Zumdahls Chapter 2 Atoms Molecules and Ions Chapter





- Slides: 13

Zumdahl’s Chapter 2 Atoms, Molecules, and Ions

Chapter Contents n n n History of Chemistry Mass & Proportions Dalton Theory Subatomic Particles Structure of the Atom Molecules and Ions n Periodic Table n n Symbols and Organization Naming Compounds n n n Binary ionic molecule Binary covalent compounds Polyatomic ions

History of Chemistry n n Democritus (5 th Century BC) atomic postulate Lucretius (1 st Century AD) “atoms and the void” Priestly (18 th Century AD) discovers oxygen Lavoisier (18 th) diamond=carbon

Conservation of Mass and Chemical Proportions n n n Mass unchanged in chemical reactions Implies atoms conserved in reactions Elements combine in definite, simple proportions by mass. n n n Molecules are atoms in definite proportions! - Dalton

John Dalton (1808) n n Elements are collections of identical, miniscule atoms. Different elements differ in their atoms. Compounds are combinations of different elements. Under reaction, compounds rearrange their atoms.

Subatomic Components n n n J. J. Thompson (1897) “cathode rays are electrons” (e–) and finds e/m ratio Robert Millikan (1909) measures e and hence melectron known at 9. 11 10 -31 kg E. Rutherford (1906) bounces (He 2+) off Au tissue proving protons (p+) in nucleus F. A. Aston (1919) “weighs” atomic ions J. Chadwick (1939) observes neutrons (no charge) by decomposition (to p+, e–, and ).

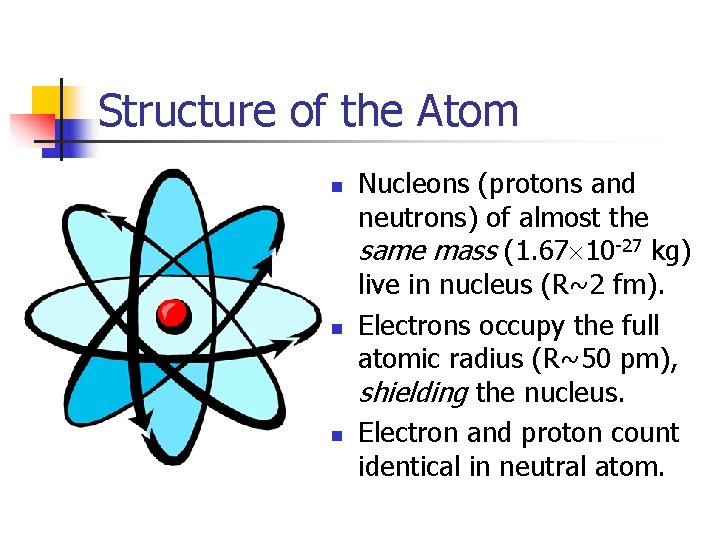
Structure of the Atom n n n Nucleons (protons and neutrons) of almost the same mass (1. 67 10 -27 kg) live in nucleus (R~2 fm). Electrons occupy the full atomic radius (R~50 pm), shielding the nucleus. Electron and proton count identical in neutral atom.

