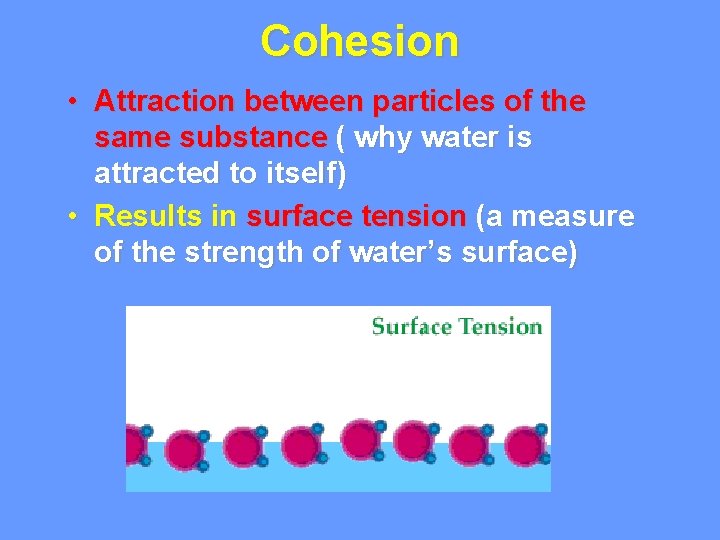
Cohesion • Attraction between particles of the same substance ( why water is attracted to itself) • Results in surface tension (a measure of the strength of water’s surface)

Cohesion … Helps insects walk across water, but how is it helpful to people?

Adhesion • Attraction between two different substances. • Water will make hydrogen bonds with other surfaces such as glass, soil, plant tissues, and cotton. • Capillary action-water molecules will “tow” each other along when in a thin glass tube.

Adhesion Causes Capillary Action Which gives water the ability to “climb” structures

High Specific Heat • Amount of heat needed to raise or lower 1 g of a substance 1° C. • Water resists temperature change, both for heating and cooling. – Would you rather walk on sand or in water on a beach on a hot summer day? – Which would be warmer that night? Why?

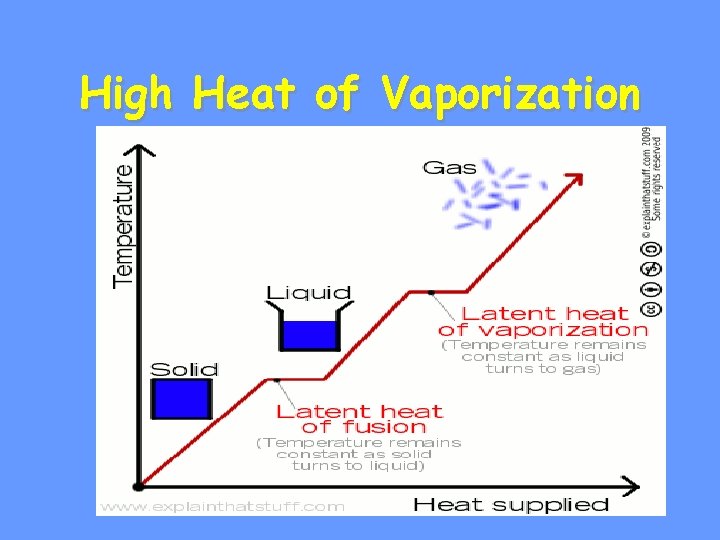
High Heat of Vaporization

• Water vapor forms a kind of global ‘‘blanket” which helps to keep the Earth warm. • Heat radiated from the sun warmed surface of the earth is absorbed and held by the vapor

Water is Less Dense as a Solid • Which is ice and which is water?

Exit Ticket 1. Draw a water molecule with labeled atoms and charges. 2. What is the property called that describes water sticking to itself? 3. Is ice les dense or more dense than liquid water? How do you know?

Do Now • Why is water’s property of high heat of vaporization important to the earth and more specifically to us? • What 2 properties of water are important for plants specifically and why? • Explain why solid water (ice) is able to float on liquid water.

Water Distribution • Water covers approximately 71% of the Earth’s surface (USGS). Most of this water (97%) is not drinkable because it is saltwater.

Water Distribution • The majority of freshwater (3%) exists in ice caps, glaciers, and oceans. • 77% of the freshwater is frozen. Of the 23% that is not frozen, approximately a half of a percent is available to supply living organisms with what they need to survive. • The availability of water varies with local geography and allows humans to utilize water as a resource.

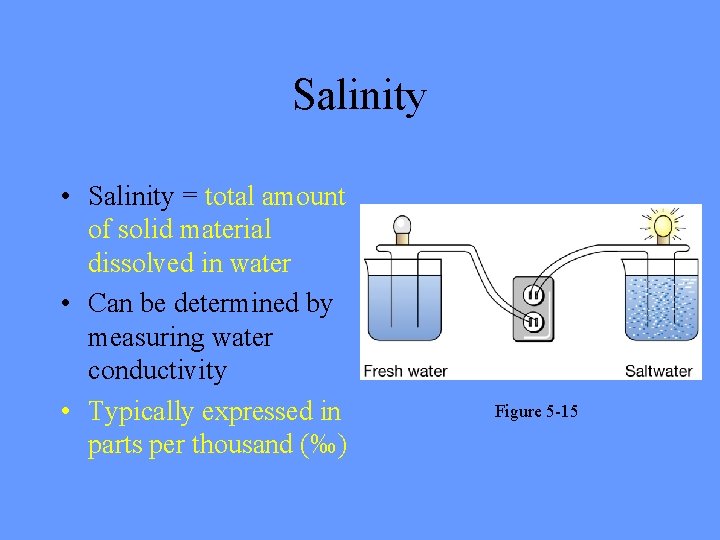
Salinity • Salinity = total amount of solid material dissolved in water • Can be determined by measuring water conductivity • Typically expressed in parts per thousand (‰) Figure 5 -15

So why is the Ocean so Salty? ! • Dissolved chemicals eroded from the Earth's crust and washed into the sea. • Solid and gaseous ejections from volcanoes, suspended particles swept to the ocean from the land by onshore winds, and materials dissolved from sediments deposited on the ocean floor have also contributed.

Salinity Cont. • Salinity in ocean waters is increased by evaporation or by freezing of sea ice and it is decreased as a result of rainfall, runoff, or the melting of ice. The average salinity of seawater is 35 parts per thousand(3. 5%). Salinities are much less than average in coastal waters, in the polar seas, and near the mouths of large rivers. • In recent years, salinity has been changing across the world’s oceans. Why do you think that is?

Processes affecting seawater salinity • Processes that decrease seawater salinity: – Precipitation – Runoff – Icebergs melting – Sea ice melting • Processes that increase seawater salinity: – Sea ice forming – Evaporation – Hydrothermal vents

What are Hydrothermal Vents?

