QKinetics Prem Sattsangi Copyright 2009 http jchemed chem

![#6 -Rate Law Equation (p. 581) • Rate = k [NH 4+]1 x [NO #6 -Rate Law Equation (p. 581) • Rate = k [NH 4+]1 x [NO](https://slidetodoc.com/presentation_image_h2/ef0f5124795cc00f65921b0a1ef33b98/image-6.jpg)
![#7 -Figuring out Order of a reaction (p. 581) [Doubling Reactant Concentration] [R]r (order #7 -Figuring out Order of a reaction (p. 581) [Doubling Reactant Concentration] [R]r (order](https://slidetodoc.com/presentation_image_h2/ef0f5124795cc00f65921b0a1ef33b98/image-7.jpg)
![#8 -Figuring out Order of a reaction [Tripling Reactant Concentration] [R]r (order “r”) is #8 -Figuring out Order of a reaction [Tripling Reactant Concentration] [R]r (order “r”) is](https://slidetodoc.com/presentation_image_h2/ef0f5124795cc00f65921b0a1ef33b98/image-8.jpg)
 0. #9 -Orders (0, 1, 2, or 3) Trial • 1 • 2 [A](M) 0.](https://slidetodoc.com/presentation_image_h2/ef0f5124795cc00f65921b0a1ef33b98/image-9.jpg)
![#11 Units of “k” [M] in Rate (p. 581) cancels M in the denominator. #11 Units of “k” [M] in Rate (p. 581) cancels M in the denominator.](https://slidetodoc.com/presentation_image_h2/ef0f5124795cc00f65921b0a1ef33b98/image-11.jpg)
- Slides: 12

Q-Kinetics Prem Sattsangi Copyright 2009 http: //jchemed. chem. wisc. edu/JCEsoft/cca/c ca 3/main/clockrx/page 1. htm

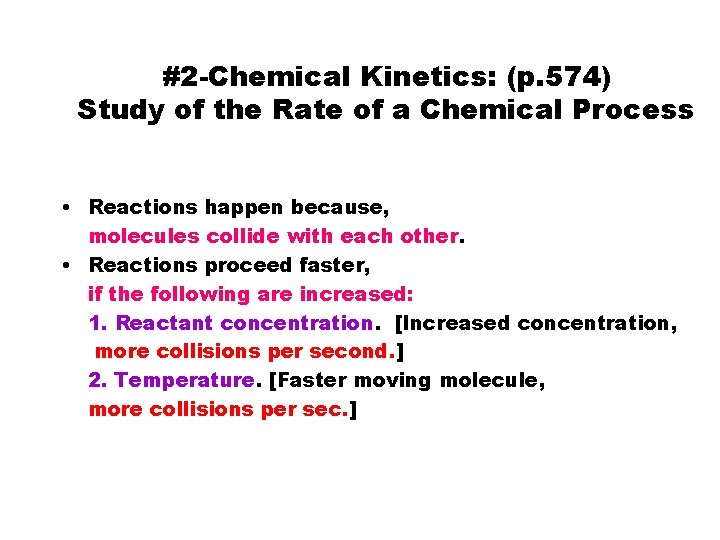
#2 -Chemical Kinetics: (p. 574) Study of the Rate of a Chemical Process • Reactions happen because, molecules collide with each other. • Reactions proceed faster, if the following are increased: 1. Reactant concentration. [Increased concentration, more collisions per second. ] 2. Temperature. [Faster moving molecule, more collisions per sec. ]

