Qualitative Analysis Chem 101 Dr Prem D Sattsangi


- Slides: 12

Qualitative Analysis Chem 101 Dr. Prem D. Sattsangi © 2010 - and Christopher L. Byers (programmer)

What is Qualitative Analysis? • No two ions behave identically in all chemical tests. • We use various tests to check for these different qualities to determine which unknown cation or anion we have. • The cations we’ll check for are Ba 2+, Ca 2+, Li+, K+, Na+, and Sr 2+.

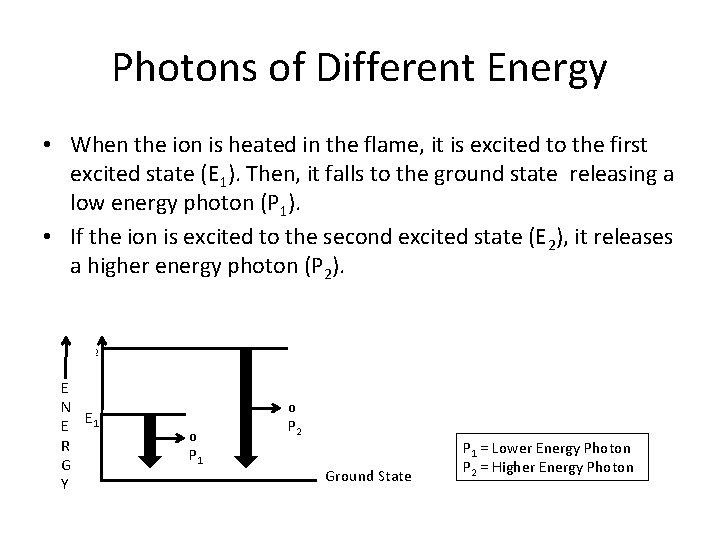
Photons of Different Energy • When the ion is heated in the flame, it is excited to the first excited state (E 1). Then, it falls to the ground state releasing a low energy photon (P 1). • If the ion is excited to the second excited state (E 2), it releases a higher energy photon (P 2). E 2 E N E 1 E R G Y o o P 1 P 2 Ground State P 1 = Lower Energy Photon P 2 = Higher Energy Photon

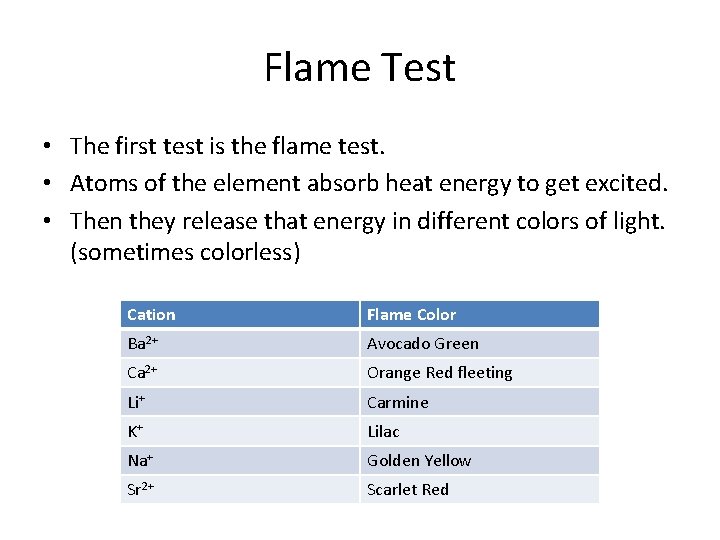
Flame Test • The first test is the flame test. • Atoms of the element absorb heat energy to get excited. • Then they release that energy in different colors of light. (sometimes colorless) Cation Flame Color Ba 2+ Avocado Green Ca 2+ Orange Red fleeting Li+ Carmine K+ Lilac Na+ Golden Yellow Sr 2+ Scarlet Red

