CORROSION Principle of Corrosion Different types of Corrosion











































- Slides: 67

CORROSION Principle of Corrosion Different types of Corrosion Coverage Stress Corrosion Cracking Pitting Corrosion Biological Corrosion Hydrogen Embrittlement Prevention & Control Lectures in Fracture Mechanics National Institute of Technology Tiruchirappalli

What is Corrosion? Corrosion is defined as deterioration or destruction of material due to its interaction with environment. It’s a reverse of metallurgy. Any material when It is kept in a certain atmosphere their will be potential difference between the material and the surroundings. To complete the circuit ions of material comes to the solution. CORROSION RATE: Rate at which ions are coming out.

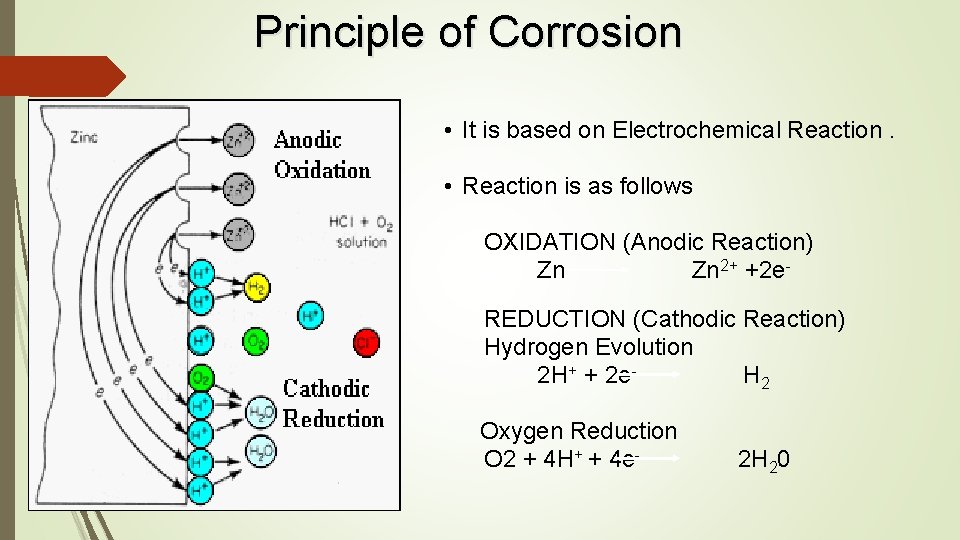
Principle of Corrosion • It is based on Electrochemical Reaction. • Reaction is as follows OXIDATION (Anodic Reaction) Zn 2+ +2 e REDUCTION (Cathodic Reaction) Hydrogen Evolution 2 H+ + 2 e- H 2 Oxygen Reduction O 2 + 4 H+ + 4 e- 2 H 20

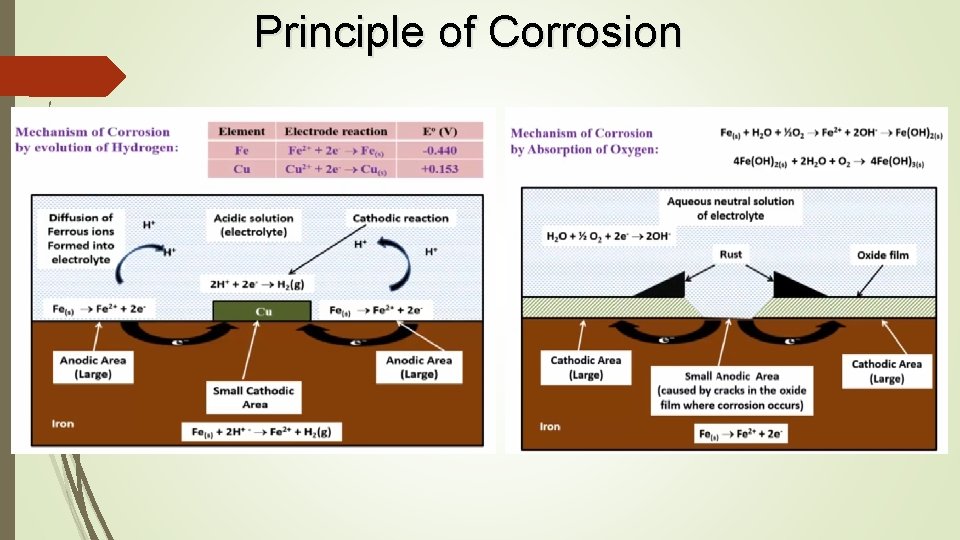
Principle of Corrosion

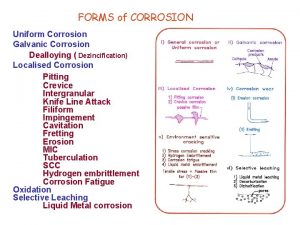
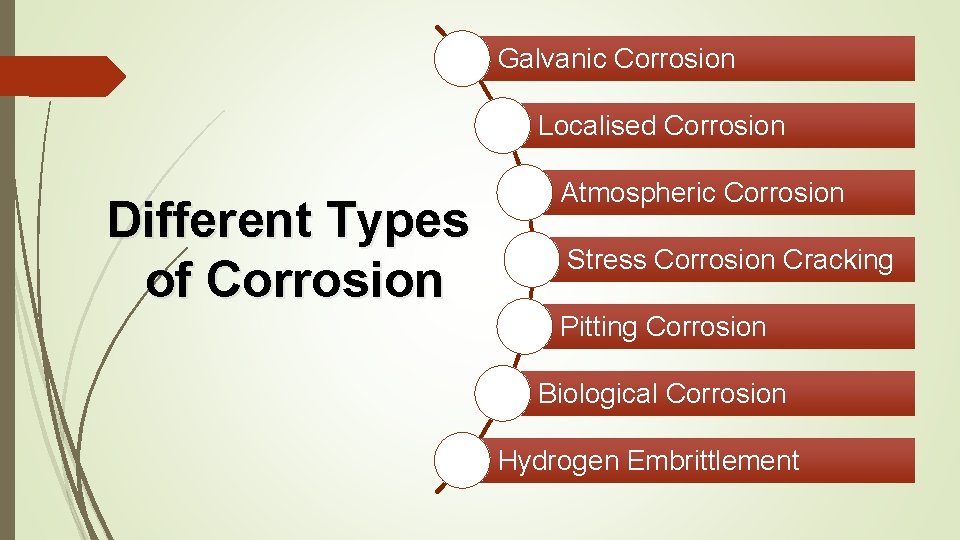
Galvanic Corrosion Localised Corrosion Different Types of Corrosion Atmospheric Corrosion Stress Corrosion Cracking Pitting Corrosion Biological Corrosion Hydrogen Embrittlement

Galvanic or Two-metal Corrosion • Galvanic Effect – If two dissimilar materials are in a contact (electrically connected or physical contact), one will be undergo corrosion (act as a anode) in the presence of corrosive medium (electrolyte). • The metal with high standard oxidation potential act as a anode and other with low reduction potential act as a cathode. • Galvanic effect can be estimated from EMF series and galvanic series of materials.

