Topic 1 Qualitative testing Must list the cation





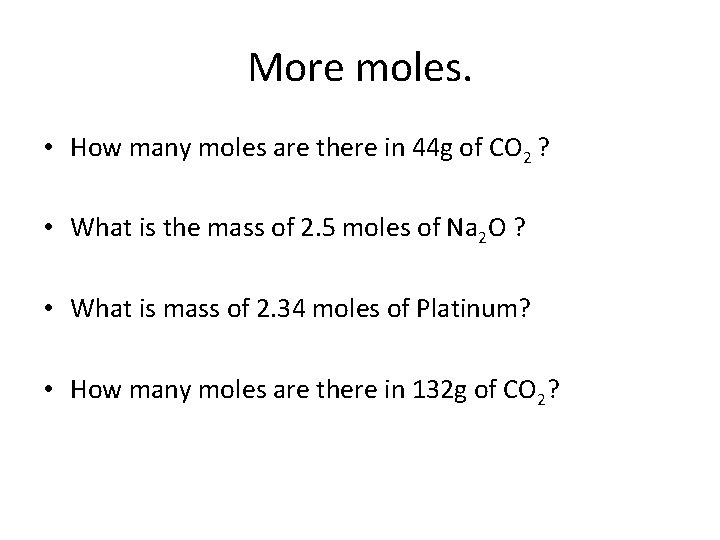
- Slides: 11

Topic 1 – Qualitative testing. • Must list the cation tests. • Should describe the anion tests. • Could work out moles using the formula given. ----------Starter: How is a flame test performed?

Cations and Anions 1) What is an ion? (1) • When ionic compounds are dissolved into a solution they form cations and anions 2) What are cations? (1) • Cations (like metals ions) have a positive charge 3) What are anions? (1) • Anions (like non-metal groups) have negative charges

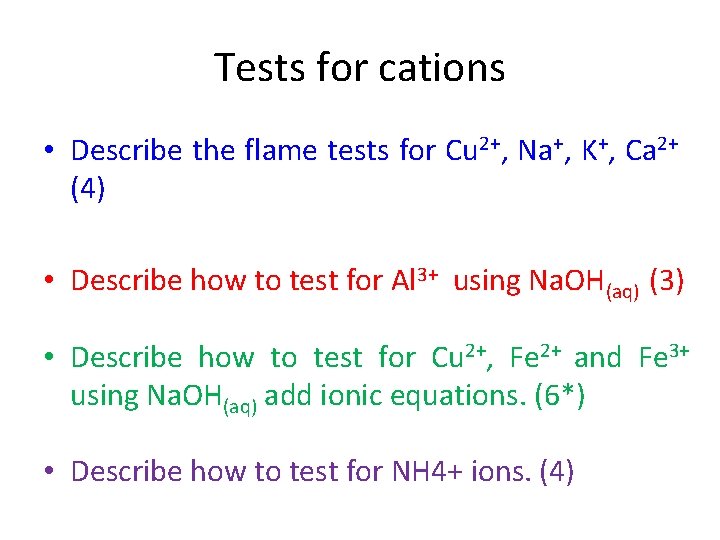
Tests for cations • Describe the flame tests for Cu 2+, Na+, K+, Ca 2+ (4) • Describe how to test for Al 3+ using Na. OH(aq) (3) • Describe how to test for Cu 2+, Fe 2+ and Fe 3+ using Na. OH(aq) add ionic equations. (6*) • Describe how to test for NH 4+ ions. (4)

