Types of Chemical Reactions Synthesis Combination reaction A
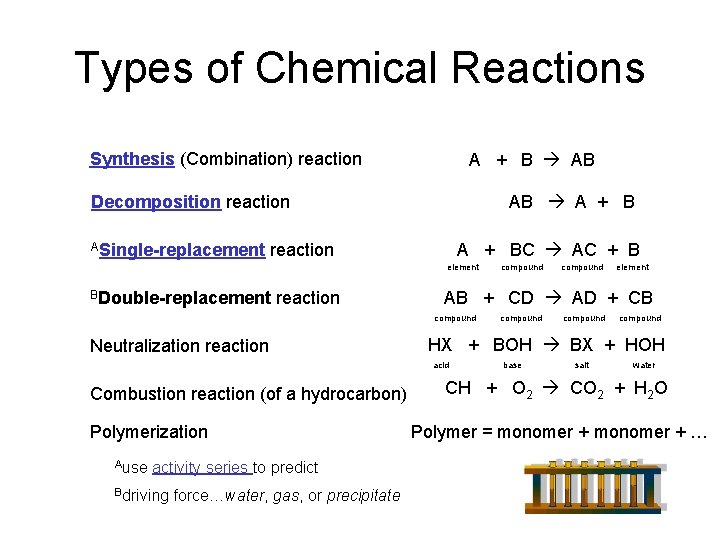
Types of Chemical Reactions Synthesis (Combination) reaction A + B AB AB A + B Decomposition reaction ASingle-replacement A + BC AC + B reaction element BDouble-replacement reaction Polymerization Ause activity series to predict Bdriving force…water, gas, or precipitate element compound HX + BOH BX + HOH acid Combustion reaction (of a hydrocarbon) compound AB + CD AD + CB compound Neutralization reaction compound base salt water CH + O 2 CO 2 + H 2 O Polymer = monomer + …
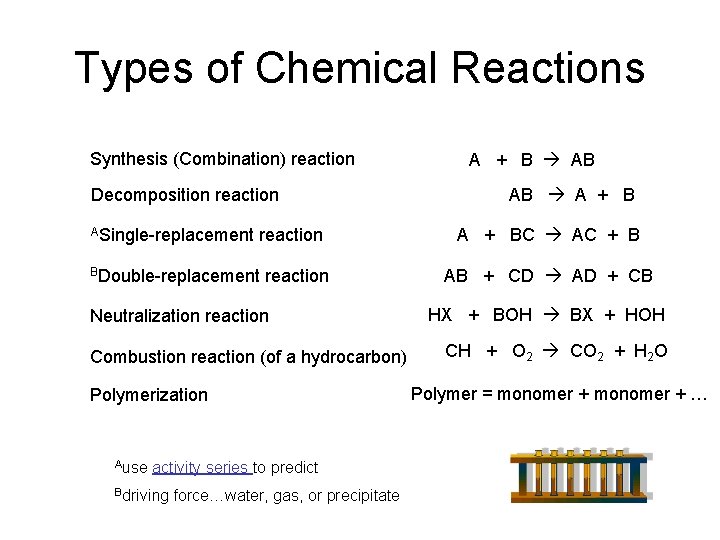