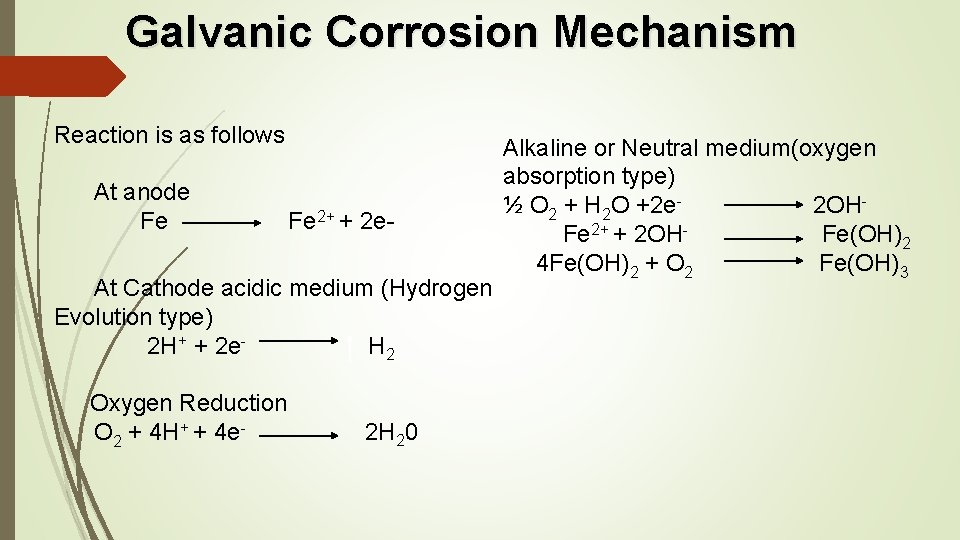
Galvanic Corrosion Mechanism Reaction is as follows At anode Fe 2+ + 2 e Alkaline or Neutral medium(oxygen absorption type) ½ O 2 + H 2 O +2 e- 2 OH Fe 2+ + 2 OH- Fe(OH)2 4 Fe(OH)2 + O 2 Fe(OH)3 At Cathode acidic medium (Hydrogen Evolution type) 2 H+ + 2 e- H 2 Oxygen Reduction O 2 + 4 H+ + 4 e- 2 H 20

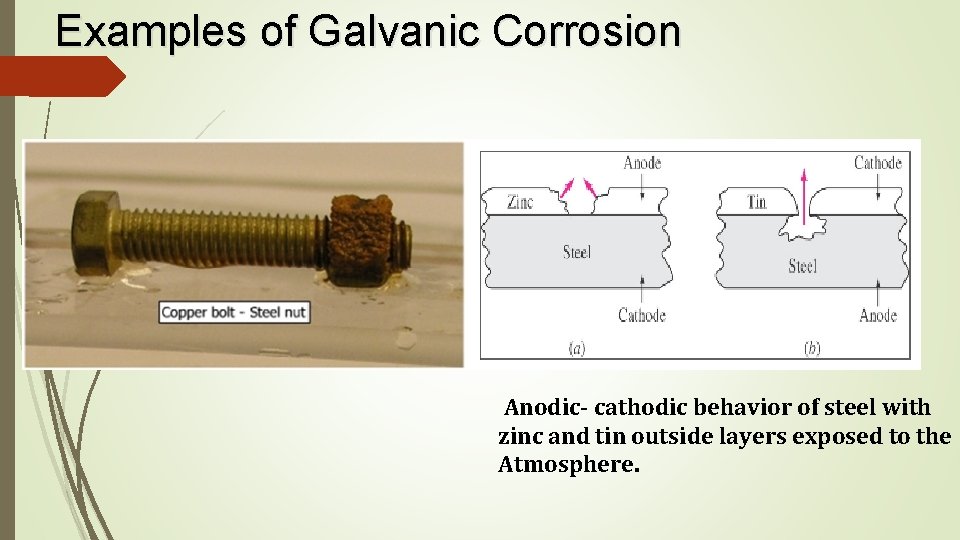
Examples of Galvanic Corrosion Anodic- cathodic behavior of steel with zinc and tin outside layers exposed to the Atmosphere.

Prevention from Galvanic Corrosion Avoid contact between dissimilar metals. For unavoidable situations Ø anodic area should be larger than the cathodic area. Ø the two metals should be as close as possible in the galvanic series. Ø an insulator may be fitted between the two metals. Ø avoid threaded joints between the metals.

Localized Corrosion • Localised Corrosion is the selective removal of metal by corrosion at a small area or zones on a metal surface in contact with corrosive environment • It takes place at a higher rate than the rest of original surface. • Occurs when corrosion works with other destructive process such as stress , fatigue , erosion and other forms of chemical attack.

Localized Corrosion… • Chemical factor: ü Initiated in the metal- intergranular, pitting ü Initiated in the environment- crevice • Chemical and mechanical factors: ü Mechanical process in metal-stress corrosion cracking, hydrogen embrittlement ü Mechanical action of metal-erosion corrosion ü Mechanical action of solid body of metal-fretting corrosion

Localized Corrosion… v Crevice corrosion: Corrosion that occurs in stagnant locations such as those found under gaskets. v Filiform corrosion: Corrosion that occurs when water gets under a coating such as paint. v Pitting corrosion: The creation of small holes in the surface of a metal. v Intergranular corrosion: Where the boundaries of crystallites of the material are more susceptible to corrosion than their insides.

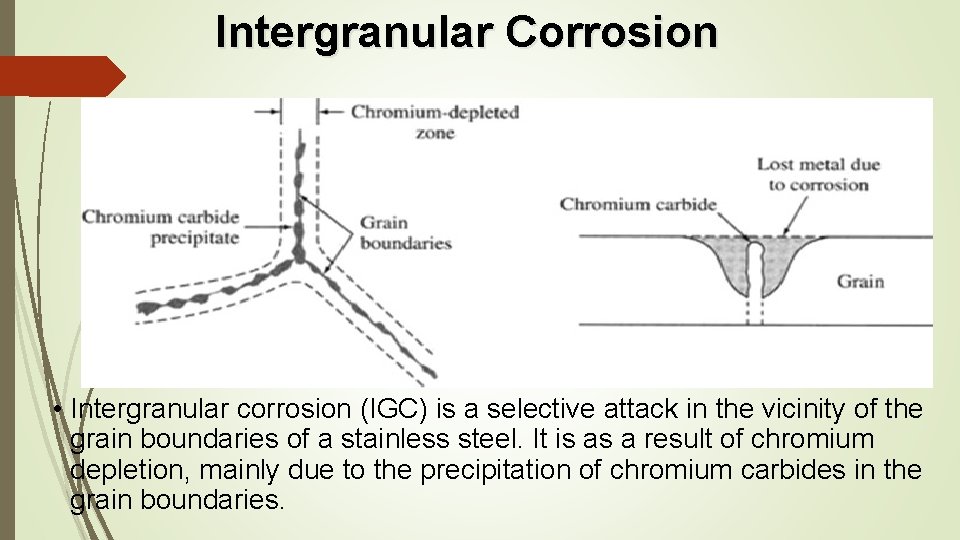
Intergranular Corrosion • Intergranular corrosion (IGC) is a selective attack in the vicinity of the grain boundaries of a stainless steel. It is as a result of chromium depletion, mainly due to the precipitation of chromium carbides in the grain boundaries.