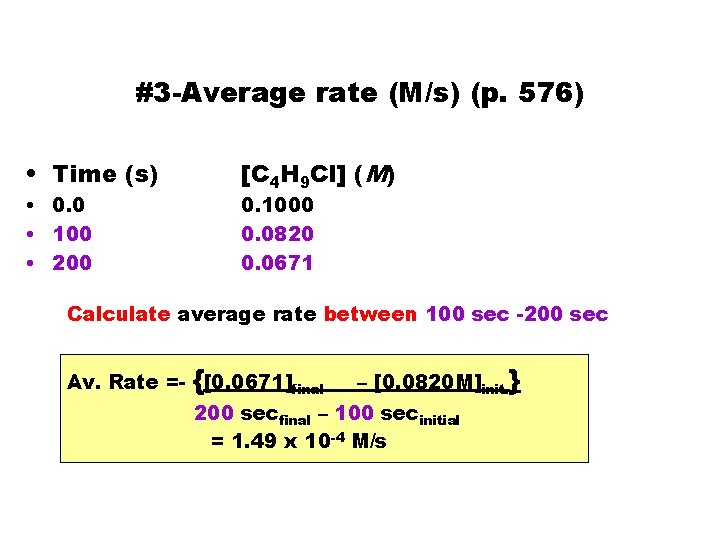
#3 -Average rate (M/s) (p. 576) • Time (s) • 0. 0 • 100 • 200 [C 4 H 9 Cl] (M) 0. 1000 0. 0820 0. 0671 Calculate average rate between 100 sec -200 sec Av. Rate =- {[0. 0671]final – [0. 0820 M]init. } 200 secfinal – 100 secinitial = 1. 49 x 10 -4 M/s

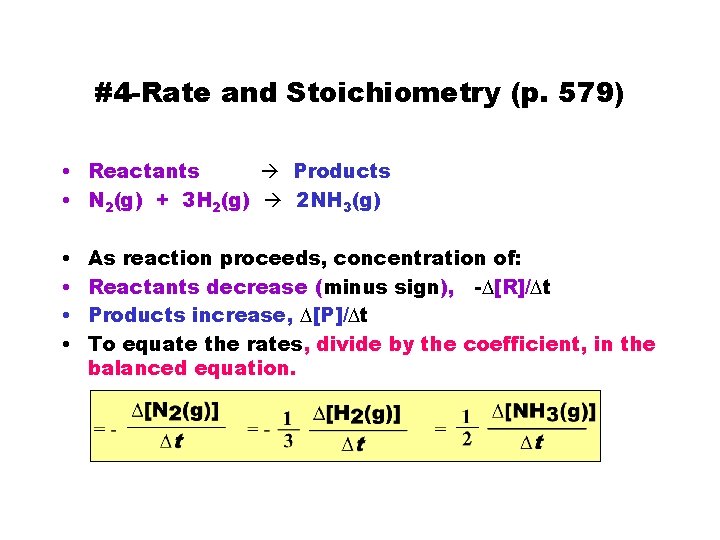
#4 -Rate and Stoichiometry (p. 579) • Reactants Products • N 2(g) + 3 H 2(g) 2 NH 3(g) • • As reaction proceeds, concentration of: Reactants decrease (minus sign), -D[R]/Dt Products increase, D[P]/Dt To equate the rates, divide by the coefficient, in the balanced equation.

