Net Ionic Equations Chem 101 Prem D Sattsangi


- Slides: 14

Net Ionic Equations Chem 101 Prem D. Sattsangi © 2010 Christopher L. Byers (programmer)

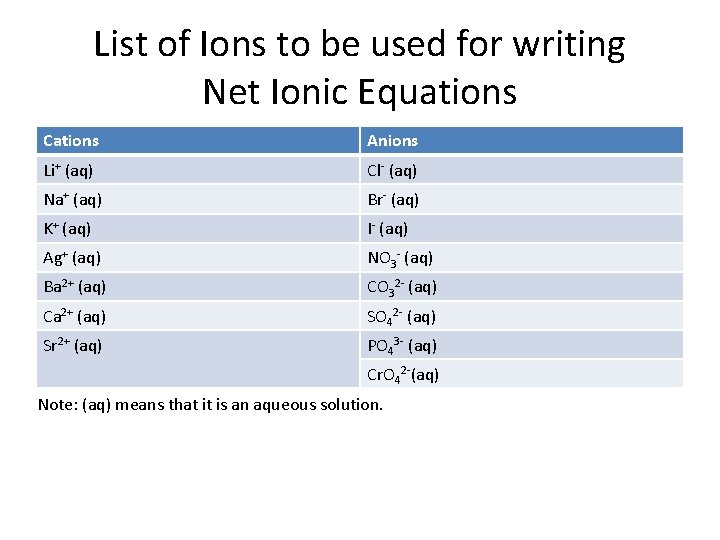
List of Ions to be used for writing Net Ionic Equations Cations Anions Li+ (aq) Cl- (aq) Na+ (aq) Br- (aq) K+ (aq) I- (aq) Ag+ (aq) NO 3 - (aq) Ba 2+ (aq) CO 32 - (aq) Ca 2+ (aq) SO 42 - (aq) Sr 2+ (aq) PO 43 - (aq) Cr. O 42 -(aq) Note: (aq) means that it is an aqueous solution.

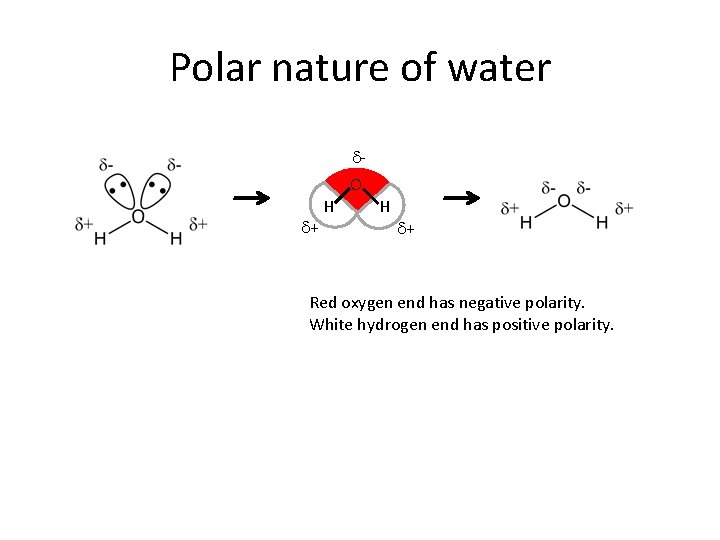
Polar nature of water d. O d+ H H d+ Red oxygen end has negative polarity. White hydrogen end has positive polarity.

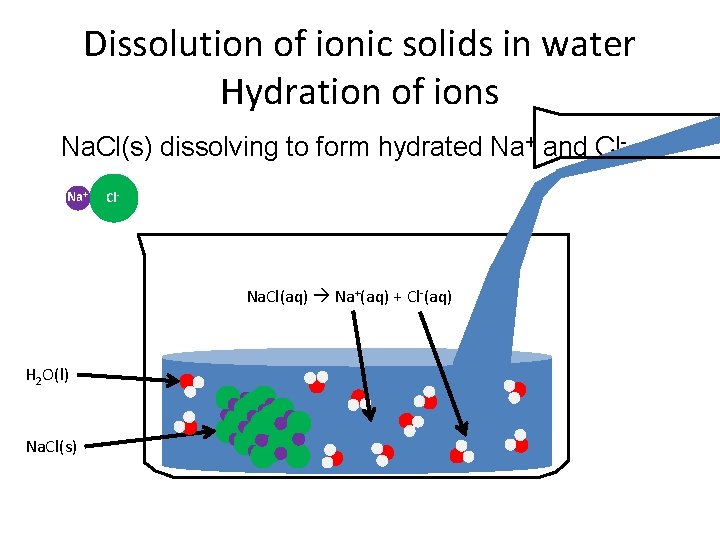
Dissolution of ionic solids in water Hydration of ions Na. Cl(s) dissolving to form hydrated Na+ and Cl-. Na+ Cl- Na. Cl(aq) Na+(aq) + Cl-(aq) H 2 O(l) Na. Cl(s)