Crevice Corrosion Electrochemical oxidation-reduction (redox) process • • It occurs within localized volumes of stagnant solution(like dust) trapped in pockets, corners or rivet heads. Crevice corrosion is much more dangerous than uniform corrosion. Crevice corrosion is highly accelerated if chloride, sulphate or bromide ions are present in the electrolyte solution. Mechanism of crevice corrosion is similar to that of Pitting corrosion: dissolution of the metal and gradual acidification of the electrolyte caused by its insufficient aeration (Oxygen penetration).

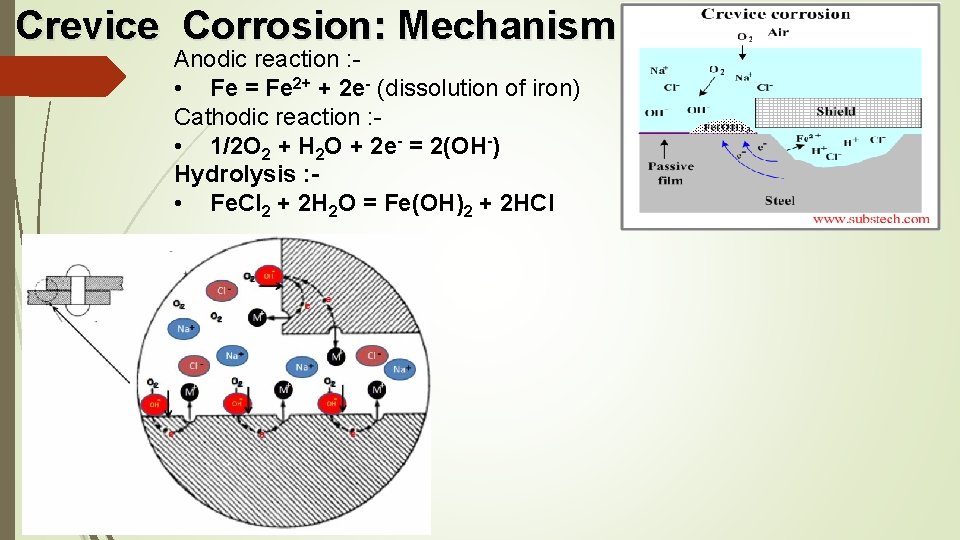
Crevice Corrosion: Mechanism Anodic reaction : - • Fe = Fe 2+ + 2 e- (dissolution of iron) Cathodic reaction : • 1/2 O 2 + H 2 O + 2 e- = 2(OH-) Hydrolysis : • Fe. Cl 2 + 2 H 2 O = Fe(OH)2 + 2 HCl

Prevention from Crevice Corrosion • • Use butt joint instead of lap-joints. Clean thoroughly to remove stagnant. Avoid sharp corner while designing Use non-absorbable solid gaskets such as Teflon.

Filiform Corrosion • It is a special type of crevice corrosion. Here the corrosion takes place under a thin film. • It appears on the steel , Magnesium and Aluminium surface covered by tin, silver, gold phosphates, etc. • The corrosion appears as thread-like filaments under the coating.

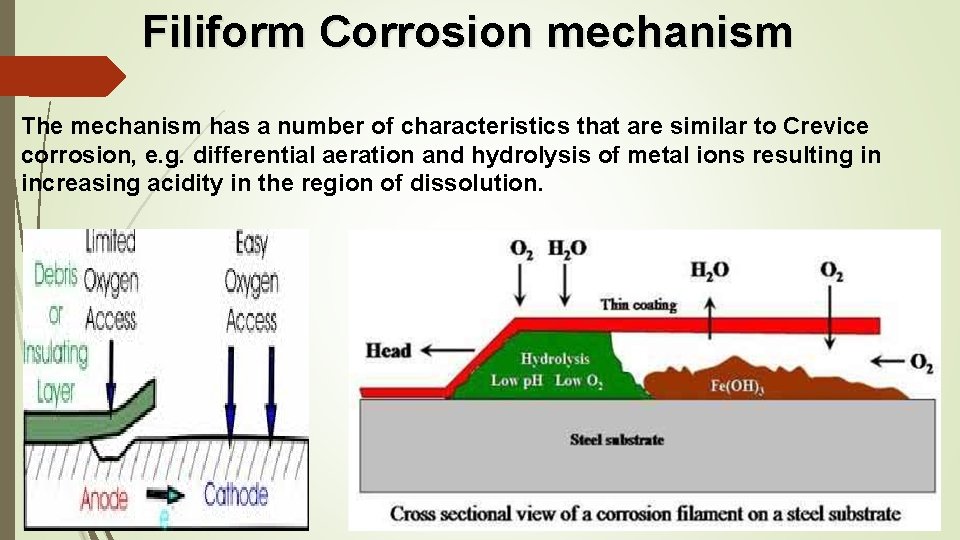
Filiform Corrosion mechanism The mechanism has a number of characteristics that are similar to Crevice corrosion, e. g. differential aeration and hydrolysis of metal ions resulting in increasing acidity in the region of dissolution.

Atmospheric Corrosion • According to this theory, corrosion on the surface of a metal is due to direct reaction of atmospheric gases like oxygen, halogens, oxides of Sulphur, oxides of nitrogen, hydrogen sulfide and fumes of chemicals with metal. • The extent of corrosion of a particular metal depends on the chemical affinity of the metal towards reactive gas. • Oxygen is mainly responsible for the corrosion of most metallic substances when compared to other gases and chemicals. • Mainly three types: (1) Oxidation corrosion (Reaction with oxygen) (2) Corrosion by other gases. (3) electrochemical

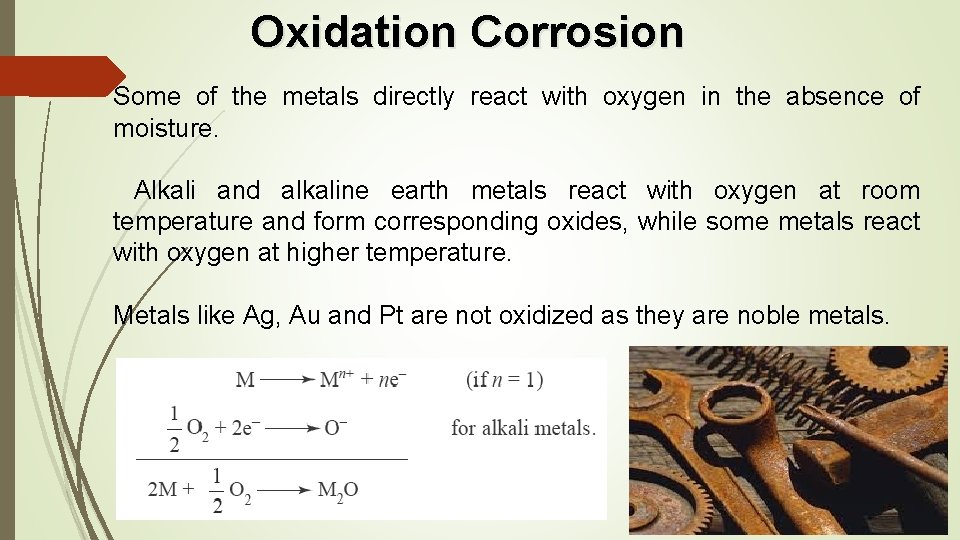
Oxidation Corrosion Some of the metals directly react with oxygen in the absence of moisture. Alkali and alkaline earth metals react with oxygen at room temperature and form corresponding oxides, while some metals react with oxygen at higher temperature. Metals like Ag, Au and Pt are not oxidized as they are noble metals.

