Chapter 2 Atoms Molecules and Ions CHAPTER 2




























- Slides: 85

Chapter 2 Atoms, Molecules and Ions

CHAPTER 2 (P. 49 -68) • Atomic number , mass number and isotopes. • The periodic table. • Molecules and ions. • Chemical formulas. • Naming compounds


. (3 : }ﻻ ﺍ ﻱ ﺍﻟ ﺍﺍ ﻻ ﻱ ﺍﻷ ﻻ ﻥ ﻻ ﻻ ﻱ ﺍ ﻳ{ )ﺳﺒﺄ Atomic Structure Elements: are composed of extremely small particles called atoms. Atom: is the basic unit of an element that can enter into chemical combination

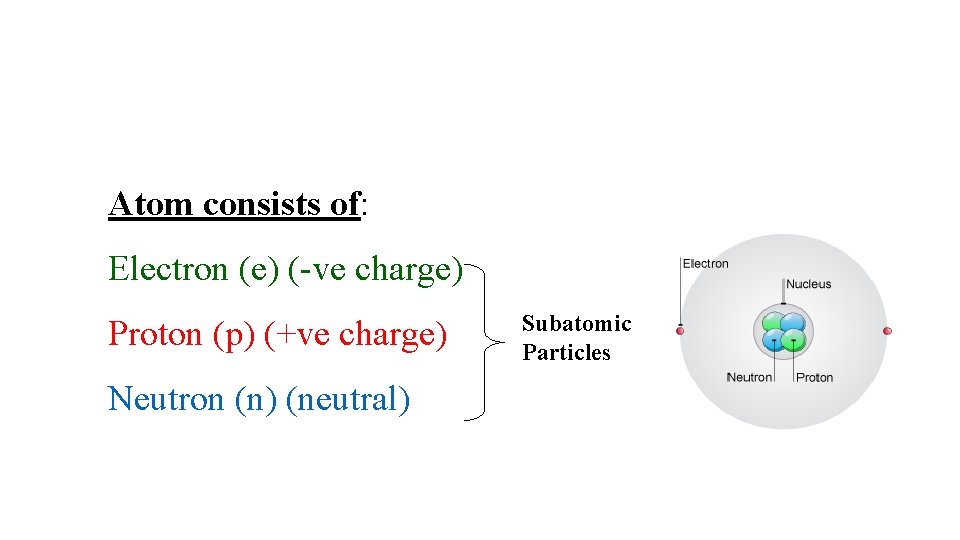
Atom consists of: Electron (e) (-ve charge) Proton (p) (+ve charge) Neutron (n) (neutral) Subatomic Particles

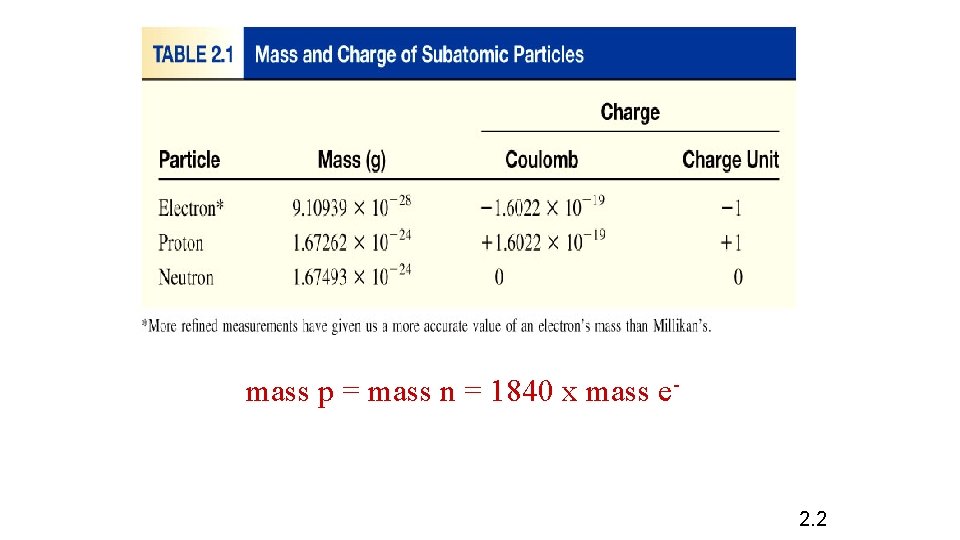
mass p = mass n = 1840 x mass e- 2. 2

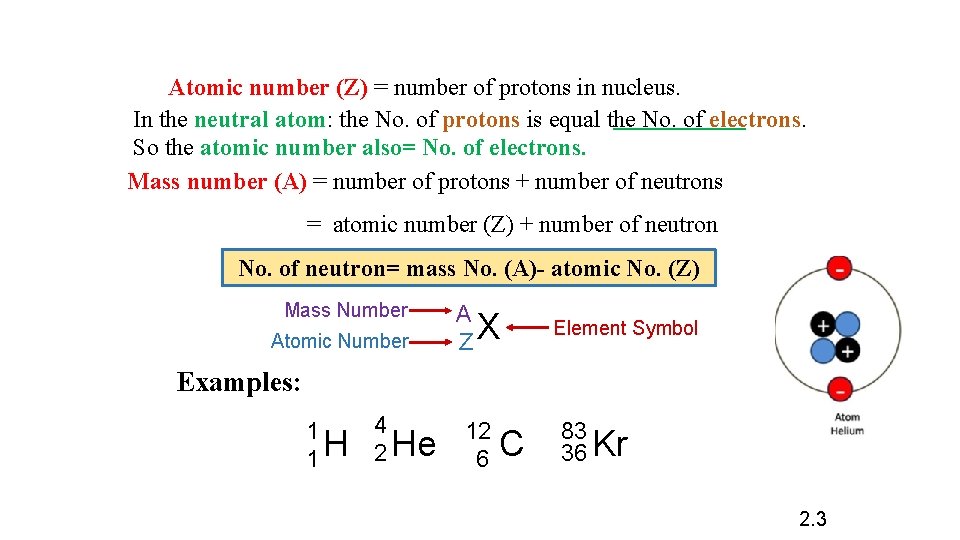
2. 3 Atomic number , mass number and isotopes.

Atomic number (Z) = number of protons in nucleus. In the neutral atom: the No. of protons is equal the No. of electrons. So the atomic number also= No. of electrons. Mass number (A) = number of protons + number of neutrons = atomic number (Z) + number of neutron No. of neutron= mass No. (A)- atomic No. (Z) Mass Number Atomic Number A ZX Element Symbol Examples: 1 1 H 4 2 He 12 6 C 83 36 Kr 2. 3

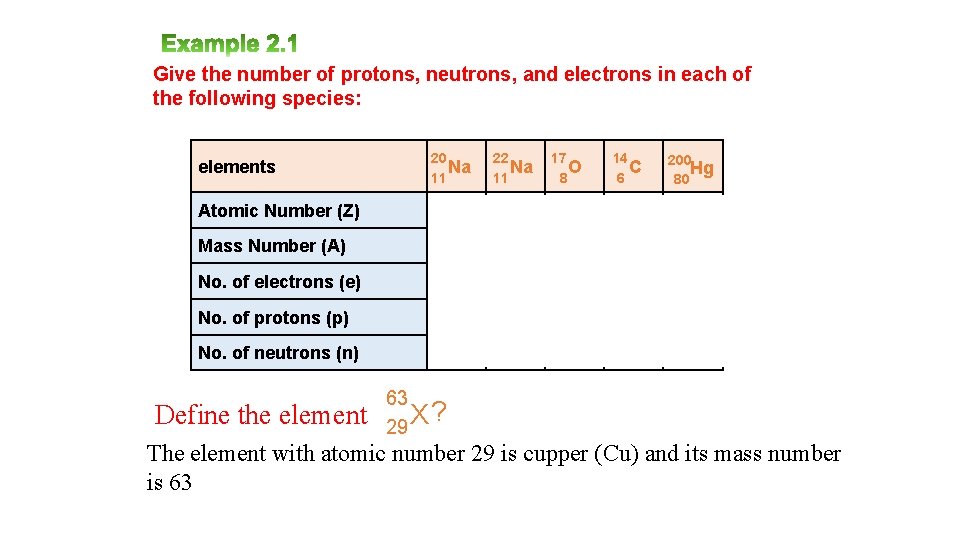
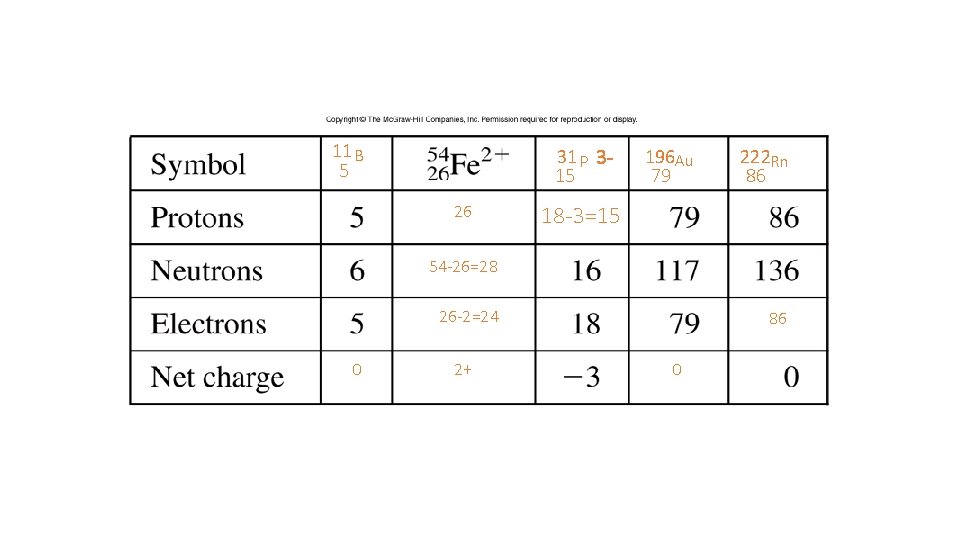
Give the number of protons, neutrons, and electrons in each of the following species: elements 20 Na 11 22 Na 11 17 O 8 14 C 6 200 Hg 80 Atomic Number (Z) 11 11 8 6 80 Mass Number (A) 20 22 17 14 200 No. of electrons (e) 11 11 8 6 80 No. of protons (p) 11 11 8 6 80 No. of neutrons (n) 9 11 9 8 120 Define the element 63 ? 29 X The element with atomic number 29 is cupper (Cu) and its mass number is 63

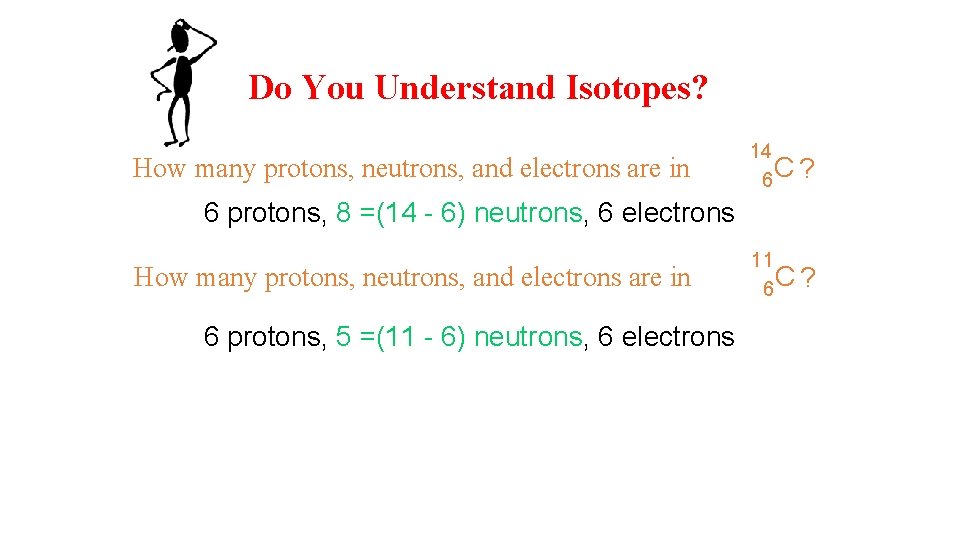
Do You Understand Isotopes? How many protons, neutrons, and electrons are in 14 6 C ? 6 protons, 8 =(14 - 6) neutrons, 6 electrons How many protons, neutrons, and electrons are in 6 protons, 5 =(11 - 6) neutrons, 6 electrons 11 6 C ?