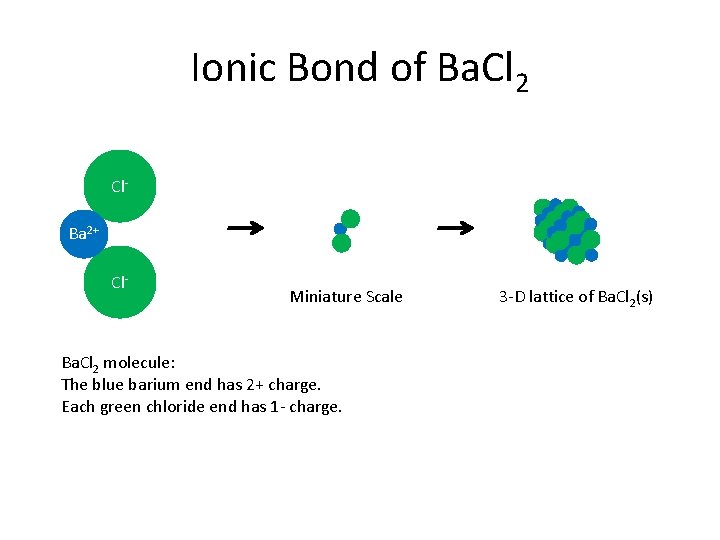
Ionic Bond of Ba. Cl 2 Cl. Ba 2+ Cl- Miniature Scale Ba. Cl 2 molecule: The blue barium end has 2+ charge. Each green chloride end has 1 - charge. 3 -D lattice of Ba. Cl 2(s)

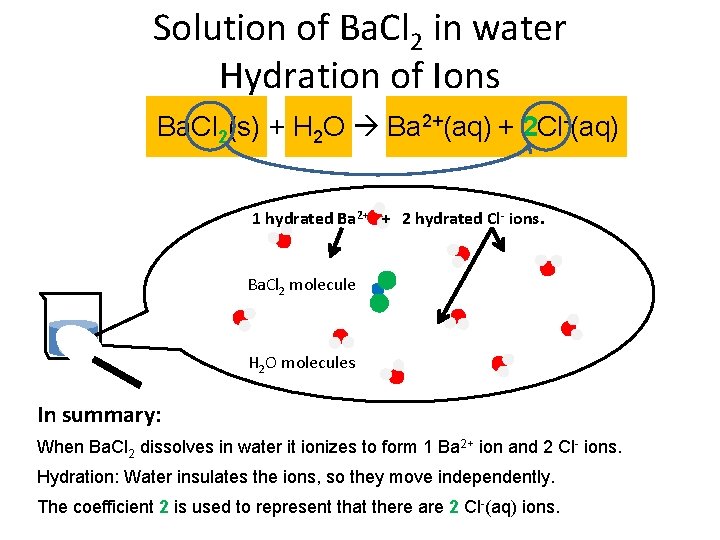
Solution of Ba. Cl 2 in water Hydration of Ions Ba. Cl 2(s) + H 2 O Ba 2+(aq) + 2 Cl-(aq) 1 hydrated Ba 2+ + 2 hydrated Cl- ions. Ba. Cl 2 molecule H 2 O molecules In summary: When Ba. Cl 2 dissolves in water it ionizes to form 1 Ba 2+ ion and 2 Cl- ions. Hydration: Water insulates the ions, so they move independently. The coefficient 2 is used to represent that there are 2 Cl-(aq) ions.

