Chapter 2 The Chemistry of Life Raw materials


























- Slides: 76

Chapter 2: The Chemistry of Life Raw materials and fuel for our bodies

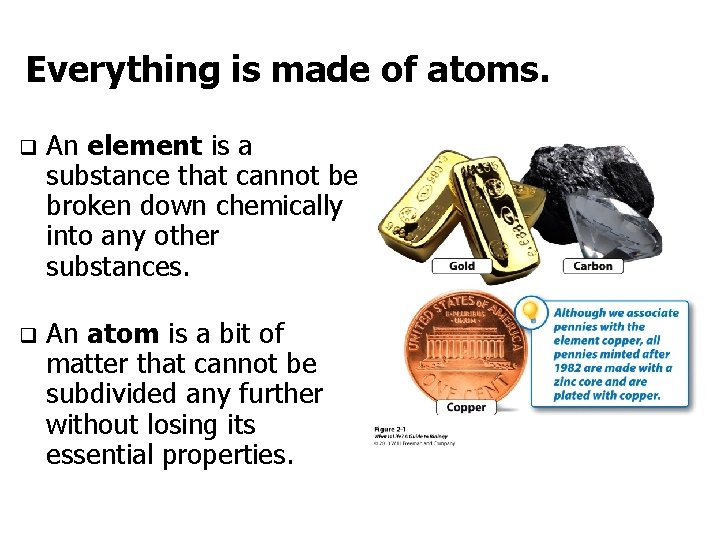
Everything is made of atoms. q An element is a substance that cannot be broken down chemically into any other substances. q An atom is a bit of matter that cannot be subdivided any further without losing its essential properties.


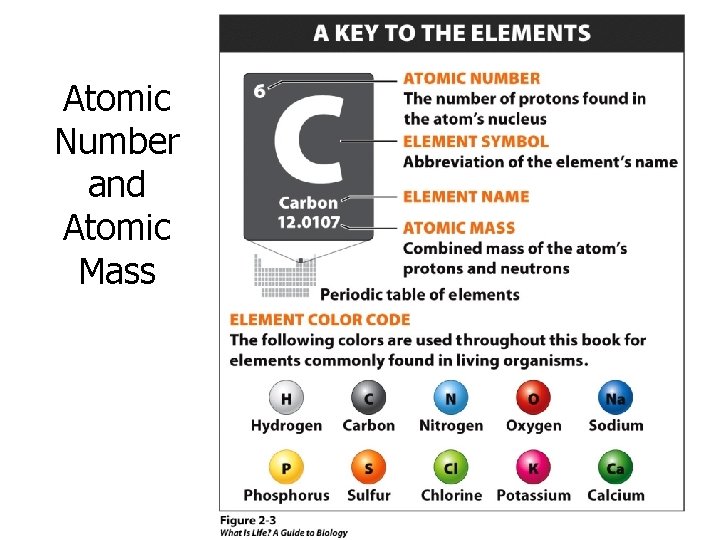
Atomic Number and Atomic Mass

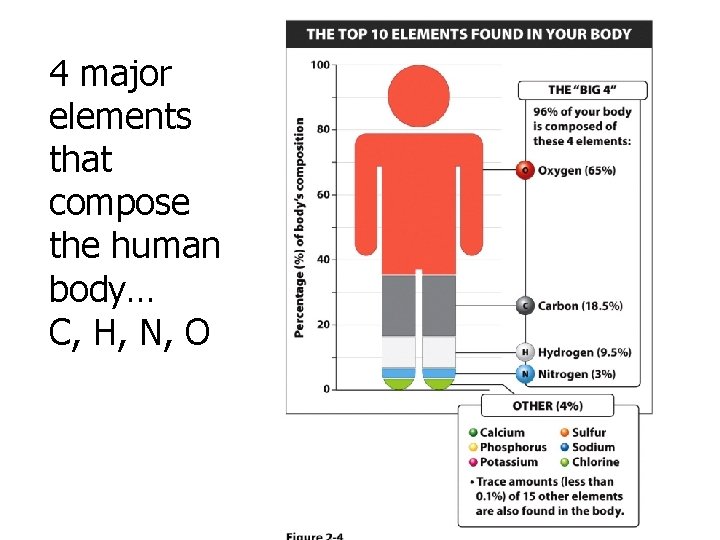
4 major elements that compose the human body… C, H, N, O

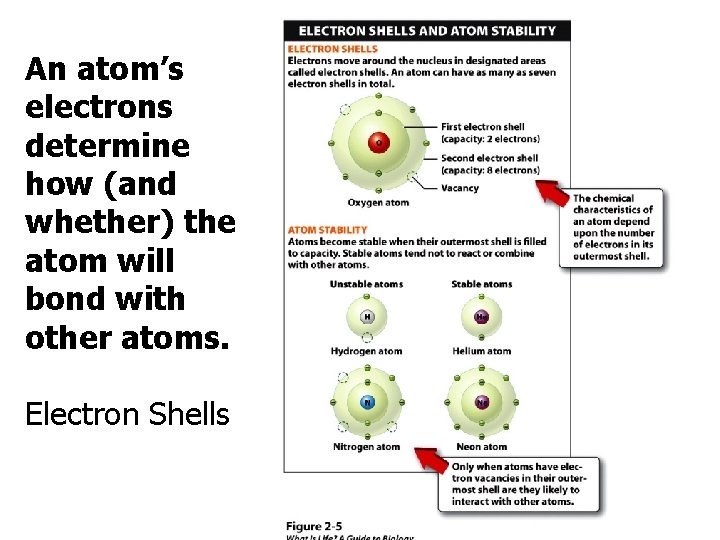
An atom’s electrons determine how (and whether) the atom will bond with other atoms. Electron Shells

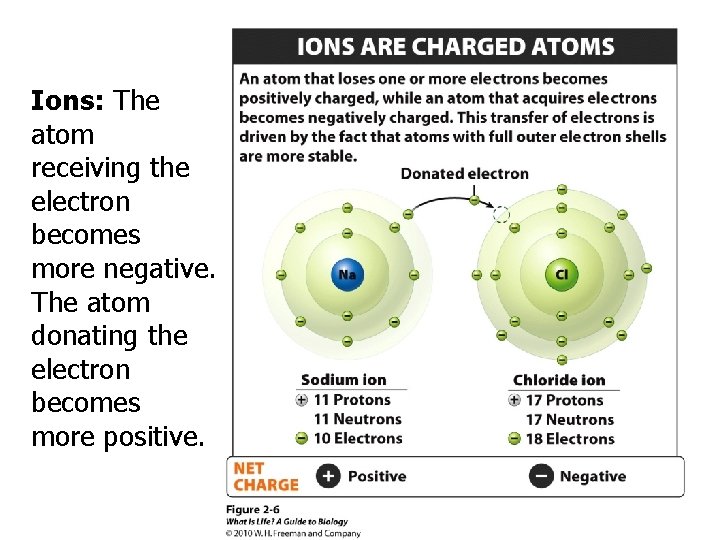
Ions: The atom receiving the electron becomes more negative. The atom donating the electron becomes more positive.

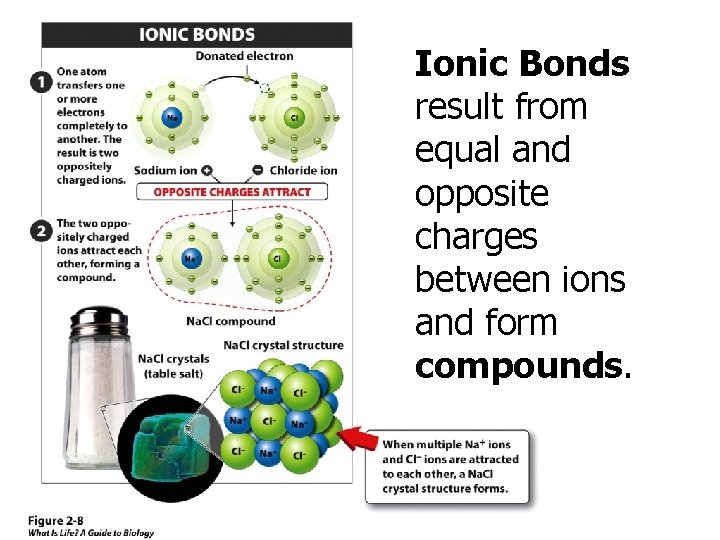
Ionic Bonds result from equal and opposite charges between ions and form compounds.