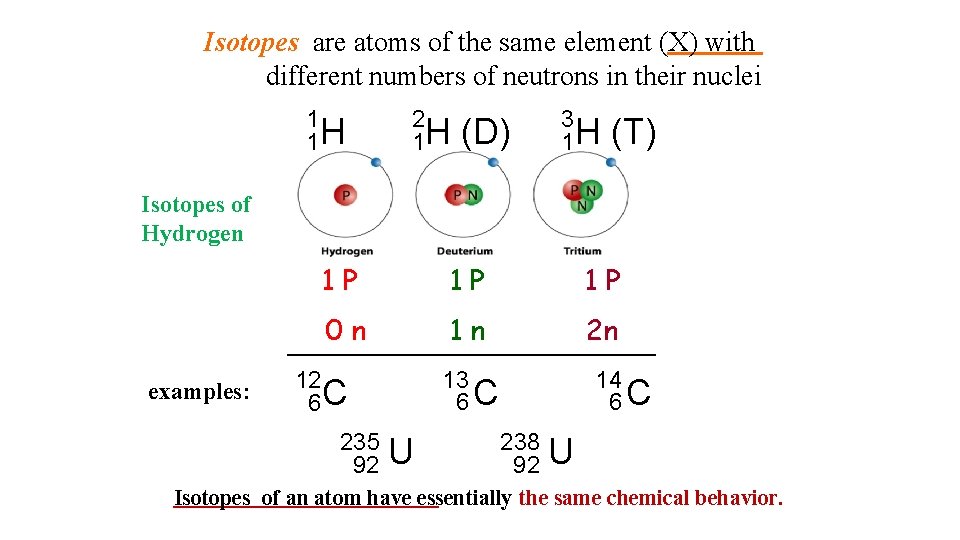
Isotopes are atoms of the same element (X) with different numbers of neutrons in their nuclei 1 1 H 2 1 H (D) 3 1 H (T) Isotopes of Hydrogen examples: 1 P 1 P 1 P 0 n 1 n 2 n 13 6 C 12 6 C 235 92 U 14 6 C 238 92 U Isotopes of an atom have essentially the same chemical behavior.

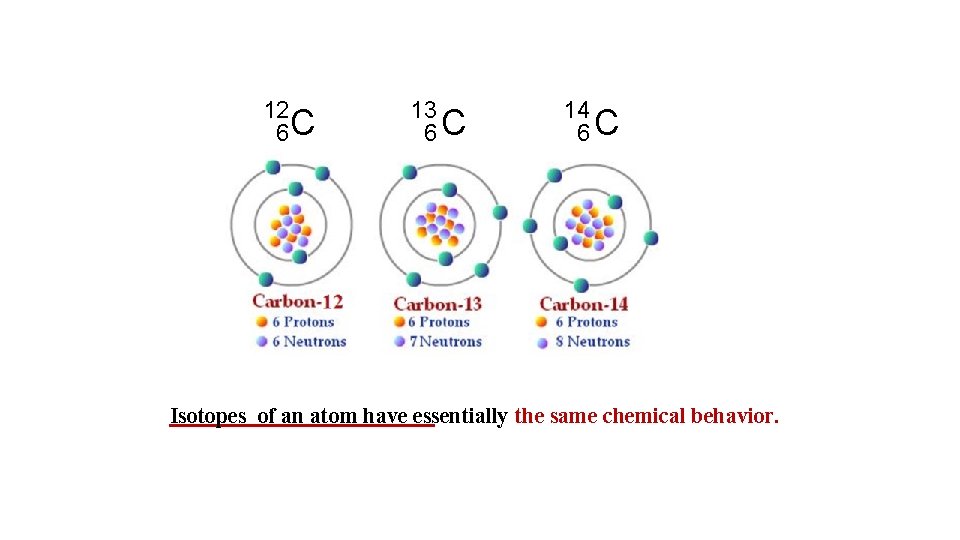
12 6 C 13 6 C 14 6 C Isotopes of an atom have essentially the same chemical behavior.

235 92 U uranium-235 238 92 U uranium-238

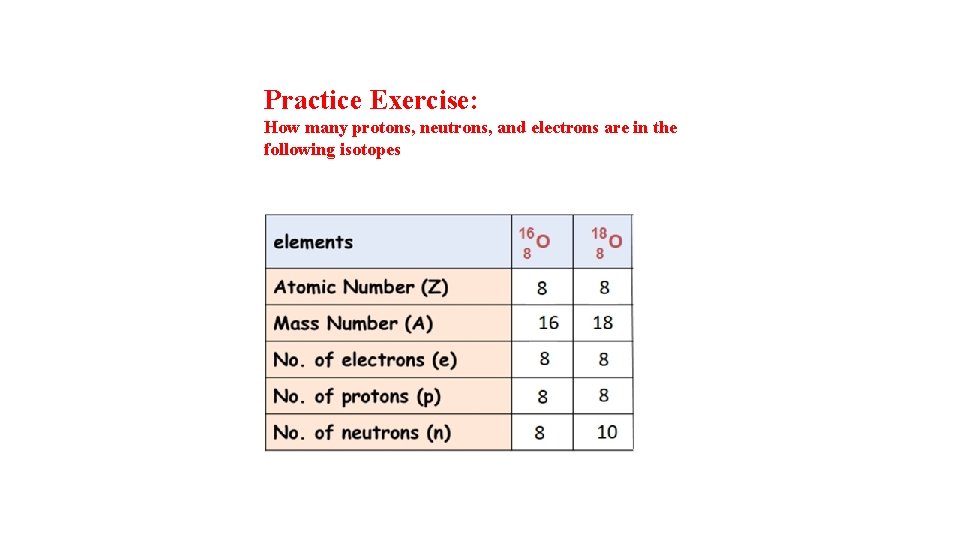
Practice Exercise: How many protons, neutrons, and electrons are in the following isotopes

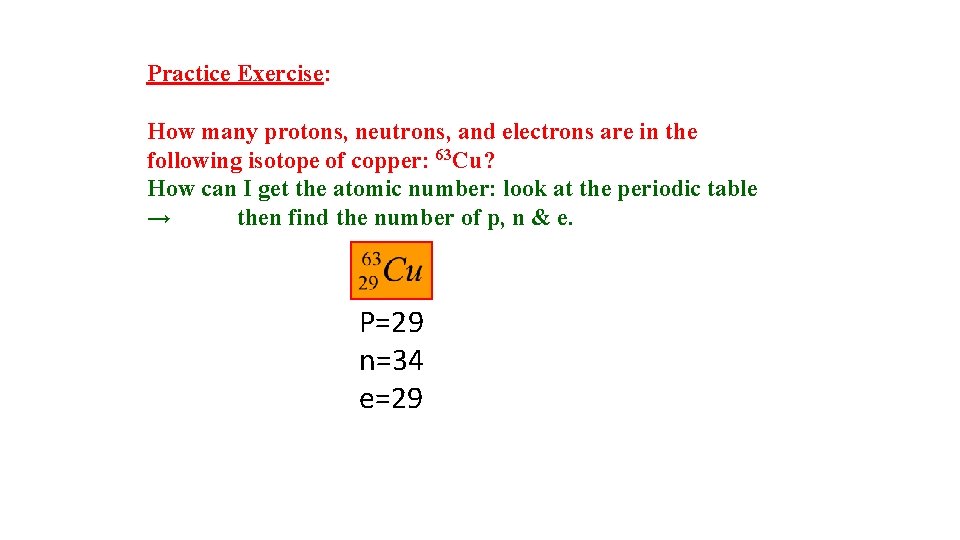
Practice Exercise: How many protons, neutrons, and electrons are in the following isotope of copper: 63 Cu? How can I get the atomic number: look at the periodic table → then find the number of p, n & e. P=29 n=34 e=29

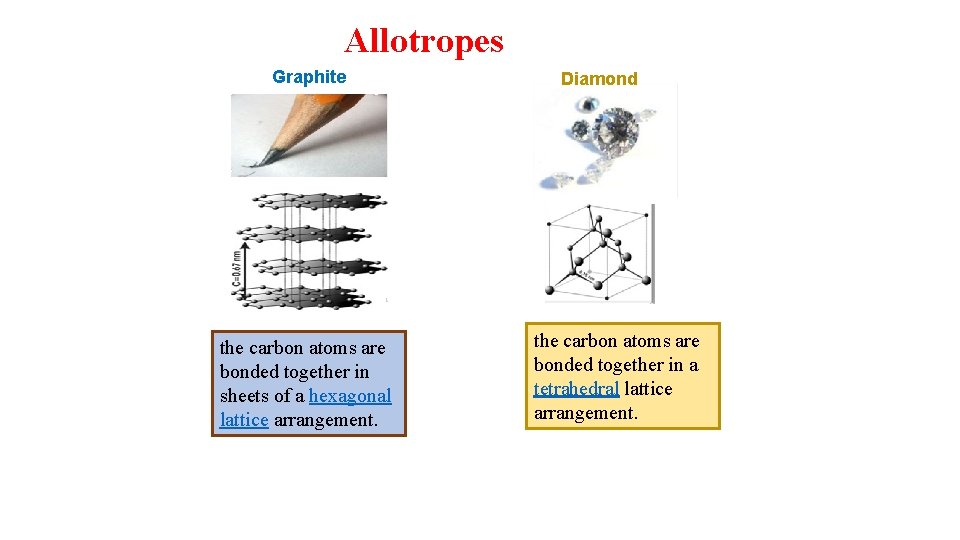
Allotropes Allotrope is one of tow or more distinct forms of an element as O 2 , O 3. O-O (O 2) and O 3 Allotropes are different structural forms of the same element and can exhibit quite different physical properties and chemical behaviors.

Allotropes Graphite the carbon atoms are bonded together in sheets of a hexagonal lattice arrangement. Diamond the carbon atoms are bonded together in a tetrahedral lattice arrangement.

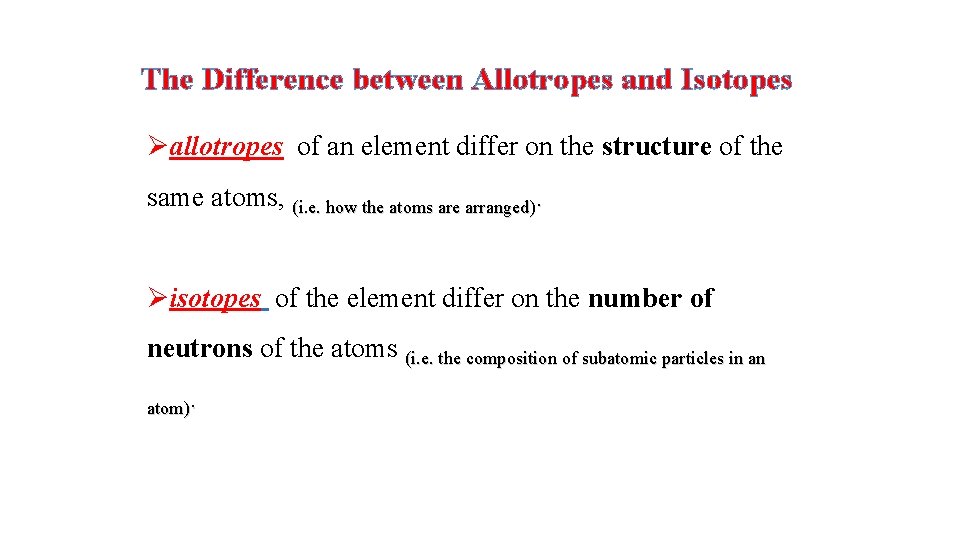
The Difference between Allotropes and Isotopes Øallotropes of an element differ on the structure of the same atoms, (i. e. how the atoms are arranged). Øisotopes of the element differ on the number of neutrons of the atoms (i. e. the composition of subatomic particles in an atom).

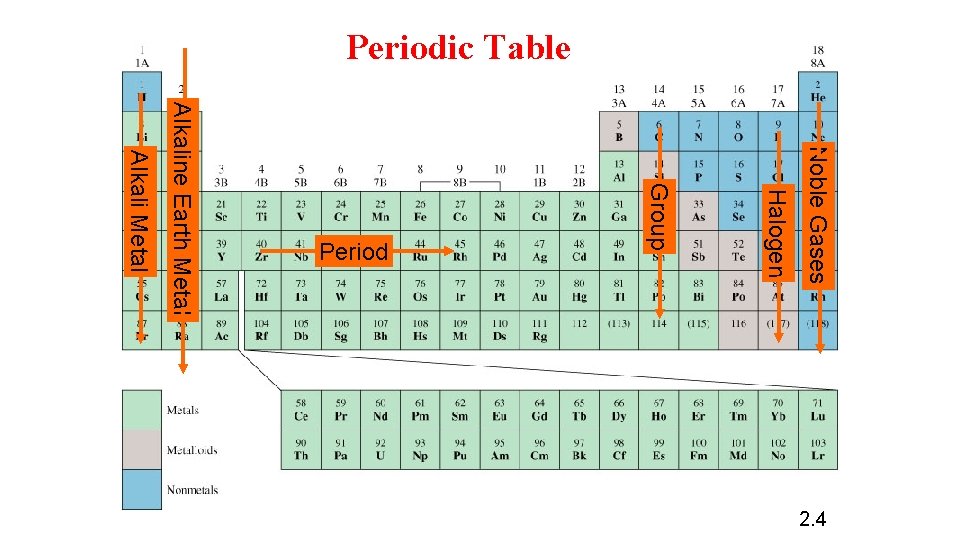
2. 4 The periodic table

Periodic Table Noble Gases Halogen Group Alkali Metal Alkaline Earth Metal Period 2. 4