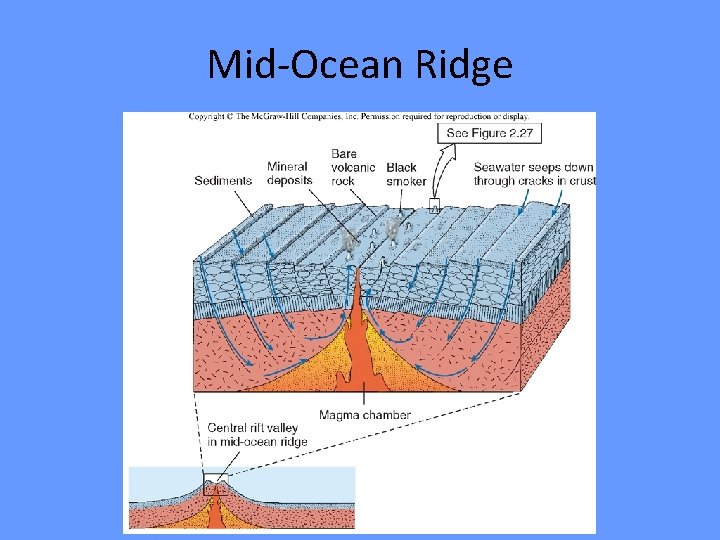
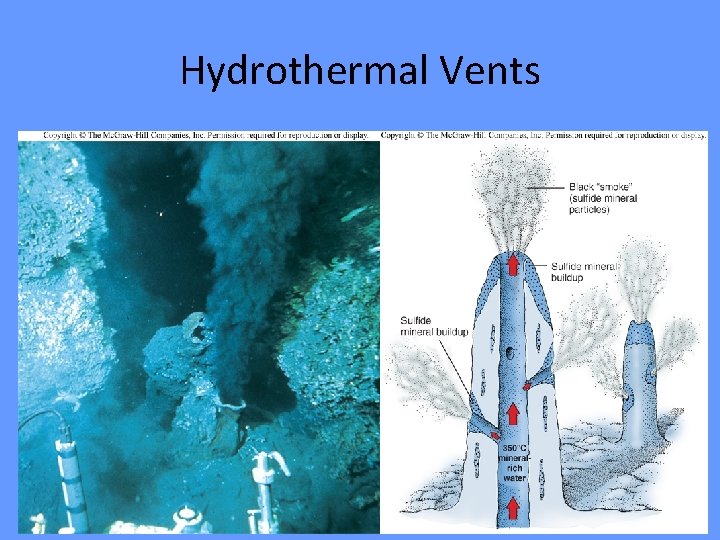
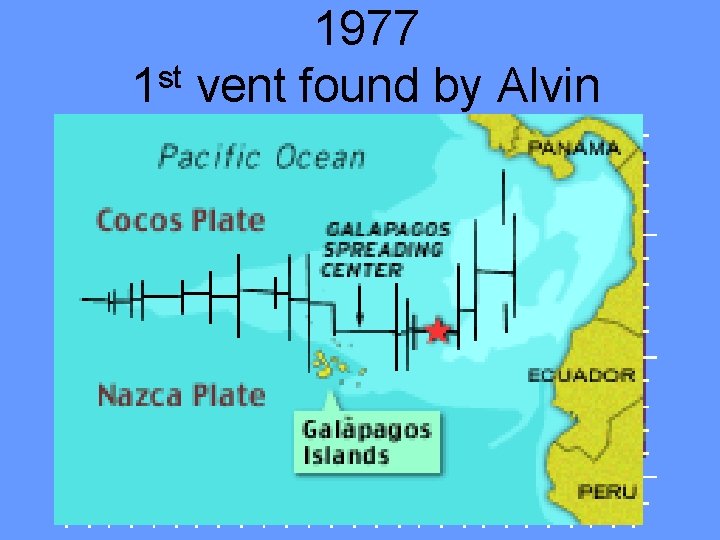
Hydrothermal Vent • Hydrothermal vent- a deep-sea hot spring where heated seawater forces its way up through the crust. • Discovered rich communities in 1977. • Temperatures range from 10 -20⁰C (50 -68⁰F) to 350⁰C (660⁰F). • Mineral particles such as sulfides and carbonates precipitate to form chimneys. • Chemosynthetic microbes form basis of food chain

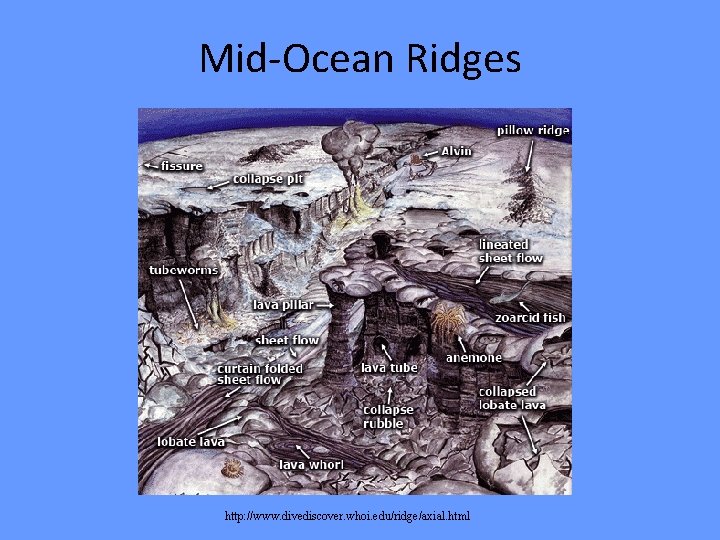
Mid-Ocean Ridge

Hydrothermal Vents

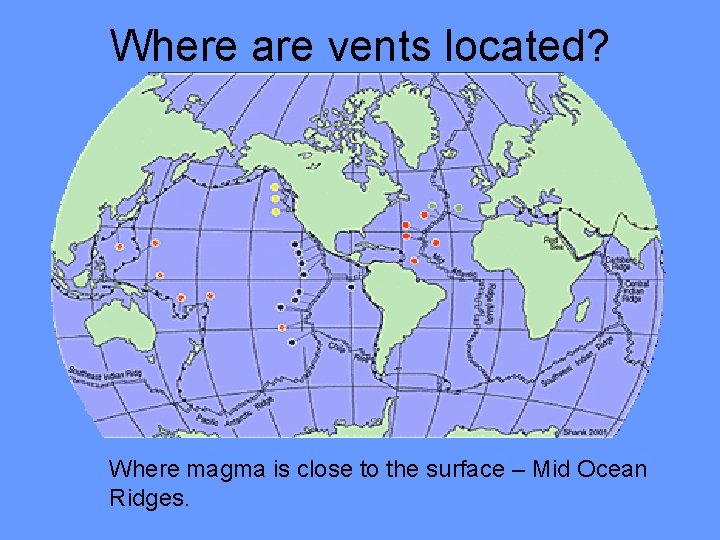
Where are vents located? Where magma is close to the surface – Mid Ocean Ridges.

Mid-Ocean Ridges http: //www. divediscover. whoi. edu/ridge/axial. html

1977 1 st vent found by Alvin

Alvin?

Alvin http: //www. youtube. com/watch? v=Xot. F 9 fzo 4 Vo

Submersibles, ROVs, & AUVs

How are vents created? STEP 1 • Cold water (2 o. C) seeps through cracks and is heated up (up to 400 o. C) http: //www. divediscover. whoi. edu/vents/vent-infomod. html

STEP 2 • Water heated to 350 -400 o. C – high temps. facilitate leaching of minerals from rock. • Oxygen is removed chemically • Picks up dissolved metals (Fe, Cu, Zn). • H 20 picks up Hydrogen sulfide.