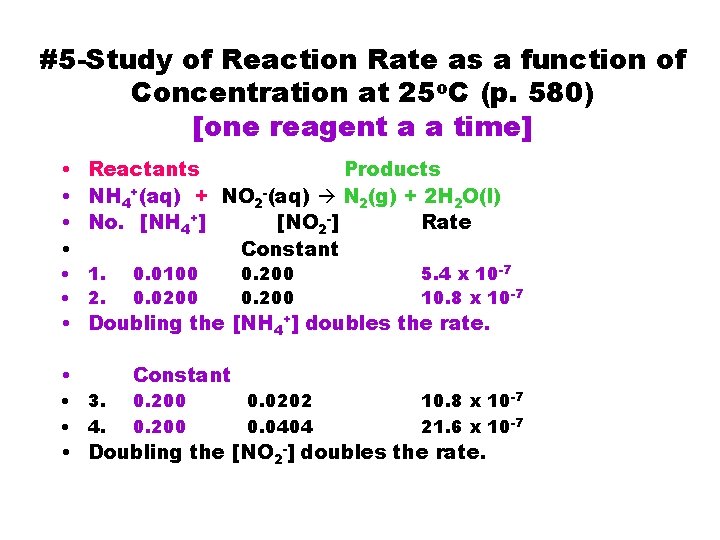
#5 -Study of Reaction Rate as a function of Concentration at 25 o. C (p. 580) [one reagent a a time] • Reactants Products • NH 4+(aq) + NO 2 -(aq) N 2(g) + 2 H 2 O(l) • No. [NH 4+] [NO 2 -] Rate • Constant • 1. • 2. 0. 0100 0. 0200 • Constant 0. 200 5. 4 x 10 -7 10. 8 x 10 -7 • Doubling the [NH 4+] doubles the rate. • 3. • 4. 0. 200 0. 0202 0. 0404 10. 8 x 10 -7 21. 6 x 10 -7 • Doubling the [NO 2 -] doubles the rate.
![6 Rate Law Equation p 581 Rate k NH 41 x NO #6 -Rate Law Equation (p. 581) • Rate = k [NH 4+]1 x [NO](https://slidetodoc.com/presentation_image_h2/ef0f5124795cc00f65921b0a1ef33b98/image-6.jpg)
#6 -Rate Law Equation (p. 581) • Rate = k [NH 4+]1 x [NO 2 -]1 • “k” = Rate constant, • (Value increases at higher temperature. ) • Exponents “ 1” are the order of reaction. • This reaction is First order in NH 4+ and • First order in NO 2 • Overall reaction order = 1 + 1 = 2
![7 Figuring out Order of a reaction p 581 Doubling Reactant Concentration Rr order #7 -Figuring out Order of a reaction (p. 581) [Doubling Reactant Concentration] [R]r (order](https://slidetodoc.com/presentation_image_h2/ef0f5124795cc00f65921b0a1ef33b98/image-7.jpg)
#7 -Figuring out Order of a reaction (p. 581) [Doubling Reactant Concentration] [R]r (order “r”) is related to Rate Doubling the concentration, “[2 R]r” and its effect on Rate: Rate Equation “r” Order UNCHANGED [2 R]0 = 1 x Rate “ 0” 2 x [2 R]1 = 2 x Rate “ 1” 4 x [2 R]2 = 4 x Rate “ 2” 8 x [2 R]3 = 8 x Rate “ 3”
![8 Figuring out Order of a reaction Tripling Reactant Concentration Rr order r is #8 -Figuring out Order of a reaction [Tripling Reactant Concentration] [R]r (order “r”) is](https://slidetodoc.com/presentation_image_h2/ef0f5124795cc00f65921b0a1ef33b98/image-8.jpg)
#8 -Figuring out Order of a reaction [Tripling Reactant Concentration] [R]r (order “r”) is related to Rate Tripling the concentration, “[3 R]r” and its effect on Rate: Rate Equation “r” Order UNCHANGED [3 R]0 = 1 x Rate “ 0” 3 x [3 R]1 = 3 x Rate “ 1” 9 x [3 R]2 = 9 x Rate “ 2” 27 x [3 R]3 = 27 x Rate “ 3”
 0.](https://slidetodoc.com/presentation_image_h2/ef0f5124795cc00f65921b0a1ef33b98/image-9.jpg)
#9 -Orders (0, 1, 2, or 3) Trial • 1 • 2 [A](M) 0. 273 [B](M) 0. 763 1. 526 Rate (M/s) 2. 83 x 10 -6 • 3 0. 819 0. 763 2. 547 x 10 -5 • In 1 and 2 [A] is constant, [B] is doubled • Rate remained same, Order in B = 0 • In 1 and 3 [B] is constant, [A] is tripled • Rate changed to 9 x or 32. • Order in A = 2

