QAlum Prem Sattsangi Copyright 2007 Corrosion of metals



- Slides: 9

Q-Alum Prem Sattsangi Copyright 2007

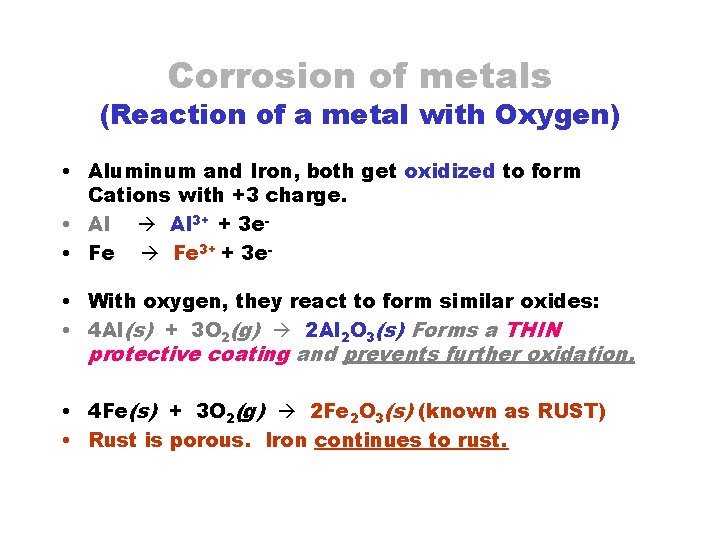
Corrosion of metals (Reaction of a metal with Oxygen) • Aluminum and Iron, both get oxidized to form Cations with +3 charge. • Al 3+ + 3 e • Fe 3+ + 3 e • With oxygen, they react to form similar oxides: • 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) Forms a THIN protective coating and prevents further oxidation. • 4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s) (known as RUST) • Rust is porous. Iron continues to rust.

#1 Alum • Explain the term alum • A Complex salt formed by combination of aluminum sulfate and a Gr. IA metal or ammonium sulfate. • Write the formula of: Ammonium alum NH 4 Al(SO 4)2. 12 H 2 O Lithium alum Li. Al(SO 4)2. 12 H 2 O Common alum KAl(SO 4)2. 12 H 2 O

#2 What is the formula of? Ammonium ion NH 4+ Sulfate ion SO 42 - Ammonium sulfate (NH 4)2 SO 4 Potassium Hydroxide KOH Aluminum hydroxide Al(OH)3 Potassium aluminum hydroxide KAl(OH)4 Potassium aluminum sulfate KAl(SO 4)2

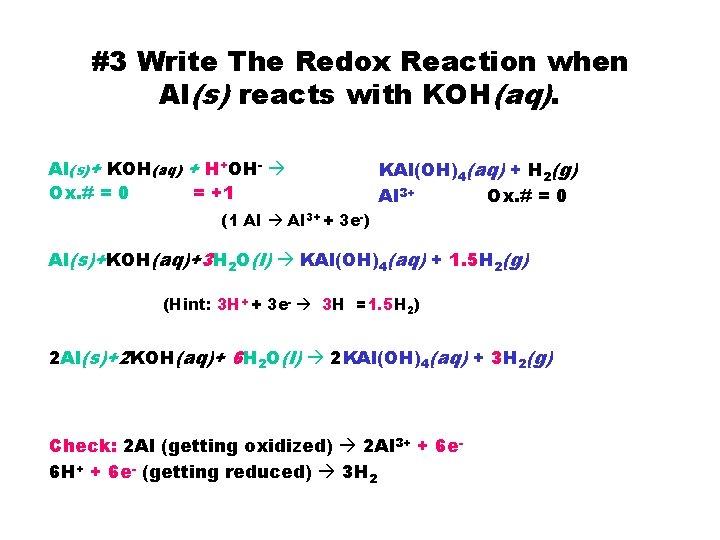
#3 Write The Redox Reaction when Al(s) reacts with KOH(aq). Al(s)+ KOH(aq) + H+OH- Ox. # = 0 = +1 KAl(OH)4(aq) + H 2(g) Al 3+ Ox. # = 0 (1 Al 3+ + 3 e-) Al(s)+KOH(aq)+3 H 2 O(l) KAl(OH)4(aq) + 1. 5 H 2(g) (Hint: 3 H+ + 3 e- 3 H =1. 5 H 2) 2 Al(s)+2 KOH(aq)+ 6 H 2 O(l) 2 KAl(OH)4(aq) + 3 H 2(g) Check: 2 Al (getting oxidized) 2 Al 3+ + 6 e 6 H+ + 6 e- (getting reduced) 3 H 2

