Synthesis of Alum Prem Sattsangi Copyright 2009Rev 2











![#17 Suction Filter Alum • Water aspirator [Removes Mother liquor] Isolation of Alum: (a)Suction #17 Suction Filter Alum • Water aspirator [Removes Mother liquor] Isolation of Alum: (a)Suction](https://slidetodoc.com/presentation_image/9ea91e1e7eb55aa0f98f88971a87d696/image-17.jpg)
![#18 Suction Filter Alum (a) Water aspirator [Removes Mother liquor] (b) 50% ethanol [Removes #18 Suction Filter Alum (a) Water aspirator [Removes Mother liquor] (b) 50% ethanol [Removes](https://slidetodoc.com/presentation_image/9ea91e1e7eb55aa0f98f88971a87d696/image-18.jpg)










- Slides: 32

Synthesis of Alum Prem Sattsangi Copyright 2009(Rev. )

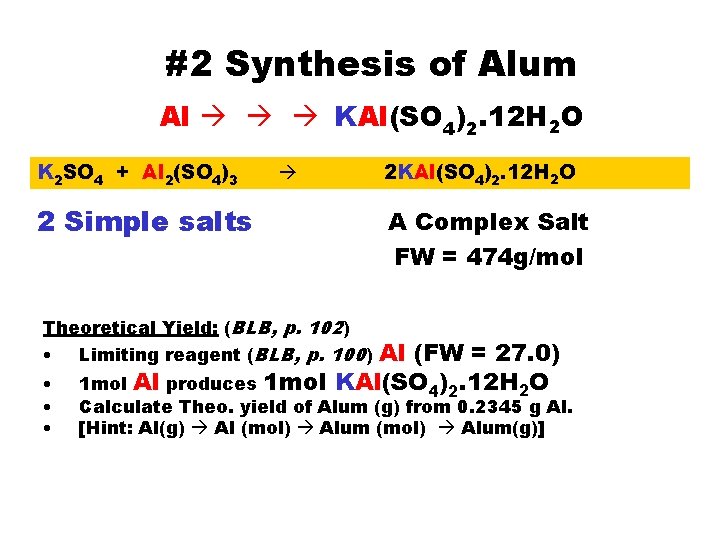
#2 Synthesis of Alum Al KAl(SO 4)2. 12 H 2 O K 2 SO 4 + Al 2(SO 4)3 2 Simple salts 2 KAl(SO 4)2. 12 H 2 O A Complex Salt FW = 474 g/mol Theoretical Yield: (BLB, p. 102) Al (FW = 27. 0) 1 mol Al produces 1 mol KAl(SO 4)2. 12 H 2 O • Limiting reagent (BLB, p. 100) • • • Calculate Theo. yield of Alum (g) from 0. 2345 g Al. [Hint: Al(g) Al (mol) Alum(g)]

#3 Analytical Balance Tare, place foil, close door, record wt. Calculate Theoretical Yield Of Alum from 0. 0566 g Al

#4 Cut Al foil in 16 squares • Length wise • Breadth wise

#5 Plastic Reaction vial with cut Aluminum foil

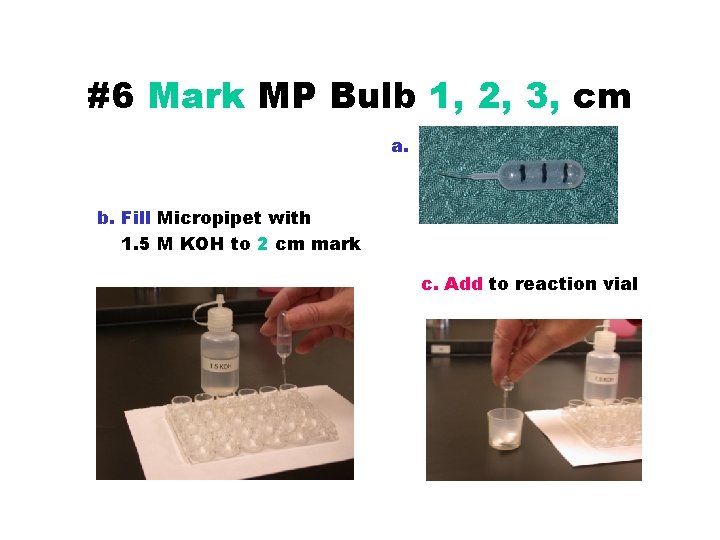
#6 Mark MP Bulb 1, 2, 3, cm a. b. Fill Micropipet with 1. 5 M KOH to 2 cm mark c. Add to reaction vial