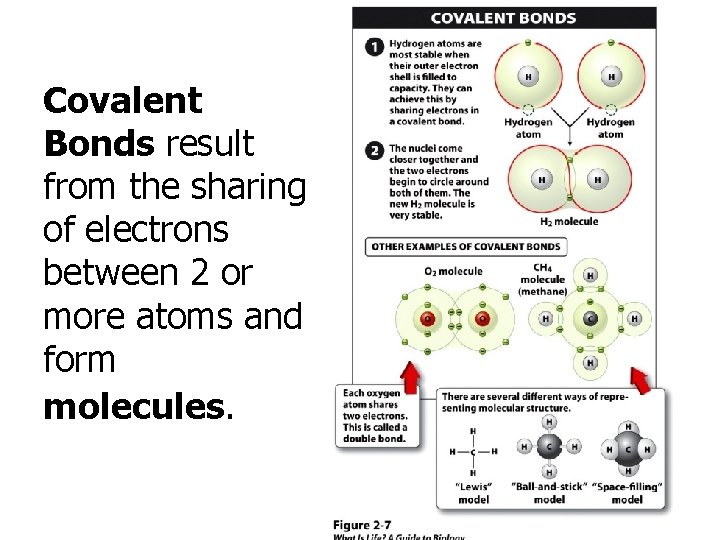
Covalent Bonds result from the sharing of electrons between 2 or more atoms and form molecules.

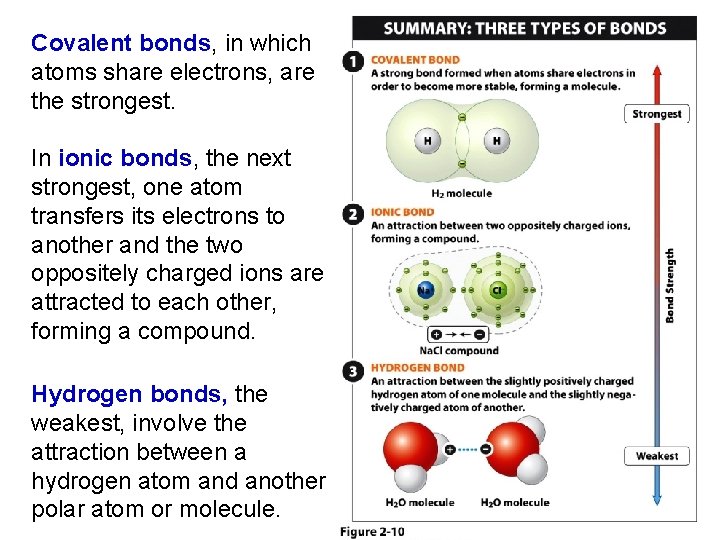
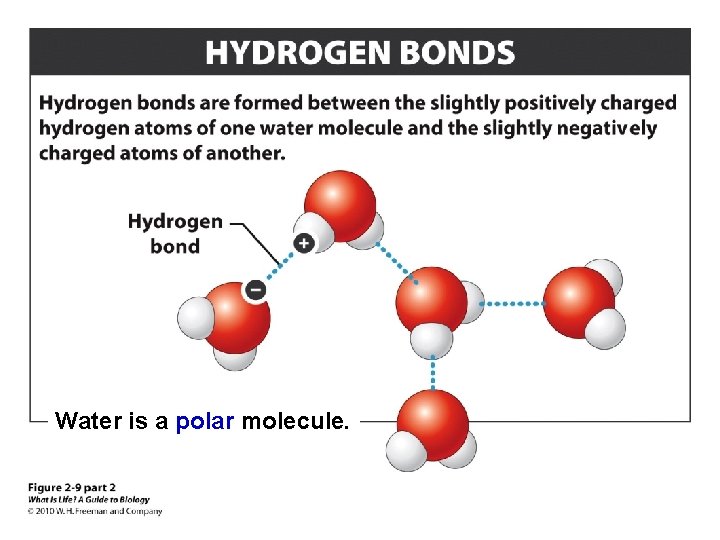
Covalent bonds, in which atoms share electrons, are the strongest. In ionic bonds, the next strongest, one atom transfers its electrons to another and the two oppositely charged ions are attracted to each other, forming a compound. Hydrogen bonds, the weakest, involve the attraction between a hydrogen atom and another polar atom or molecule.

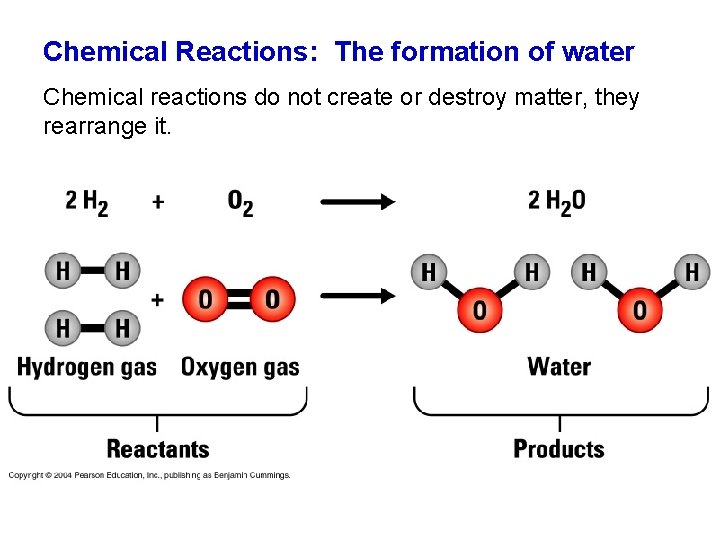
Chemical Reactions: The formation of water Chemical reactions do not create or destroy matter, they rearrange it. 02 -UNp 27 -Chemical. React-L. jpg

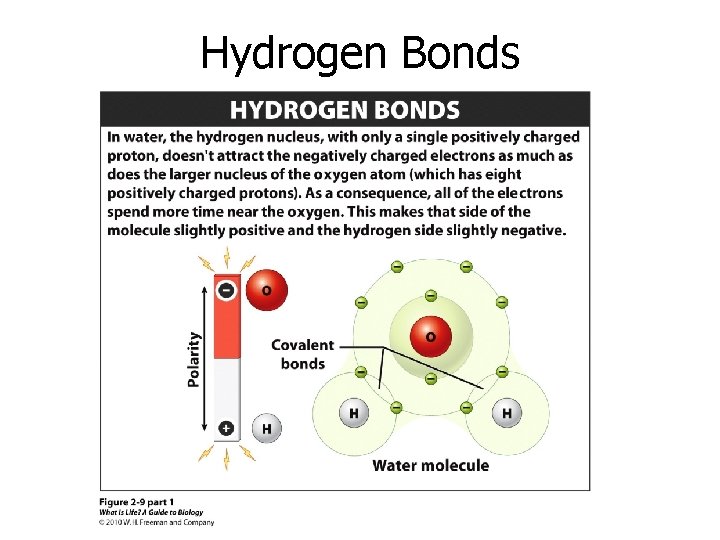
Hydrogen Bonds

Water is a polar molecule.

1. Cohesion/Adhesion 2. Good solvent 3. Regulates Temperature 4. Low density as a solid

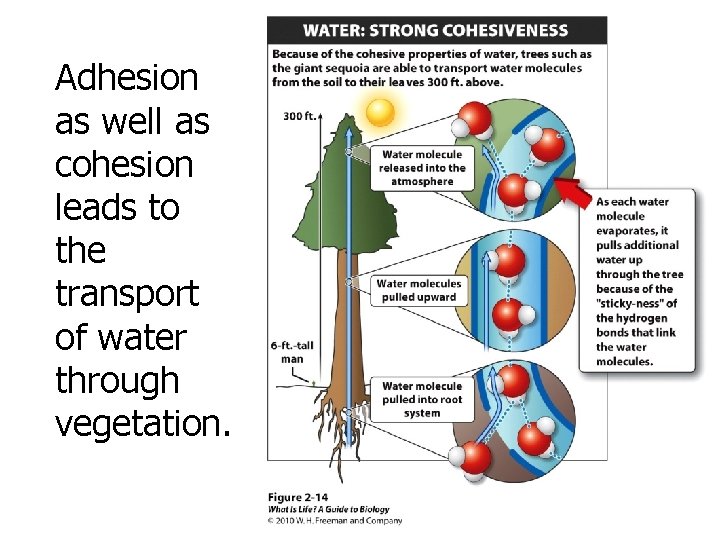
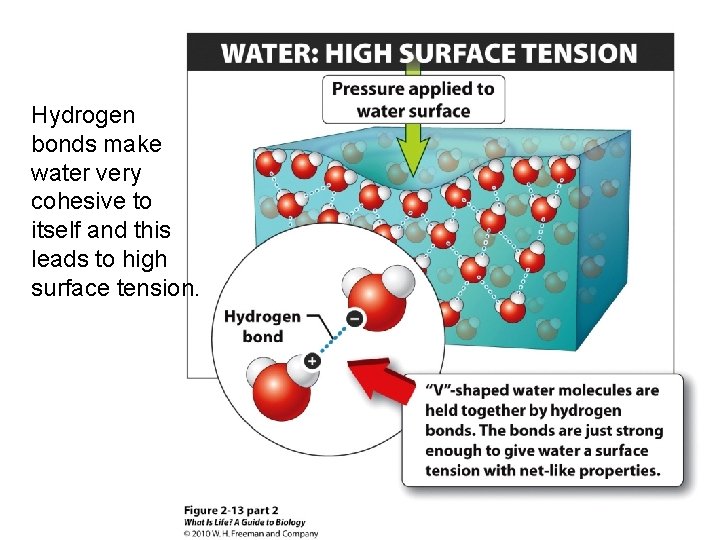
Hydrogen bonds make water cohesive and adhesive. • Cohesion: water molecules sticking to each other – Water has a high water tension. Molecules are cohesive with their neighbors in the liquid not with the air molecules. • Adhesion: water sticking to other things