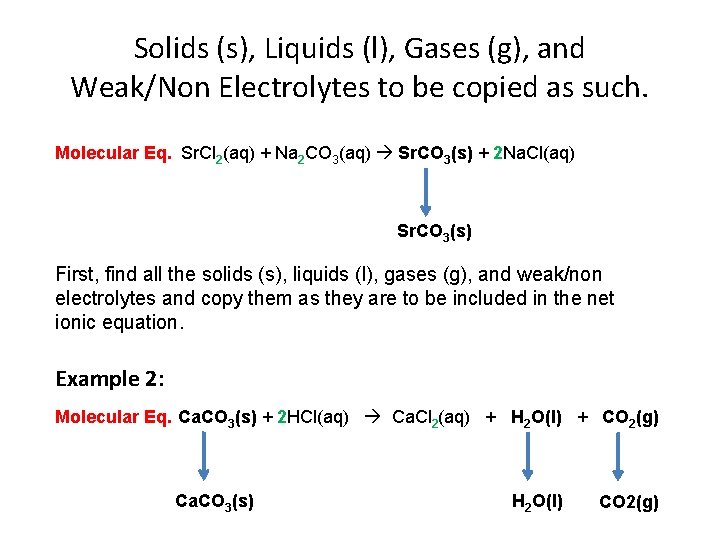
Solids (s), Liquids (l), Gases (g), and Weak/Non Electrolytes to be copied as such. Molecular Eq. Sr. Cl 2(aq) + Na 2 CO 3(aq) Sr. CO 3(s) + 2 Na. Cl(aq) Sr. CO 3(s) First, find all the solids (s), liquids (l), gases (g), and weak/non electrolytes and copy them as they are to be included in the net ionic equation. Example 2: Molecular Eq. Ca. CO 3(s) + 2 HCl(aq) Ca. Cl 2(aq) + H 2 O(l) + CO 2(g) Ca. CO 3(s) H 2 O(l) CO 2(g)

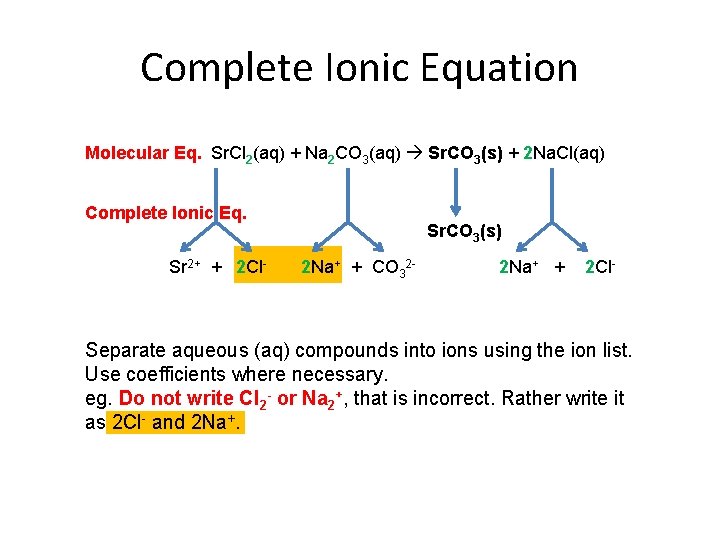
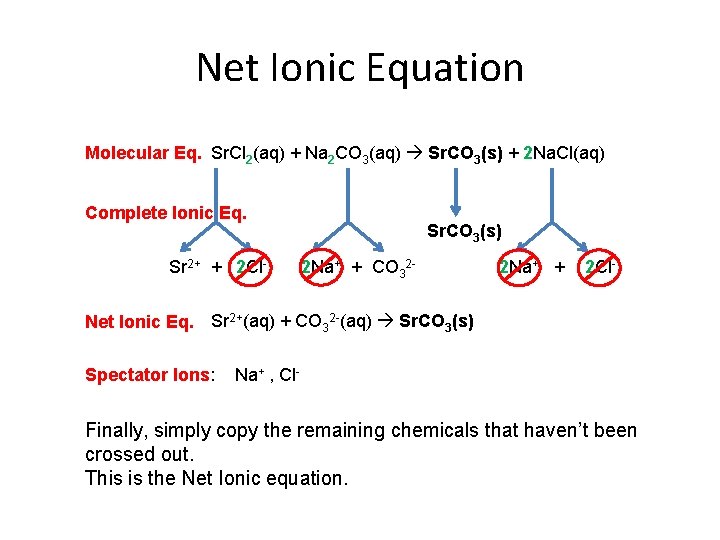
Complete Ionic Equation Molecular Eq. Sr. Cl 2(aq) + Na 2 CO 3(aq) Sr. CO 3(s) + 2 Na. Cl(aq) Complete Ionic Eq. Sr 2+ + 2 Cl- Sr. CO 3(s) 2 Na+ + CO 32 - 2 Na+ + 2 Cl- Separate aqueous (aq) compounds into ions using the ion list. Use coefficients where necessary. eg. Do not write Cl 2 - or Na 2+, that is incorrect. Rather write it as 2 Cl- and 2 Na+.