#7 Add more KOH (1 cm) a. Fill KOH to 1 cm mark b. Add to reaction vial

#8 Start reaction, In fume hood, Label 250 m. L beaker with Stn. # (a) Take hot tap water up to 2 cm mark in the coffee cup. (b) Support the coffee cup in 250 m. L beaker (Stn. #__) STN# 4 (c) FLOAT reaction vial in the coffee cup using forceps.

#9 Oxidation Numbers HELP KEEP TRACK OF ELECTRONS • BLB p. 128 -129 • Ox. No. of elements in standard states = 0 • Ox. No. of an ION = Charge on it Ox. No. • • • Aluminum (foil) Aluminum ion (Al 3+) Hydrogen (H 2(g) Hydrogen ion (H+) Hydride ion (H: -) H in water [H+ OH-] LEO the lion says GER Al foil 0 +3 Loss of Electron = Oxidation 0 +1 -1 Gain of electron = Reduction +1

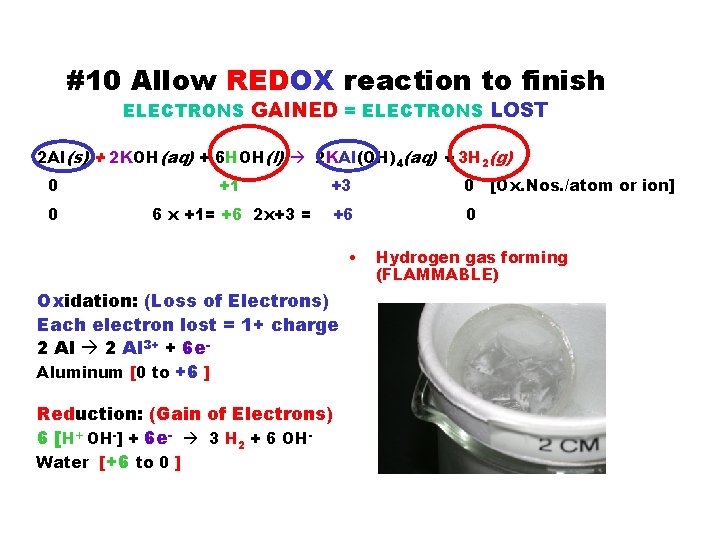
#10 Allow REDOX reaction to finish ELECTRONS GAINED = ELECTRONS LOST 2 Al(s) + 2 KOH(aq) + 6 HOH(l) 2 KAl(OH)4(aq) + 3 H 2(g) 0 +1 +3 0 [Ox. Nos. /atom or ion] 0 6 x +1= +6 2 x+3 = +6 0 • Oxidation: (Loss of Electrons) Each electron lost = 1+ charge 2 Al 3+ + 6 e. Aluminum [0 to +6 ] Reduction: (Gain of Electrons) 6 [H+ OH-] + 6 e- 3 H 2 + 6 OHWater [+6 to 0 ] Hydrogen gas forming (FLAMMABLE)

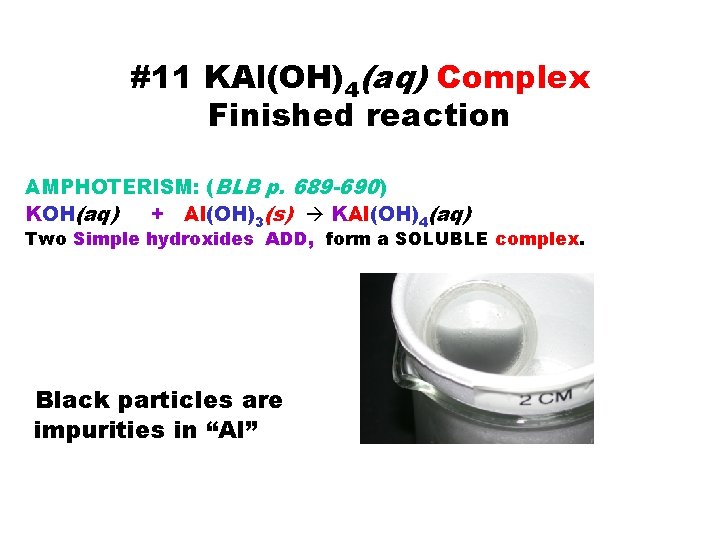
#11 KAl(OH)4(aq) Complex Finished reaction AMPHOTERISM: (BLB p. 689 -690) KOH(aq) + Al(OH)3(s) KAl(OH)4(aq) Two Simple hydroxides ADD, form a SOLUBLE complex. Black particles are impurities in “Al”