Adhesion as well as cohesion leads to the transport of water through vegetation.

Hydrogen bonds make water very cohesive to itself and this leads to high surface tension.

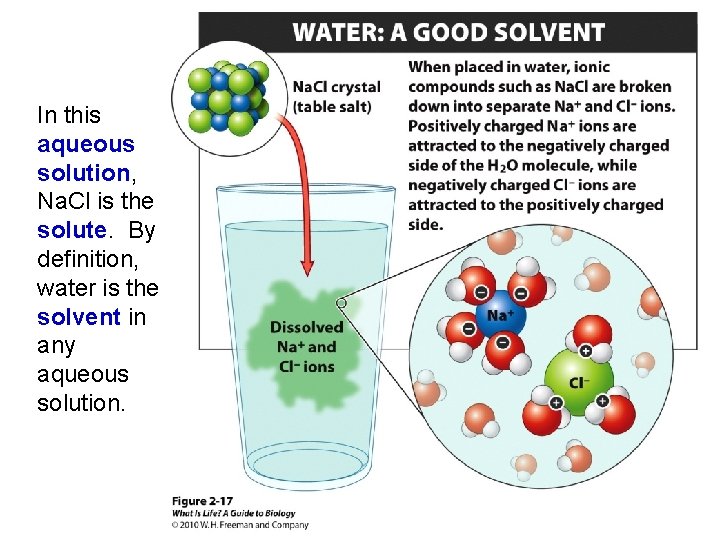
In this aqueous solution, Na. Cl is the solute. By definition, water is the solvent in any aqueous solution.

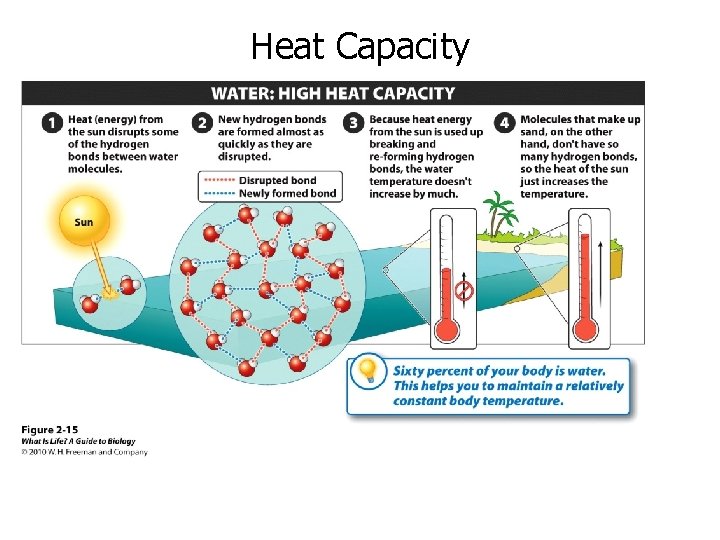
Heat Capacity

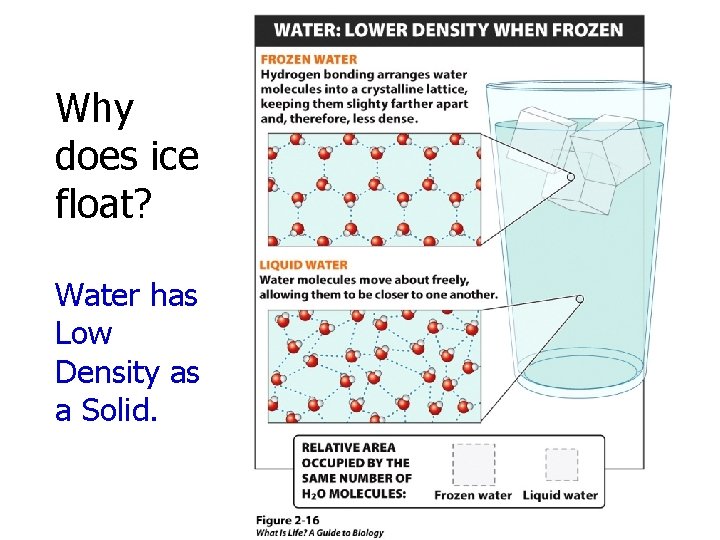
Why does ice float? Water has Low Density as a Solid.

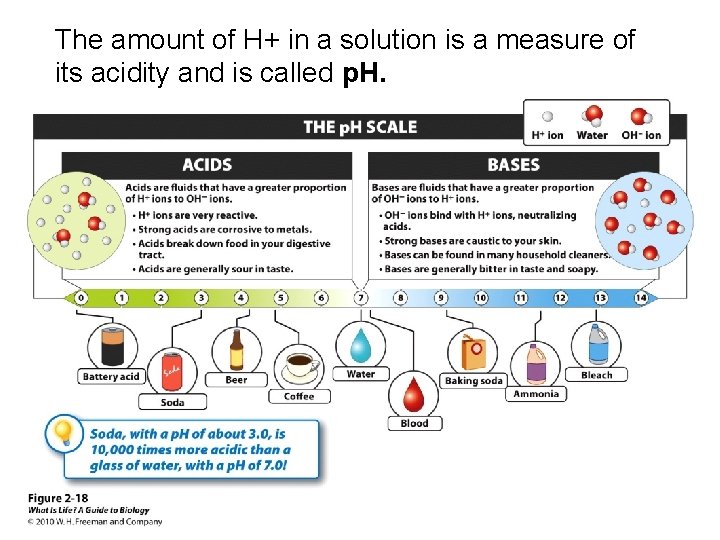
The amount of H+ in a solution is a measure of its acidity and is called p. H.

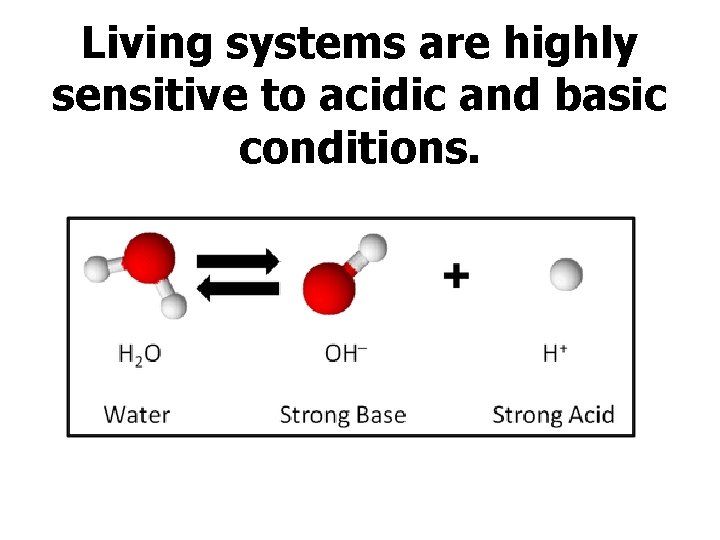
Living systems are highly sensitive to acidic and basic conditions.