• During oxidation of a metal, metal oxide is formed as a thin film on the metallic surface which protects the metal from further corrosion. • If diffusion of either oxygen or metal is across this layer, further corrosion is possible. Thus, the layer of metal oxide plays an important role in the process of corrosion. • Oxides of Pb, Al and Sn are stable and hence inhibit further corrosion. They form a stable, tightly adhering oxide film. • In case of porous oxide film, atmospheric gases pass through the pores and react with the metal and the process of corrosion continues to occur till the entire metal is converted into oxide. • Porous oxide layer is formed by alkali and alkaline earth metals. Molybdenum forms a volatile oxide film of Mo. O 3 and accelerates corrosion. • Au, Ag, Pt form unstable oxide layer which decomposes soon after the formation, thereby preventing further corrosion.

CORROSION BY OTHER GASES • In dry atmosphere, these gases react with metal and form corrosion products which may be protective or non-protective. • Dry Cl 2 reacts with Ag and forms Ag. Cl which is a protective layer, while Sn. Cl 4 is volatile. • In petroleum industries at high temperatures, H 2 S attacks steel forming Fe. S scale which is porous and interferes with normal operations.

Stress Corrosion Cracking • Stress corrosion occurs due to tensile stresses (applied and residual stresses) on the susceptible material in the presence of a specific corrosive environment, resulting In the formation of propagating cracks. Susceptibl e Material SCC Corrosive Environ. Tensile Stress

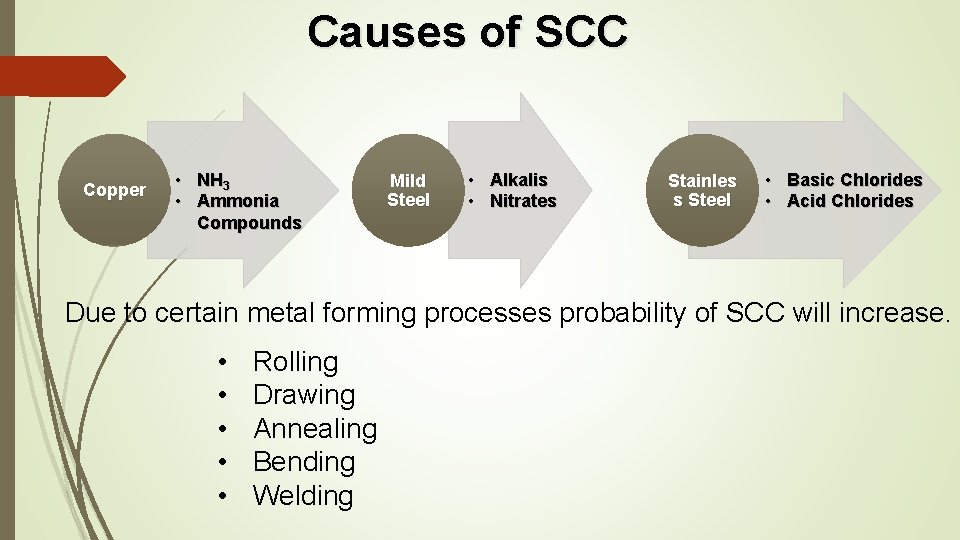
Causes of SCC Copper • NH 3 • Ammonia Compounds Mild Steel • Alkalis • Nitrates Stainles s Steel • Basic Chlorides • Acid Chlorides Due to certain metal forming processes probability of SCC will increase. • • • Rolling Drawing Annealing Bending Welding

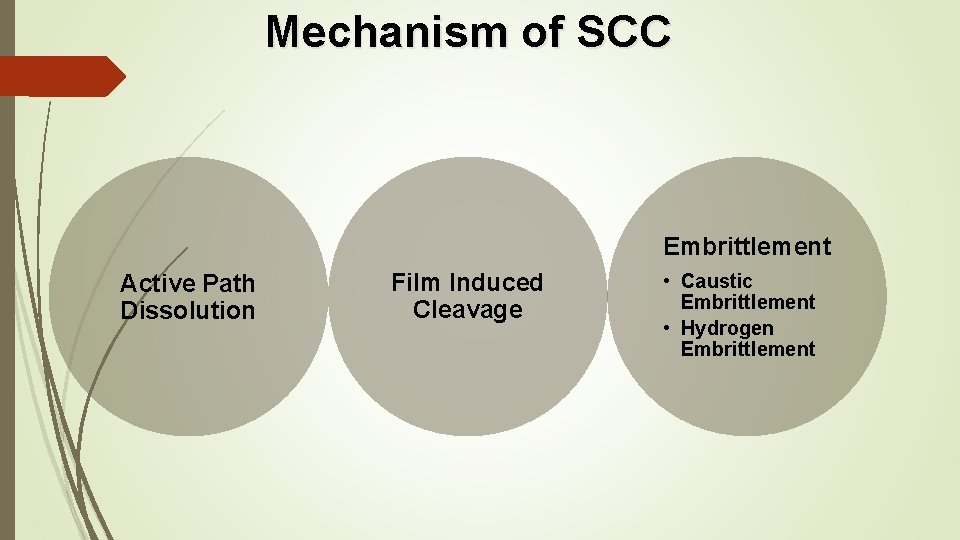
Mechanism of SCC Embrittlement Active Path Dissolution Film Induced Cleavage • Caustic Embrittlement • Hydrogen Embrittlement

Active Path Dissolution • Active path dissolution is a material failure due to stress corrosion cracking (SCC) that is characterized by the progression of corrosion along an intermolecular path of least resistance with high concentrations of tensile strength. • Active path dissolution is characterized by the significantly increased rate of corrosion along an intergranular sub-section or boundary that presents above average susceptibility to corrosion. Such granular boundaries on the molecular level within metals tend to display segregation of impure elements, while the bulk layer of the material contains a passive tendency. This makes it more difficult for passivation and protection of the bulk material. occur.