#12 Remove Black Particles • Insert a wad of cotton in a glass pipet. • clamp to a stand, wet, remove water, filter. • May need 2 times filtration on the same pipet to get a filtrate. clear KAl(OH)4(aq)

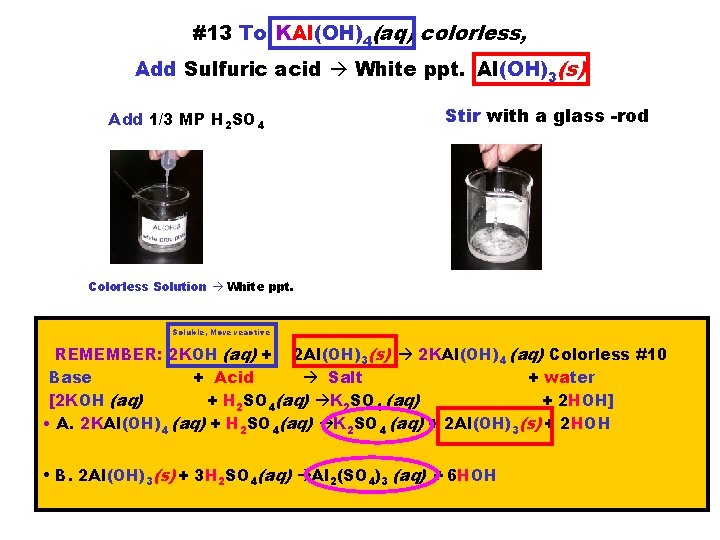
#13 To KAl(OH)4(aq) colorless, Add Sulfuric acid White ppt. Al(OH)3(s) Add 1/3 MP H 2 SO 4 Stir with a glass -rod Colorless Solution White ppt. Soluble, More reactive REMEMBER: 2 KOH (aq) + 2 Al(OH)3(s) 2 KAl(OH)4 (aq) Colorless #10 Base + Acid Salt + water [2 KOH (aq) + H 2 SO 4(aq) K 2 SO 4 (aq) + 2 HOH] • A. 2 KAl(OH)4 (aq) + H 2 SO 4(aq) K 2 SO 4 (aq) + 2 Al(OH)3(s) + 2 HOH • B. 2 Al(OH)3(s) + 3 H 2 SO 4(aq) Al 2(SO 4)3 (aq) + 6 HOH

#14 White precipitate dissolves. Alum forms. (a)Stir with a glass rod. (b)Heat on hot plate • K 2 SO 4(aq) + Al 2(SO 4)3 (aq) 2 KAl(SO 4)2. 12 H 2 O • Simple salts ADD, form a COMPLEX salt

#15 Crystallization of Alum • Room temperature • (15 minutes or more) SEEDING? • Ice bath • (10 minutes)