Types of Chemical Reactions Synthesis (Combination) reaction Decomposition reaction ASingle-replacement reaction BDouble-replacement reaction Neutralization reaction Combustion reaction (of a hydrocarbon) Polymerization Ause activity series to predict Bdriving force…water, gas, or precipitate A + B AB AB A + BC AC + B AB + CD AD + CB HX + BOH BX + HOH CH + O 2 CO 2 + H 2 O Polymer = monomer + …
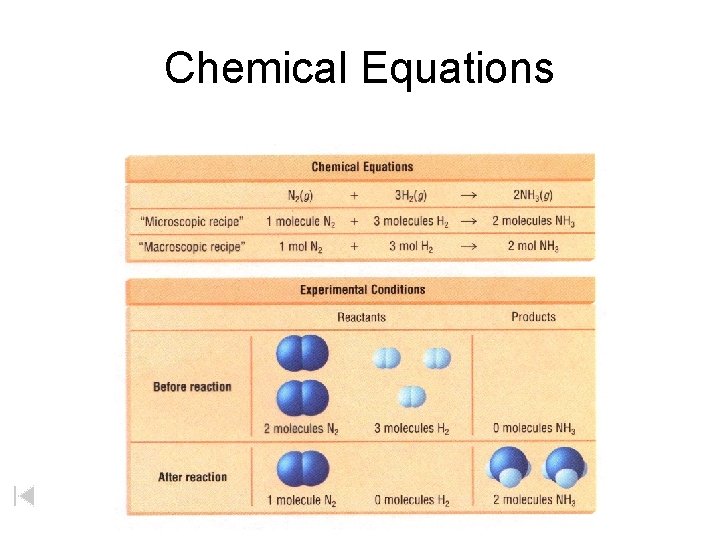
Chemical Equations
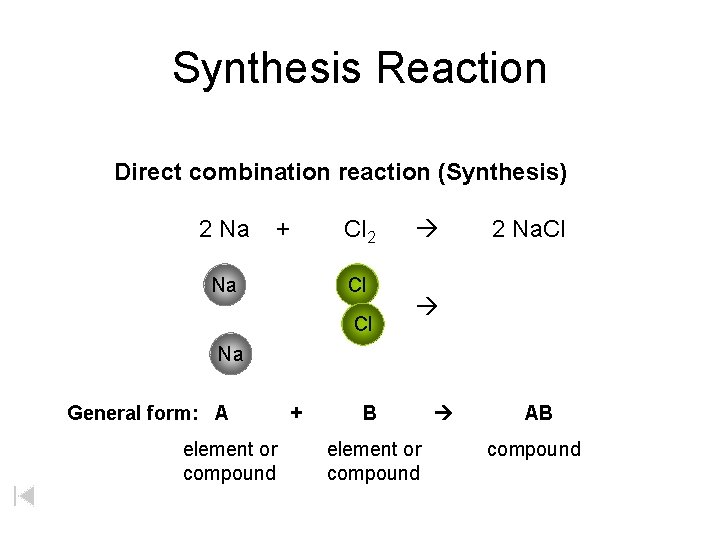
Synthesis Reaction Direct combination reaction (Synthesis) 2 Na + Na Cl 2 Cl Cl 2 Na. Cl Na General form: A element or compound + B element or compound AB compound
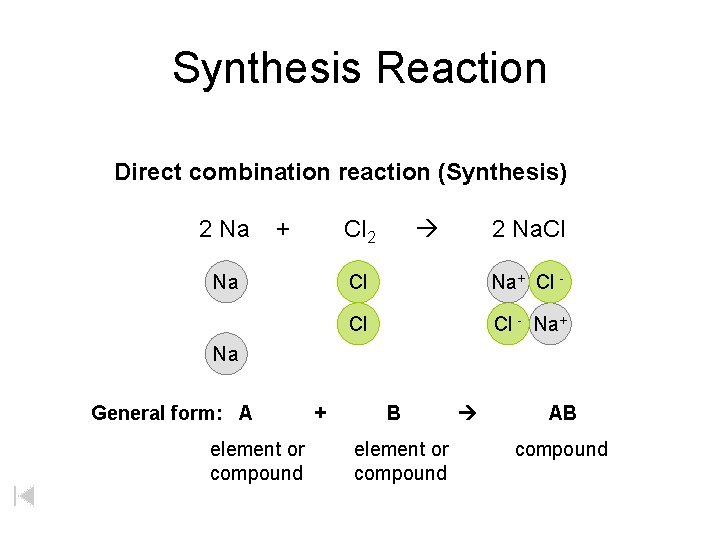
Synthesis Reaction Direct combination reaction (Synthesis) 2 Na + Cl 2 Na. Cl Cl Na+ Cl - Cl Cl - Na+ Na General form: A element or compound + B element or compound AB compound