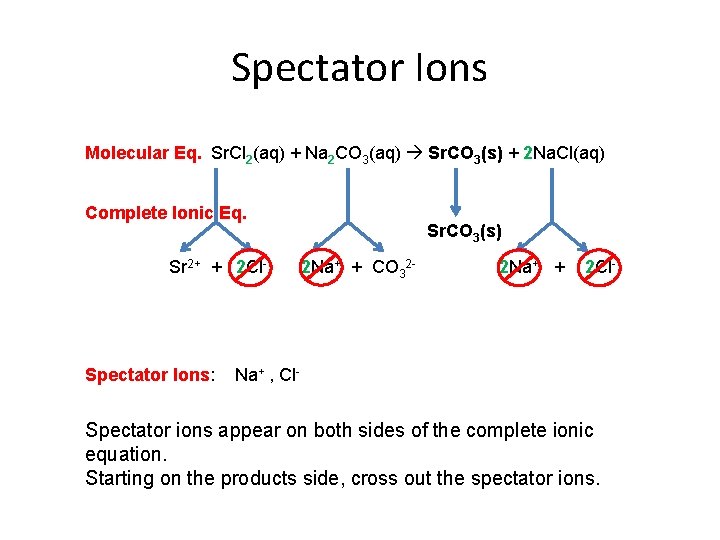
Spectator Ions Molecular Eq. Sr. Cl 2(aq) + Na 2 CO 3(aq) Sr. CO 3(s) + 2 Na. Cl(aq) Complete Ionic Eq. Sr 2+ + 2 Cl- Spectator Ions: Sr. CO 3(s) 2 Na+ + CO 32 - 2 Na+ + 2 Cl- Na+ , Cl- Spectator ions appear on both sides of the complete ionic equation. Starting on the products side, cross out the spectator ions.

Net Ionic Equation Molecular Eq. Sr. Cl 2(aq) + Na 2 CO 3(aq) Sr. CO 3(s) + 2 Na. Cl(aq) Complete Ionic Eq. Sr 2+ + 2 Cl- Sr. CO 3(s) 2 Na+ + CO 32 - 2 Na+ + 2 Cl- Net Ionic Eq. Sr 2+(aq) + CO 32 -(aq) Sr. CO 3(s) Spectator Ions: Na+ , Cl- Finally, simply copy the remaining chemicals that haven’t been crossed out. This is the Net Ionic equation.

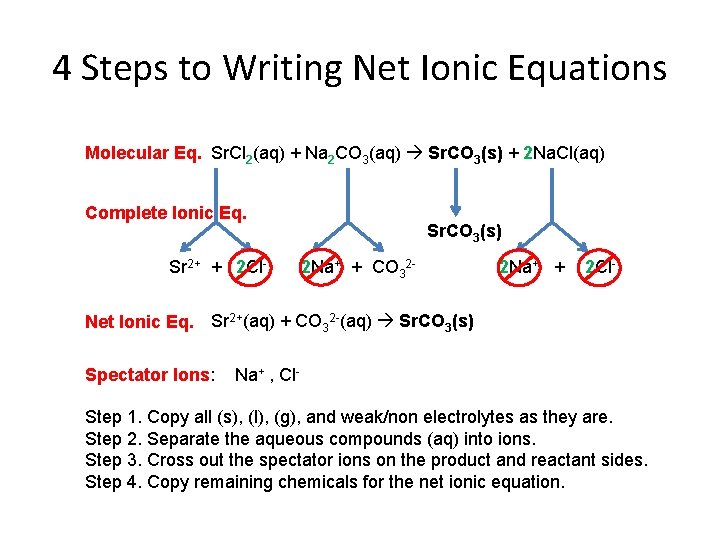
4 Steps to Writing Net Ionic Equations Molecular Eq. Sr. Cl 2(aq) + Na 2 CO 3(aq) Sr. CO 3(s) + 2 Na. Cl(aq) Complete Ionic Eq. Sr 2+ + 2 Cl- Sr. CO 3(s) 2 Na+ + CO 32 - 2 Na+ + 2 Cl- Net Ionic Eq. Sr 2+(aq) + CO 32 -(aq) Sr. CO 3(s) Spectator Ions: Na+ , Cl- Step 1. Copy all (s), (l), (g), and weak/non electrolytes as they are. Step 2. Separate the aqueous compounds (aq) into ions. Step 3. Cross out the spectator ions on the product and reactant sides. Step 4. Copy remaining chemicals for the net ionic equation.