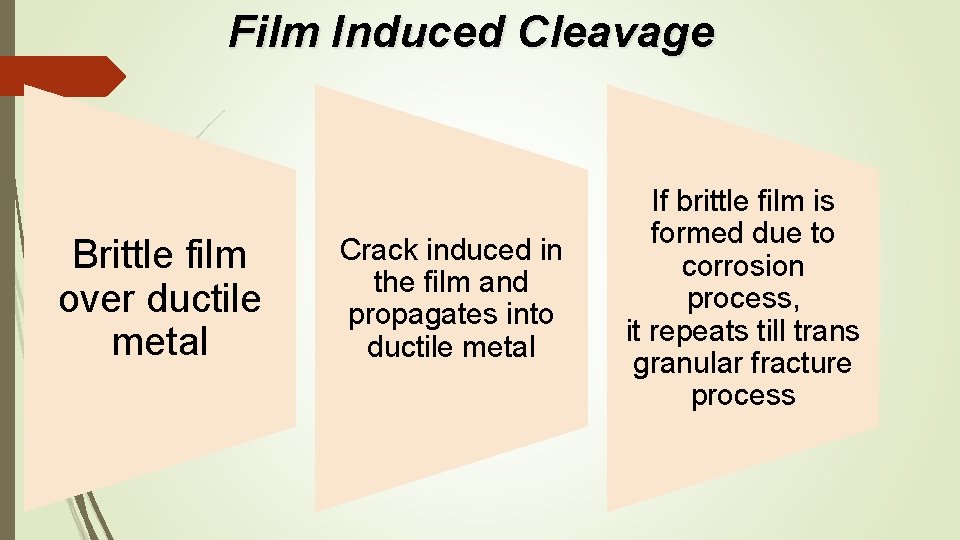
Film Induced Cleavage Brittle film over ductile metal Crack induced in the film and propagates into ductile metal If brittle film is formed due to corrosion process, it repeats till trans granular fracture process

Prevention • Control over operating temperature. • Control stresses while machining. • Avoid specific chemical environment. • Choose an inert material, that does not reacts with the specific environment.

Pitting Corrosion • Pitting corrosion is a localized attack in the metal resulting in the formation of pit, hole, cavity. (confined to a point or small area) • Reason: – When the anodic area is small and cathodic area is large, it leads to drastic corrosion at the anode to form a pit or a hole.

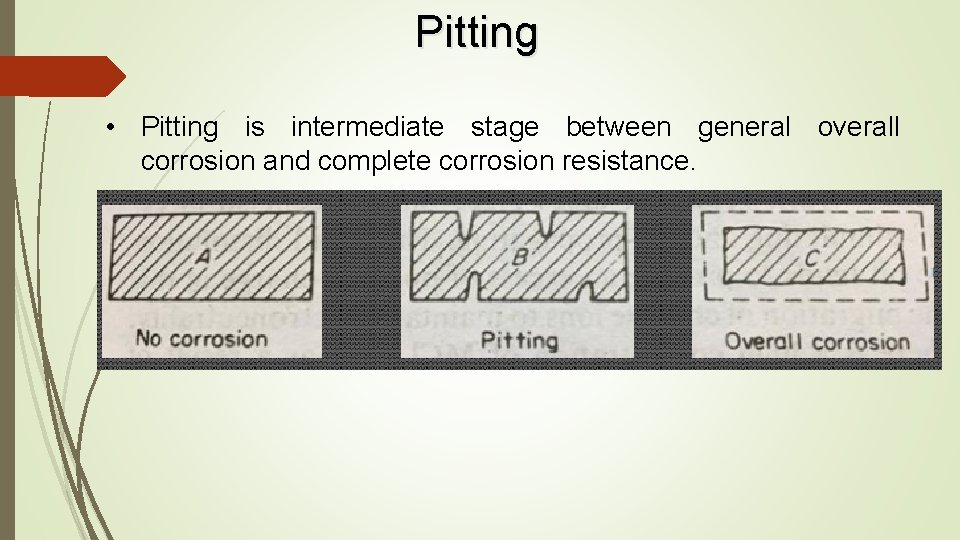
Pitting • Pitting is intermediate stage between general overall corrosion and complete corrosion resistance.

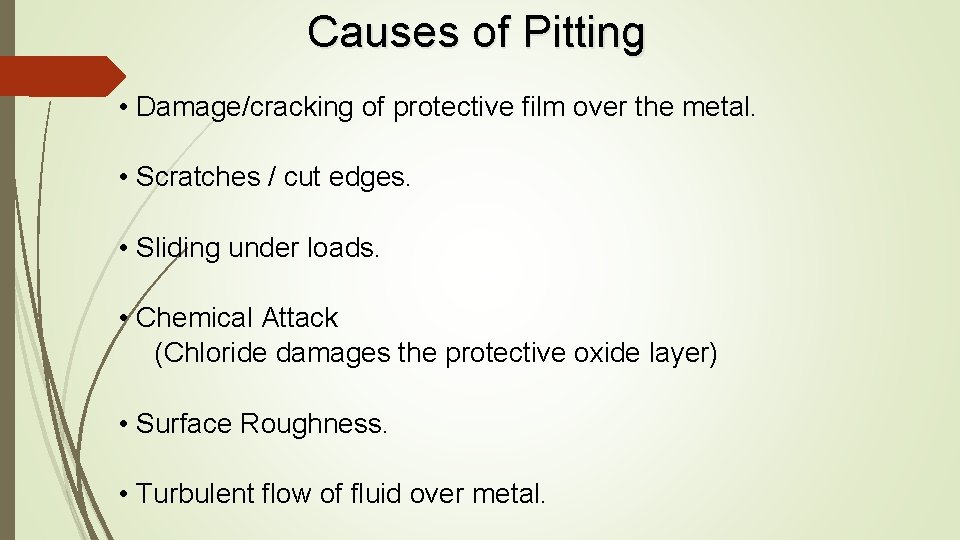
Causes of Pitting • Damage/cracking of protective film over the metal. • Scratches / cut edges. • Sliding under loads. • Chemical Attack (Chloride damages the protective oxide layer) • Surface Roughness. • Turbulent flow of fluid over metal.

Pit Shape and Growth • Pits usually grow in direction in gravity. • Lesser number start on vertical surface and rarely do they grow upward. • Pits tend to undermine or undercut the surface as they grow. • Subsurface damage is much more severe than is indicated by surface appearances. • Pitting requires an extended initiation period , once started pit penetrates at an ever increasing rate.

Mechanism of Pit Formation • At the interface between the pit and the adjacent surface, iron hydroxide forms due to interaction between the OH- produced by cathodic reaction and pit corrosion product. • This is further oxidised by the dissolved oxygen in the solution to Fe(OH)3 , Fe 304 and other oxides. • This rust rim grows in the form of a tube.

Environmental Factors • Most pitting corrosions are caused by chloride and chloride containing ions. • Most pitting is associated with halide ions, with chlorides, bromides and hypo chlorites being the most prevalent. • Fluorides and Iodides have comparatively little pitting tendencies. • Even most corrosion resistant alloys can be pitted by Copper Chlorides and Iron Chlorides.

Biological Corrosion • The interaction of organisms with corrosion processes, is a complex subject encompassing contributions from several disciplines ranging from chemistry and surface science to microbiology and bacteriology. • They are caused by microbes and fungi. • Aerobic bacteria decreases the concentration of oxygen in the medium in contact with the metal surface. • The main product of corrosion is Iron Sulphide.