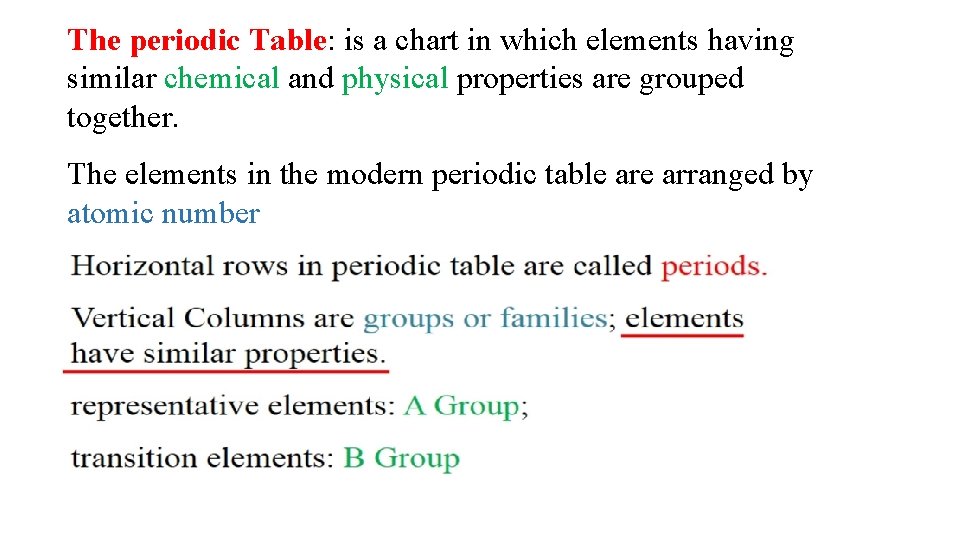
The periodic Table: is a chart in which elements having similar chemical and physical properties are grouped together. The elements in the modern periodic table arranged by atomic number

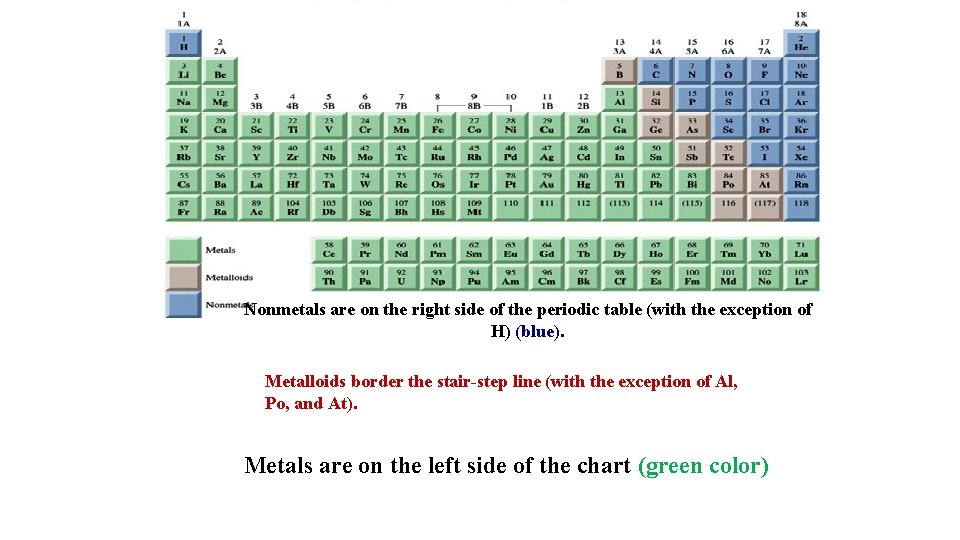
Metal . Nonmetal . Metalloid.

• Positive ions are called: a. positrons. b. anions. c. cations. d. nucleons. • The elements located in group 7 A of the periodic table are called: a. alkali metals. b. noble gases. c. chalcogens. d. halogens. What are the ions present in the compound (NH 4)2 SO 4 ? � NH 3, H 2, and SO 2 � N 3–, H+, S 2–, O 2– � NH 42+ and SO 4– � NH 4+ and SO 42– (3 IONS) � (NH 4+)2 and SO 42–

(a) a nonmetal 3 - Which of the following sets of elements is expected to have similar chemical properties? (b) found in group 6 A a) Sulfur and phosphorous (c) both a and b b) Sulfur and oxygen 1 - Selenium (34 Se) element is: c) Sulfur and argon 2 - Gallium (Ga) element is found in the periodic table in (a) period 3, group 1 B (b) period 3 A, group 4 (c) period 4, group 1 A (d) period 4, group 3 A 4 - Which of these elements is most likely to be a good conductor of electricity? Which of the following is metal? A. B. C. D. N S He Fe

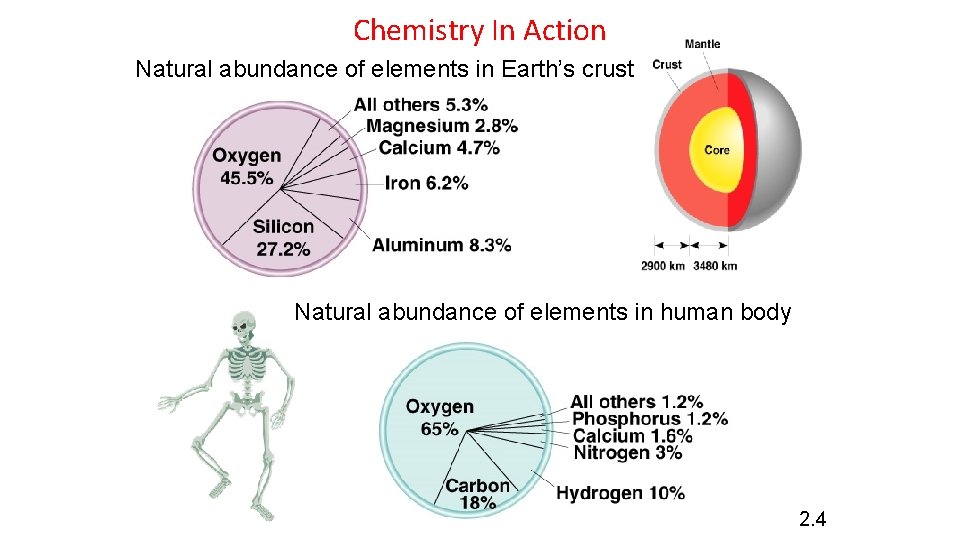
Chemistry In Action Natural abundance of elements in Earth’s crust Natural abundance of elements in human body 2. 4

2. 5 MOLECULES AND IONS.

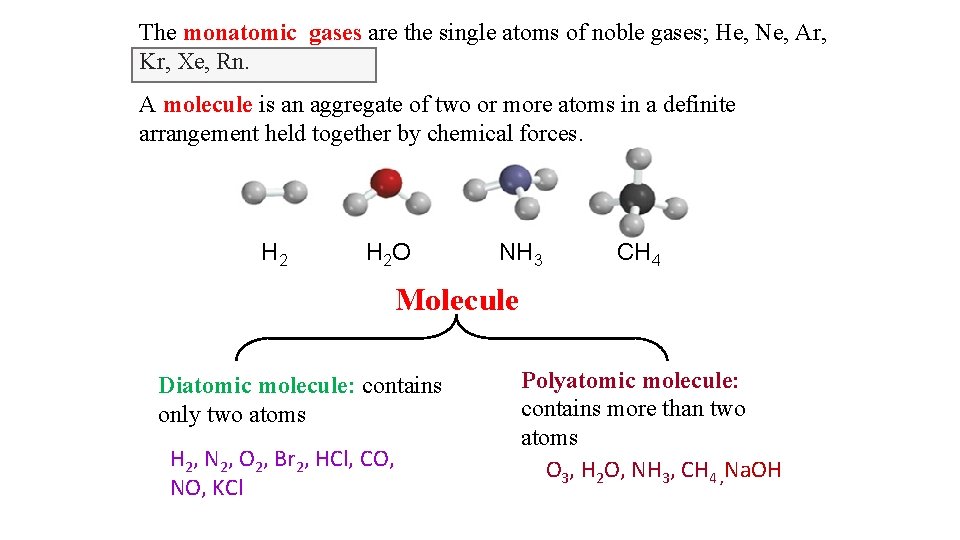
The monatomic gases are the single atoms of noble gases; He, Ne, Ar, Kr, Xe, Rn. A molecule is an aggregate of two or more atoms in a definite arrangement held together by chemical forces. H 2 O NH 3 CH 4 Molecule Diatomic molecule: contains only two atoms H 2, N 2, O 2, Br 2, HCl, CO, NO, KCl Polyatomic molecule: contains more than two atoms O 3, H 2 O, NH 3, CH 4 , Na. OH

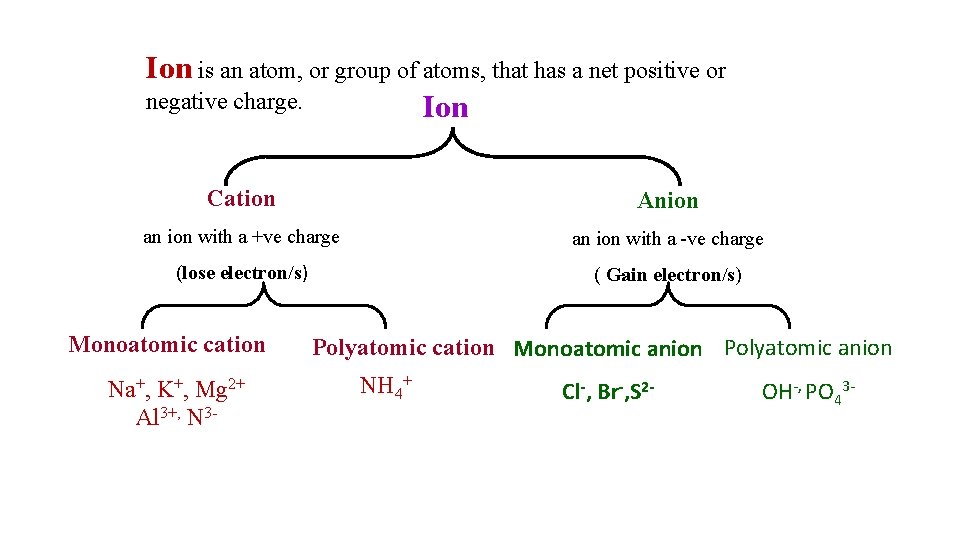
Ion is an atom, or group of atoms, that has a net positive or negative charge. Ion Cation Anion an ion with a +ve charge an ion with a -ve charge (lose electron/s) ( Gain electron/s) Monoatomic cation Na+, K+, Mg 2+ Al 3+, N 3 - Polyatomic cation Monoatomic anion Polyatomic anion NH 4+ Cl-, Br-, S 2 - OH-, PO 43 -

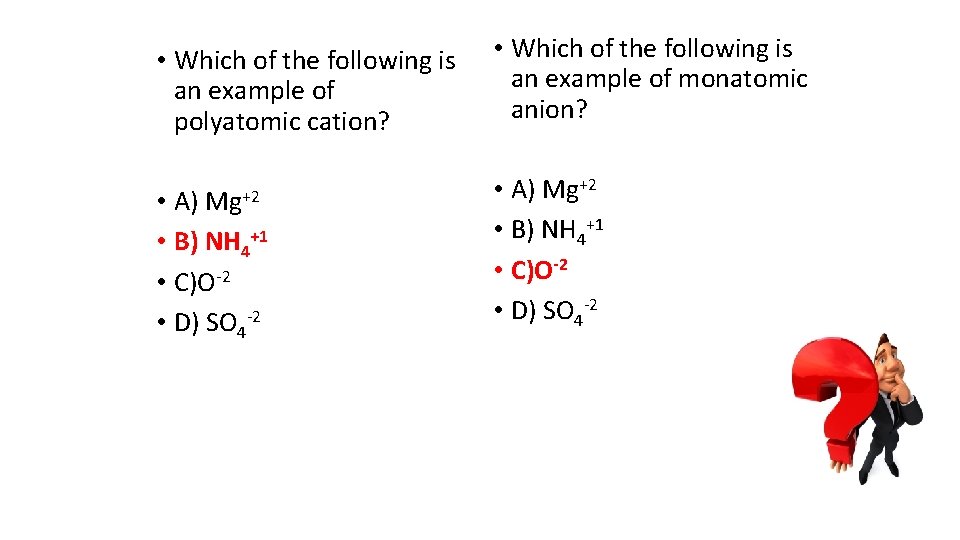
• Which of the following is an example of polyatomic cation? Mg+2 • A) • B) NH 4+1 • C)O-2 • D) SO 4 -2 • Which of the following is an example of monatomic anion? • A) Mg+2 • B) NH 4+1 • C)O-2 • D) SO 4 -2