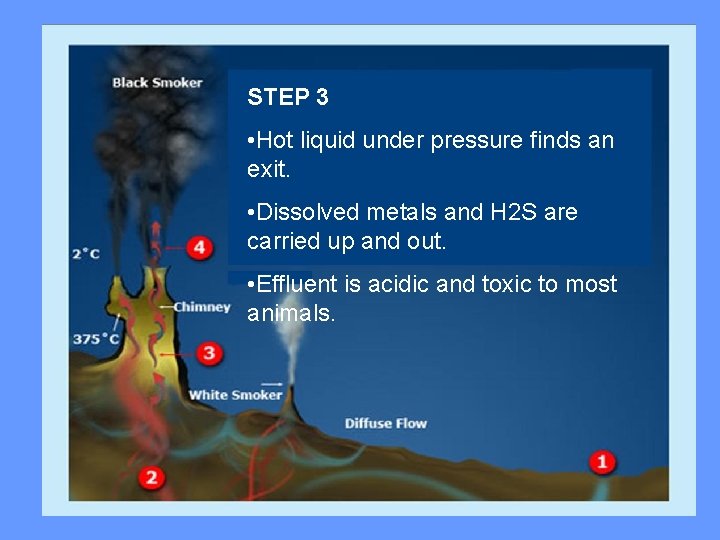
STEP 3 • Hot liquid under pressure finds an exit. • Dissolved metals and H 2 S are carried up and out. • Effluent is acidic and toxic to most animals.

White and Black Smokers

Black Smoker Hottest of all vents

monosulfide. This compound gives the smoker its black color. They spew mostly iron and sulfide, which combine to form iron monosulfide. This compound gives the smoker its black color.


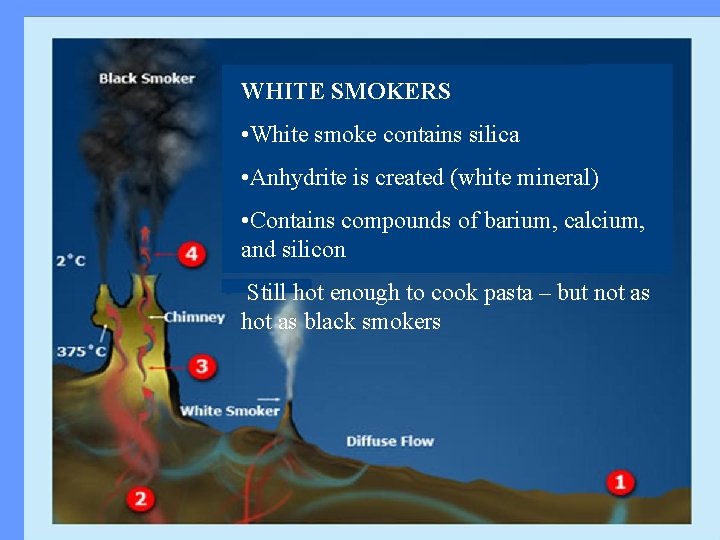
WHITE SMOKERS • White smoke contains silica • Anhydrite is created (white mineral) • Contains compounds of barium, calcium, and silicon Still hot enough to cook pasta – but not as hot as black smokers



Vent crabs

Zooarcid Fish (Eelpout)

Giant Vent Mussel

Lepetodrilus- limpets

Pompeii Worm

Giant Hydrothermal Vent Clam

Anemones

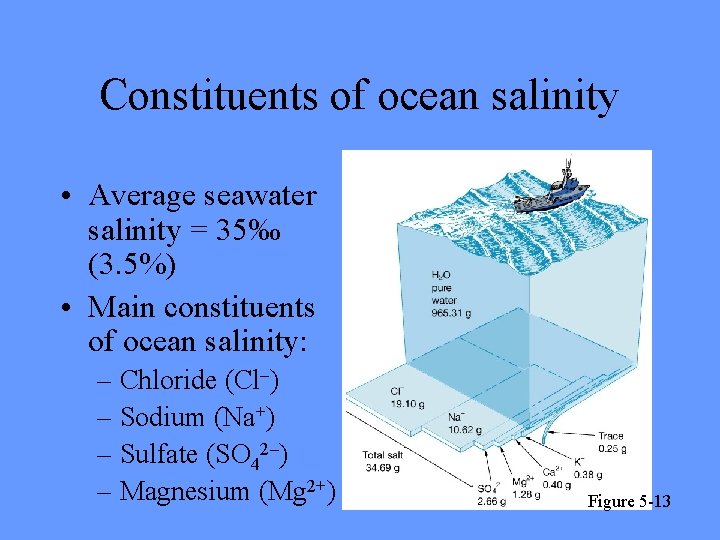
Constituents of ocean salinity • Average seawater salinity = 35‰ (3. 5%) • Main constituents of ocean salinity: – Chloride (Cl–) – Sodium (Na+) – Sulfate (SO 42–) – Magnesium (Mg 2+) Figure 5 -13

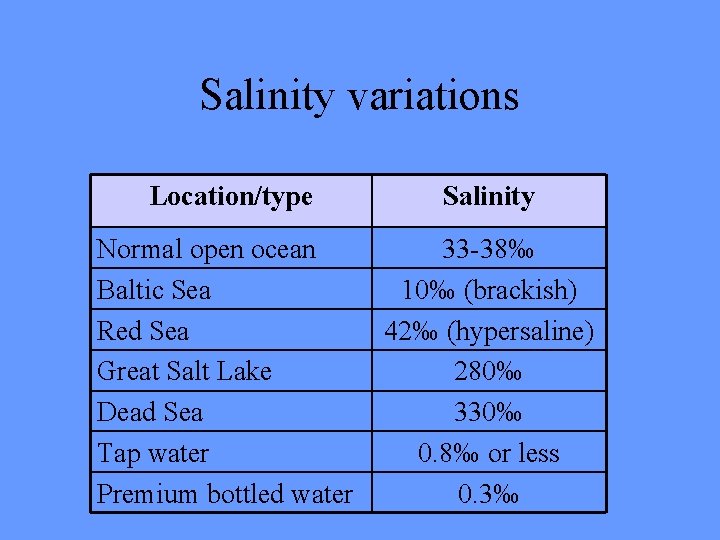
Salinity variations Location/type Salinity Normal open ocean Baltic Sea Red Sea Great Salt Lake Dead Sea Tap water Premium bottled water 33 -38‰ 10‰ (brackish) 42‰ (hypersaline) 280‰ 330‰ 0. 8‰ or less 0. 3‰

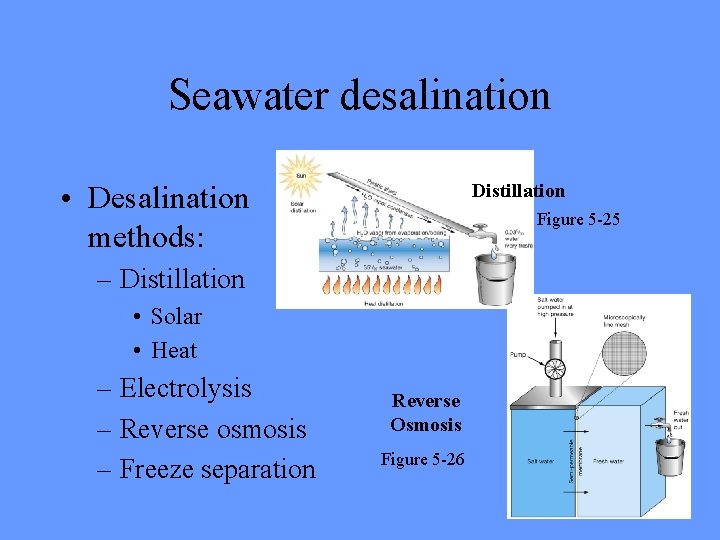
Seawater desalination Distillation • Desalination methods: Figure 5 -25 – Distillation • Solar • Heat – Electrolysis – Reverse osmosis – Freeze separation Reverse Osmosis Figure 5 -26

But why do we really care about salinity anyway? • The ocean currents are affected by salinity and ocean currents regulate climate around the world. • The earths oceans and seas are also the biggest recyclers of gasses in the atmosphere. • Finally the oceans store heat that allows us to have the seasons.