Net Ionic Equations: Example 2 Molecular Eq. 3 Ca. Cl 2(aq) + 2 Na 3 PO 4(aq) Ca 3(PO 4)2(s) + 6 Na. Cl(aq) Complete Ionic Eq. 3 Ca 2+ + 6 Cl(3 x 2) Net Ionic Eq. Spectator Ions: Ca 3(PO 4)2(s) 6 Na+ + 2 PO 43 - 6 Na+ + 6 Cl- 3 Ca 2+(aq) + 2 PO 43 -(aq) Ca 3(PO 4)2(s) Na+ , Cl- Step 1. Copy all (s), (l), (g), and weak/non electrolytes as they are. Step 2. Separate the aqueous compounds (aq) into ions. Step 3. Cross out the spectator ions on the product and reactant sides. Step 4. Copy remaining chemicals for the net ionic equation.

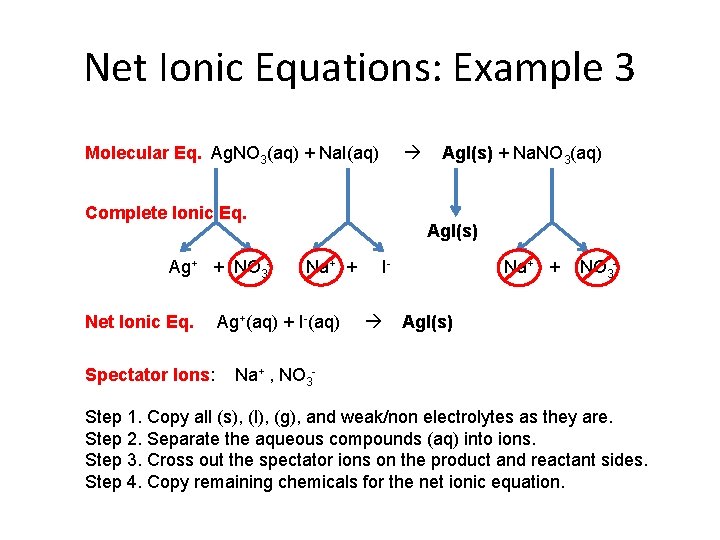
Net Ionic Equations: Example 3 Molecular Eq. Ag. NO 3(aq) + Na. I(aq) Complete Ionic Eq. Ag+ + NO 3 Net Ionic Eq. Spectator Ions: Ag. I(s) + Na. NO 3(aq) Ag. I(s) Na+ + Ag+(aq) + I-(aq) I Na+ + NO 3 - Ag. I(s) Na+ , NO 3 - Step 1. Copy all (s), (l), (g), and weak/non electrolytes as they are. Step 2. Separate the aqueous compounds (aq) into ions. Step 3. Cross out the spectator ions on the product and reactant sides. Step 4. Copy remaining chemicals for the net ionic equation.

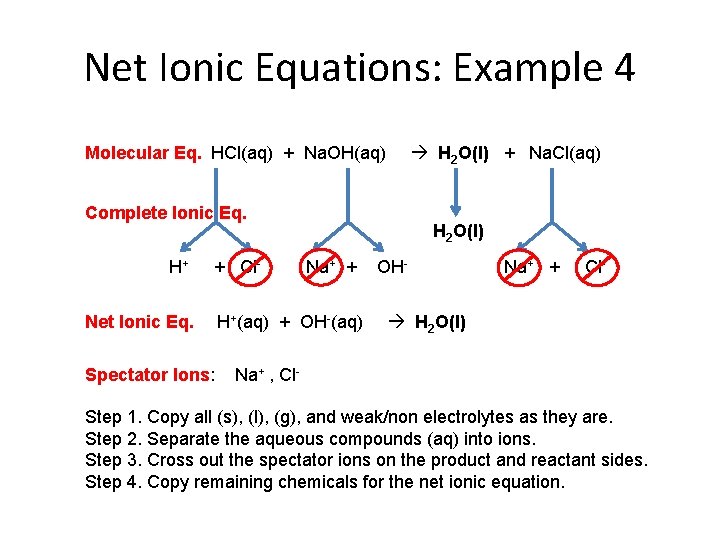
Net Ionic Equations: Example 4 Molecular Eq. HCl(aq) + Na. OH(aq) H 2 O(l) + Na. Cl(aq) Complete Ionic Eq. H+ + Cl- Net Ionic Eq. Spectator Ions: H 2 O(l) Na+ + H+(aq) + OH-(aq) OH- Na+ + Cl- H 2 O(l) Na+ , Cl- Step 1. Copy all (s), (l), (g), and weak/non electrolytes as they are. Step 2. Separate the aqueous compounds (aq) into ions. Step 3. Cross out the spectator ions on the product and reactant sides. Step 4. Copy remaining chemicals for the net ionic equation.