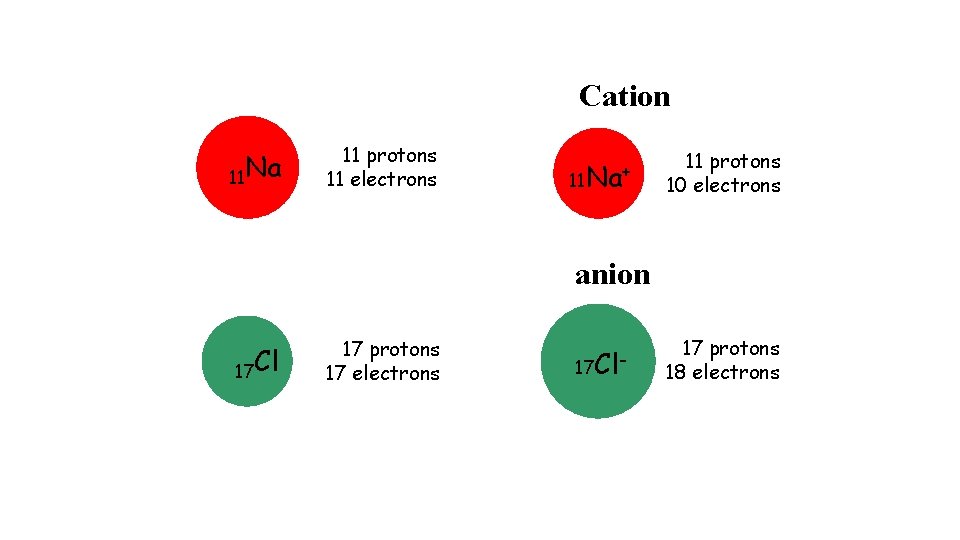
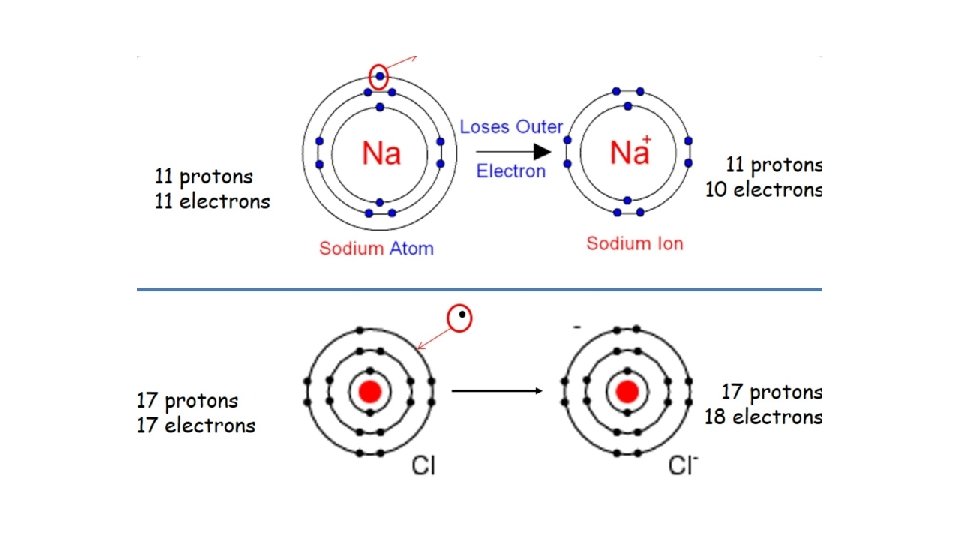
Cation 11 Na 11 protons 11 electrons 11 Na+ 11 protons 10 electrons anion 17 Cl 17 protons 17 electrons 17 Cl- 17 protons 18 electrons

Cation 11 Na 11 protons 11 electrons 11 Na+ 11 protons 10 electrons anion 17 Cl 17 protons 17 electrons 17 Cl- 17 protons 18 electrons


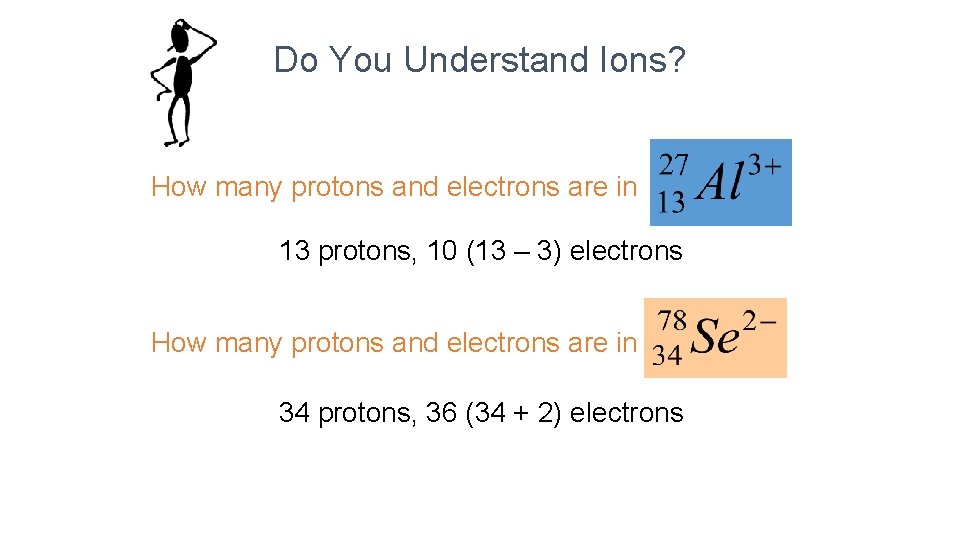
Do You Understand Ions? How many protons and electrons are in 27 3+ 13 Al ? 13 protons, 10 (13 – 3) electrons How many protons and electrons are in 78 2 Se ? 34 34 protons, 36 (34 + 2) electrons

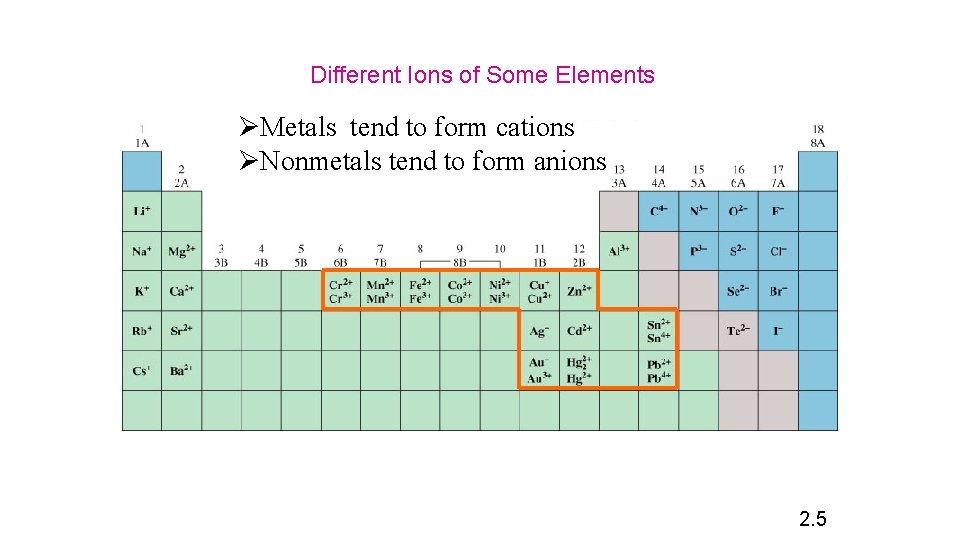
Different Ions of Some Elements ØMetals tend to form cations ØNonmetals tend to form anions 2. 5

Nonmetals are on the right side of the periodic table (with the exception of H) (blue). Metalloids border the stair-step line (with the exception of Al, Po, and At). Metals are on the left side of the chart (green color)

11 B 5 31 P 315 26 196 Au 79 222 Rn 86 18 -3=15 54 -26=28 26 -2=24 0 2+ 86 0

2. 6 CHEMICAL FORMULAS.



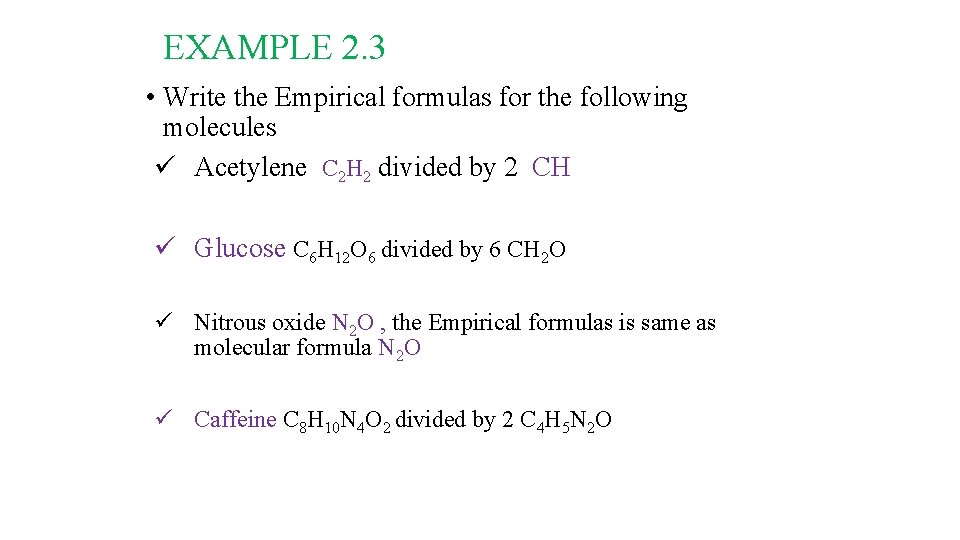
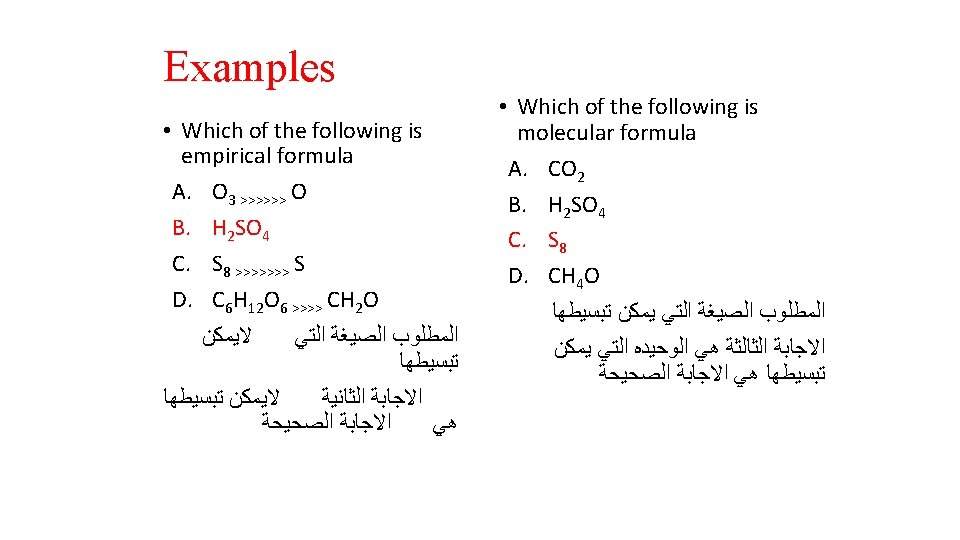
It must be noted that: • The Empirical Formula may the same or different from the Molecular Formula • How can we get the Empirical Formula? • Answer: Divide all the numbers by the largest Common devisor. • e. g. The empirical formula of H 2 O is H 2 O • e. g. the empirical formula of C 6 H 12 O 6 is CH 2 O (divide the numbers 6, 12 and 6 by the largest common devisor 6)

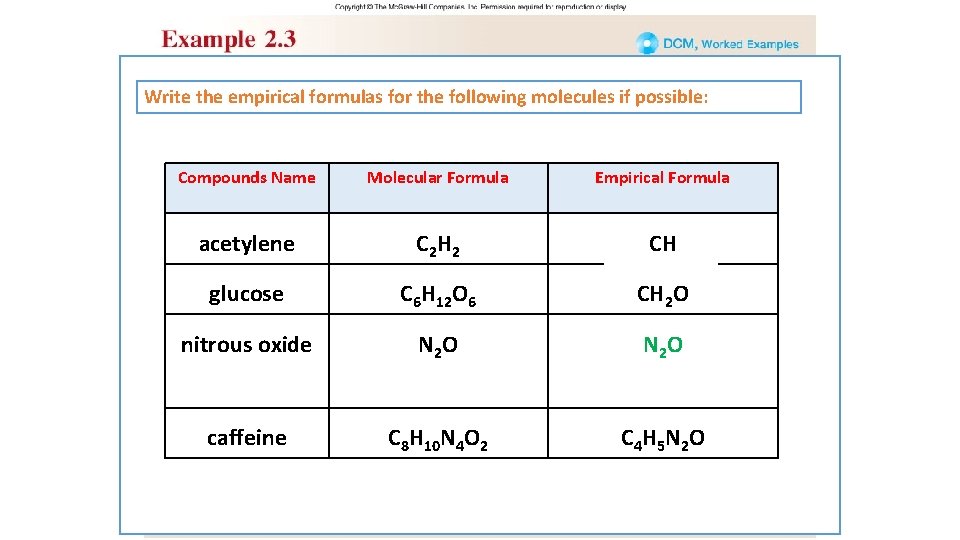
EXAMPLE 2. 3 • Write the Empirical formulas for the following molecules ü Acetylene C 2 H 2 divided by 2 CH ü Glucose C 6 H 12 O 6 divided by 6 CH 2 O ü Nitrous oxide N 2 O , the Empirical formulas is same as molecular formula N 2 O ü Caffeine C 8 H 10 N 4 O 2 divided by 2 C 4 H 5 N 2 O