What are Currents? • Currents are the slow movement of sea water by different factors – Surface currents are caused by wind and temperature variations – Deeper currents are caused by changes in density (salinity) and temperature – http: //www. youtube. com/watch? v=qe. Zg. Jzt 3 m 0 4

Heat Transport by Currents • Surface currents play significant roles in transport heat energy from equatorial waters towards the poles • May serve as “heat sources” to cooler overlying air, “heat sinks” from warmer • Evaporation and condensation participate in latent heat exchanges

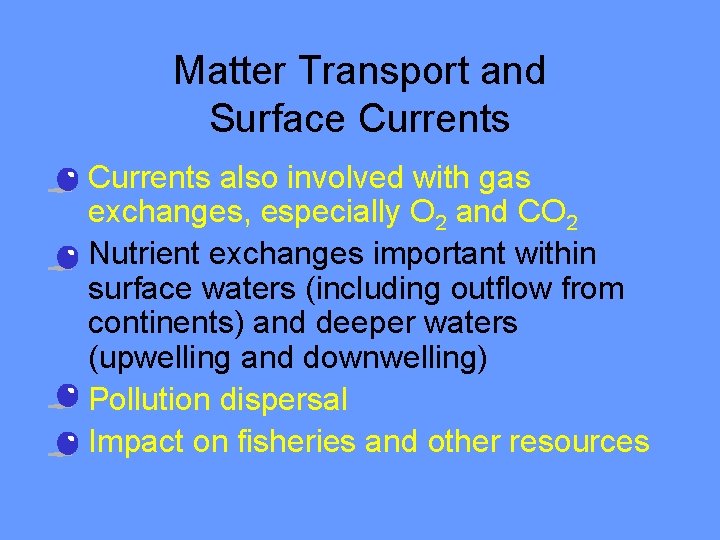
Matter Transport and Surface Currents • Currents also involved with gas exchanges, especially O 2 and CO 2 • Nutrient exchanges important within surface waters (including outflow from continents) and deeper waters (upwelling and downwelling) • Pollution dispersal • Impact on fisheries and other resources

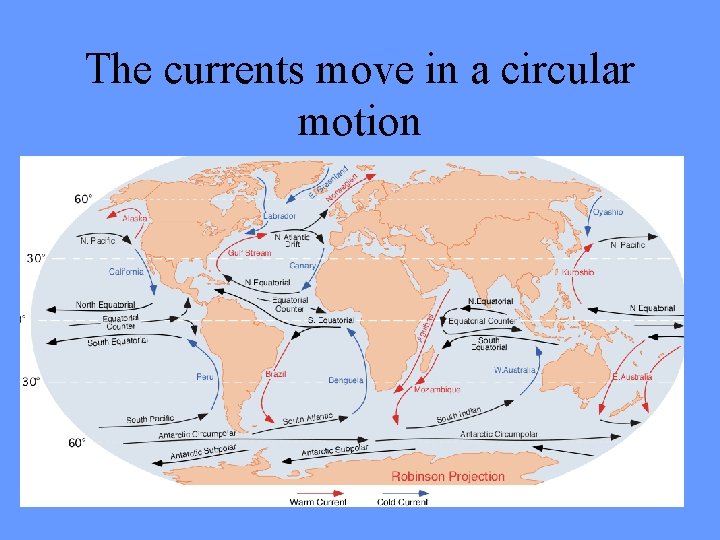
The currents move in a circular motion

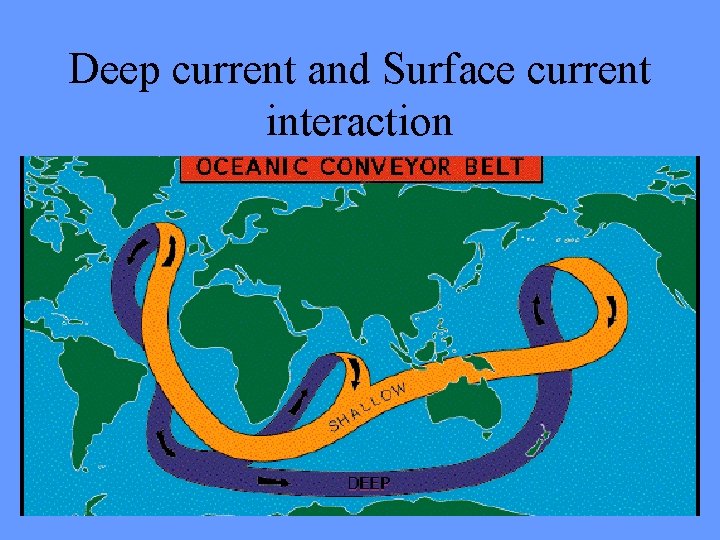
Deep current and Surface current interaction

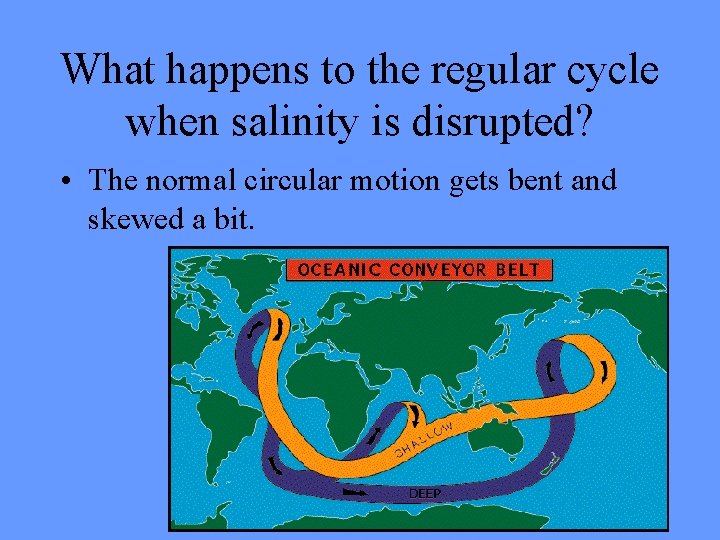
What happens to the regular cycle when salinity is disrupted? • The normal circular motion gets bent and skewed a bit.

Do Now • Sit SILENTLY and answer the following questions in your notes. • What are the 4 main properties of water? • Give a definition in your own words describing each of the properties listed in the previous question. • Explain why England has a similar climate to ours despite being so much farther north.

Current Gyres - large circular-moving loops of water Five main gyres (one in each ocean basin): • North Pacific • South Pacific • North Atlantic • South Atlantic • Indian • Generally 4 currents in each gyre • Centered about 30 o north or south latitude

North Pacific Subtropical Gyre • “Great Pacific Garbage Patch” • Estimate: 46, 000 pieces of floating garbage/mi 2.