Cells Have an Optimum p. H • Protein enzymes work best at certain p. Hs within the cell

Amoeba Sisters: Properties of Water • https: //www. youtube. com/watch? v=3 jw. AG Wky 98 c

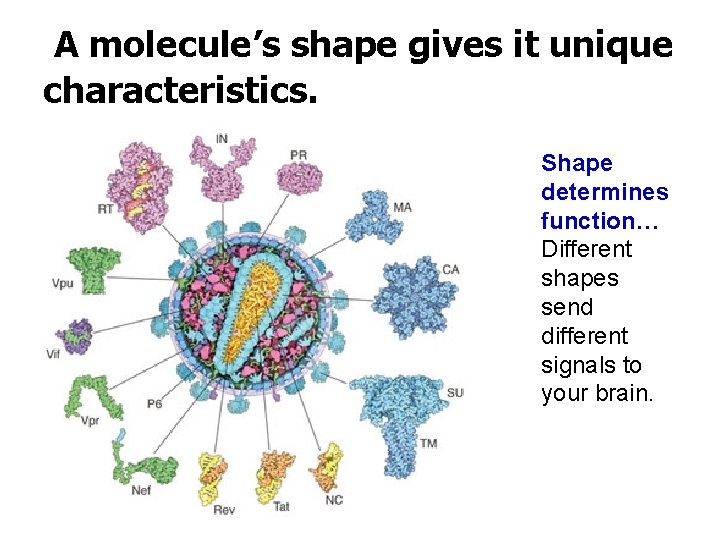
A molecule’s shape gives it unique characteristics. Shape determines function… Different shapes send different signals to your brain.

Biomolecules – Shapes and Functions

Amoeba Sisters: Biomolecules • https: //www. youtube. com/watch? v=YO 244 P 1 e 9 QM

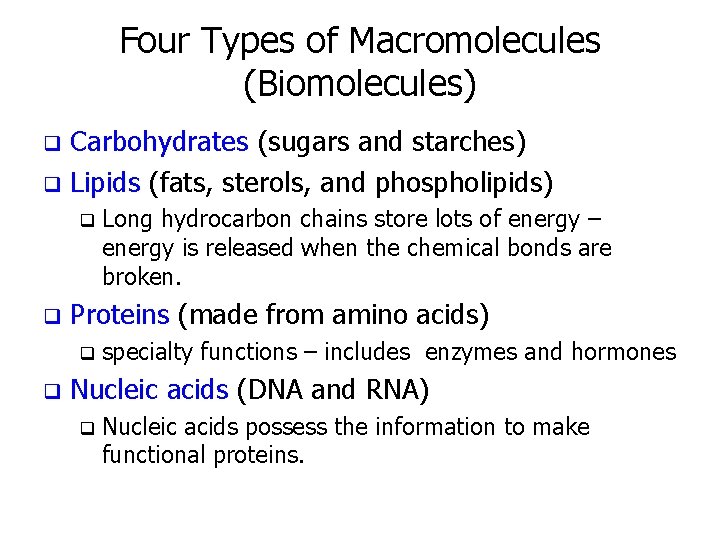
Four Types of Macromolecules (Biomolecules) Carbohydrates (sugars and starches) q Lipids (fats, sterols, and phospholipids) q q q Proteins (made from amino acids) q q Long hydrocarbon chains store lots of energy – energy is released when the chemical bonds are broken. specialty functions – includes enzymes and hormones Nucleic acids (DNA and RNA) q Nucleic acids possess the information to make functional proteins.

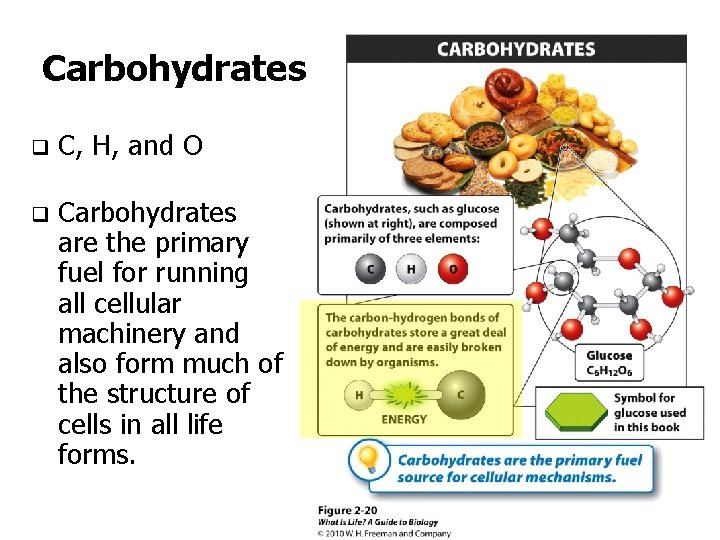
Carbohydrates q C, H, and O q Carbohydrates are the primary fuel for running all cellular machinery and also form much of the structure of cells in all life forms.

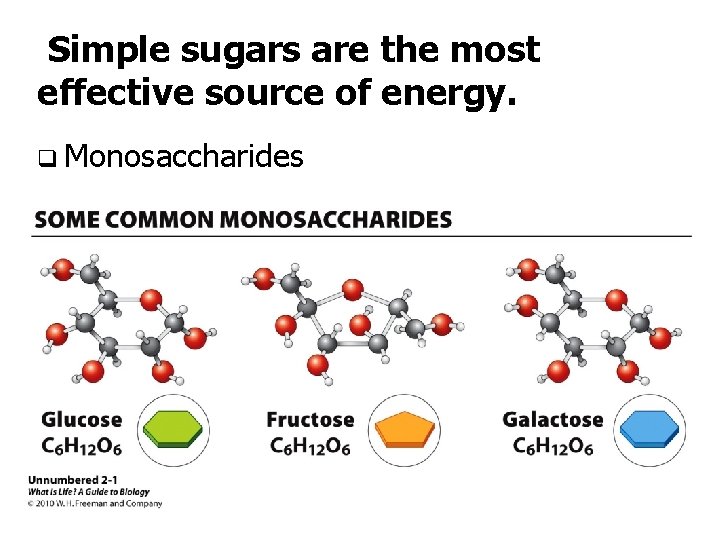
Simple sugars are the most effective source of energy. q Monosaccharides

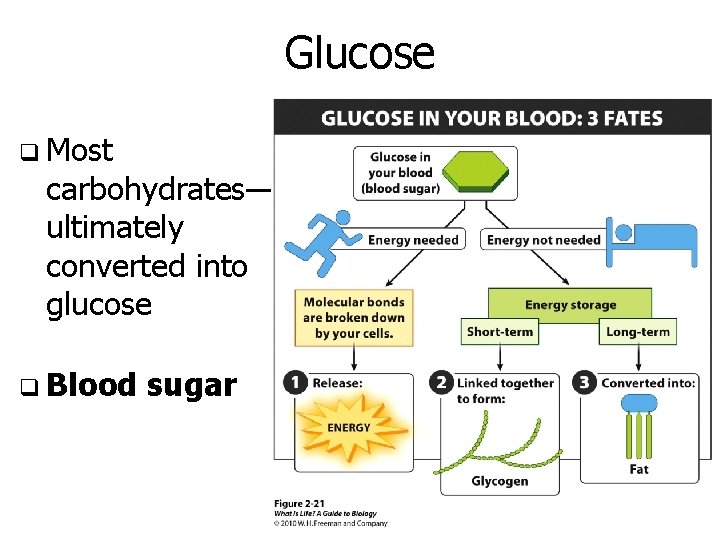
Glucose q Most carbohydrates— ultimately converted into glucose q Blood sugar


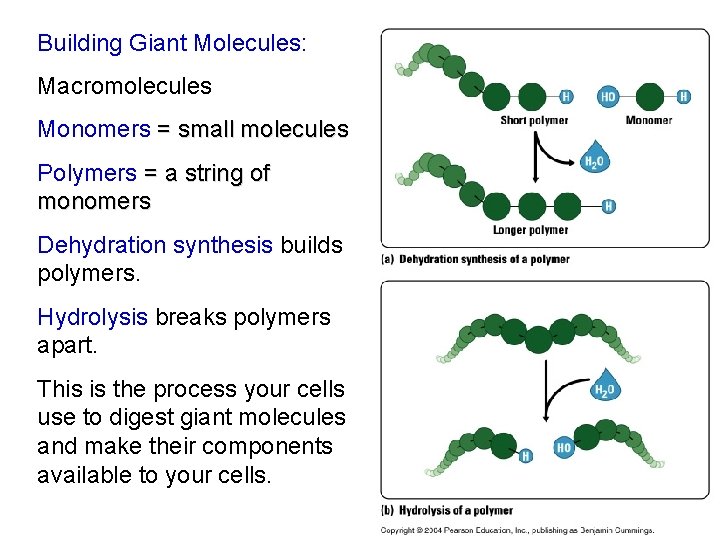
Building Giant Molecules: Macromolecules Monomers = small molecules Polymers = a string of monomers Dehydration synthesis builds polymers. Hydrolysis breaks polymers apart. This is the process your cells use to digest giant molecules and make their components available to your cells.