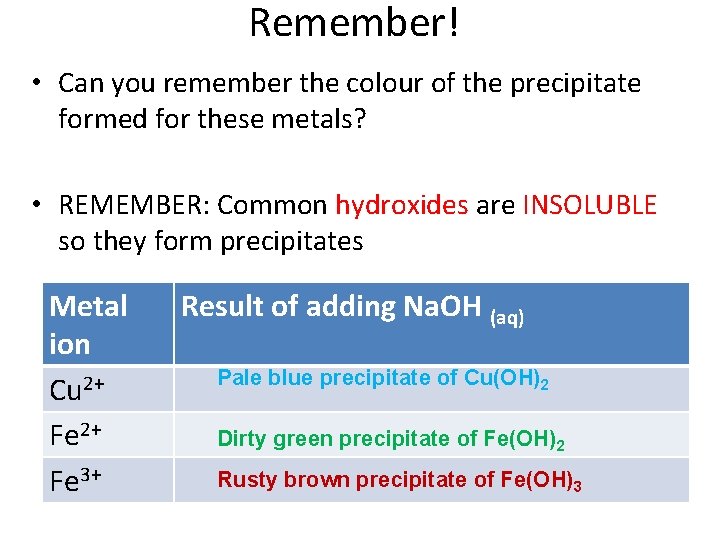
Remember! • Can you remember the colour of the precipitate formed for these metals? • REMEMBER: Common hydroxides are INSOLUBLE so they form precipitates Metal ion Cu 2+ Fe 3+ Result of adding Na. OH (aq) Pale blue precipitate of Cu(OH)2 Dirty green precipitate of Fe(OH)2 Rusty brown precipitate of Fe(OH)3

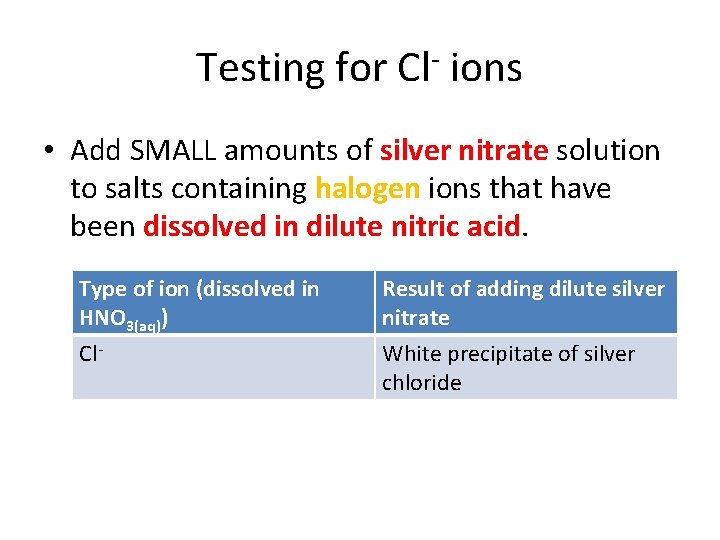
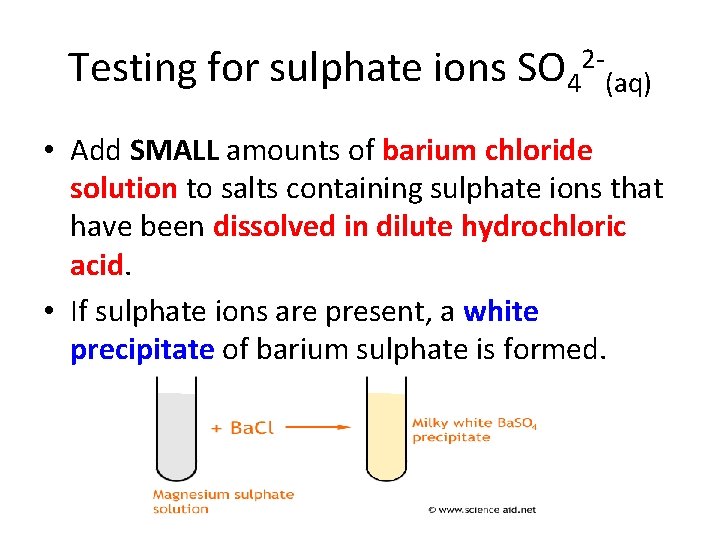
Testing for anions • Describe how to test for Chloride (Cl-) ions (3) Using dilute nitric acid and silver nitrate solution…white ppt • Describe how to test for Sulphate (SO 42 -) ions (3) Using dilute hydrochloric acid and barium chloride solution…white ppt • Describe how to test for Carbonate (CO 32 -) ions (3) using dilute hydrochloric acid and bubbling through limewater…white ppt.