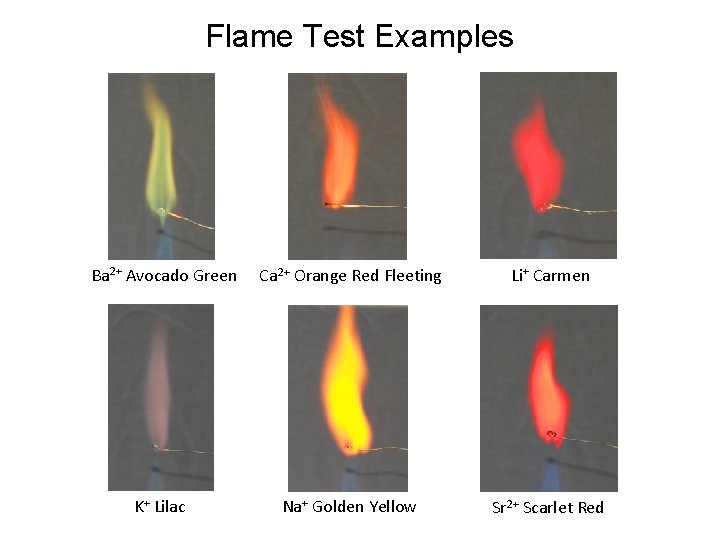
Flame Test Examples Ba 2+ Avocado Green Ca 2+ Orange Red Fleeting Li+ Carmen K+ Lilac Na+ Golden Yellow Sr 2+ Scarlet Red

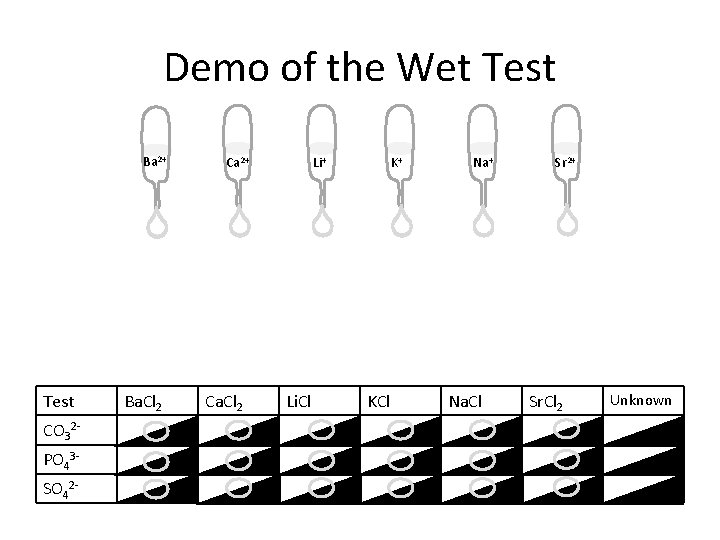
Wet Tests Test Ba. Cl 2 Ca. Cl 2 Li. Cl KCl Na. Cl Sr. Cl 2 Unknown CO 32 PO 43 SO 42 In your Lab Book you have a chart that looks like this for use in wet tests. Directions: 1. Add 1 drop of the cation to each of the 3 spaces below it with ½ of the drop being on the black and ½ being on the white. 2. Add 1 drop of the testing reagent (anion) to each of the cations. 3. Record your results with each test. 4. Add 1 drop of the unknown in each of the 3 spaces. 5. Add 1 drop of the anion testing reagent to 1 drop of each unknown. 6. Record your results. 7. Compare your results to the cations to determine the identity of your unknown.

Demo of the Wet Test Ba 2+ Test CO 32 PO 43 SO 42 - Ba. Cl 2 Ca 2+ Ca. Cl 2 Li+ Li. Cl K+ KCl Na+ Na. Cl Sr 2+ Sr. Cl 2 Unknown