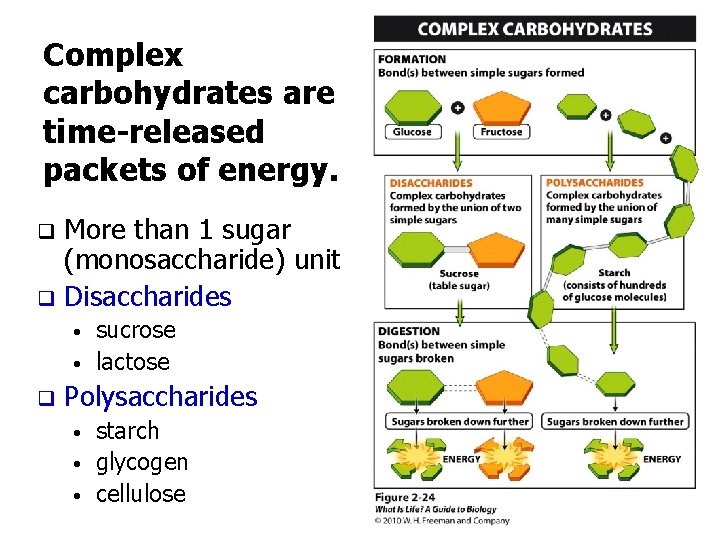
Complex carbohydrates are time-released packets of energy. More than 1 sugar (monosaccharide) unit q Disaccharides q • • q sucrose lactose Polysaccharides • • • starch glycogen cellulose


Ted-Ed Carbohydrates • https: //www. youtube. com/watch? v=wxzc_ 2 c 6 GMg

Ted-Ed Fats • https: //www. youtube. com/watch? v=Qh. Urc 4 Bn. Pgg

Not all carbohydrates are digestible. q Chitin q Cellulose

The Cellulose in our diet is fiber. As it moves through our digestive system, it stimulates the more rapid passage of food and possibly harmful products of digestion through our intestines. Fiber reduces the risk of colon cancer.

Lipids are macromolecules with several functions, including energy storage.

Why does a salad dressing made with vinegar and oil separate into two layers shortly after you shake it? q Hydrophobic – non-polar, “water fearing” q Hydrophilic – polar, “water loving” q Lipids are non-soluble in water and greasy to the touch. q They are valuable to organisms in longterm energy storage and insulation, membrane formation, and as hormones.

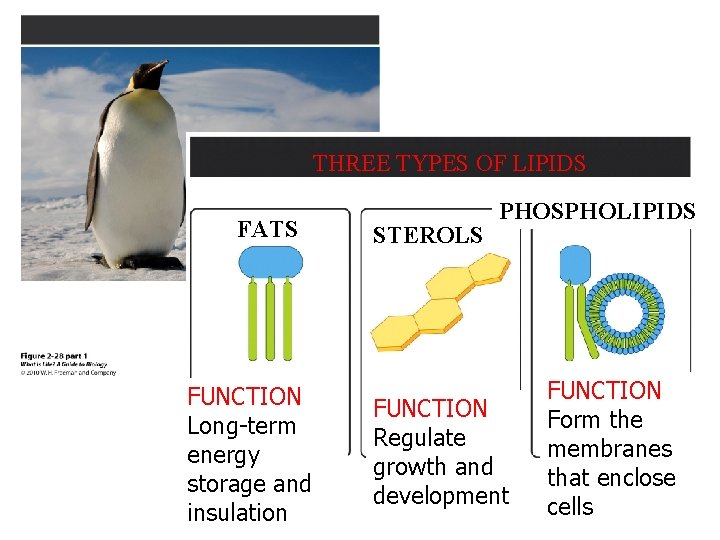
THREE TYPES OF LIPIDS FATS FUNCTION Long-term energy storage and insulation STEROLS PHOSPHOLIPIDS FUNCTION Regulate growth and development FUNCTION Form the membranes that enclose cells

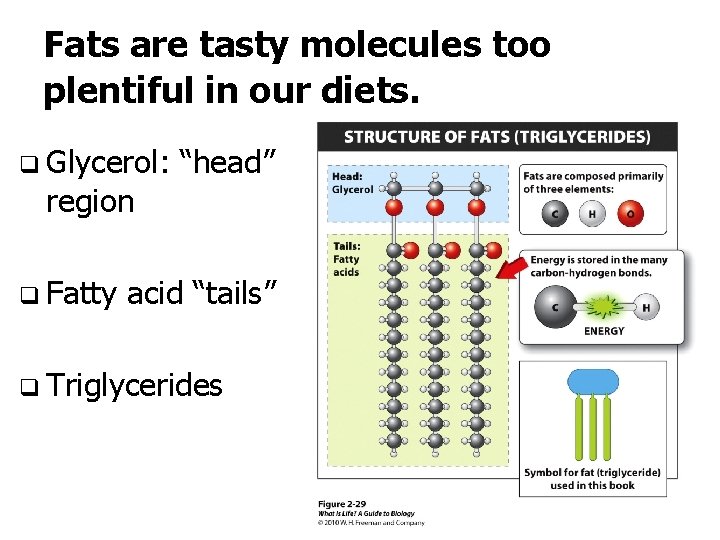
Fats are tasty molecules too plentiful in our diets. q Glycerol: region q Fatty “head” acid “tails” q Triglycerides

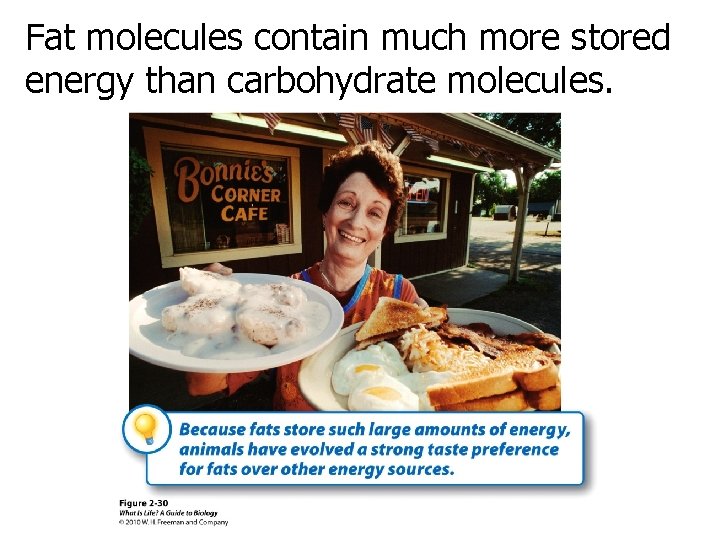
Fat molecules contain much more stored energy than carbohydrate molecules.

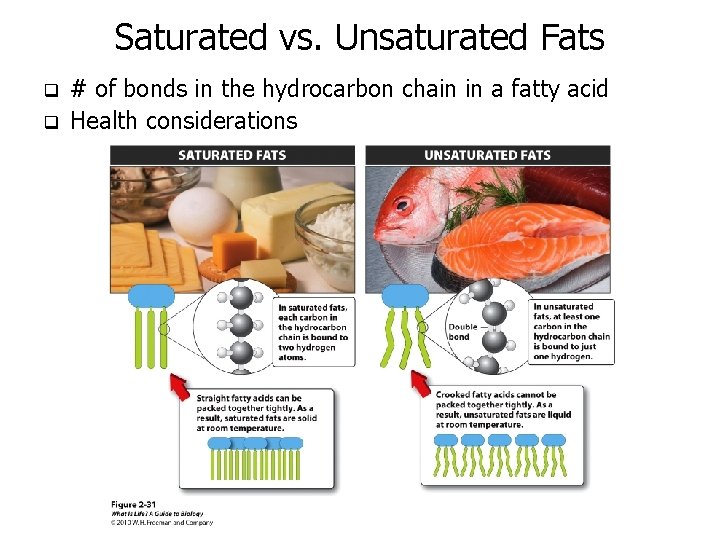
Saturated vs. Unsaturated Fats q q # of bonds in the hydrocarbon chain in a fatty acid Health considerations