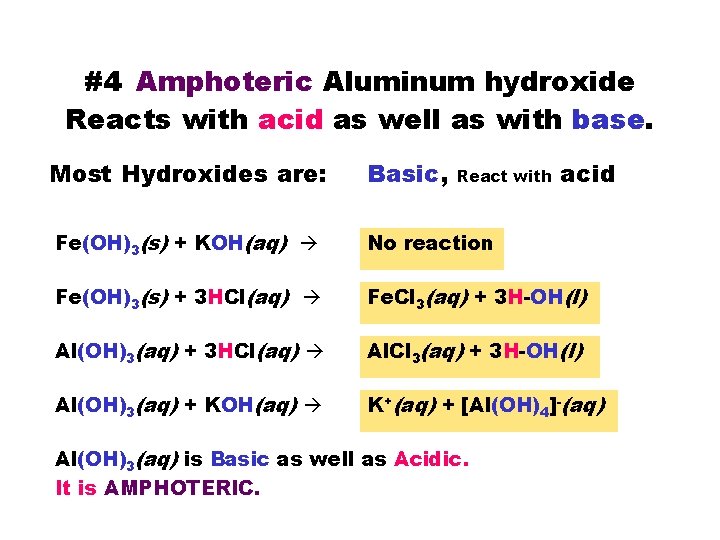
#4 Amphoteric Aluminum hydroxide Reacts with acid as well as with base. Most Hydroxides are: Basic, Fe(OH)3(s) + KOH(aq) No reaction Fe(OH)3(s) + 3 HCl(aq) Fe. Cl 3(aq) + 3 H-OH(l) Al(OH)3(aq) + 3 HCl(aq) Al. Cl 3(aq) + 3 H-OH(l) Al(OH)3(aq) + KOH(aq) K+(aq) + [Al(OH)4]-(aq) React with Al(OH)3(aq) is Basic as well as Acidic. It is AMPHOTERIC. acid

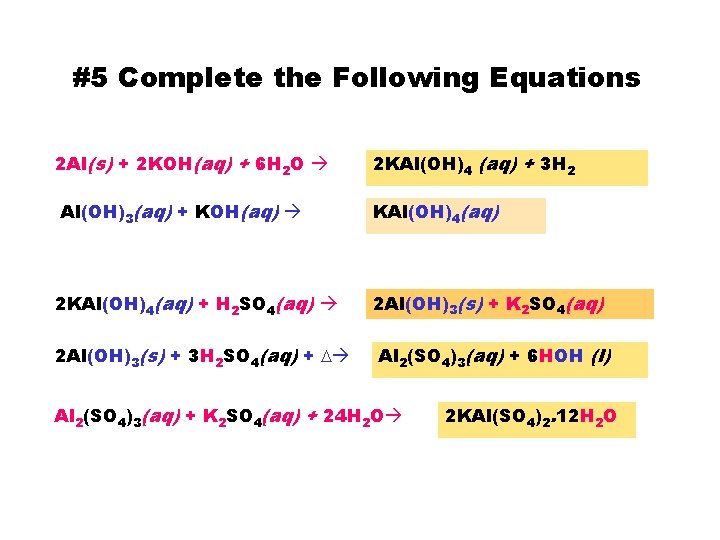
#5 Complete the Following Equations 2 Al(s) + 2 KOH(aq) + 6 H 2 O Al(OH)3(aq) + KOH(aq) 2 KAl(OH)4(aq) + H 2 SO 4(aq) 2 Al(OH)3(s) + 3 H 2 SO 4(aq) + D 2 KAl(OH)4 (aq) + 3 H 2 KAl(OH)4(aq) 2 Al(OH)3(s) + K 2 SO 4(aq) Al 2(SO 4)3(aq) + 6 HOH (l) Al 2(SO 4)3(aq) + K 2 SO 4(aq) + 24 H 2 O 2 KAl(SO 4)2. 12 H 2 O