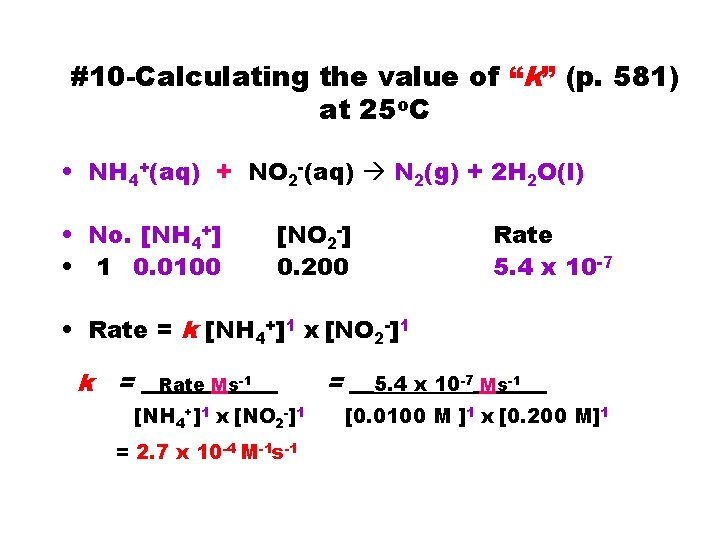
#10 -Calculating the value of “k” (p. 581) at 25 o. C • NH 4+(aq) + NO 2 -(aq) N 2(g) + 2 H 2 O(l) • No. [NH 4+] • 1 0. 0100 [NO 2 -] 0. 200 Rate 5. 4 x 10 -7 • Rate = k [NH 4+]1 x [NO 2 -]1 k = __Rate Ms-1___ [NH 4+]1 x [NO 2 -]1 = 2. 7 x 10 -4 M-1 s-1 = __ 5. 4 x 10 -7 Ms-1___ [0. 0100 M ]1 x [0. 200 M]1
![11 Units of k M in Rate p 581 cancels M in the denominator #11 Units of “k” [M] in Rate (p. 581) cancels M in the denominator.](https://slidetodoc.com/presentation_image_h2/ef0f5124795cc00f65921b0a1ef33b98/image-11.jpg)
#11 Units of “k” [M] in Rate (p. 581) cancels M in the denominator. • Rate law k= Unit • Rate = k[A]1 x [B]1 __Rate Ms-1___ [A M]1 x [B M]1 M-1 s-1 • Rate = k[A]1 x [B]2 __Rate Ms-1___ [A M]1 x [B M]2 M-2 s-1 • Rate = k[A]2 x [B]1 __Rate Ms-1___ [A M]2 x [B M]1 M-2 s-1 • Rate = k[A]2 x [B]2 __Rate Ms-1___ [A M]2 x [B M]2 M-3 s-1

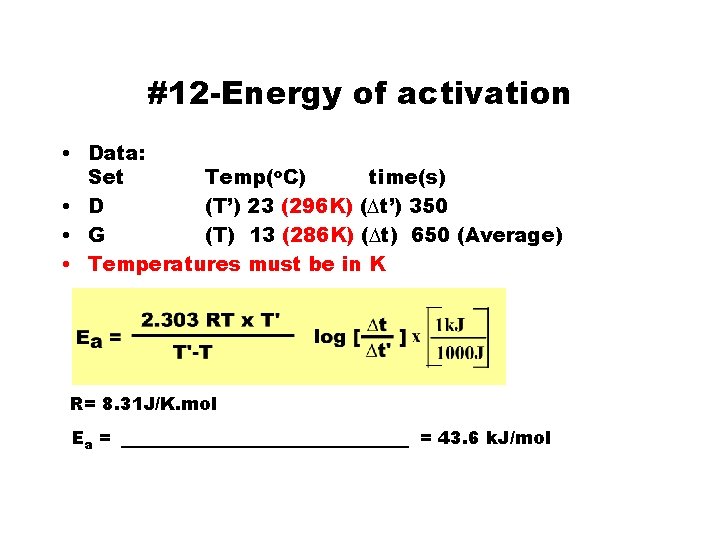
#12 -Energy of activation • Data: Set Temp(o. C) time(s) • D (T’) 23 (296 K) (Dt’) 350 • G (T) 13 (286 K) (Dt) 650 (Average) • Temperatures must be in K R= 8. 31 J/K. mol Ea = _________ = 43. 6 k. J/mol
 Diffrazione zanichelli
Diffrazione zanichelli Copyright 2009 pearson education inc
Copyright 2009 pearson education inc Copyright international color consortium, 2009
Copyright international color consortium, 2009 Copyright 2009
Copyright 2009 Copyright 2009 pearson education inc
Copyright 2009 pearson education inc Dell all rights reserved copyright 2009
Dell all rights reserved copyright 2009 Zanichelli
Zanichelli 2009 pearson education inc
2009 pearson education inc Copyright 2009 pearson education inc
Copyright 2009 pearson education inc Copyright 2009 pearson education inc
Copyright 2009 pearson education inc Copyright 2009 pearson education inc
Copyright 2009 pearson education inc Who is the father of indian english novel
Who is the father of indian english novel Raj rani vs prem adib
Raj rani vs prem adib