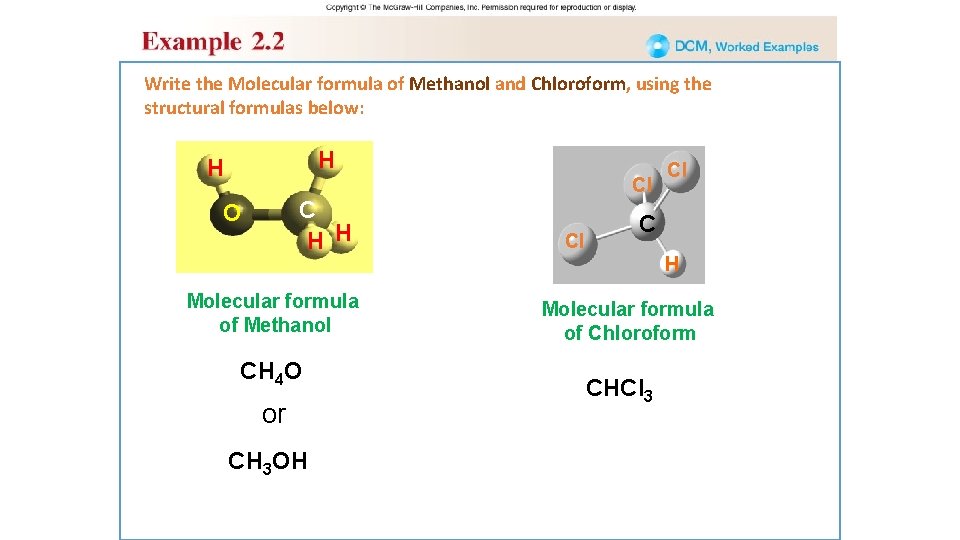
Write the Molecular formula of Methanol and Chloroform, using the structural formulas below: H H Cl C H H O Molecular formula of Methanol CH 4 O or CH 3 OH Cl Cl C H Molecular formula of Chloroform CHCl 3

Write the empirical formulas for the following molecules if possible: Compounds Name Molecular Formula Empirical Formula acetylene C 2 H 2 CH glucose C 6 H 12 O 6 CH 2 O nitrous oxide N 2 O caffeine C 8 H 10 N 4 O 2 C 4 H 5 N 2 O

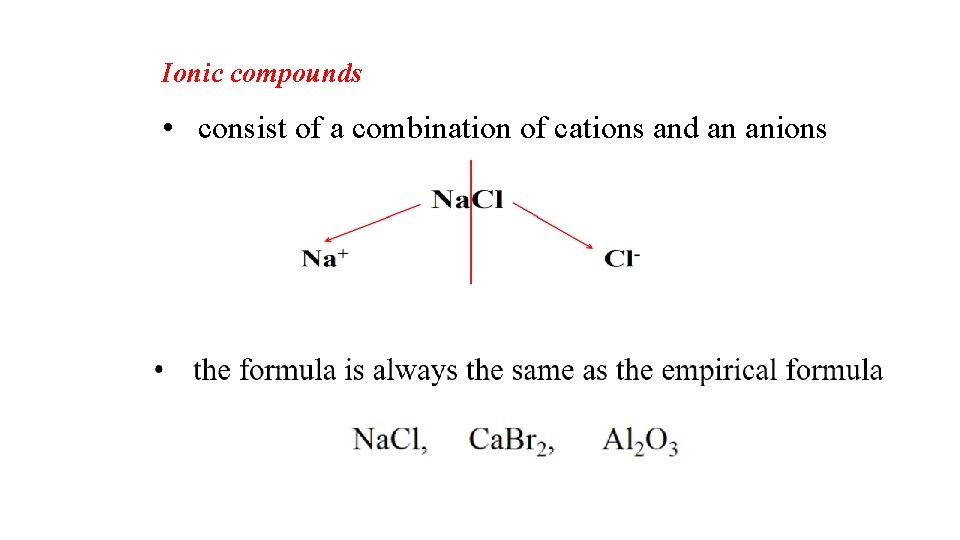
Ionic compounds • consist of a combination of cations and an anions

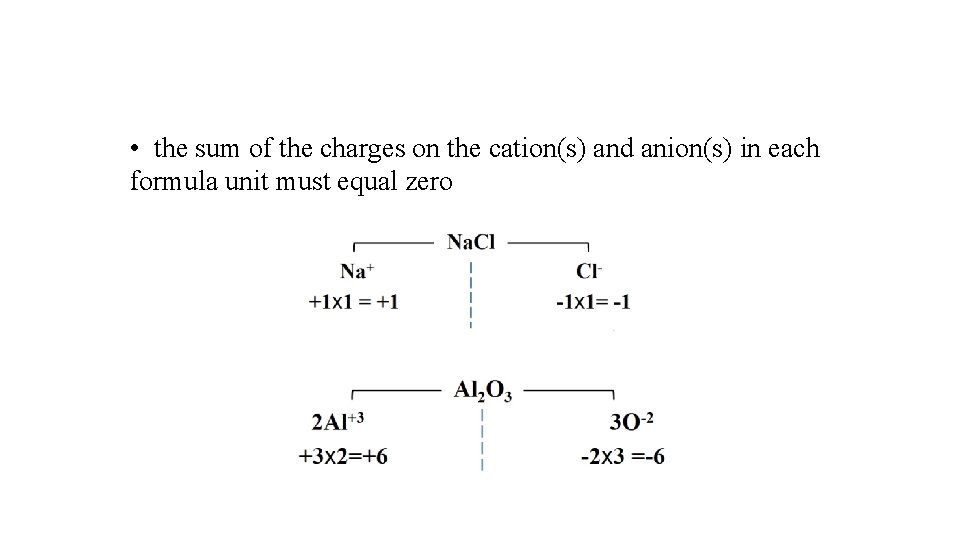
• the sum of the charges on the cation(s) and anion(s) in each formula unit must equal zero


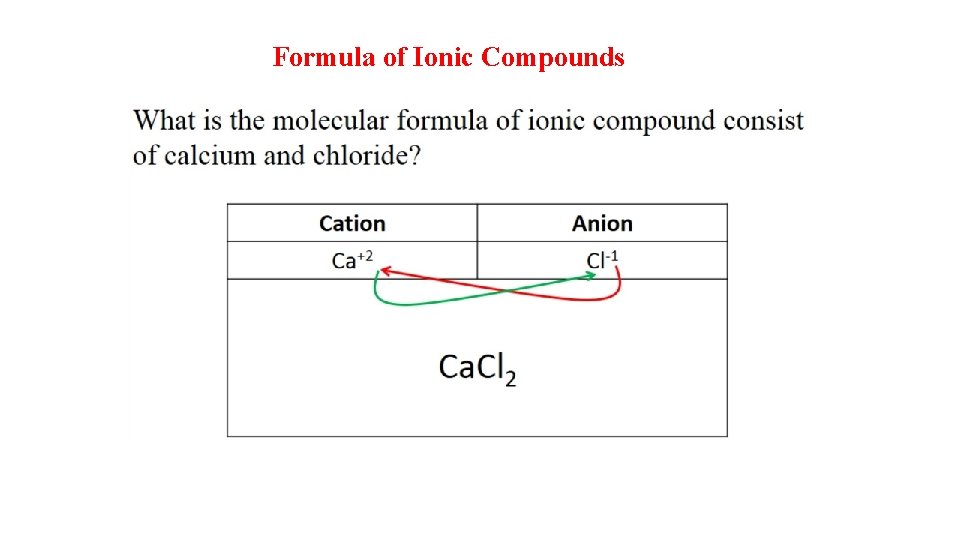
Formula of Ionic Compounds

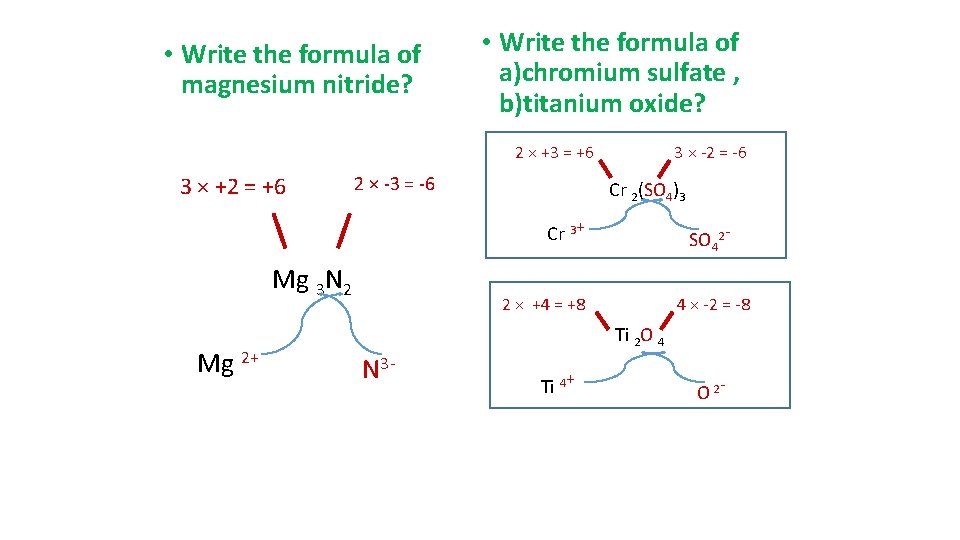
• Write the formula of magnesium nitride? • Write the formula of a)chromium sulfate , b)titanium oxide? 2 × +3 = +6 3 × +2 = +6 2 × -3 = -6 3 × -2 = -6 Cr 2(SO 4)3 Cr 3+ Mg 3 N 2 Mg 2+ SO 42 - 2 × +4 = +8 4 × -2 = -8 Ti 2 O 4 N 3 - Ti 4+ O 2 -

1. An anion is defined as A. B. C. D. a charged atom or group of atoms with a net negative charge. a stable atom. a group of stable atoms. an atom or group of atoms with a net positive charge. 2. Atoms of the same element with different mass numbers are called A. B. C. D. E. ions. neutrons. allotropes. chemical families. isotopes. 3. How many neutrons are there in an atom of lead A. B. C. D. E. 82 126 208 290 none of them 82 Pb whose mass number is 208?

4. An atom of the isotope sulfur-31 consists of how many protons, neutrons, and electrons? (p = proton, n = neutron, e = electron) A. B. C. D. E. 15 p, 16 n, 15 e 16 p, 15 n, 16 e 16 p, 31 n, 16 e 32 p, 31 n, 32 e 16 p, 16 n, 15 e 5. A magnesium ion, Mg 2+, has A. B. C. D. E. 12 protons and 13 electrons. 24 protons and 26 electrons. 12 protons and 10 electrons. 24 protons and 22 electrons. 12 protons and 14 electrons. 6. A sulfide ion, S 2 - , has: A. B. C. D. E. 16 protons and 16 electrons 32 protons and 16 electrons 16 protons and 14 electrons 16 protons and 18 electrons 32 protons and 18 electrons

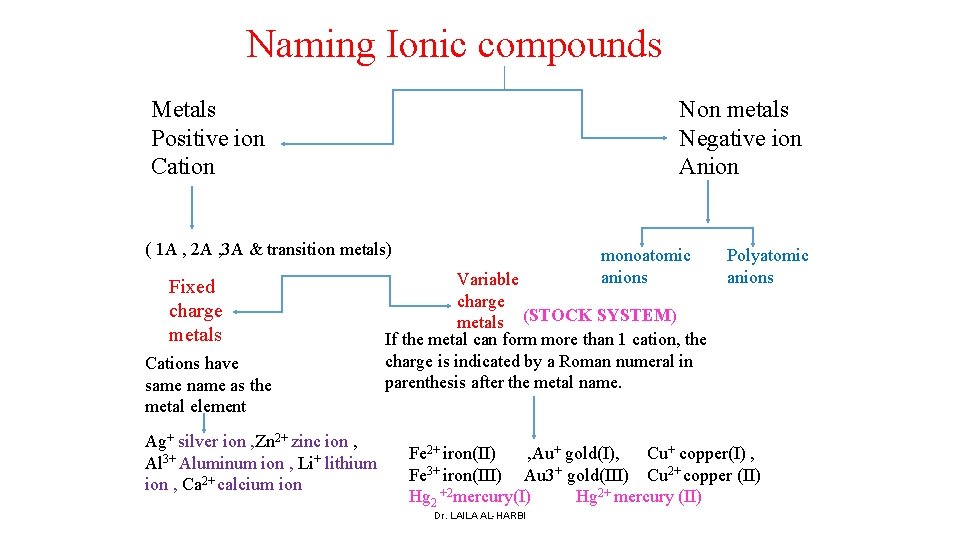
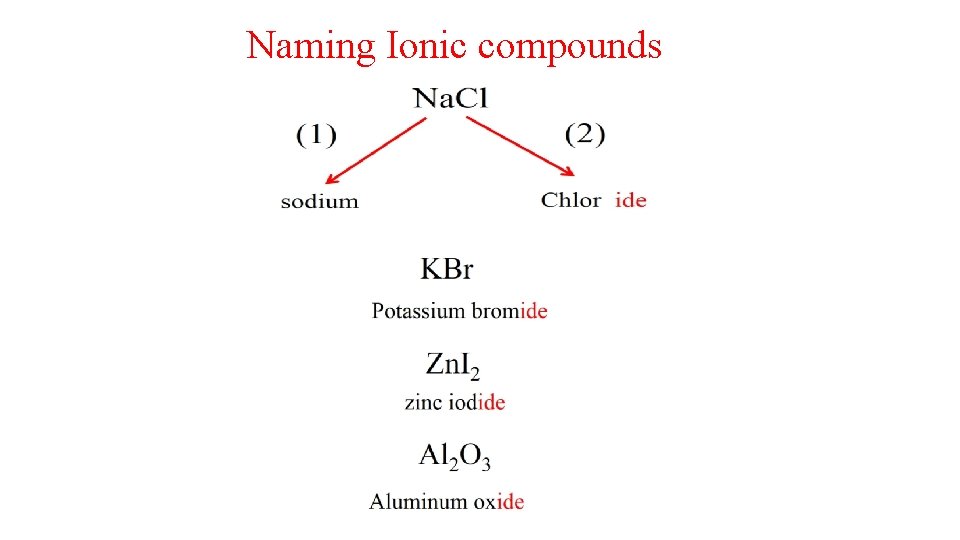
2. 7 NAMING COMPOUNDS.