Testing for Cl- ions • Add SMALL amounts of silver nitrate solution to salts containing halogen ions that have been dissolved in dilute nitric acid. Type of ion (dissolved in HNO 3(aq)) Result of adding dilute silver nitrate Cl- White precipitate of silver chloride

Testing for sulphate ions SO 42 -(aq) • Add SMALL amounts of barium chloride solution to salts containing sulphate ions that have been dissolved in dilute hydrochloric acid. • If sulphate ions are present, a white precipitate of barium sulphate is formed.

Testing for NH 4+ (“ammonium ion”) • • Add Na. OH (aq) Warm gently Test gas given off with damp red litmus paper Ammonia gas is given off which turns the damp red litmus paper BLUE Does anyone know why the litmus paper changes colour?

Testing for carbonate ions CO 32 -(aq) • Add dilute hydrochloric acid • See if it is effervescent (fizzes due to carbon dioxide gas being given off) • Gas makes limewater milky.

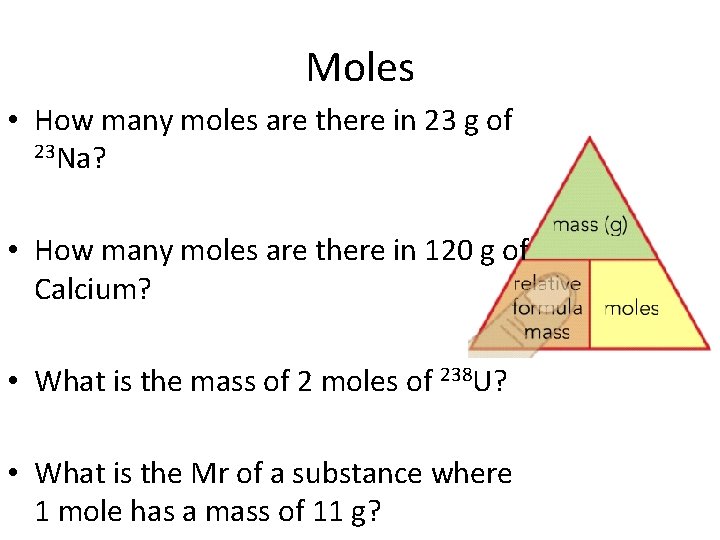
Moles • How many moles are there in 23 g of 23 Na? • How many moles are there in 120 g of Calcium? • What is the mass of 2 moles of 238 U? • What is the Mr of a substance where 1 mole has a mass of 11 g?
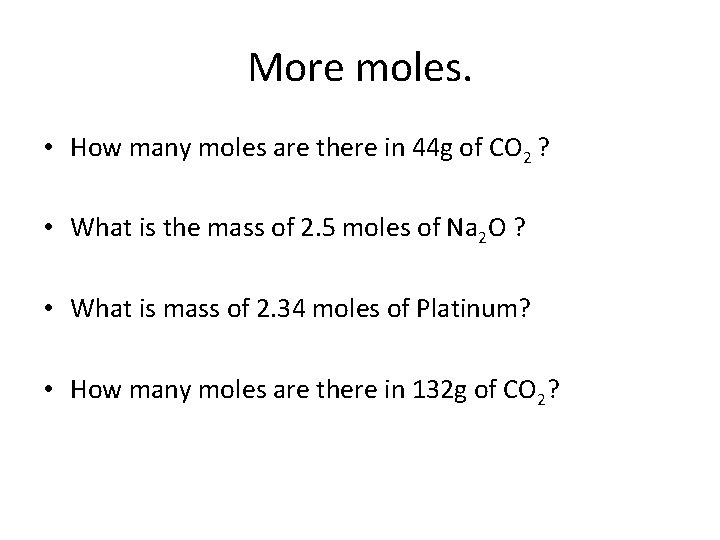
More moles. • How many moles are there in 44 g of CO 2 ? • What is the mass of 2. 5 moles of Na 2 O ? • What is mass of 2. 34 moles of Platinum? • How many moles are there in 132 g of CO 2?