#16 Alum crystals Typical result Single
![17 Suction Filter Alum Water aspirator Removes Mother liquor Isolation of Alum aSuction #17 Suction Filter Alum • Water aspirator [Removes Mother liquor] Isolation of Alum: (a)Suction](https://slidetodoc.com/presentation_image/9ea91e1e7eb55aa0f98f88971a87d696/image-17.jpg)
#17 Suction Filter Alum • Water aspirator [Removes Mother liquor] Isolation of Alum: (a)Suction filter. (b)Rinse 2 x with 15 drs 50% ethanol(0 o. C) (c) Rinse 2 x with 95% ethanol(0 o. C) (d)Continue suction ~5 more minutes to dry the crystals.
![18 Suction Filter Alum a Water aspirator Removes Mother liquor b 50 ethanol Removes #18 Suction Filter Alum (a) Water aspirator [Removes Mother liquor] (b) 50% ethanol [Removes](https://slidetodoc.com/presentation_image/9ea91e1e7eb55aa0f98f88971a87d696/image-18.jpg)
#18 Suction Filter Alum (a) Water aspirator [Removes Mother liquor] (b) 50% ethanol [Removes Al 3+(aq), K+(aq), SO 42 -(aq)] (c) 95% ethanol. [Removes water] (d) Continue suction ~5 minutes. [To air dry the crystals]

#19 Dry on a pre-weighed paper Weight of paper_____ g Transfer Alum on PAPER. Cover with a Funnel. Allow to dry.

#20 Weigh crystals, Calculate %Yield Wt of crystals + paper = 0. 9802 g Wt of paper = – 0. 4181 g Wt of crystals = 0. 5621 g Exp. Yield = 0. 5621 g • %Yield • = Exp. Yield x 100 Theo. Yield (Slide #2) Please calculate %Yield now: =

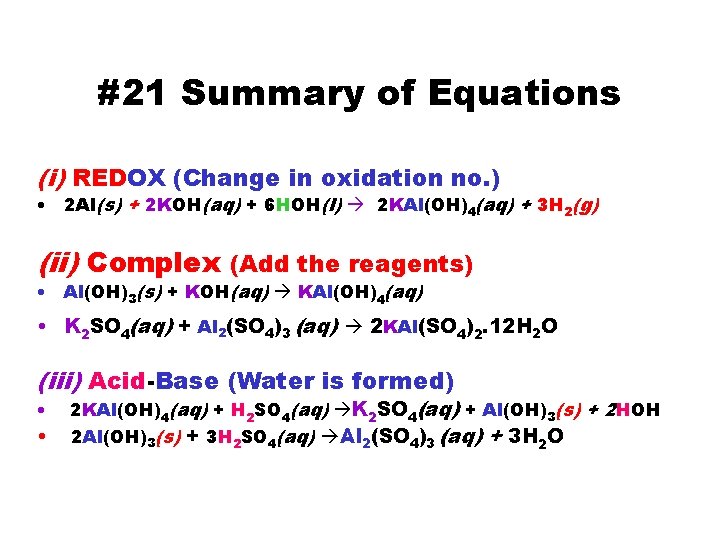
#21 Summary of Equations (i) REDOX (Change in oxidation no. ) • 2 Al(s) + 2 KOH(aq) + 6 HOH(l) 2 KAl(OH)4(aq) + 3 H 2(g) (ii) Complex (Add the reagents) • Al(OH)3(s) + KOH(aq) KAl(OH)4(aq) • K 2 SO 4(aq) + Al 2(SO 4)3 (aq) 2 KAl(SO 4)2. 12 H 2 O (iii) Acid-Base (Water is formed) • • 2 KAl(OH)4(aq) + H 2 SO 4(aq) K 2 SO 4(aq) + Al(OH)3(s) + 2 HOH 2 Al(OH)3(s) + 3 H 2 SO 4(aq) Al 2(SO 4)3 (aq) + 3 H 2 O