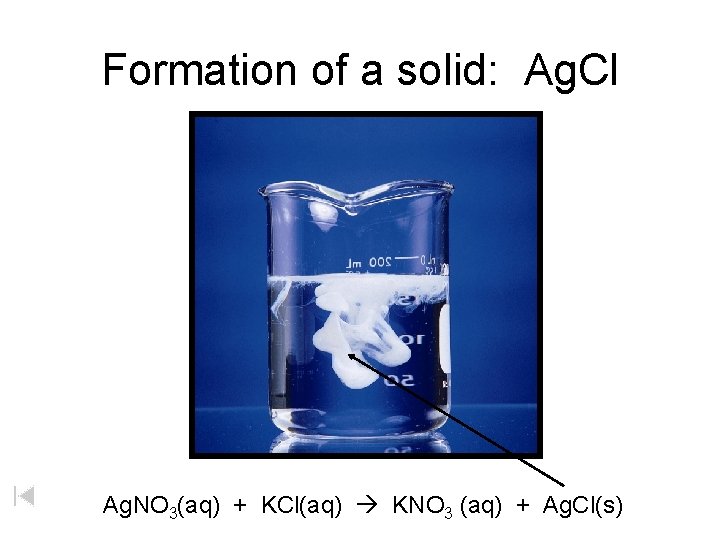
Formation of a solid: Ag. Cl Ag. NO 3(aq) + KCl(aq) KNO 3 (aq) + Ag. Cl(s)
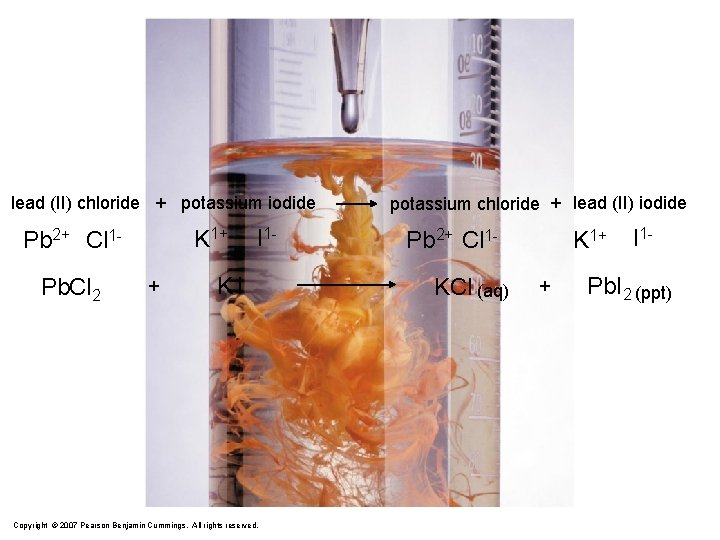
lead (II) chloride + potassium iodide K 1+ Pb 2+ Cl 1 Pb. Cl 2 + I 1 - KI Copyright © 2007 Pearson Benjamin Cummings. All rights reserved. potassium chloride + lead (II) iodide Pb 2+ Cl 1 KCl (aq) K 1+ + I 1 - Pb. I 2 (ppt)
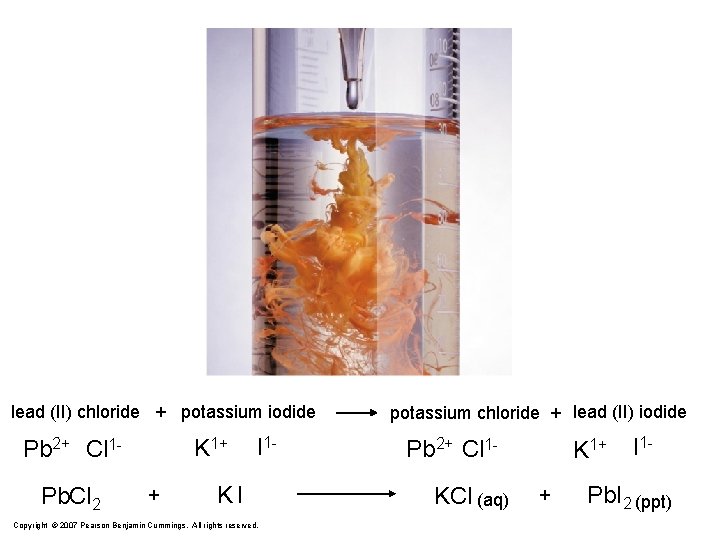
lead (II) chloride + potassium iodide K 1+ Pb 2+ Cl 1 Pb. Cl 2 + I 1 - KI Copyright © 2007 Pearson Benjamin Cummings. All rights reserved. potassium chloride + lead (II) iodide Pb 2+ Cl 1 KCl (aq) K 1+ + I 1 - Pb. I 2 (ppt)
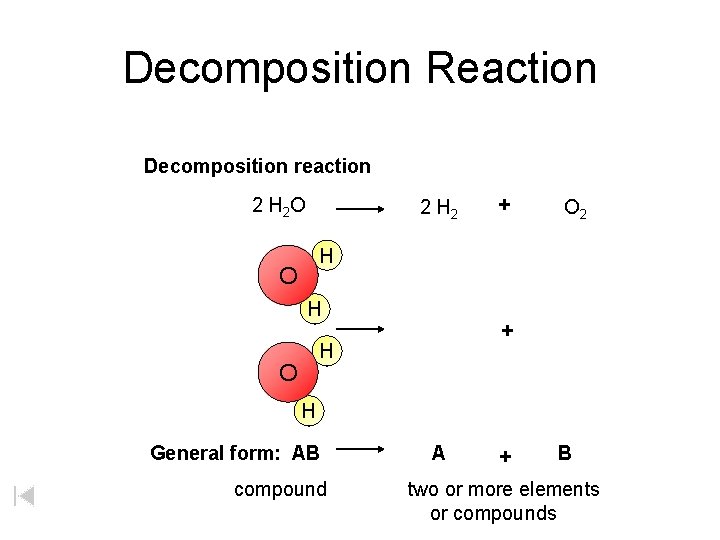