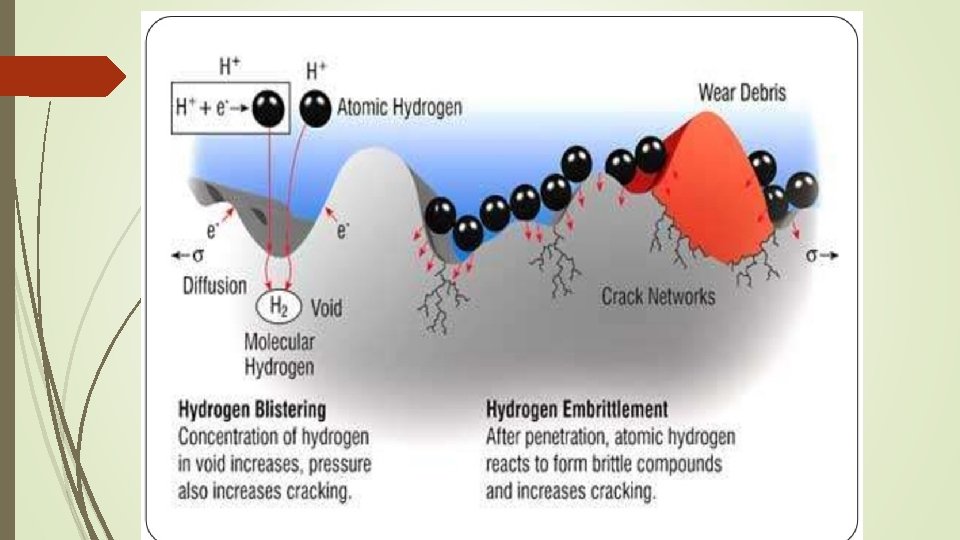
HYDROGEN EMBRITTLEMENT Hydrogen Embrittlement is the degradation of structural properties of solid due to hydrogen. Primary impact on the metals takes the form of loss of ductility and reduced load carrying capacity. This is often a result of accidental introduction of hydrogen during forming and finishing operations. Occurs in most metal, but not usually in copper, gold, silver and tungsten.

During hydrogen embrittlement, hydrogen is introduced to the surface of a metal and individual hydrogen atoms diffuse through the metal. Solubility of hydrogen increases at higher temperatures, raising the temperature can increase the diffusion of hydrogen. If there is significantly more hydrogen outside the metal than inside, hydrogen diffusion can occur even at lower temperatures. Individual hydrogen atoms within the metal gradually recombine to form hydrogen molecules, creating pressure from within the metal. This pressure can increase to levels where the metal has reduced


HYDROGEN EMBRITTLEMENT THEORIES Decohesion Theory • The absorption of hydrogen decreases the atomic binding forces of the metal lattice. • This results in the premature brittle-material fracture along the grain boundaries or network levels. Reduced Surface Theory • The absorption of hydrogen decreases the surface free energy of the metal. • Propagation of the crack tip is enhanced. • Explains crack propagation of high-strength steels in lowpressure hydrogen environments. Planar Pressure Theory • Occurs when metals are charged with hydrogen during solidification. • High-pressure hydrogen can form in micro voids. • Same mechanism as hydrogen blistering.

Occurrences Hydrogen embrittlement can occur during various manufacturing operations or operational use anywhere that the metal comes into contact with atomic or molecular hydrogen. Processes that can lead to this include cathodic protection, pickling, phosphating and electroplating.

Preventive Measures v Prevention in Design: High-strength metals and alloys are more susceptible to embrittlement. Common mistake is to overcompensate on strength requirements for service. At ambient conditions: Minimize the stress acting on the material!

Preventive Measures… v Prevention in processing: Embrittlement can originate from poor production techniques. Problems arise when hydrogen is allowed into production environment. Remedies: Maintain low hydrogen atmosphere Heat treatment

Preventive Measures… v Prevention in Welding: Embrittlement can be localized around a weld. Welding rods containing hydrogen are the source. Remedies: Low hydrogen welding rods stored in a dry place Local heat treatment before & after welding

Preventive Measures… v Using Clean Steal: Rimmed steel tend to have numerous voids. Substitution of killed steel greatly increases the resistance to hydrogen interstitials for embrittlement. Because of less number of voids in this material.

Preventive Measures… v Reducing Corrosion Rate: Hydrogen embrittlement occurs frequently during pickling operations. Where corrosion of the base metal produces vigorous hydrogen evolution. By adding inhibitor, base metal corrosion can be eliminated during pickling by subsequent decrease in hydrogen pickup.

Preventive Measures… v Baking: Hydrogen embrittlement is an almost reversible process, especially in steels. If hydrogen is removed, the mechanical properties of treated material are almost equal to Hydrogen free steel. Common way of removing hydrogen in steel is by baking at low temperatures at 200 -300 Fo.

Preventive Measures… v Substituting Alloys: High strength steels are more susceptible to hydrogen embrittlement. Alloying with Ni or Mo reduces susceptibility. Nickel containing steel and Nickel base alloys have very low hydrogen diffusion rates. Best way to prevent from hydrogen embrittlement.

Testing There are two ASTM standards for testing embrittlement due to hydrogen gas. Standard Test Method for Determination of the Susceptibility of Metallic Materials to Hydrogen Gas Embrittlement (HGE): - Uses a diaphragm loaded with differential pressure. If hydrogen containing environment at high pressure , high temperature, or both: - Uses a cylindrical tensile specimen tested into an enclosure pressurized with hydrogen or helium.

Example In 2013, six months prior to opening, the East Span of the Oakland Bay Bridge failed during testing. Catastrophic failures occurred in shear bolts in the span, after only two weeks of service, with the failure attributed to embrittlement, possibly from the environment.

Corrosion Prevention o o o o Material Selection Design Alteration of Environment Coating Cathodic Protection Electroplating Hot Dipping § Galvanising § Tinning

Material Selection • Most important method: – • Generally people believe that SS is good , but in chlorine containing medium and stressed structures , these are more susceptible than ordinary steels. • Localised such as Pitting and Crevice corrosion attack more on SS than MS. • General combination of alloy and medium for better resistance is given below : • SS – Nitric Acid • Tin – Distilled Water • Steel – Conc. Sulphuric Acid and many more……

As riveted joints are sites for crevice corrosion, welding is preferred more than riveting tanks. Excessive mechanical stresses or residual stresses are avoided to reduce stress corrosion cracking. Design Eliminate electrical contact between dissimilar to prevent galvanic corrosion by using similar material or using insulate materials. Avoid sharp bends in piping systems when high velocities abrasion materials are involved to prevent erosion corrosion. Tank bottoms must be designed in some inclination towards holes for easy draining otherwise the left out liquids results in severe corrosion.