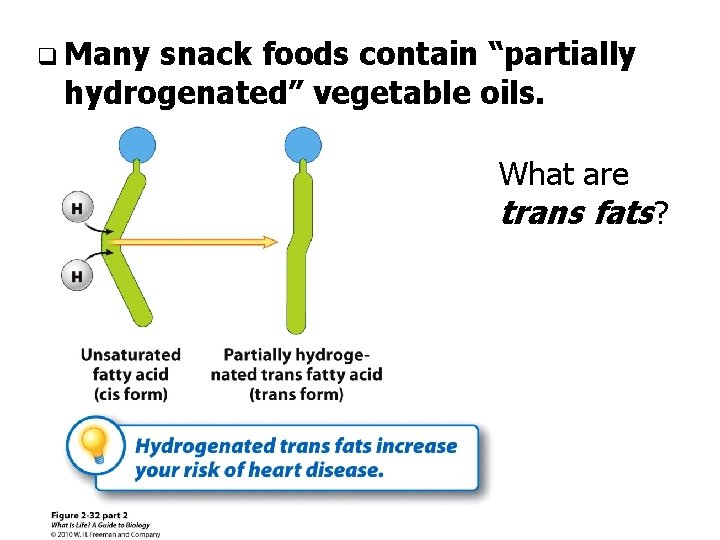
q Many snack foods contain “partially hydrogenated” vegetable oils. What are trans fats?



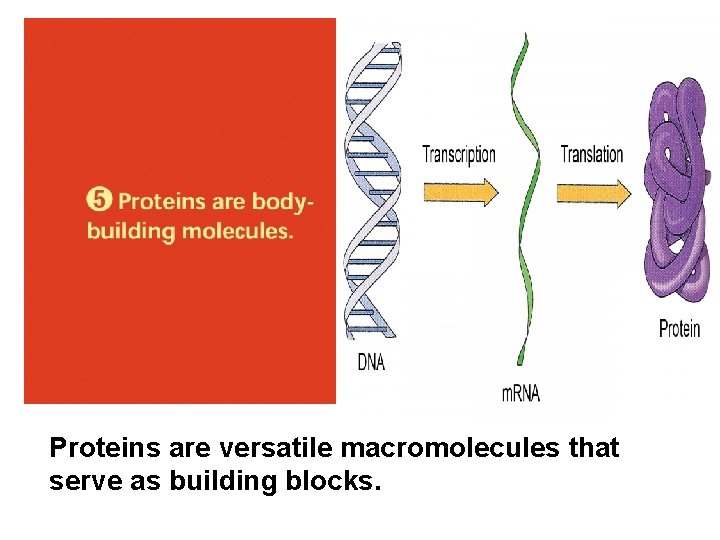
Proteins are versatile macromolecules that serve as building blocks.

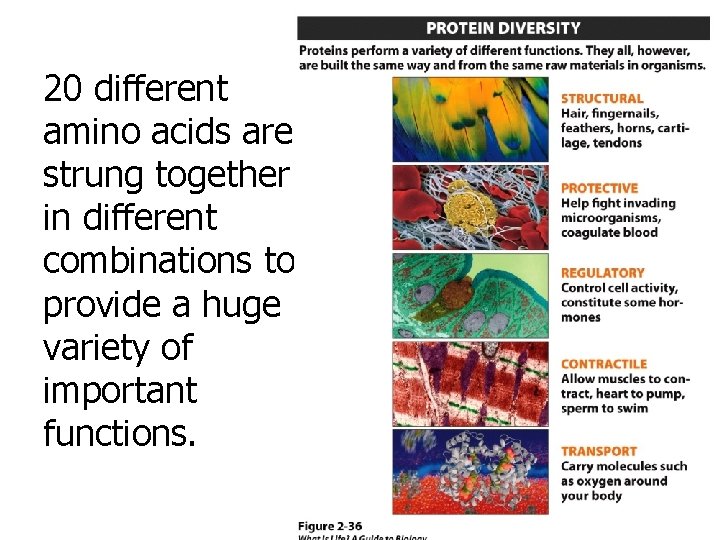
20 different amino acids are strung together in different combinations to provide a huge variety of important functions.


Proteins are an essential dietary component. q Growth q Repair q Replacement Food labels indicate an item’s protein content. Why is this insufficient for you to determine whether you are protein deficient, even if your protein intake exceeds your recommended daily amount?

Complete Proteins Have all 8 essential amino acids – Cannot be made by our bodies and must be consumed.

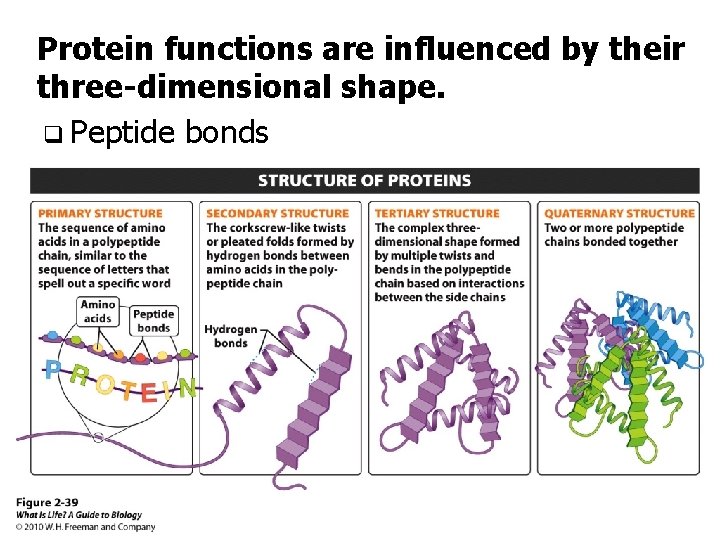
Protein functions are influenced by their three-dimensional shape. q Peptide bonds

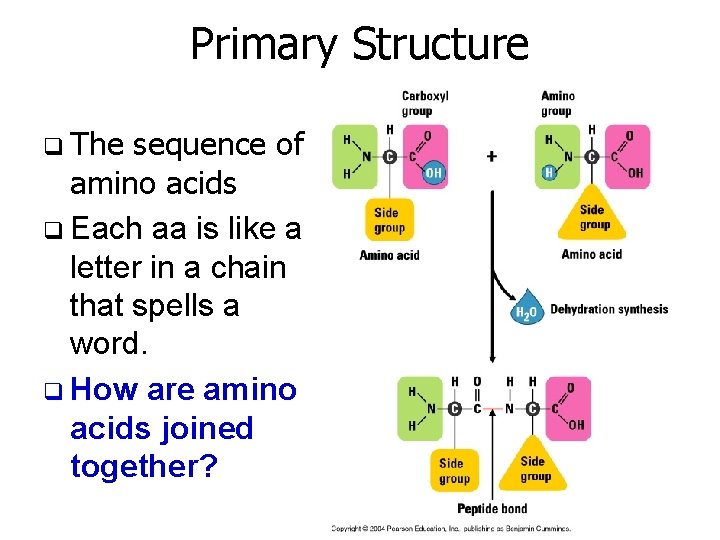
Primary Structure q The sequence of amino acids q Each aa is like a letter in a chain that spells a word. q How are amino acids joined together?

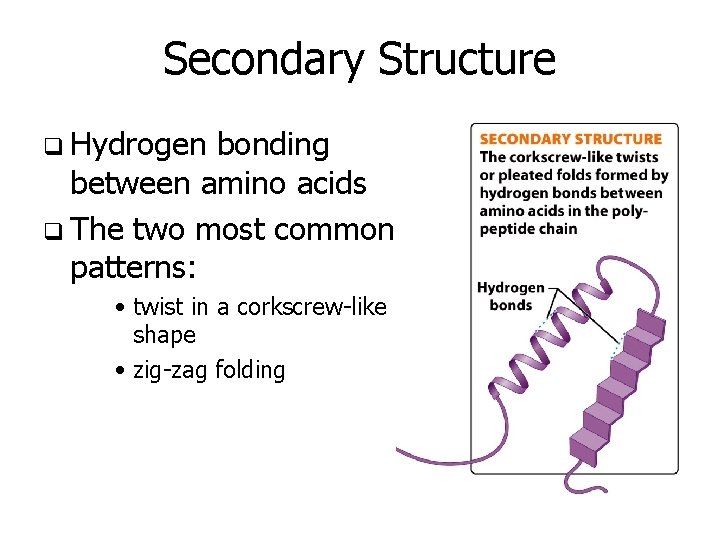
Secondary Structure q Hydrogen bonding between amino acids q The two most common patterns: • twist in a corkscrew-like shape • zig-zag folding