Stop and Check • Why does trash collect in the center of gyres? • Infer why you think that the pacific gyre has a particularly large amount of garbage.

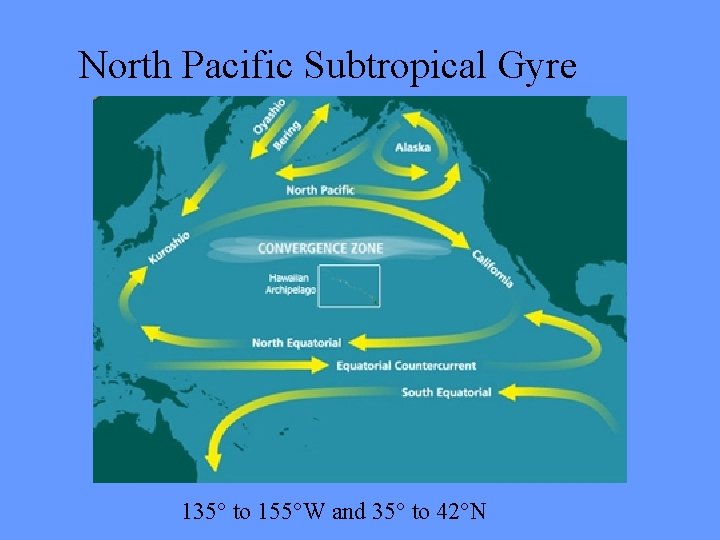
North Pacific Subtropical Gyre 135° to 155°W and 35° to 42°N

North Pacific Subtropical Gyre Great Pacific Garbage Patch- Good Morning America 2010 http: //www. youtube. com/watch? v=Co 43 TXJXry. I http: //marinedebris. noaa. gov/info/patch. html#6

Lost at Sea

Duckie Progress • January 1992 - shipwrecked in the Pacific Ocean, off the coast of China • November 1992 - half had drifted north to the Bering Sea and Alaska; the other half went south to Indonesia and Australia • 1995 to 2000 - spent five years in the Arctic ice floes, slowly working their way through the glaciers 2001 - the duckies bobbed over the place where the Titanic had sunk • 2003 - they were predicted to begin washing up onshore in New England, but only one was spotted in Maine • 2007 - a couple duckies and frogs were found on the beaches of Scotland southwest England.

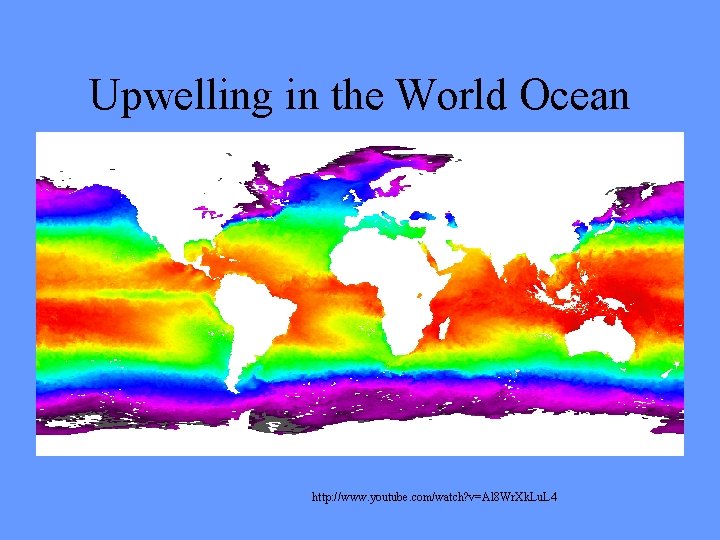
Upwelling in the World Ocean http: //www. youtube. com/watch? v=Al 8 Wr. Xk. Lu. L 4

Upwelling • Upwelling is the process that brings cold nutrient rich water to the surface. • It comes to the surface, because winds move water away forcing the water below it to move up, much like the process of convection.

Stop and check • Why does water move from the bottom to the surface?

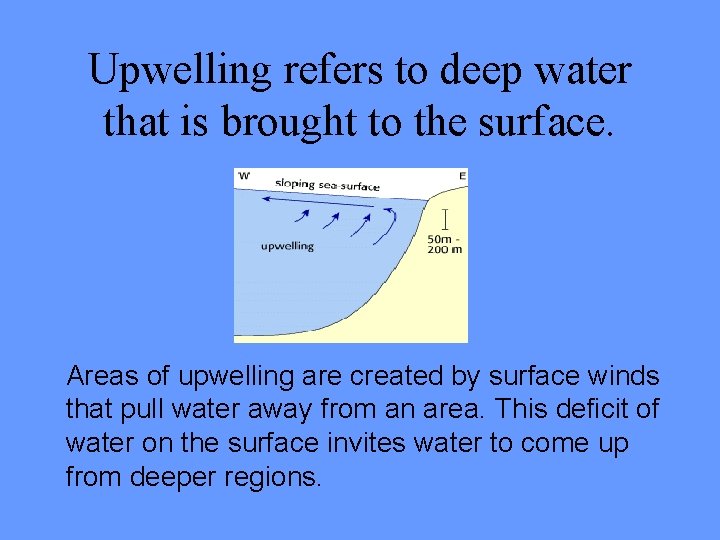
Upwelling refers to deep water that is brought to the surface. Areas of upwelling are created by surface winds that pull water away from an area. This deficit of water on the surface invites water to come up from deeper regions.

Types of Upwelling • Equatorial • Coastal • Seasonal

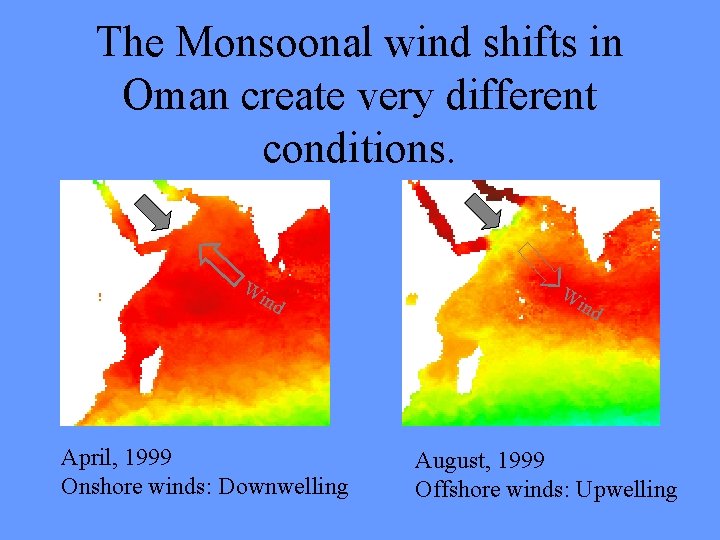
The Monsoonal wind shifts in Oman create very different conditions. Wi nd April, 1999 Onshore winds: Downwelling Wi nd August, 1999 Offshore winds: Upwelling