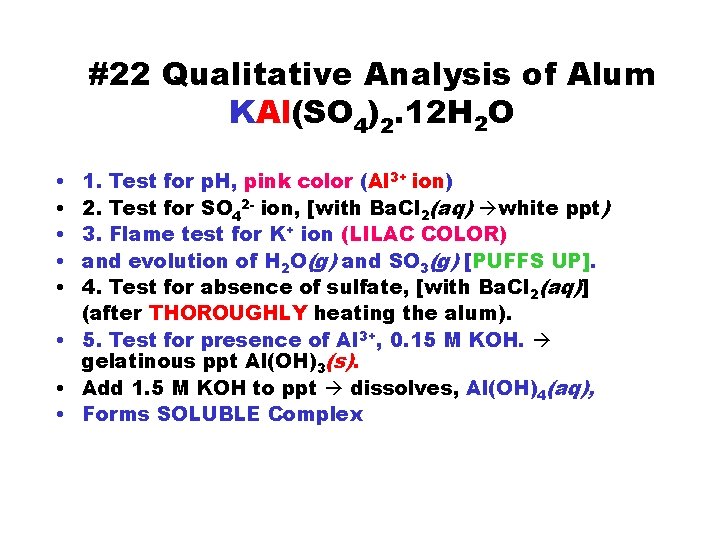
#22 Qualitative Analysis of Alum KAl(SO 4)2. 12 H 2 O • • • 1. Test for p. H, pink color (Al 3+ ion) 2. Test for SO 42 - ion, [with Ba. Cl 2(aq) white ppt) 3. Flame test for K+ ion (LILAC COLOR) and evolution of H 2 O(g) and SO 3(g) [PUFFS UP]. 4. Test for absence of sulfate, [with Ba. Cl 2(aq)] (after THOROUGHLY heating the alum). • 5. Test for presence of Al 3+, 0. 15 M KOH. gelatinous ppt Al(OH)3(s). • Add 1. 5 M KOH to ppt dissolves, Al(OH)4(aq), • Forms SOLUBLE Complex

#23 Prepare saturated Alum solution. • Take 2 mm mound of Alum, KAl(SO 4)2. 12 H 2 O, with 3 drops of water on the plastic sheet. Crush alum with a glass rod.

#24 p. H Test for Al 3+ • Alum, KAl(SO 4)2. 12 H 2 O, contains Al 3+ ion. • Al 3+ ion has: • a. High charge. • b. Small radius. • Such cations, may rip apart the water molecule to produce H+. • Al 3+ + HOH Al. OH 2+ + H+

#25 Chemical Test for Al 3+ 0. 15 M KOH Gel (soluble in 1. 5 M KOH) • In the 24 well tray, mix 1 dr. 1. 5 M KOH + 9 drs. Distilled water to make 0. 15 M KOH • Alum contains Al 3+. • 1 drop Alum(aq) + 1 drop 0. 15 M KOH Gel • Al 3+ + 3 KOH(0. 15 M) Al(OH)3(gel) + 3 K+ Insol(s) • Add 1 drop 1. 5 M KOH to gel Dissolves. • Al(OH)3(gel) +KOH(1. 5 M) KAl(OH)4(aq) • Simple hydroxides ADD COMPLEX

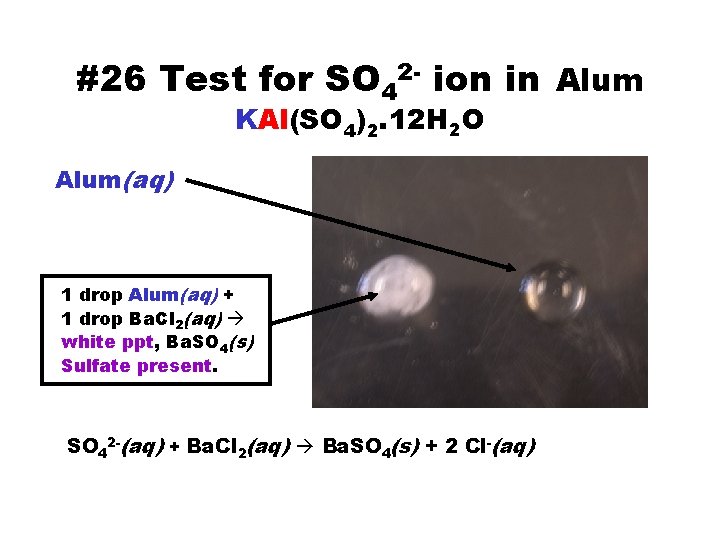
#26 Test for SO 42 - ion in Alum KAl(SO 4)2. 12 H 2 O Alum(aq) 1 drop Alum(aq) + 1 drop Ba. Cl 2(aq) white ppt, Ba. SO 4(s) Sulfate present. SO 42 -(aq) + Ba. Cl 2(aq) Ba. SO 4(s) + 2 Cl-(aq)

#27 Flame test sample prep. • Place some Alum on a watch glass. • Heat nail. • Touch Alum with Red Hot Nail

#28 Evolution of gases and Lilac flame, K+ ion. • Slowly bring close to the flame. • Watch it swell as gases H 2 O(g) and SO 3(g) evolve. • Bring it into the flame. • Lilac color, “K+” ion.

