IONS and E config u Ions are atoms



















- Slides: 23

IONS and E config u Ions are atoms that have lost or gained electrons. u Remember that the number of protons cannot change for an element. u When an atom gains more electrons than the number of protons it has it will have a negative charge.

u When an element loses more electrons than the number of protons it has, it will have a positive charge. u What drives atoms to give away electrons or take on more?

Driving Force u Full Energy Levels are very low energy u Noble Gases have full orbitals u Atoms behave in ways to achieve noble gas configuration

Ionic Size u Cations are positive ions u Cations form by losing electrons u Cations are smaller than the atom they come from u Metals form cations u Cations of representative elements have noble gas configuration.

Ionic size u Anions are negative ions u Anions form by gaining electrons u Anions are bigger than the atom they come from u Nonmetals form anions u Anions of representative elements have noble gas configuration.

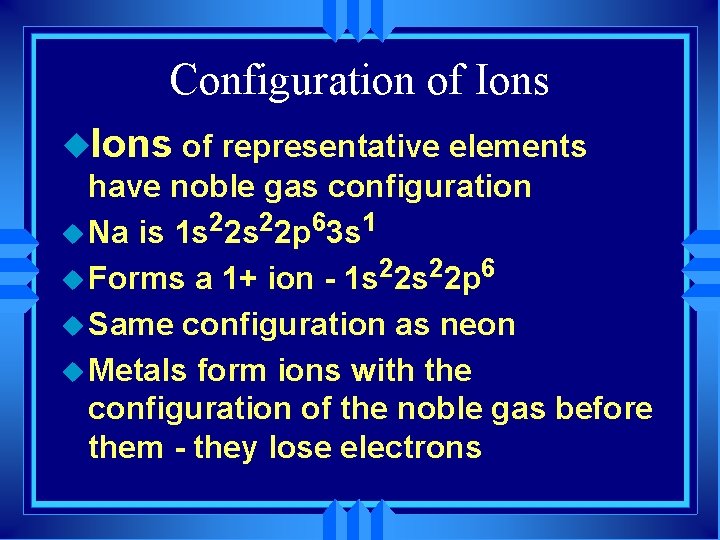
Configuration of Ions u. Ions of representative elements have noble gas configuration is 1 s 22 p 63 s 1 u Forms a 1+ ion - 1 s 22 p 6 u Same configuration as neon u Metals form ions with the configuration of the noble gas before them - they lose electrons u Na

Configuration of Ions u Non-metals form ions by gaining electrons to achieve noble gas configuration. u They end up with the configuration of the noble gas after them.

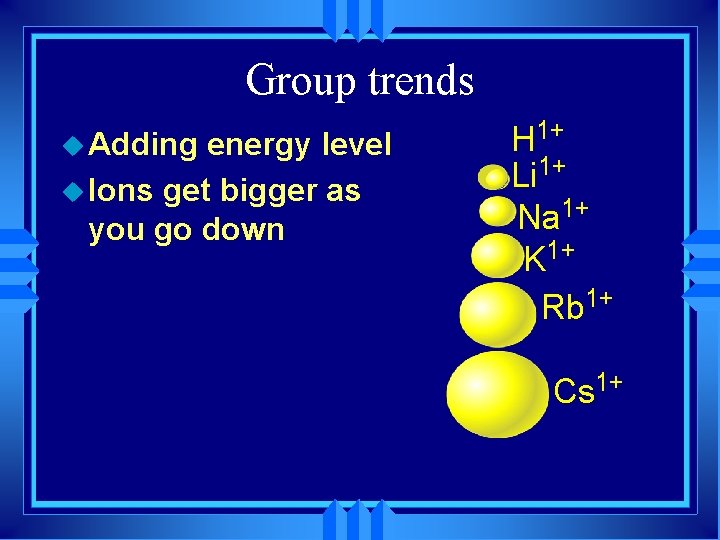
Group trends u Adding energy level u Ions get bigger as you go down H 1+ Li 1+ Na 1+ K 1+ Rb 1+ Cs 1+