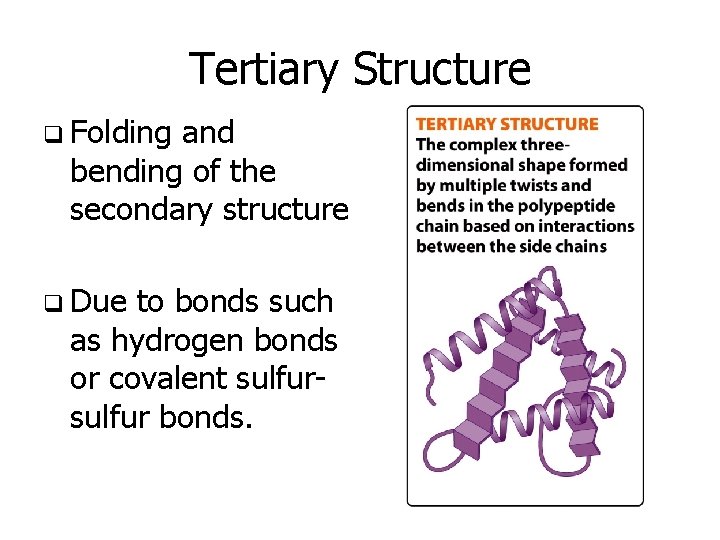
Tertiary Structure q Folding and bending of the secondary structure q Due to bonds such as hydrogen bonds or covalent sulfur bonds.

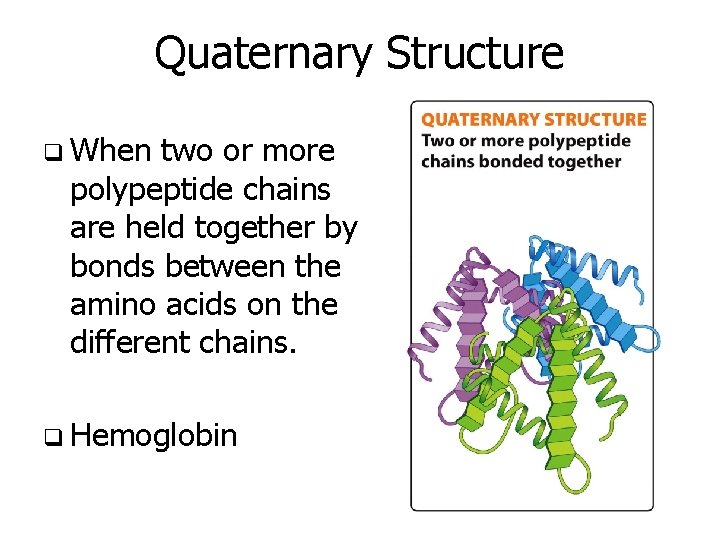
Quaternary Structure q When two or more polypeptide chains are held together by bonds between the amino acids on the different chains. q Hemoglobin


q Egg whites contain a lot of protein. q Why does cooking them change their texture and color?

Summary: Protein Structure and Function q The particular amino acid sequence of a protein determines how it folds into a particular shape. q This shape determines the protein's functions, such as which molecules it will interact with. q When a protein's shape is deformed (denaturation), the protein usually loses its ability to function.

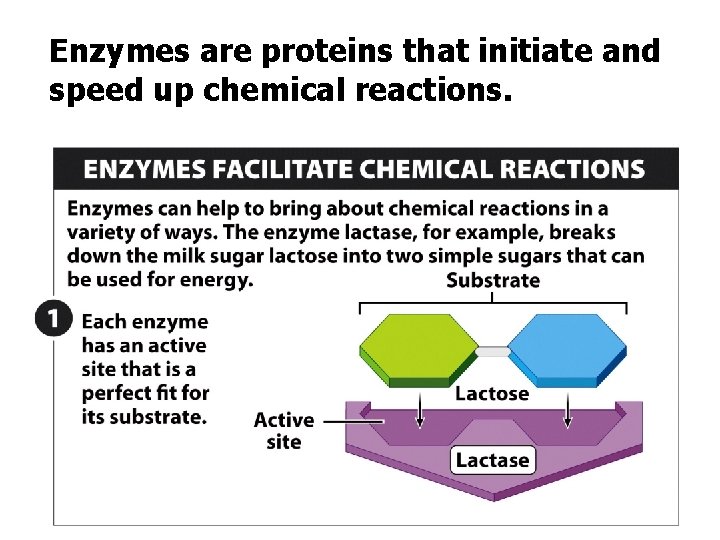
Enzymes are proteins that initiate and speed up chemical reactions.


Amoeba Sisters Enzymes • https: //www. youtube. com/watch? v=qg. VFk Rn 8 f 10

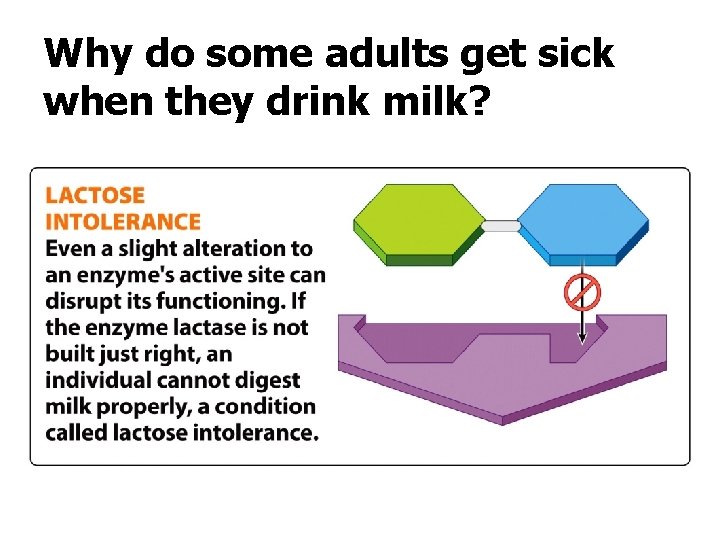
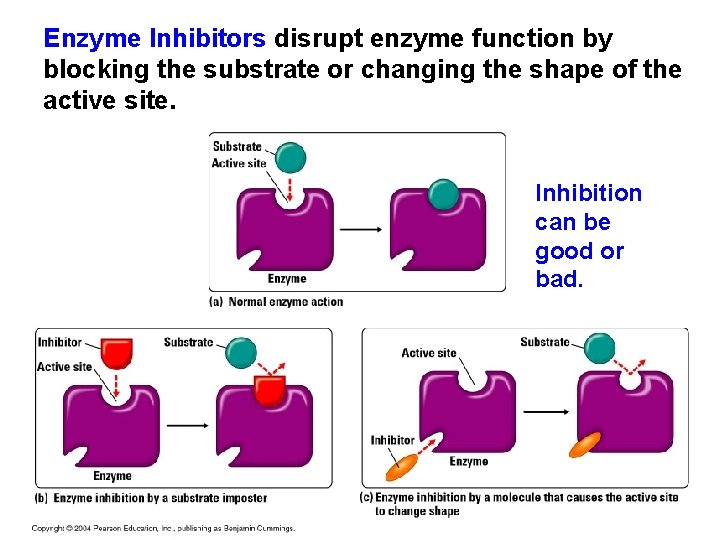
“Misspelled” Proteins and Enzyme Inhibitors q Mutations in a person’s DNA or mistakes in the translation process can lead to an incorrect amino acid sequence. q This may result in active site disruptions. q Chemicals called inhibitors can also disrupt the active site. q Directly or indirectly

Why do some adults get sick when they drink milk?

Enzyme Inhibitors disrupt enzyme function by blocking the substrate or changing the shape of the active site. Inhibition can be good or bad.


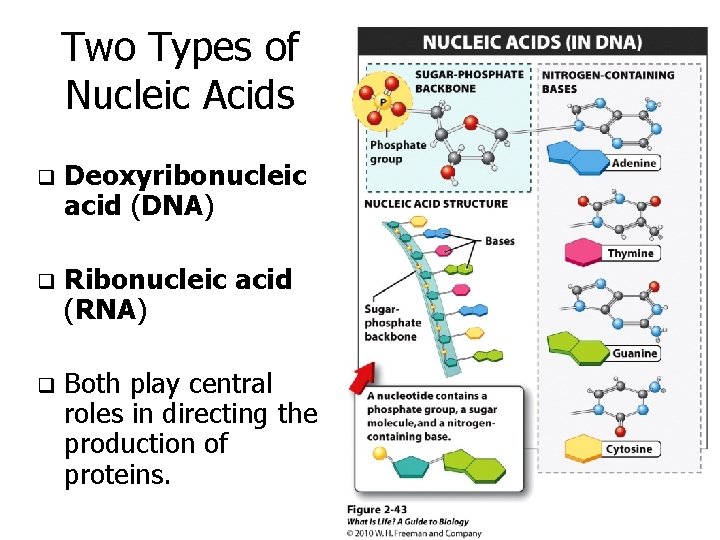
Two Types of Nucleic Acids q Deoxyribonucleic acid (DNA) q Ribonucleic acid (RNA) q Both play central roles in directing the production of proteins.