Decomposition Reaction Decomposition reaction 2 H 2 O 2 H 2 + O 2 H O H + H O H General form: AB compound A + B two or more elements or compounds
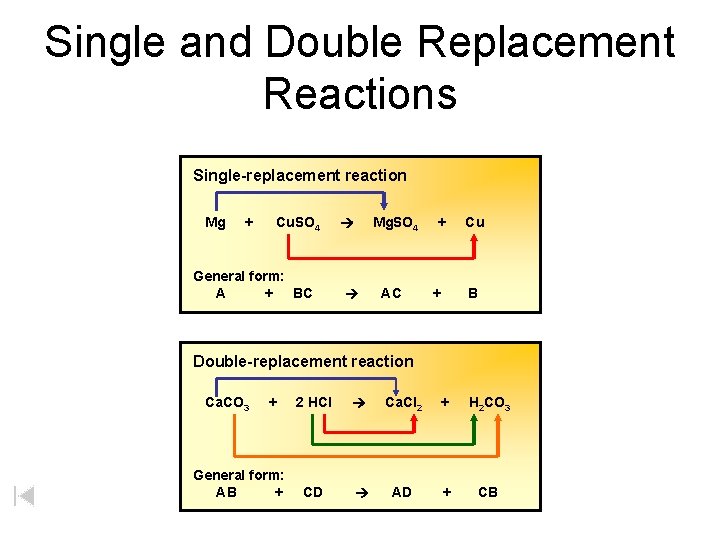
Single and Double Replacement Reactions Single-replacement reaction Mg + Cu. SO 4 General form: A + BC Mg. SO 4 AC + + Cu B Double-replacement reaction Ca. CO 3 + General form: AB + 2 HCl Ca. Cl 2 + H 2 CO 3 CD AD + CB
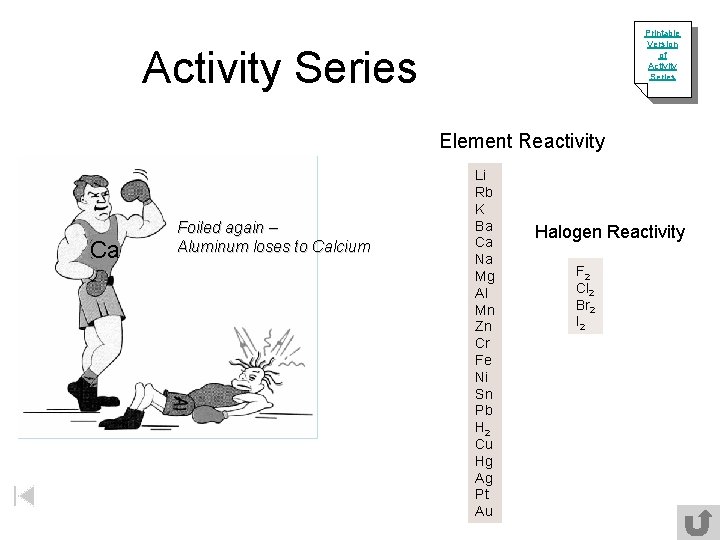
Printable Version of Activity Series Element Reactivity Ca Foiled again – Aluminum loses to Calcium Li Rb K Ba Ca Na Mg Al Mn Zn Cr Fe Ni Sn Pb H 2 Cu Hg Ag Pt Au Halogen Reactivity F 2 Cl 2 Br 2 I 2
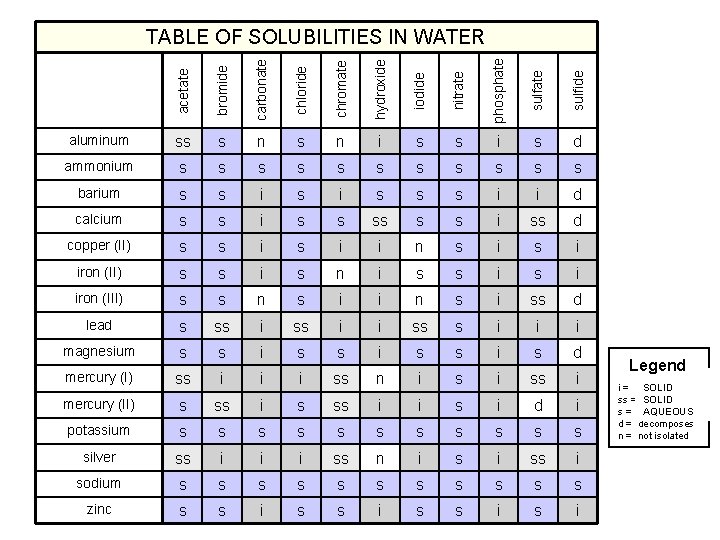