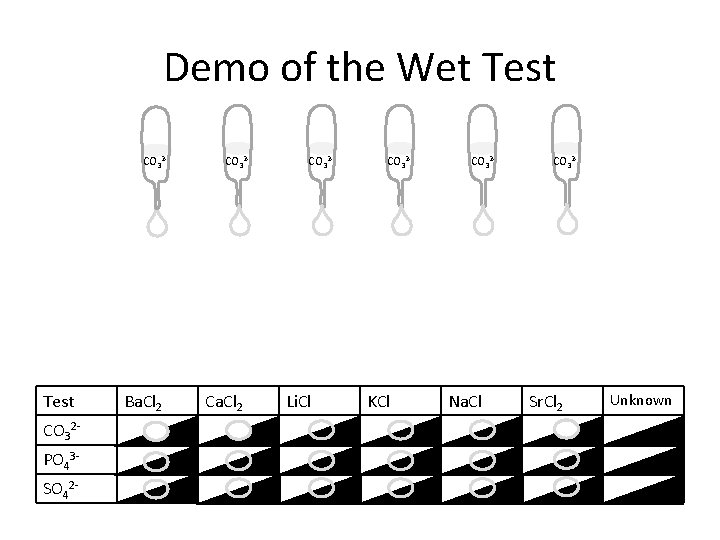
Demo of the Wet Test CO 32 - Test CO 32 PO 43 SO 42 - Ba. Cl 2 CO 32 - Ca. Cl 2 CO 32 - Li. Cl CO 32 - KCl CO 32 - Na. Cl CO 32 - Sr. Cl 2 Unknown

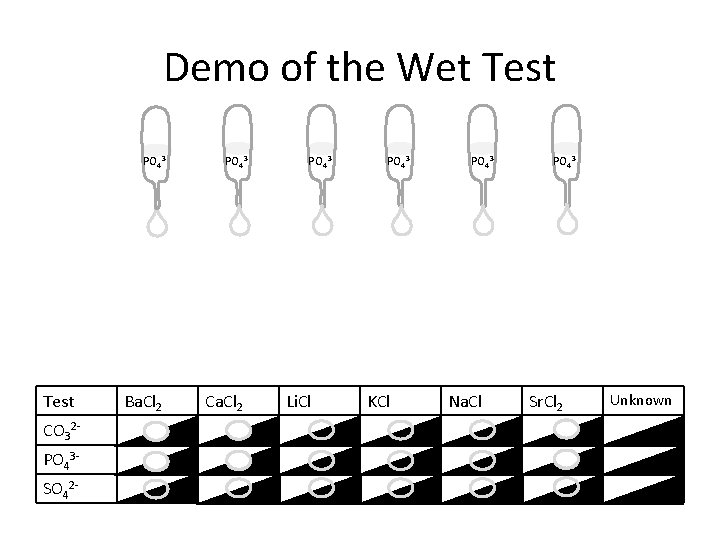
Demo of the Wet Test PO 43 - Test CO 32 PO 43 SO 42 - Ba. Cl 2 PO 43 - Ca. Cl 2 PO 43 - Li. Cl PO 43 - KCl PO 43 - Na. Cl PO 43 - Sr. Cl 2 Unknown

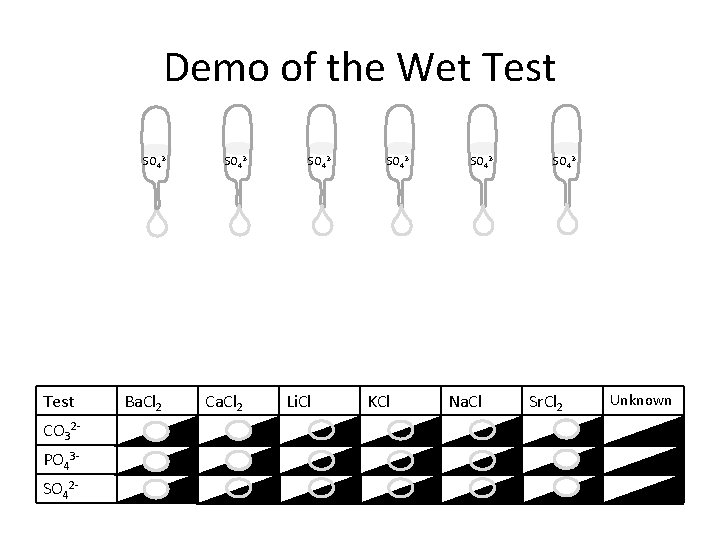
Demo of the Wet Test SO 42 - Test CO 32 PO 43 SO 42 - Ba. Cl 2 SO 42 - Ca. Cl 2 SO 42 - Li. Cl SO 42 - KCl SO 42 - Na. Cl SO 42 - Sr. Cl 2 Unknown