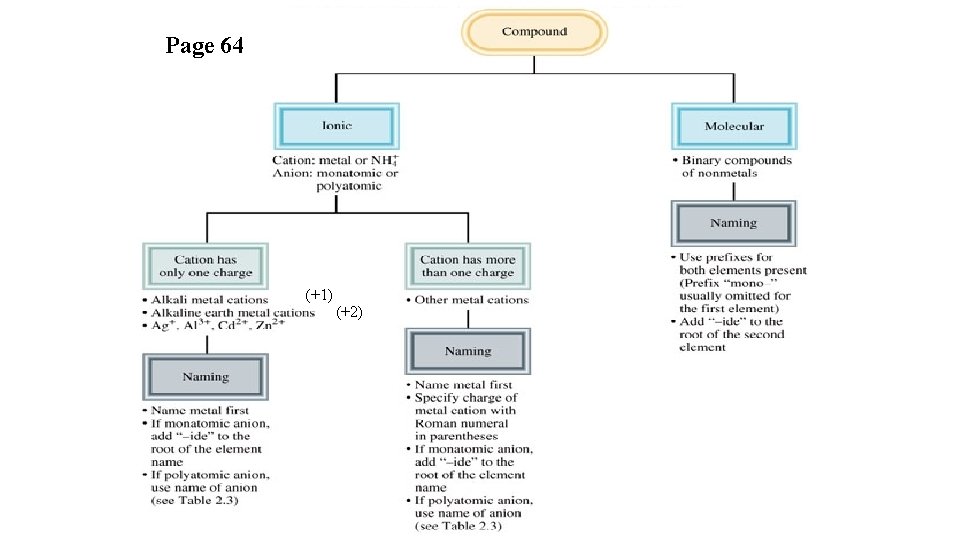
Page 64 (+1) (+2)

Naming Ionic compounds Metals Positive ion Cation Non metals Negative ion Anion ( 1 A , 2 A , 3 A & transition metals) Fixed charge metals Cations have same name as the metal element Ag+ silver ion , Zn 2+ zinc ion , Al 3+ Aluminum ion , Li+ lithium ion , Ca 2+ calcium ion monoatomic anions Variable charge metals (STOCK SYSTEM) If the metal can form more than 1 cation, the charge is indicated by a Roman numeral in parenthesis after the metal name. Polyatomic anions Fe 2+ iron(II) , Au+ gold(I), Cu+ copper(I) , Fe 3+ iron(III) Au 3+ gold(III) Cu 2+ copper (II) Hg 2 +2 mercury(I) Hg 2+ mercury (II) Dr. LAILA AL-HARBI

Naming Ionic compounds

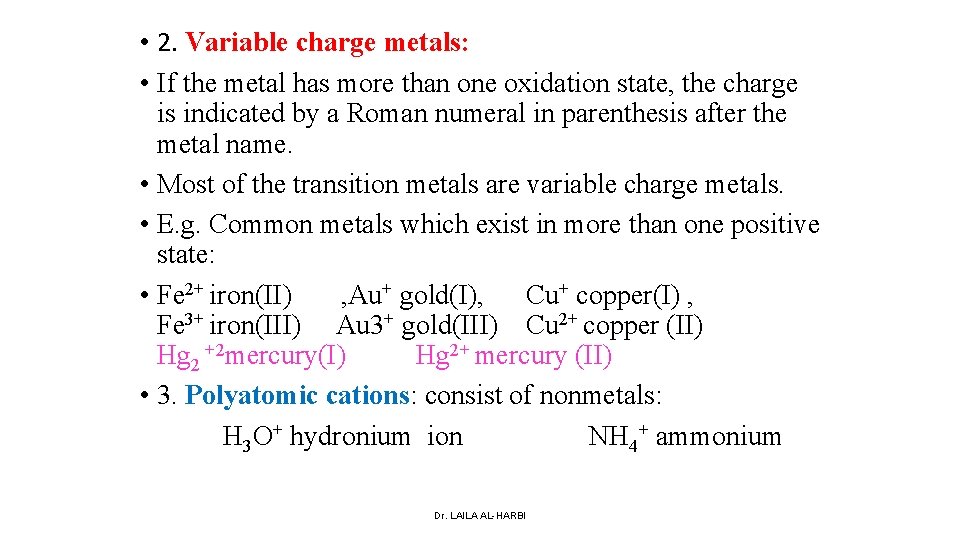
• 2. Variable charge metals: • If the metal has more than one oxidation state, the charge is indicated by a Roman numeral in parenthesis after the metal name. • Most of the transition metals are variable charge metals. • E. g. Common metals which exist in more than one positive state: • Fe 2+ iron(II) , Au+ gold(I), Cu+ copper(I) , Fe 3+ iron(III) Au 3+ gold(III) Cu 2+ copper (II) Hg 2 +2 mercury(I) Hg 2+ mercury (II) • 3. Polyatomic cations: consist of nonmetals: H 3 O+ hydronium ion NH 4+ ammonium Dr. LAILA AL-HARBI

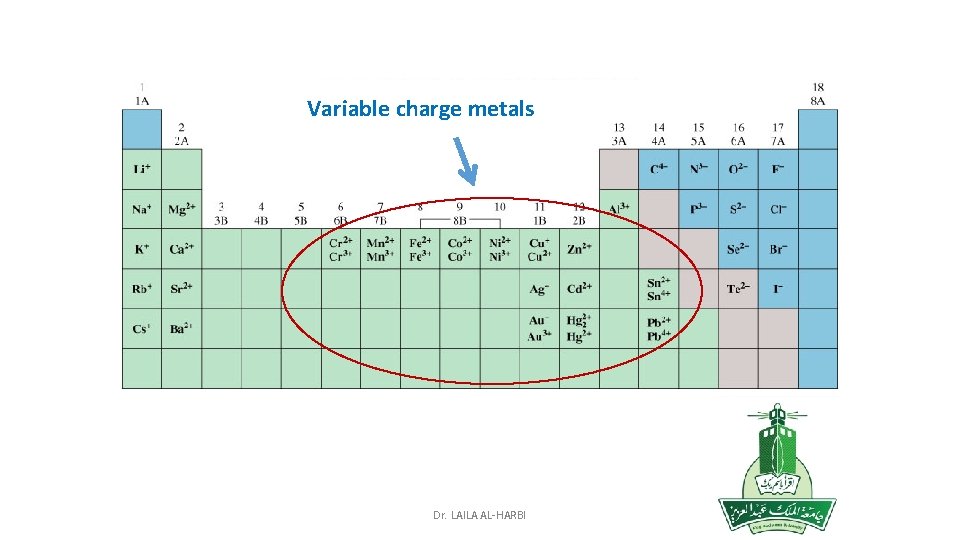
Variable charge metals Dr. LAILA AL-HARBI

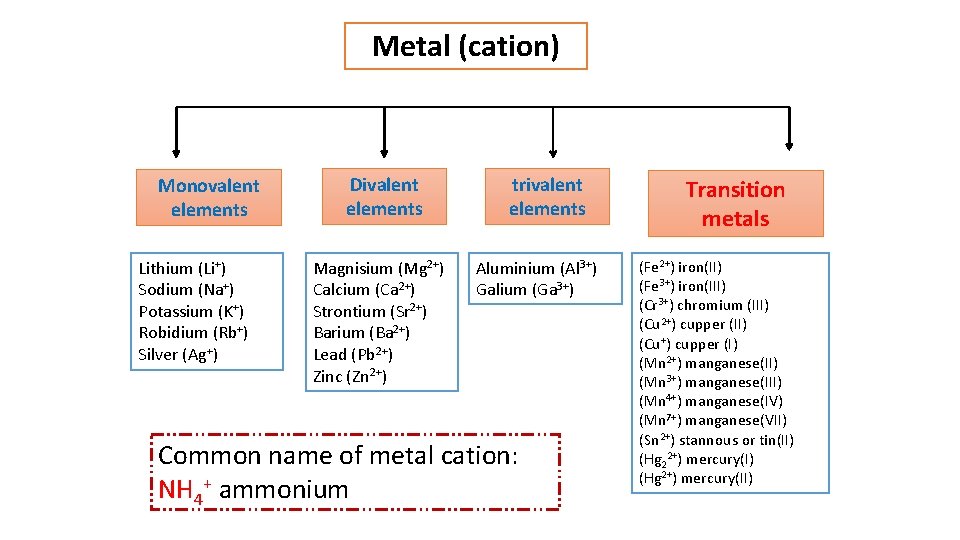
Metal (cation) Monovalent elements Lithium (Li+) Sodium (Na+) Potassium (K+) Robidium (Rb+) Silver (Ag+) Divalent elements Magnisium (Mg 2+) Calcium (Ca 2+) Strontium (Sr 2+) Barium (Ba 2+) Lead (Pb 2+) Zinc (Zn 2+) trivalent elements Aluminium (Al 3+) Galium (Ga 3+) Common name of metal cation: NH 4+ ammonium Transition metals (Fe 2+) iron(II) (Fe 3+) iron(III) (Cr 3+) chromium (III) (Cu 2+) cupper (II) (Cu+) cupper (I) (Mn 2+) manganese(II) (Mn 3+) manganese(III) (Mn 4+) manganese(IV) (Mn 7+) manganese(VII) (Sn 2+) stannous or tin(II) (Hg 22+) mercury(I) (Hg 2+) mercury(II)

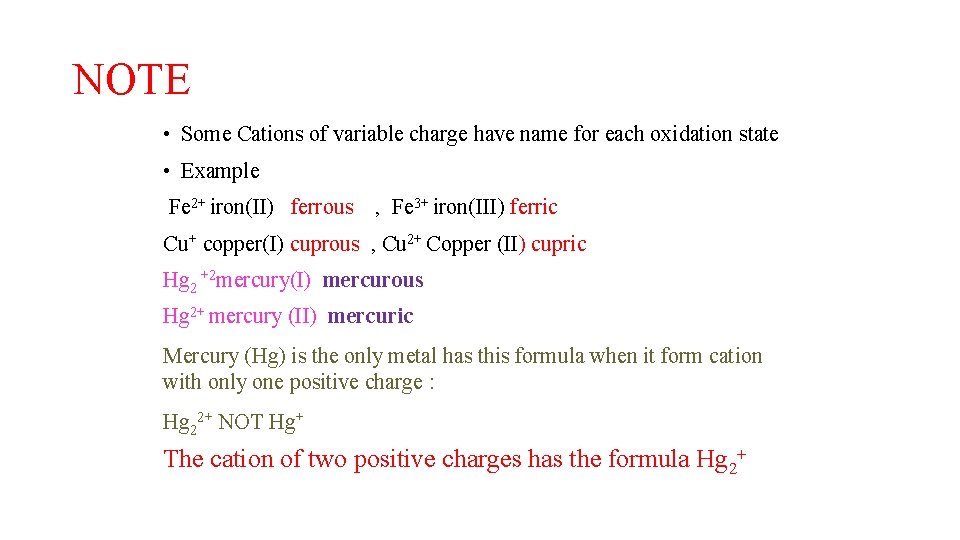
NOTE • Some Cations of variable charge have name for each oxidation state • Example Fe 2+ iron(II) ferrous , Fe 3+ iron(III) ferric Cu+ copper(I) cuprous , Cu 2+ Copper (II) cupric Hg 2 +2 mercury(I) mercurous Hg 2+ mercury (II) mercuric Mercury (Hg) is the only metal has this formula when it form cation with only one positive charge : Hg 22+ NOT Hg+ The cation of two positive charges has the formula Hg 2+ Dr. LAIL A AL HAR BI