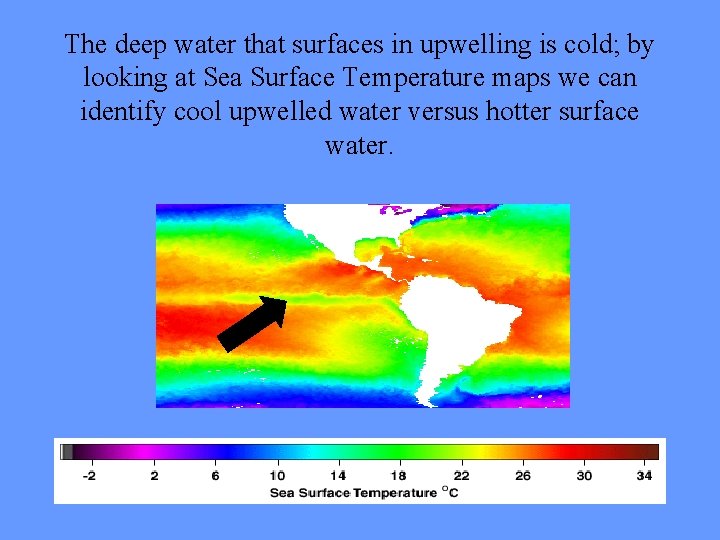
The deep water that surfaces in upwelling is cold; by looking at Sea Surface Temperature maps we can identify cool upwelled water versus hotter surface water.

http: //www. youtube. com/watch? v=H 7 s. ACT 0 Dx 0 Q Upwelled water also contains nutrients (nitrate, phosphate, silicate) and dissolved gases (oxygen and carbon dioxide) that are not utilized at depth because of a lack of sunlight. Now on the surface, these nutrients and gases help to fuel photosynthesis by small algae called phytoplankton.

Stop and check • Determine why there are more nutrients in the colder darker water than in the warm surface water. Discuss with your table partner and prepare to share.

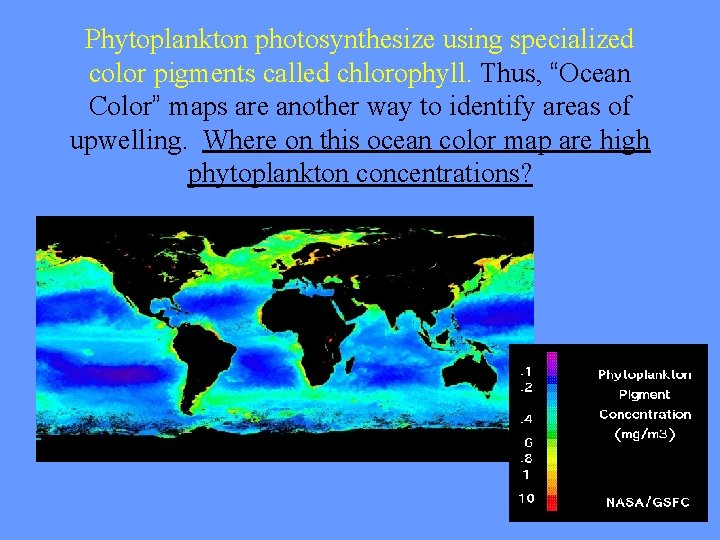
Phytoplankton photosynthesize using specialized color pigments called chlorophyll. Thus, “Ocean Color” maps are another way to identify areas of upwelling. Where on this ocean color map are high phytoplankton concentrations?

Ecological and Economic effects of upwelling: • Upwelling leads to more phytoplankton • More phytoplankton leads to more fish • More fish lead to commercial fishing jobs and to more seafood

Stop and check • Why is upwelling important to the ocean and us?

Phytoplankton come in many shapes and forms. Collectively they form the base of oceanic food webs. Without upwelling many of the world’s fisheries would not thrive.

Even though upwelling areas account for only 1% of the ocean surface, they support 50% of the worlds fisheries.

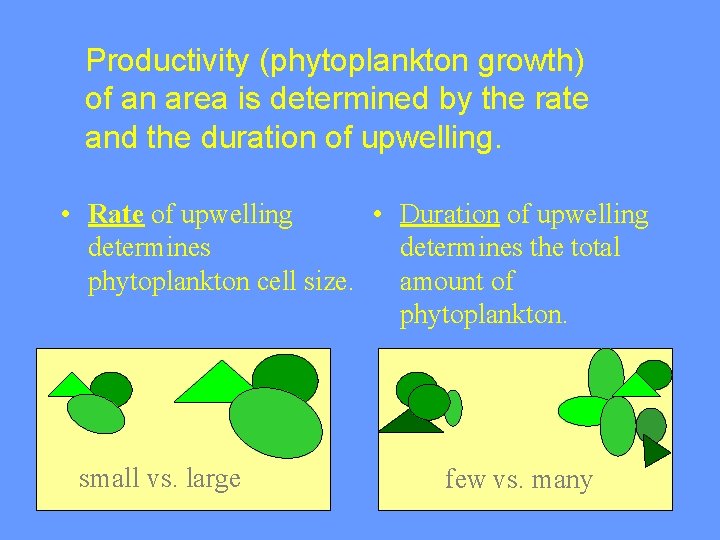
Productivity (phytoplankton growth) of an area is determined by the rate and the duration of upwelling. • Rate of upwelling • Duration of upwelling determines the total phytoplankton cell size. amount of phytoplankton. small vs. large few vs. many

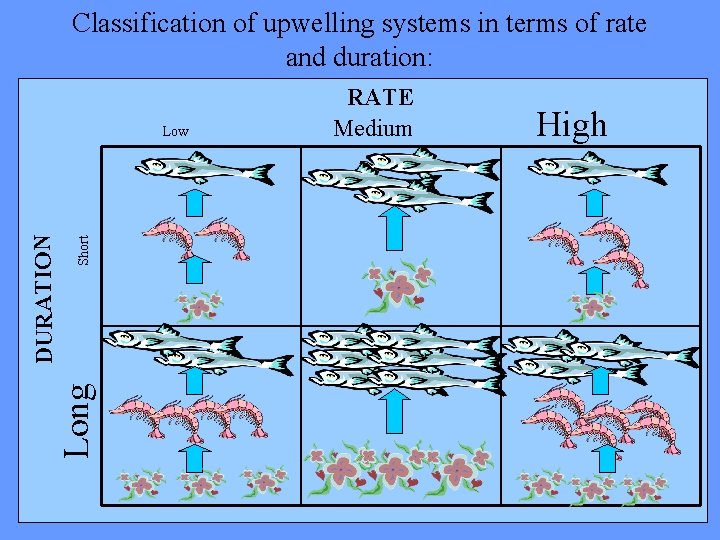
Classification of upwelling systems in terms of rate and duration: Short High Long DURATION Low RATE Medium After Thurman, H. V. (1994)

• Moderates of upwelling for long duration (8 months or longer) provide the ultimate combination for a large fishery. • With too low or too high a rate, phytoplankton are small, so there is a trophic level between the algae and the fish…. therefore the fish receive less energy.