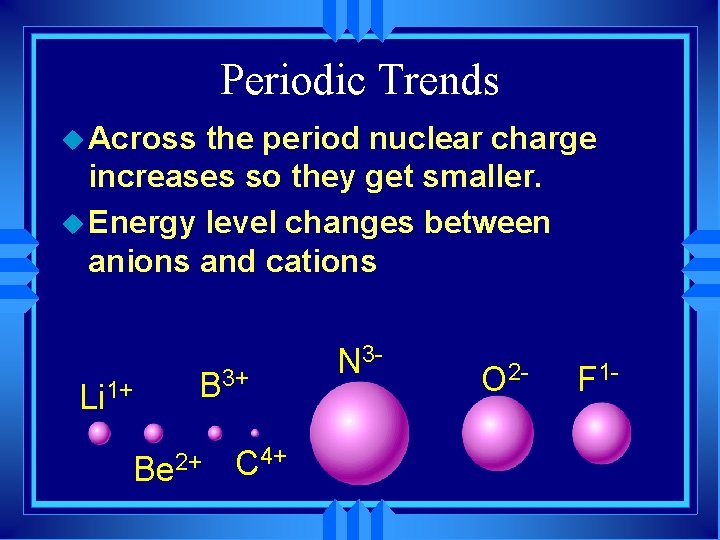
Periodic Trends u Across the period nuclear charge increases so they get smaller. u Energy level changes between anions and cations Li 1+ B 3+ Be 2+ C 4+ N 3 - O 2 - F 1 -

Keeping Track of Electrons u The electrons responsible for the chemical properties of atoms are those in the outer energy level. u Valence electrons - The s and p electrons in the outer energy level. u Core electrons -those in the energy levels below. u Basis for shorthand

Keeping Track of Electrons u Atoms in the same column u Have the same properties because u Have the same outer electron configuration. u Have the same valence electrons. u Found by looking up the group number on the periodic table. u Group 2 A - Be, Mg, Ca, etc. u 2 valence electrons

Lewis Dot diagrams help us determine the charge

Electron Dot diagrams u. A way of keeping track of valence electrons. u How to write them u Write the symbol. u Put one dot for each valence electron u Don’t pair up until they have to X

The Electron Dot diagram for Nitrogen § Nitrogen has 5 valence electrons. § First we write the symbol. §Then add 1 electron at a time to each side. §Until they are forced to pair up. N

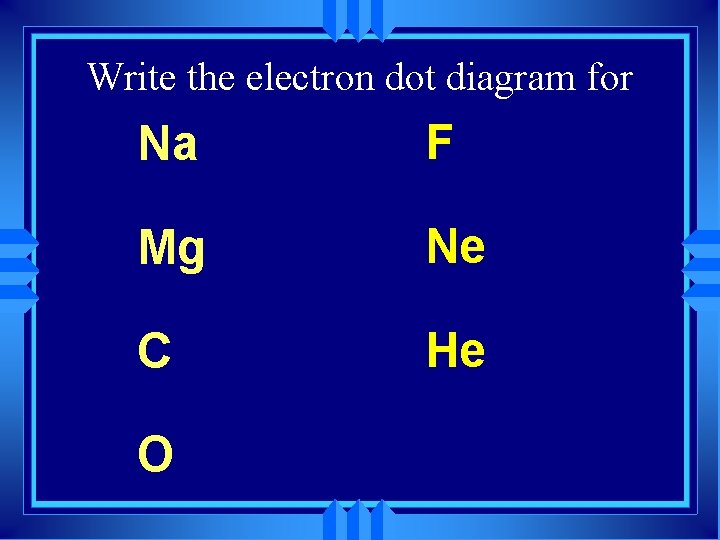
Write the electron dot diagram for Na F Mg Ne C He O

Electron Configurations for Cations u Metals lose electrons to attain noble gas configuration. u They make positive ions. u Na 1 s 22 p 63 s 1 - 1 valence electron u Na+ 1 s 22 p 6 -noble gas configuration

Electron Dots For Cations u Metals will have few valence electrons Ca

Electron Dots For Cations u Metals will have few valence electrons u These will come off Ca

Electron Dots For Cations u Metals will have few valence electrons u These will come off u Forming positive ions 2+ Ca

Electron Configurations for Anions u Nonmetals gain electrons to attain noble gas configuration. u They make negative ions. u S 1 s 22 p 63 s 23 p 4 - 6 valence electrons u S 2 - 1 s 22 p 63 s 23 p 6 -noble gas configuration.

Electron Dots For Anions u Nonmetals will have many valence . electrons. u They will gain electrons to fill outer shell. P 3 P

Practice u Use electron dot diagrams to show the following form ions u Al u Cl u. C

Stable Electron Configurations u All atoms react to achieve noble gas configuration. u Noble gases have 2 s and 6 p electrons. u 8 valence electrons. u Also called the octet rule. Ar