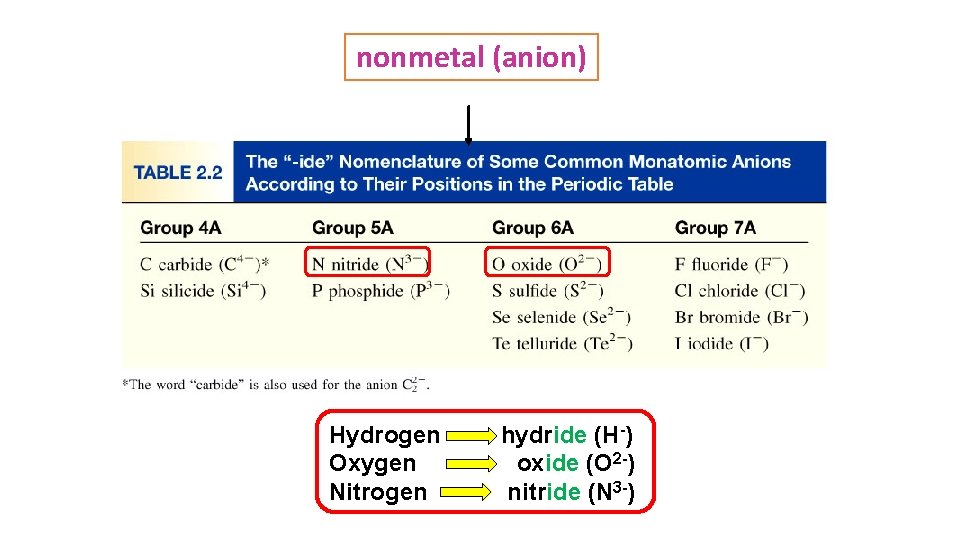
nonmetal (anion) Hydrogen Oxygen Nitrogen hydride (H-) oxide (O 2 -) nitride (N 3 -)

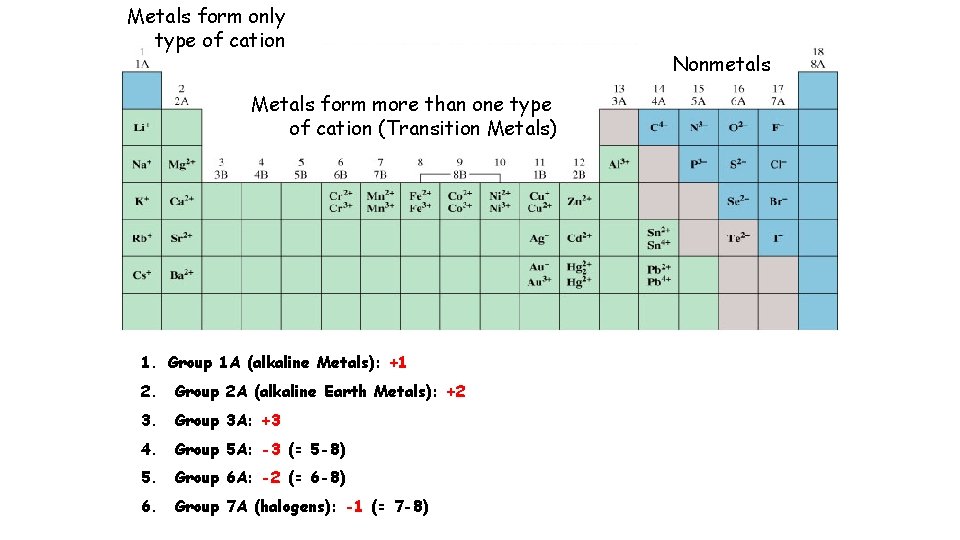
Metals form only type of cation Metals form more than one type of cation (Transition Metals) 1. Group 1 A (alkaline Metals): +1 2. Group 2 A (alkaline Earth Metals): +2 3. Group 3 A: +3 4. Group 5 A: -3 (= 5 -8) 5. Group 6 A: -2 (= 6 -8) 6. Group 7 A (halogens): -1 (= 7 -8) Nonmetals

B. Naming Anions • 1. monoatomic anions: change ending to -ide • E. g. Oxygen→ Oxide Sulfur → Sulfide Hydrogen →Hydride chlorine → Chloride Florine → Floride Bromine → Bromide • Polyatomic anions: most end in -ate or -ite; usually contain O (oxy) • Know polyatomic anions on handout. • Carbonate CO 3 -2 , Nitrate NO 3 - , Sulfate SO 4 -2 , • Phosphate PO 4 -3 Cyanide CN- , Hydroxide OH- , Oxide O 2 -2 See table 2. 3

Nonmetal polyatomic anions ﺍﺕ ﻳﺖ (SO 42 -) sulfate , (SO 32 -) sulfite (HSO 4 -) hydrogen sulfate or bisulfate (PO 43 -) phosphate (PO 33 - ) phosphite (HPO 42 -) hydrogen phosphate or biphosphate (H 2 PO 4 -) dihydrogen phosphate (NO 3 -) nitrate (NO 2 -) nitrite (Cl. O 4– ) perchlorate (Cl. O 3 - ) chlorate (CO 32–) carbonate (HCO 3– ) hydrogen carbonate or bicarbonate ﻛﺒﺮﻳﺘﺎﺕ ﻛﺒﺮﻳﺘﻴﺖ

• Ionic compounds names start with the positive ion (metal) (include Roman numeral in parenthesis ONLY IF metal has variable charge) followed by the negative ion (nonmetal). • Na. Cl Sodium Chloride • Ba. Cl 2 Barium Chloride • K 2 O Potassium oxide • KNO 3 Potassium Nitrate • Na 2 CO 3 Sodium Carbonate • Fe. Cl 2 Iron(II) Chloride → ferrous Chloride • Fe. Cl 3 Iron(III) Chloride → ferric Chloride • Cr 2 S 3 Chromium(III) Sulfide • (NH 4)3 PO 4 Ammonium Phosphate • Cu(NO 3)2 Cupper(II)nitrate • Pb. O Lead(II) oxide



Example 2. 5 p 61: Name the following compounds: (a) Cu(NO 3)2 1. Cation: Copper cation (can form two types of cation →Stock system) → Copper (II) 2. Anion: NO 3 - anion has a common name Nitrate ﻗﺎﻋﺪﺓ ﺗﺒﺎﺩﻝ ﺍﻟﺘﻜﺎﻓﺆﺎﺕ Thus: the name of the compound is: Copper (II) nitrate Dr. LAILA AL-HARBI

(b) KH 2 PO 4 1. Cation: K form only one type of cation → Potassium Note: not potassium (I) 2. Anion: H 2 PO 4 - has a common name dihydrogen phosphate Thus: the name of the compound is: Potasium dihydroen phosphate

• Write the chemical formula for the following compounds • Mercury(I)nitrite Hg 2 (NO 2)2 • Cesium sulfide Ce 2 S ØCalcium phosphate Ø Ca 3 (PO 4)2 • Write the chemical formula for the following compounds • Rubidium sulfate • Rb 2 SO 4 • Barium hydride • Ba. H 2 Dr. LAILA AL-HARBI

Name the following ionic compounds: Cu(NO 3)2 Cupper(II) nitrate KH 2 PO 4 Potassium dihydrogen phosphate NH 4 SCN Ammonium thiocyanate H 2 O 2 Hydrogen peroxide Li 2 SO 3 Lithium sulfite Ca 3(PO 3)2 Calcium phosphite KMn. O 4 Potassium permenganate Hg 2 Cl 2 Mercury(I) chloride K 2 Cr. O 4 Potassium chromate Br 2 O 7 Dibromine heptoxide

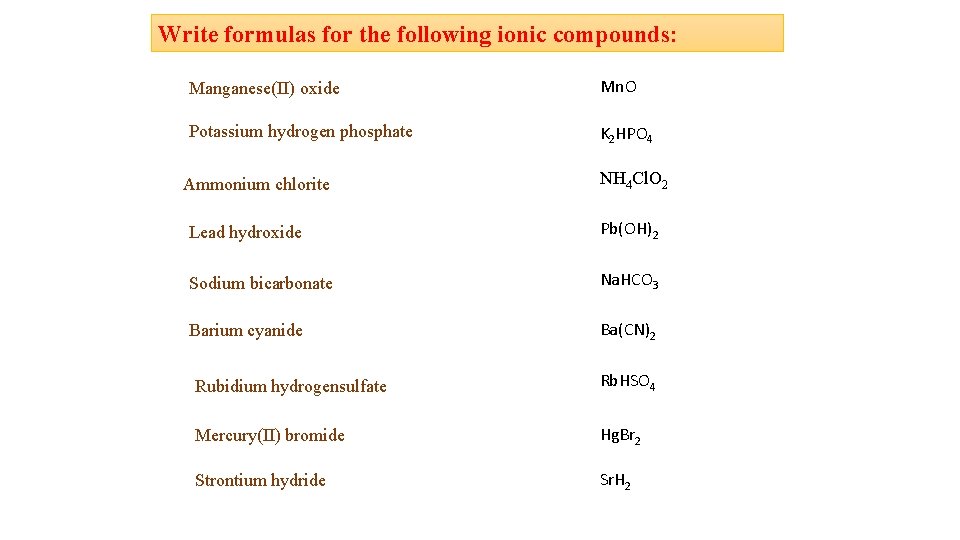
Write formulas for the following ionic compounds: Manganese(II) oxide Mn. O Potassium hydrogen phosphate K 2 HPO 4 Ammonium chlorite NH 4 Cl. O 2 Lead hydroxide Pb(OH)2 Sodium bicarbonate Na. HCO 3 Barium cyanide Ba(CN)2 Rubidium hydrogensulfate Rb. HSO 4 Mercury(II) bromide Hg. Br 2 Strontium hydride Sr. H 2

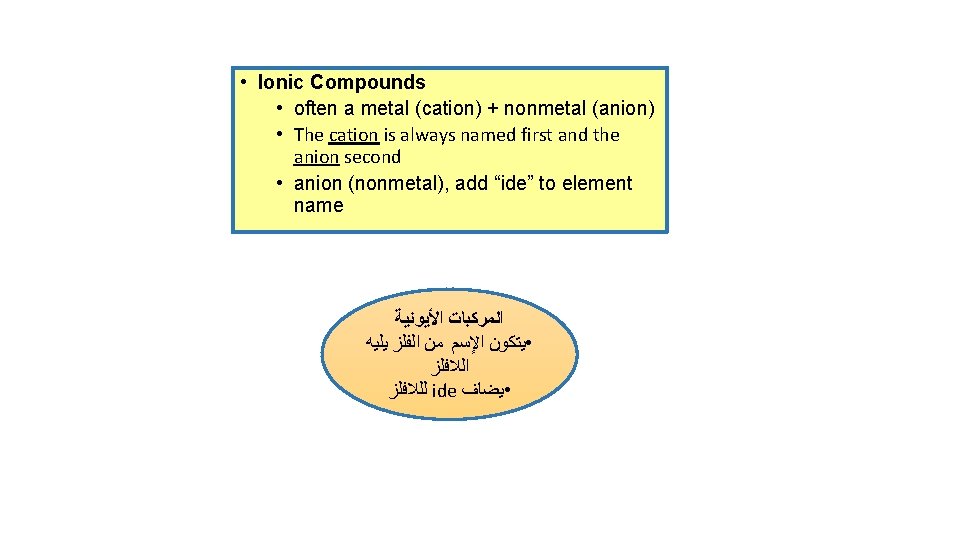
• Ionic Compounds • often a metal (cation) + nonmetal (anion) • The cation is always named first and the anion second • anion (nonmetal), add “ide” to element name ﺍﻟﻤﺮﻛﺒﺎﺕ ﺍﻷﻴﻮﻧﻴﺔ • ﻳﺘﻜﻮﻥ ﺍﻹﺳﻢ ﻣﻦ ﺍﻟﻔﻠﺰ ﻳﻠﻴﻪ ﺍﻟﻼﻓﻠﺰ ﻟﻠﻼﻓﻠﺰ ide • ﻳﻀﺎﻑ

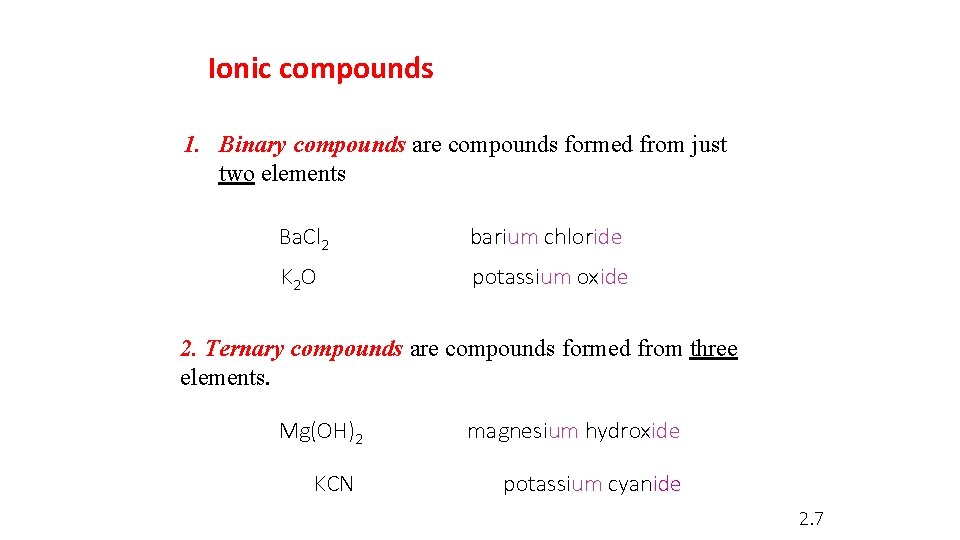
Ionic compounds 1. Binary compounds are compounds formed from just two elements Ba. Cl 2 barium chloride K 2 O potassium oxide 2. Ternary compounds are compounds formed from three elements. Mg(OH)2 KCN magnesium hydroxide potassium cyanide 2. 7