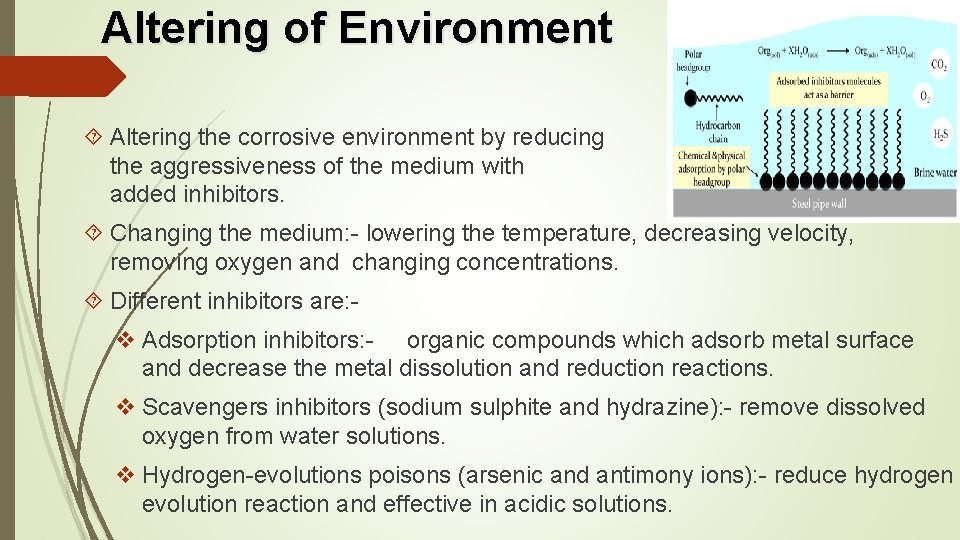
Altering of Environment Altering the corrosive environment by reducing the aggressiveness of the medium with added inhibitors. Changing the medium: - lowering the temperature, decreasing velocity, removing oxygen and changing concentrations. Different inhibitors are: v Adsorption inhibitors: - organic compounds which adsorb metal surface and decrease the metal dissolution and reduction reactions. v Scavengers inhibitors (sodium sulphite and hydrazine): - remove dissolved oxygen from water solutions. v Hydrogen-evolutions poisons (arsenic and antimony ions): - reduce hydrogen evolution reaction and effective in acidic solutions.

Coatings Metallic or inorganic & organic coatings! • Metallic coating can be applied easily very easily and they are quite deformable. eg: -silverware, galvanized steel, tin cans. • Inorganic coating are to be applied by diffusion or chemical conversion and these are brittle. eg: - chromized steel, anodized aluminium • Organic coating protect more than the metallic coating on a tonnage basis. Eg: -paints, varnishes, lacquers.

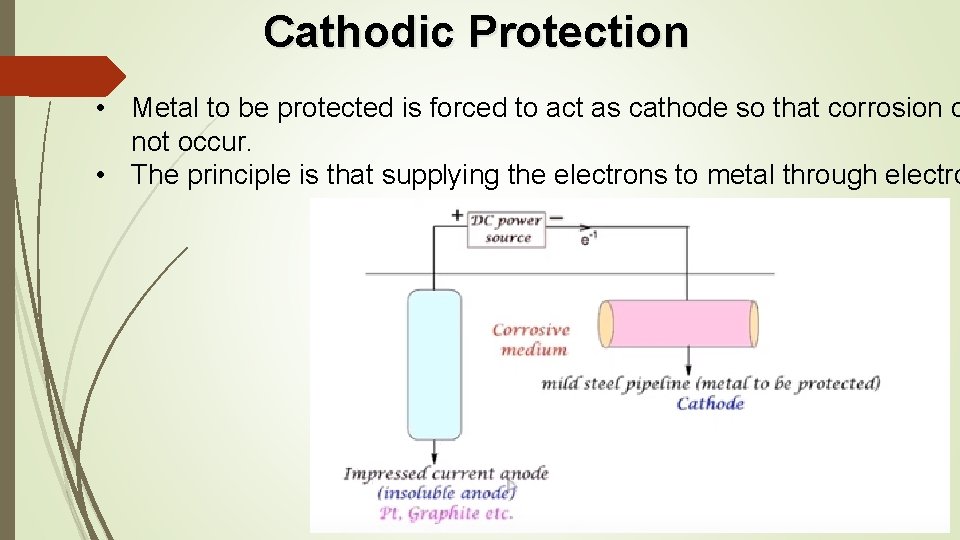
Cathodic Protection • Metal to be protected is forced to act as cathode so that corrosion d not occur. • The principle is that supplying the electrons to metal through electro

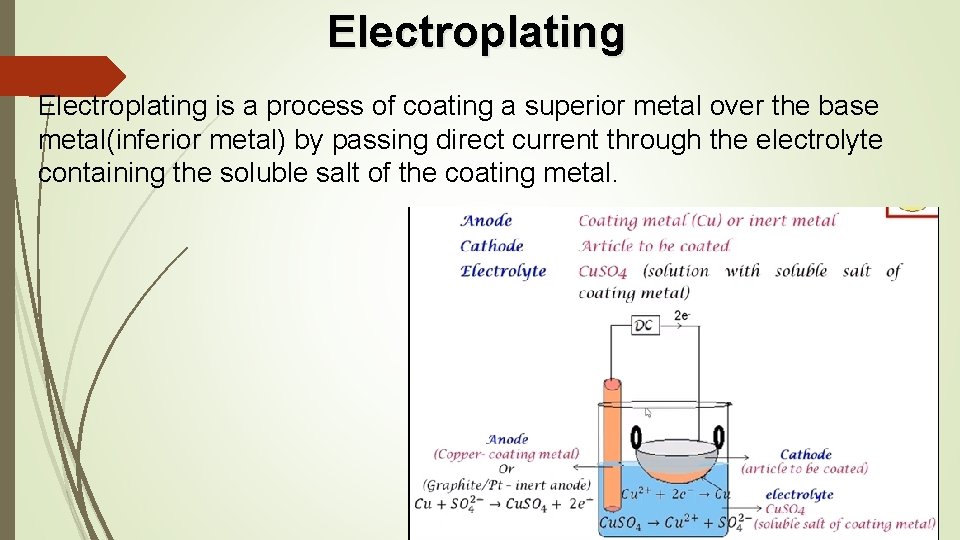
Electroplating is a process of coating a superior metal over the base metal(inferior metal) by passing direct current through the electrolyte containing the soluble salt of the coating metal.

Hot Dipping • It is the process of coating the base metal (metal with higher melting point ) dipping into a bath containing molten coating metal (metal with low melting point). • It is of two types: (A) galvanising (anodic coating) : - coating of more active metal, Zn over Cu. (B) tinning (Cathodic Coating): - coating of less active material, Tin over Cu.