The Water Cycle By Mr. Miller

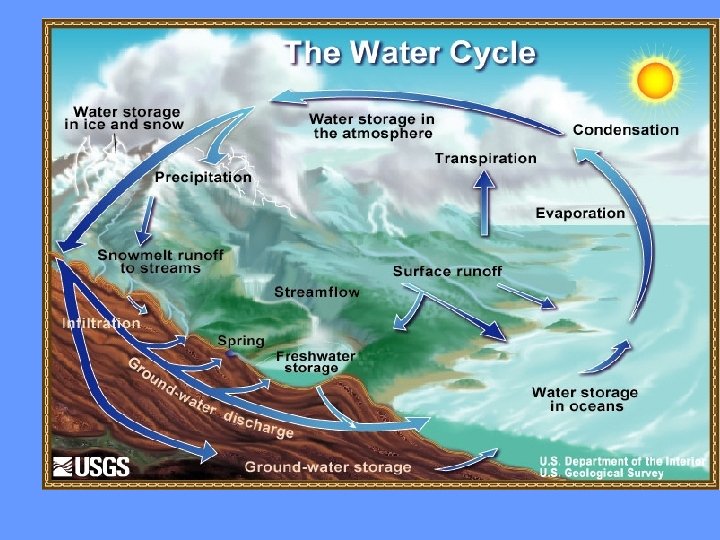
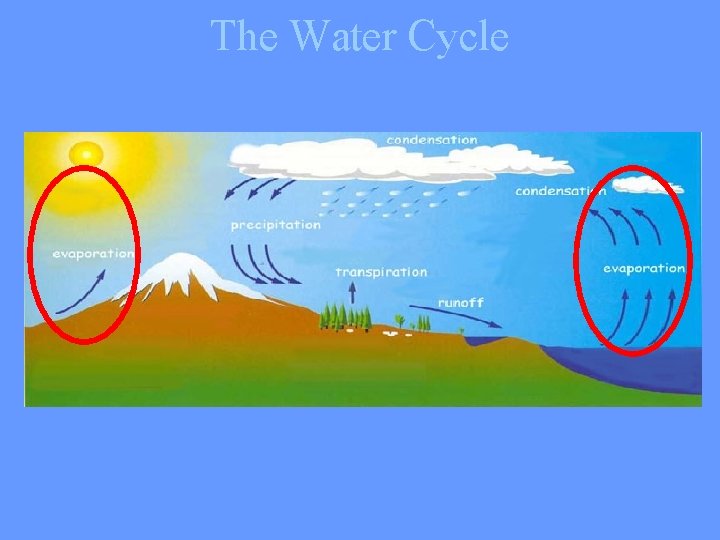
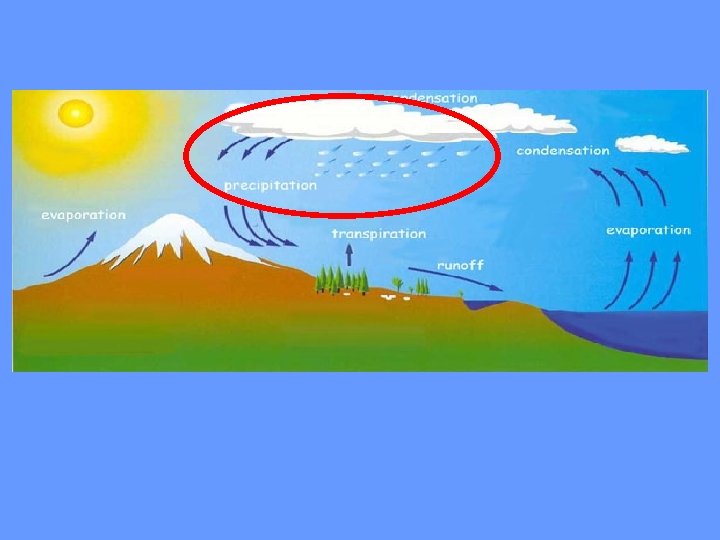
Water never leaves the Earth. It is constantly being cycled through the atmosphere, ocean, and land. This process, known as the water cycle, is driven by energy from the sun. The water cycle is crucial to the existence of life on our planet.

http: //www. youtube. com/watch? v=i 3 Ne. MVBc. XXU Consider the following… • Watch the following clip and while its playing define the following in your notes • Evaporation • Condensation • Precipitation • Transpiration • Evection • Runoff


The Water Cycle

During part of the water cycle, the sun heats up liquid water and changes it to a gas by the process of evaporation. Water that evaporates from Earth’s oceans, lakes, rivers, and moist soil rises up into the atmosphere.


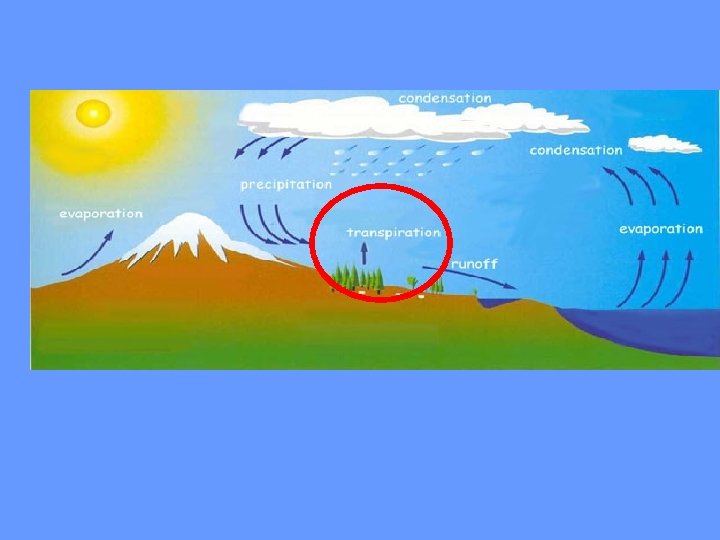
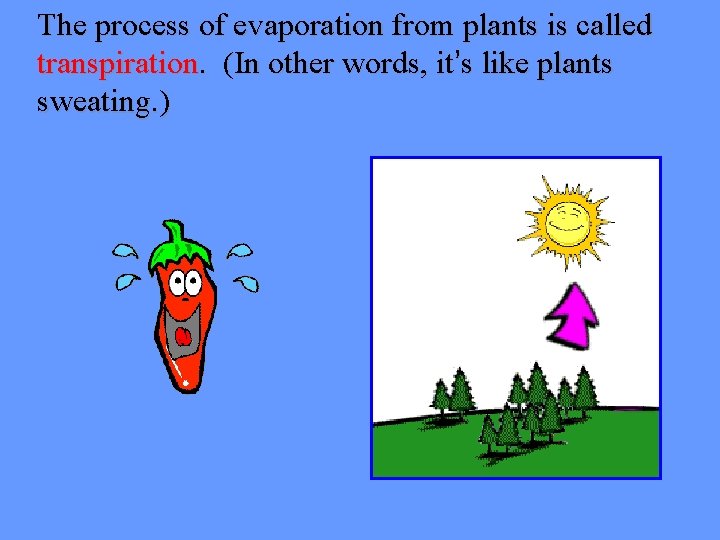
The process of evaporation from plants is called transpiration. (In other words, it’s like plants sweating. )


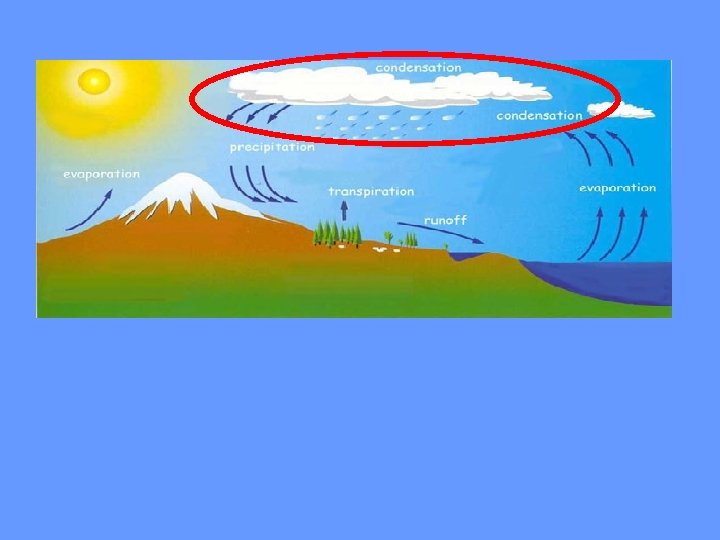
As water (in the form of gas) rises higher in the atmosphere, it starts to cool and become a liquid again. This process is called condensation. When a large amount of water vapor condenses, it results in the formation of clouds.