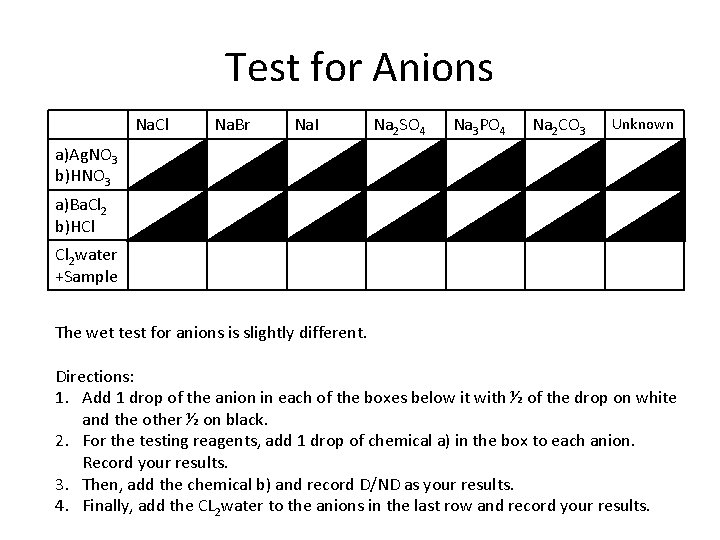
Test for Anions Na. Cl Na. Br Na. I Na 2 SO 4 Na 3 PO 4 Na 2 CO 3 Unknown a)Ag. NO 3 b)HNO 3 a)Ba. Cl 2 b)HCl Cl 2 water +Sample The wet test for anions is slightly different. Directions: 1. Add 1 drop of the anion in each of the boxes below it with ½ of the drop on white and the other ½ on black. 2. For the testing reagents, add 1 drop of chemical a) in the box to each anion. Record your results. 3. Then, add the chemical b) and record D/ND as your results. 4. Finally, add the CL 2 water to the anions in the last row and record your results.

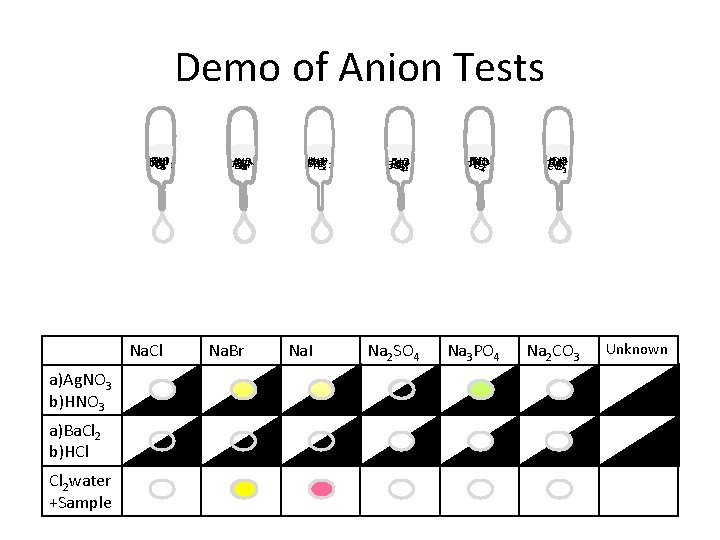
Demo of Anion Tests HCl Cl HNO Ba Ag Cl 22++-3 Na. Cl a)Ag. NO 3 b)HNO 3 a)Ba. Cl 2 b)HCl Cl 2 water +Sample +HNO Ag Cl 22+ HCl Ba 3 Br Na. Br HNO HCl Ag Ba Cl I-2+2+3 Na. I +2 HNO Ag Cl 42+ HCl Ba SO 23 Na 2 SO 4 +3 Ba Cl 2+ HCl HNO Ag PO 42 3 Na 3 PO 4 + HNO Cl 2+ Ag Ba HCl 2 -3 CO 3 Na 2 CO 3 Unknown