Prevention and Control of Corrosion Ø Corrosion prevention techniques can be generally classified into 6 groups: Environmental Modifications Metal Selection and Surface Conditions Cathodic Protection Corrosion Inhibitors Coating Plating

Environmental Modification- Methods to reduce the sulfur, chloride or oxygen content in the surrounding environment can limit the speed of metal corrosion. For example, feed water for water boilers can be treated with softeners or other chemical media to adjust the hardness, alkalinity or oxygen content in order to reduce corrosion on the interior of the unit. Metal Selection and Surface Conditionsv. Proper monitoring and the elimination of unnecessarily vulnerable surface conditions, along with taking steps to ensure that systems are designed to avoid reactive metal combinations and that corrosive agents are not used in the cleaning or maintenance of metal parts are all also part of effective corrosion reduction program. v. The development of new alloys, designed to protect against corrosion in specific environments are constantly under production. Nickel alloys and titanium alloys are all examples of alloys designed for corrosion prevention.

Cathodic Protection- This method, known as a sacrificial system, uses metal anodes, introduced to the electrolytic environment, to sacrifice themselves (corrode) in order to protect the cathode. In a sacrificial system, metallic ions move from the anode to the cathode, which leads the anode to corrode more quickly than it otherwise would. As a result, the anode must regularly be replaced. Example: Using Mg blocks in ships

Ø Corrosion Inhibitors- Corrosion inhibitors are chemicals that react with the metal's surface or the environmental gasses causing corrosion, thereby, interrupting the chemical reaction that causes corrosion. Ø The effectiveness of a corrosion inhibitor depends on fluid composition, quantity of water, and flow regime. Ø A common mechanism for inhibiting corrosion involves formation of a coating, often a passivation layer, which prevents access of the corrosive substance to the metal Ø Volatile amines are used in boilers to minimize the effects of acid. Ø Corrosion inhibitors are often added to paints.


Coatings- Ø Paints and other organic coatings are used to protect metals from the degradative effect of environmental gasses. Ø Anti-corrosion coatings allow for added protection of metal surfaces and acts as a barrier to inhibit the contact between chemical compounds or corrosive materials. Ceramic coatings - Ceramic coatings provide corrosion, erosion and thermal protection. Polymer coatings - Polymer coatings/paints are composed of binders (major polymer constituent of the coating), solvents (water or Hydrocarbon solvents) for viscosity control, pigments and additives (eg. , Corrosion inhibitors). Oil and grease coating

Plating- Ø Metallic coatings, or plating, can be applied to inhibit corrosion as well as provide aesthetic, decorative finishes. Ø There are four common types of metallic coatings: 1 -Electroplating: A thin layer of metal - often nickel, tin, or chromium - is deposited on the substrate metal (generally steel) in an electrolytic bath. The electrolyte usually consists of a water solution containing salts of the metal to be deposited. 2 -Hot dipping: When immersed in a molten bath of the protective, coating metal a thin layer adheres to the substrate metal.

3 -Electroless plating: also known as chemical or auto-catalytic plating, is a non-galvanic plating method that involves several simultaneous reactions in an aqueous solution. It is mainly different from electroplating by not using external electrical power. In the manufacture of printed circuit boards, electroless plating is used to form the conductive part. The non-conductive part is treated with palladium catalyst and then made conductive by electroless copper plating

4 -Mechanical plating: also known as peen plating, mechanical deposition, or impact plating It is a plating process that imparts the coating by cold welding fine metal particles to a workpiece. It is commonly used to overcome hydrogen embrittlement problems. Commonly plated workpieces include nails, screws, nuts, washers, stampings, springs, etc.

Process The process begins with a descaling and removing soil from the workpiece. After cleaning, the parts are prepared by combining them with water, medium, and a surface conditioner. The surface conditioner lightly coats the workpiece in copper, while the medium removes any residual mill scale or oxides. Finally, accelerators, promoters and metal powder are added to the mix. The accelerators and promoters provide the proper chemical environment for the plating to occur, such as the maintenance of a p. H level of 1 to 2 to prevent oxidation and promote adhesion. The medium that is already in the mixture cold welds the metal powder to the workpiece through impacts that are induced by the tumbling action of the tumbler (Due to the rotation of tumbler) The surface finish can be improved with a water polish. The time required for the above process is approximately 50 minutes.
 Differentiate between dry corrosion and wet corrosion
Differentiate between dry corrosion and wet corrosion Dry corrosion and wet corrosion
Dry corrosion and wet corrosion Oxidation
Oxidation Types of electrochemical corrosion
Types of electrochemical corrosion Why do different polymers have different properties?
Why do different polymers have different properties? Flame test principle
Flame test principle Sound will travel at different speeds in different mediums.
Sound will travel at different speeds in different mediums. Sound will travel at different speeds in different mediums.
Sound will travel at different speeds in different mediums. Cultural relativism
Cultural relativism Different angle different story
Different angle different story Acids and bases song
Acids and bases song Different materials have different
Different materials have different What things make us special
What things make us special Venn diagram different same different
Venn diagram different same different What are different types of love
What are different types of love Three different types of irony
Three different types of irony Type of serial killers
Type of serial killers Different types of graphs
Different types of graphs Mac and tosh are resting on top of the water near
Mac and tosh are resting on top of the water near Different types of vegetarian
Different types of vegetarian 20kn
20kn Shadow box window display
Shadow box window display Transitory group definition sociology
Transitory group definition sociology Different types of sae programs
Different types of sae programs What are the two types of roots?
What are the two types of roots? Types of redox reactions
Types of redox reactions Different types of reactions
Different types of reactions English essay hooks
English essay hooks Types of home work
Types of home work News headlines examples
News headlines examples 3 types of claims examples
3 types of claims examples Different types of chemical bonds
Different types of chemical bonds Different kind of characters
Different kind of characters Irony devices
Irony devices Different types of irony
Different types of irony Formal letter types
Formal letter types Theater staging
Theater staging Different types of errors
Different types of errors Different types of testicles
Different types of testicles Swellgarfo
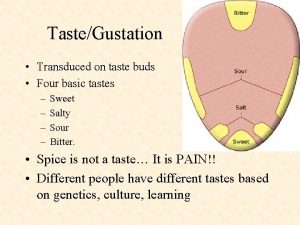
Swellgarfo Basic tastes
Basic tastes Metallic tapes are made up of
Metallic tapes are made up of Biotic and abiotic components of an ecosystem
Biotic and abiotic components of an ecosystem Different types of sports
Different types of sports Mother sauces
Mother sauces Different types of social groups
Different types of social groups The six types of conflict
The six types of conflict Give the seven types of feature article
Give the seven types of feature article Transduction of hearing
Transduction of hearing Scalar waves
Scalar waves Types of unions
Types of unions Different types of leverage
Different types of leverage Types of study design
Types of study design Quadrilateral with 2 acute angles
Quadrilateral with 2 acute angles Different types of syntax
Different types of syntax Primary and secondary tillage implements
Primary and secondary tillage implements Bed position in nursing
Bed position in nursing Types of population pyramids
Types of population pyramids Two types of third person
Two types of third person 3 sound devices in poetry
3 sound devices in poetry Sequence pattern of development example
Sequence pattern of development example T piece oxygen delivery
T piece oxygen delivery Overproduction in natural selection
Overproduction in natural selection Neuronal pool
Neuronal pool Types of narrative voice
Types of narrative voice How internet connection works
How internet connection works External motivation
External motivation Different types of solutions
Different types of solutions