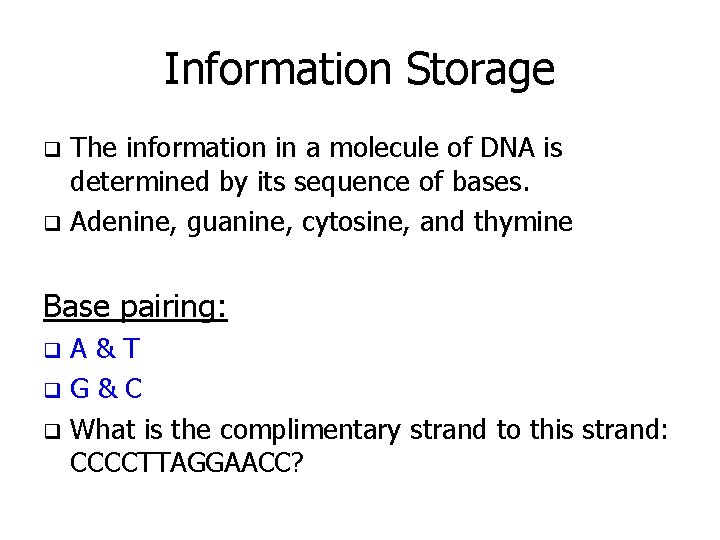
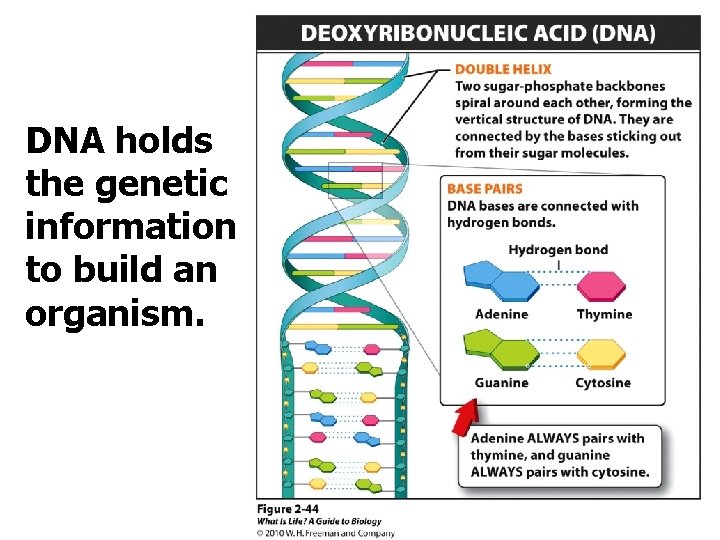
Information Storage The information in a molecule of DNA is determined by its sequence of bases. q Adenine, guanine, cytosine, and thymine q Base pairing: A&T q. G & C q What is the complimentary strand to this strand: CCCCTTAGGAACC? q

DNA holds the genetic information to build an organism.

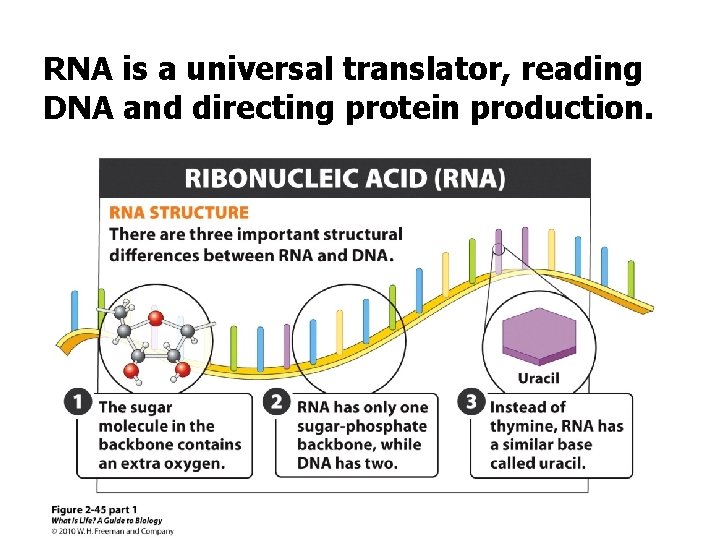
RNA is a universal translator, reading DNA and directing protein production.


In what three important ways does RNA differ from DNA structurally? q The sugar molecule of the sugarphosphate backbone: ribose vs. deoxyribose q Single-stranded q Uracil (U) replaces thymine (T)

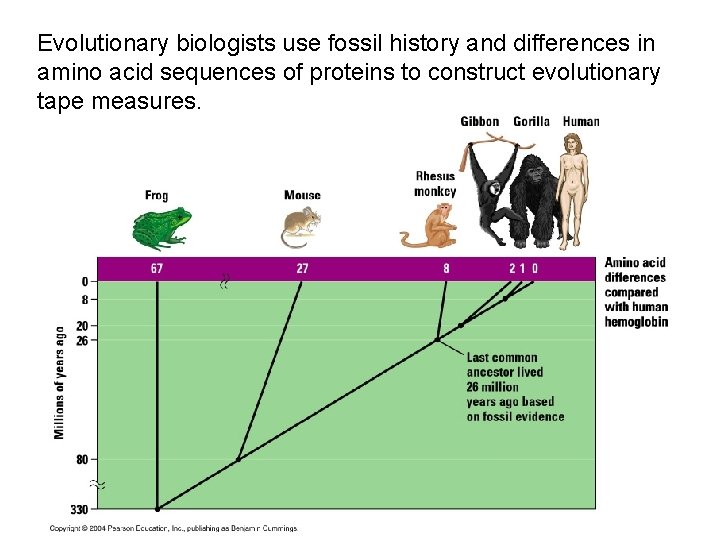
Evolutionary biologists use fossil history and differences in amino acid sequences of proteins to construct evolutionary tape measures.
 Bullets smacking the belly out of the air meaning
Bullets smacking the belly out of the air meaning Raw material research and development council salary scale
Raw material research and development council salary scale Raw material quality control
Raw material quality control Green plants make their own food by photosynthesis
Green plants make their own food by photosynthesis Two raw materials necessary for photosynthesis
Two raw materials necessary for photosynthesis Section through a leaf
Section through a leaf Raw materials examples
Raw materials examples Raw materials characteristics
Raw materials characteristics Raw materials budget example
Raw materials budget example Starting materials for cellular respiration
Starting materials for cellular respiration Polyurethane raw materials
Polyurethane raw materials Types of raw materials
Types of raw materials Raw materials initiative
Raw materials initiative Costing
Costing Weight loss raw materials
Weight loss raw materials Raw materials of sulphuric acid
Raw materials of sulphuric acid Quality control of raw materials
Quality control of raw materials Kic eit raw materials
Kic eit raw materials Recycle package
Recycle package Location planning analysis
Location planning analysis Chapter 2 the chemistry of life section 2-3 answer key
Chapter 2 the chemistry of life section 2-3 answer key Cant stop the feeling go noodle
Cant stop the feeling go noodle How would you differentiate useful from harmful materials?
How would you differentiate useful from harmful materials? Natural materials and man made materials
Natural materials and man made materials Adopting materials
Adopting materials Direct materials budget with multiple materials
Direct materials budget with multiple materials Between us raw chapter 30
Between us raw chapter 30 Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp tư thế worms-breton
Chụp tư thế worms-breton Hát lên người ơi
Hát lên người ơi Các môn thể thao bắt đầu bằng tiếng nhảy
Các môn thể thao bắt đầu bằng tiếng nhảy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V cc cc
V cc cc Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Số nguyên tố là
Số nguyên tố là Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi You light up my life lab answers
You light up my life lab answers Chemical kinetics half life
Chemical kinetics half life Chemistry
Chemistry Concept 2 chemistry of life
Concept 2 chemistry of life Concept 2 chemistry of life
Concept 2 chemistry of life Chemistry of life summary
Chemistry of life summary