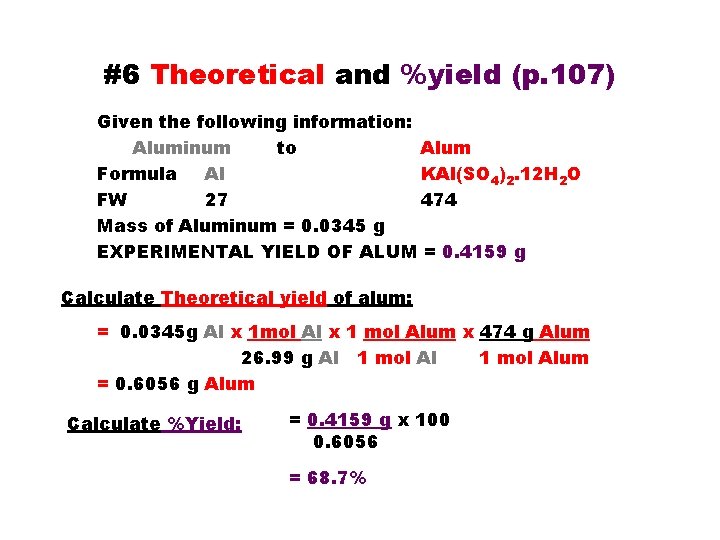
#6 Theoretical and %yield (p. 107) Given the following information: Aluminum to Alum Formula Al KAl(SO 4)2. 12 H 2 O FW 27 474 Mass of Aluminum = 0. 0345 g EXPERIMENTAL YIELD OF ALUM = 0. 4159 g Calculate Theoretical yield of alum: = 0. 0345 g Al x 1 mol Al x 1 mol Alum x 474 g Alum 26. 99 g Al 1 mol Alum = 0. 6056 g Alum Calculate %Yield: = 0. 4159 g x 100 0. 6056 = 68. 7%

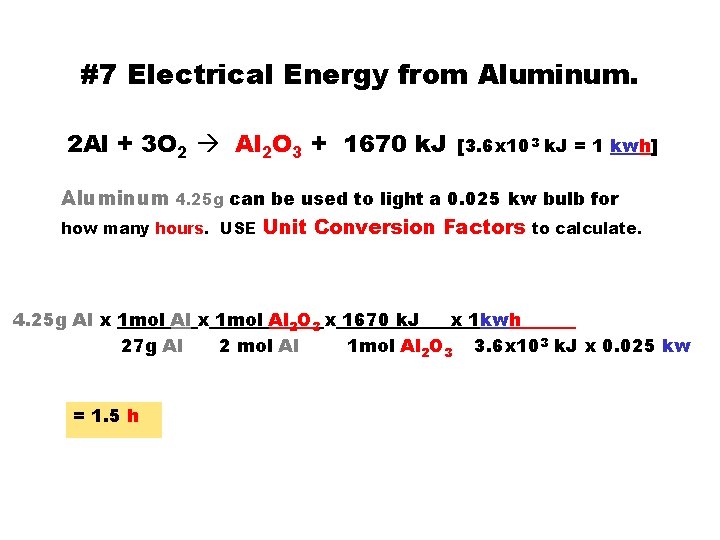
#7 Electrical Energy from Aluminum. 2 Al + 3 O 2 Al 2 O 3 + 1670 k. J [3. 6 x 103 k. J = 1 kwh] Aluminum 4. 25 g can be used to light a 0. 025 kw bulb for how many hours. USE Unit Conversion Factors to calculate. 4. 25 g Al x 1 mol Al 2 O 3 x 1670 k. J x 1 kwh______ 27 g Al 2 mol Al 1 mol Al 2 O 3 3. 6 x 103 k. J x 0. 025 kw = 1. 5 h

