4 - element further left in periodic table is 1 st (less electronegative) (HCl) H(1 A) and Cl(7 A), so hydrogen is 1 st, the name is hydrogen chloride 5 - element closest to bottom of group is 1 st (less electronegative) (Si. C) Si(4 A) and C also belongs to (4 A) , but (Si) is closest to bottom of the group, so silicon is 1 st , the name is silicon carbide • ﺗﻜﺘﺐ ﺍﻟﻌﻨﺎﺻﺮ ﺍﻟﻤﻮﺟﻮﺩﻩ ﻳﺴﺎﺭ ﺍﻟﺠﺪﻭﻝ ﺍﻟﺪﻭﺭﻱ ﺃﻮ ﺍﻷﻘﺮﺏ . ﻟﻠﻴﺴﺎﺭ ﺃﻮﻻ • ﺍﻟﻤﺮﻛﺒﺎﺕ ﺍﻟﻤﺤﺘﻮﻳﺔ ﻋﻠﻰ ﺃﻜﺜﺮ ﻣﻦ ﺫﺭﺓ ﻣﻦ ﻧﻔﺲ ﺍﻟﻌﻨﺼﺮ ﻳﻌﺒﺮ ﻋﻦ ﻋﺪﺩ ﺍﻟﺬﺭﺍﺕ ﻟﻠﻌﻨﺼﺮ ﻟﻬﺎ ﺑﺎﺳﺘﺨﺪﺍﻡ ﺍﻟﺒﺎﺩﺋﺎﺕ ﺍﻟﻴﻮﻧﺎﻧﻴﺔ

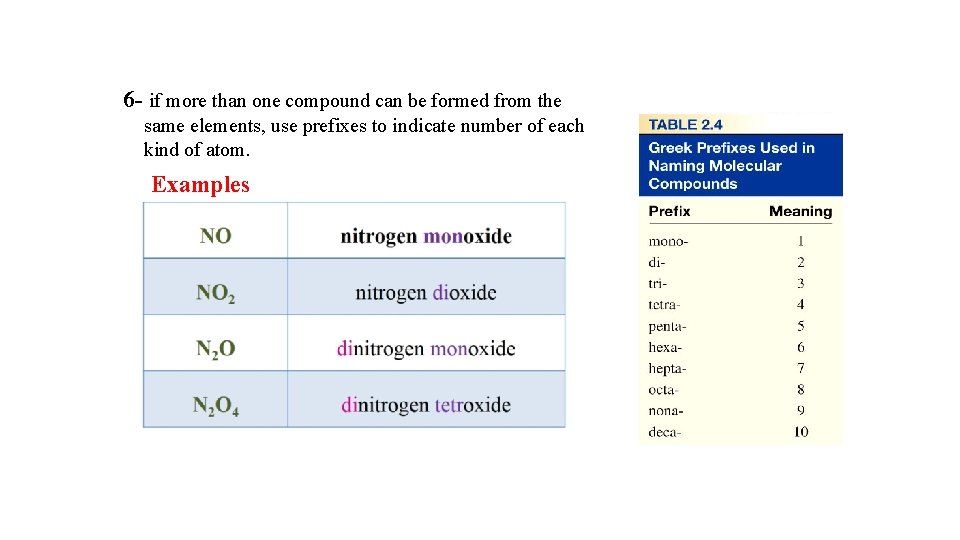
6 - if more than one compound can be formed from the same elements, use prefixes to indicate number of each kind of atom. Examples

Note • Note that mono- is never used for the first element • For oxides, the ending “a” in the prefix is omitted. • N 2 O 4 dinitrogen tetroxide not (dinitrogen tetraoxide) • For oxides, the ending “o” in the prefix is omitted. • N 2 O dinitrogen monoxide not (dinitrogen monooxide ) Dr. LAILA AL-HARBI

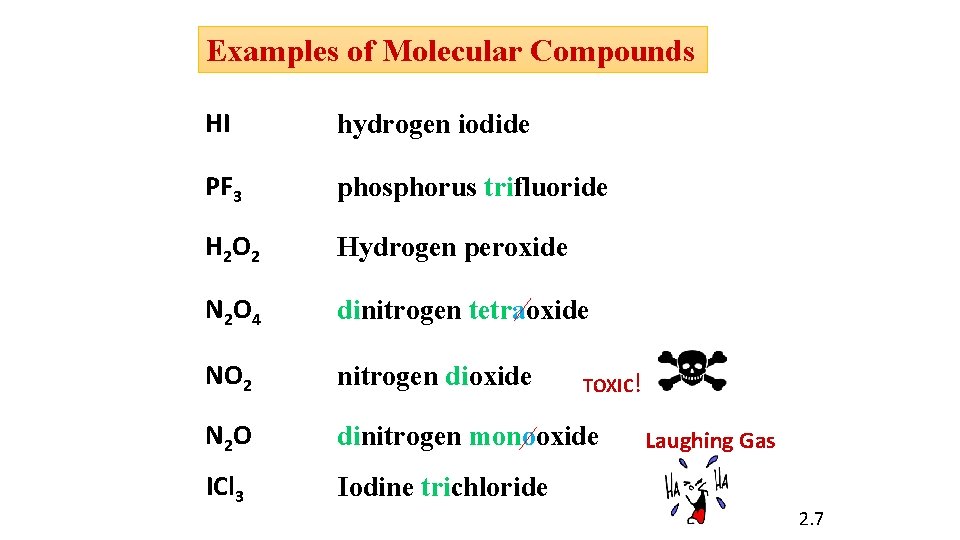
Examples of Molecular Compounds HI hydrogen iodide PF 3 phosphorus trifluoride H 2 O 2 Hydrogen peroxide N 2 O 4 dinitrogen tetraoxide NO 2 nitrogen dioxide N 2 O dinitrogen monooxide ICl 3 Iodine trichloride TOXIC! Laughing Gas 2. 7

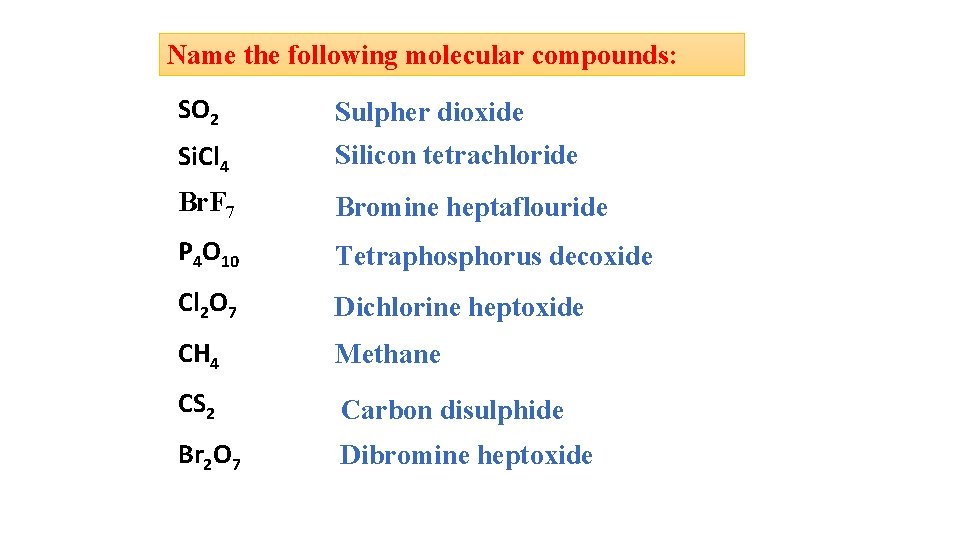
Name the following molecular compounds: SO 2 Sulpher dioxide Si. Cl 4 Silicon tetrachloride Br. F 7 Bromine heptaflouride P 4 O 10 Tetraphosphorus decoxide Cl 2 O 7 Dichlorine heptoxide CH 4 Methane CS 2 Carbon disulphide Br 2 O 7 Dibromine heptoxide

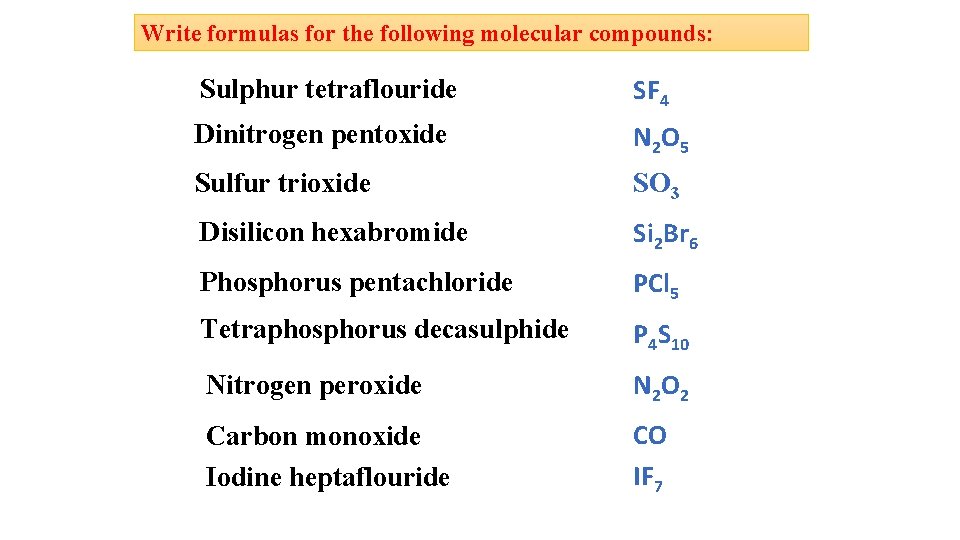
Write formulas for the following molecular compounds: Sulphur tetraflouride SF 4 Dinitrogen pentoxide N 2 O 5 Sulfur trioxide SO 3 Disilicon hexabromide Si 2 Br 6 Phosphorus pentachloride PCl 5 Tetraphosphorus decasulphide P 4 S 10 Nitrogen peroxide N 2 O 2 Carbon monoxide Iodine heptaflouride CO IF 7

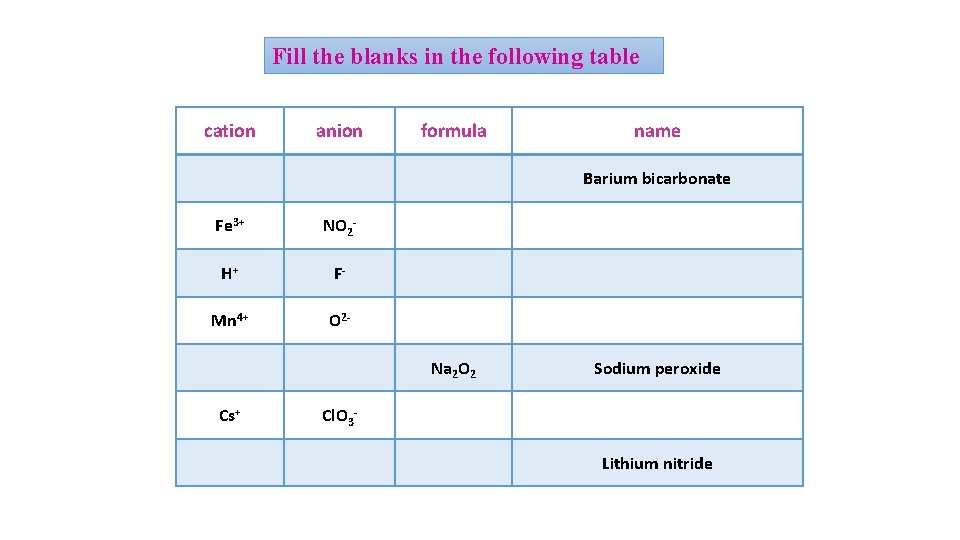
Fill the blanks in the following table cation anion formula name Ba 2+ HCO 3 - Ba(HCO 3)2 Barium bicarbonate Fe 3+ NO 2 - Fe (NO 2)3 Iron(III)nitrite H+ F- HF Hyrdogen flouride Mn 4+ O 2 - Mn. O 2 Manganise(IV)oxide Na+ O 22 - Na 2 O 2 Sodium peroxide Cs+ Cl. O 3 - Cs. Cl. O 3 Cesium chlorate Li+ N 3 - Li 3 N Lithium nitride

• Which of these pairs of elements would be most likely to form an ionic compound? (a) P and Br (b) Cu and K (c) C and O (d) O and Zn (e) Al and Rb • Which of these pairs of elements would be most likely to form a molecular compound? (a) Na and Br (b) C and O (c) Ca and O (d) Zn and O (e) Mg and Cl

8. 9. Which of the following compounds is named lithium carbonate? A) Na 2 CO 3 B) Li. HCO 3 C) Li. CO D) Li 2 CO 3 What is the name of KCl. O? A) potassium chlorite B) potassium chloride C) potassium hypochlorite D) potassium oxide 10. What is the formula for ammonium sulfate? A) NH 4 SO 4 B) NH 4(SO 4)2 C) (NH 4)2 SO 4 D) NH 4 S