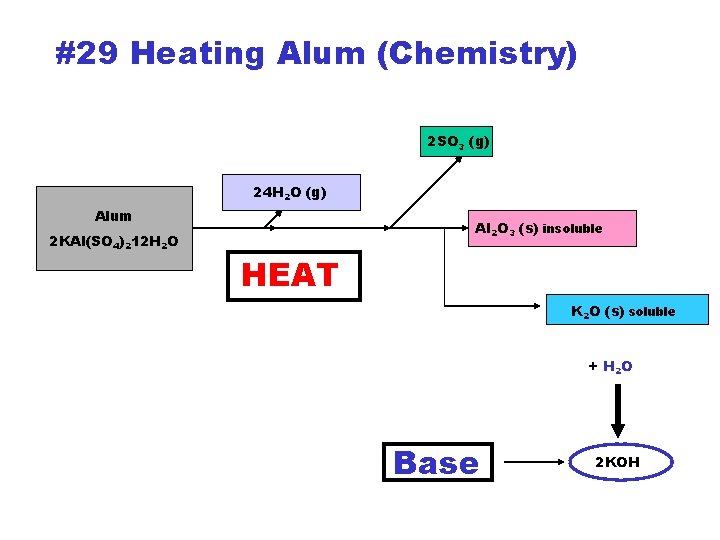
#29 Heating Alum (Chemistry) 2 SO 3 (g) 24 H 2 O (g) Alum 2 KAl(SO 4)212 H 2 O Al 2 O 3 (s) insoluble HEAT K 2 O (s) soluble + H 2 O Base 2 KOH

#30 Tests in heated Alum sample • 2 KAl(SO 4)2. 12 H 2 O 24 H 2 O(g) + 4 SO 3(g) + Al 2 O 3(s) + K 2 O(s) Remove Alum RESIDUE Crush it in a ~2 cm pool of distilled water.

#31 Tests with HEATED Alum sample • Check p. H, Basic. WHY? • K 2 O(s) + H 2 O(g) 2 KOH(aq) Filter over cotton. Test with Ba. Cl 2(aq) No SO 42 -, No ppt. CAUTION: Any Undecomposed alum may give positive test with Ba. Cl 2.

Acknowledgements Experiments: Ms. Virginia Roll Power Point Assistance: Mr. Bill Hager Christopher Byers
 Synthesis decomposition single replacement
Synthesis decomposition single replacement Double salts and coordination compounds
Double salts and coordination compounds Nalgonda technique
Nalgonda technique How does budhiya die in the shroud
How does budhiya die in the shroud Raja rani vs prem adib
Raja rani vs prem adib Munshi prem chandra
Munshi prem chandra Voltage securemail cloud
Voltage securemail cloud On prem
On prem Prem
Prem Aareon wiki
Aareon wiki Prem earth
Prem earth Logic synthesis
Logic synthesis Kiliani fischer synthesis
Kiliani fischer synthesis Gravitation and newton's synthesis
Gravitation and newton's synthesis Peptide synthesis
Peptide synthesis Protein synthesis
Protein synthesis Holistic synthesis
Holistic synthesis Sucrose synthesis
Sucrose synthesis Peptide bond dehydration synthesis
Peptide bond dehydration synthesis Lipid synthesis.
Lipid synthesis. Subtractive colour theory
Subtractive colour theory Thyroid storm mnemonic
Thyroid storm mnemonic Sources of carbon and nitrogen in pyrimidine ring
Sources of carbon and nitrogen in pyrimidine ring Strecker synthesis
Strecker synthesis Texture synthesis by non-parametric sampling
Texture synthesis by non-parametric sampling Optimal synthesis inc
Optimal synthesis inc Hydration reaction equation
Hydration reaction equation Application synthesis question
Application synthesis question Catalytic functions
Catalytic functions Robert van engelen
Robert van engelen Organic synthesis via enolates
Organic synthesis via enolates Question stem meaning
Question stem meaning Population genetics
Population genetics