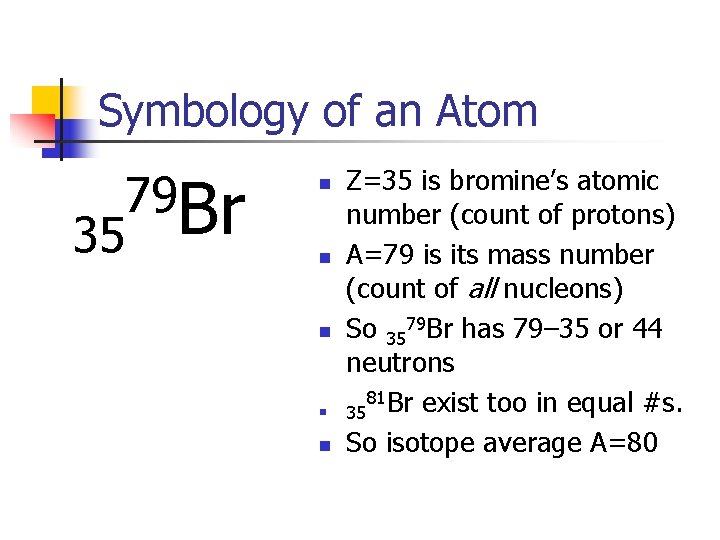
Symbology of an Atom 35 79 Br n n n Z=35 is bromine’s atomic number (count of protons) A=79 is its mass number (count of all nucleons) So 3579 Br has 79– 35 or 44 neutrons 81 Br exist too in equal #s. 35 So isotope average A=80

Molecules and Ions n n Atoms in molecules share (covalent) or steal (ionic) electrons to bond. Stolen electrons lead to attraction of unlike charged ions (ionic bonding) Directional electron “clouds” lead to molecular shapes. Molecules can be ionic as well as atoms.

Periodic Table n n Z increases linearly from 1 H upper left Groups (columns) have similar chemistry n Alkali metals, alkaline earths, transition metals, halogens and noble gases

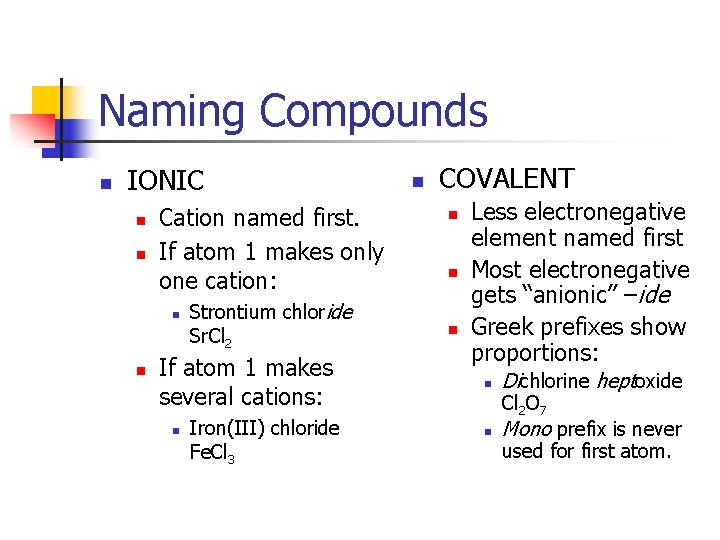
Naming Compounds n IONIC n n Cation named first. If atom 1 makes only one cation: n n Strontium chloride Sr. Cl 2 If atom 1 makes several cations: n Iron(III) chloride Fe. Cl 3 n COVALENT n n n Less electronegative element named first Most electronegative gets “anionic” –ide Greek prefixes show proportions: n Dichlorine heptoxide n Mono prefix is never Cl 2 O 7 used for first atom.

Polyatomic Ions n Few polyatomic cations n n Most common: ammonium (NH 4)+ Many polyatomic anions n n NO 3– nitrate, C 2 O 42– oxalate, HSO 4– hydrogen sulfate, H 2 PO 4– dihydrogen phosphate, Cr 2 O 72– dichromate Cl. O– hypochlorite, Cl. O 2– chlorite, Cl. O 3– chlorate, Cl. O 4– perchlorate

Naming Exercise n n n n Al 2(S 2 O 3)3 P 4 O 10 Cu(NO 2)2 Na. Mn. O 4 CS 2 Fe 2(Cr. O 4)3 HCl (gas) PH 4 Br. O 2 n n n n Aluminum thiosulfate Tetraphosphorous decaoxide Copper(II) nitrite Sodium permanganate Carbon disulfide Iron(III) chromate Hydrogen chloride Phosphonium bromite