When the water in the clouds gets too heavy, the water falls back to the earth. This is called precipitation.


When rain falls on the land, some of the water is absorbed into the ground forming pockets of water called groundwater. Most groundwater eventually returns to the ocean. Other precipitation runs directly into streams or rivers. Water that collects in rivers, streams, and oceans is called runoff.


Eutrophication • The process by which a body of water acquires a high concentration of nutrients, especially phosphates and nitrates. • These typically promote excessive growth of algae. As the algae die and decompose, high levels of organic matter and the decomposing organisms deplete the water of available oxygen, causing the death of other organisms, such as fish. • Eutrophication is a natural, slow-aging process for a water body, but human activity greatly speeds up the process

Do Now • Sit SILENTLY and answer the following questions in your notes. • Define eutropihcation, how it is caused and whether it is good or bad for the ecosystem it is in. • What is transpiration? • List the steps of the water cycle and describe each.

What is an estuary? • Partially enclosed body of water • Salt water estuaries – Saltwater from the ocean mixes with fresh water • Fresh water estuaries – Lake water mixes with stream/river water – Freshwater the concentration of salts, or salinity, is nearly zero

Commercial Economic Benefits Fancy steamed shrimp or fried catfish for dinner? • Estuaries provide habitat for over 75% of the U. S. commercial sea catch. • Estuaries support jobs and income for many Americans each year. • Many estuaries are important centers of transportation and international commerce.

Why are estuaries Important? • Ecosystem Services – Water filtration – Habitat protection • Ecosystem services are difficult to put a value on; they are essentially priceless.

What Role do Estuaries Play in Earth’s Cycle? • Nutrient Cycle – Elements are recycled and made available to living organisms. • Water Cycle – Evaporation occurs, and also serve to recharge groundwater. • Cycle of Life – Estuaries provide shelter, food and nursery grounds for animals.

Stormwater Runoff and Water Quality

Stormwater Runoff G Definition: Rainfall that, instead of soaking into the ground, flows into water bodies picking up pollutants along the way http: //www. kytc. state. ky. us/Env. Analysis/Stormwaterquality/Gen_info_edu. htm

How does runoff become groundwater? • As long as water is able to run through permeable rock, it can be stored naturally as ground water. • How do you think humans prevent this?

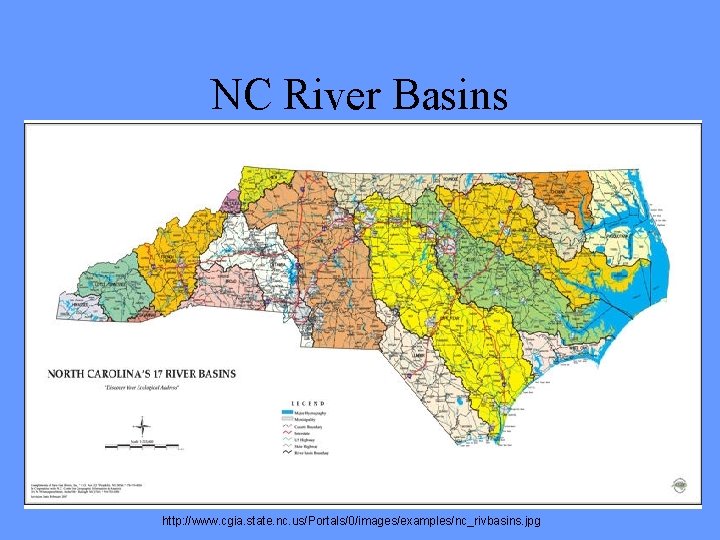
River Basin G Definition: defined area where all water within the area flows into a common water body G There are 17 different river basins in NC G You live in the Catawba River Basin, which has the largest number of people so what implications do you think there are for the environment here?

NC River Basins http: //www. cgia. state. nc. us/Portals/0/images/examples/nc_rivbasins. jpg

Pollution G As stormwater flows across streets and lawns, it picks up chemical pollutants G All pollution in a river basin accumulates in the common water body

Types of Pollution Point Source Pollution G Definition-localized source of pollution, usually industrial and coming from a pipe G Examples G Industry Nonpoint Source Pollution G Definition- general and indirect water pollution from many diffuse sources G Examples G G http: //www. liverpool. nsw. gov. au/LCC/INTERNET/me. get? site. sectionshow&PAGE 1230&BODY Agricultural Runoff Animal Waste Residential Property Runoff Parking Lot and Road Runoff

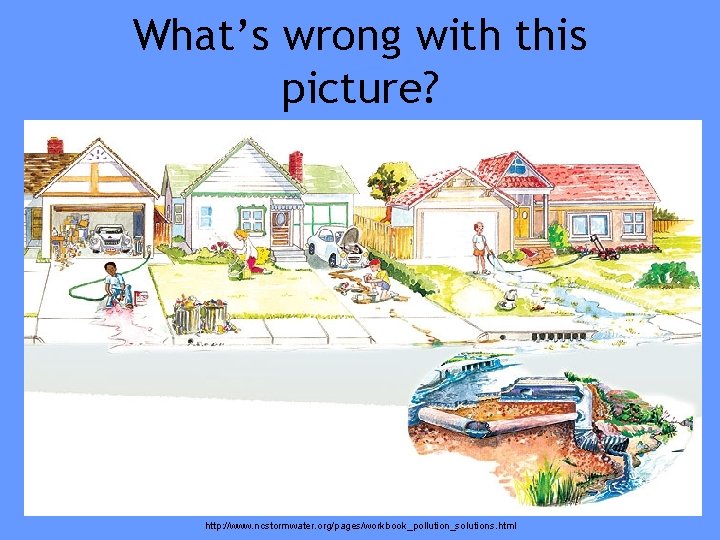
What’s wrong with this picture? http: //www. ncstormwater. org/pages/workbook_pollution_solutions. html

Visual Indicators of Water Quality G Diverse Organisms G Bugs as Indicators of Water Quality Mayflies and Caddisflies cannot tolerate water pollution, therefore their presence in a water body indicates good water quality. Mayfly larvae http: //swittersb. files. wordpress. com/2008/08/mayfly 20 nymph. jpg Caddisfly larvae http: //www-staff. lboro. ac. uk/~gymfj 2/Caddisfly. htm

Measuring Water Quality G Dissolved Oxygen G Water Temperature G p. H G Extremely high or low levels of any of these indicate poor water quality A YSI Meter is used to measure these quantities.

How do we cleanse our waters? ! G Riparian buffers G A vegetated area near a stream, usually forested which helps shade and partially protect a stream from the impact of adjacent land uses such as farming G Wetlands G Areas that stay saturated and support vegetation that helps control water and pollution from urban centers