acetate bromide carbonate chloride chromate hydroxide iodide nitrate phosphate sulfide TABLE OF SOLUBILITIES IN WATER aluminum ss s n i s s i s d ammonium s s s barium s s i s s s i i d calcium s s i ss d copper (II) s s i i n s i iron (II) s s i s n i s s i iron (III) s s n s i i n s i ss d lead s ss i i ss s i i i magnesium s s i s d mercury (I) ss i i i ss n i ss i mercury (II) s ss i i s i d i potassium s s silver ss i i i ss n i ss i sodium s s s zinc s s i Legend SOLID i = insoluble SOLIDsoluble ss = slightly AQUEOUS s = soluble d = decomposes n = not isolated
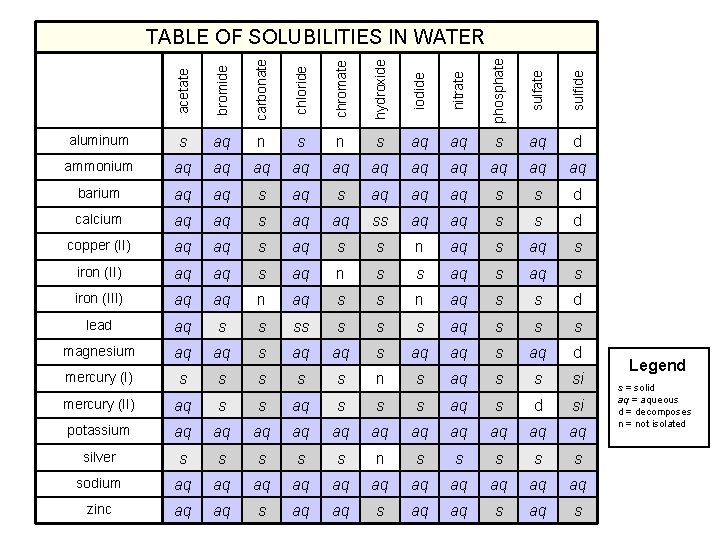
acetate bromide carbonate chloride chromate hydroxide iodide nitrate phosphate sulfide TABLE OF SOLUBILITIES IN WATER aluminum s aq n s aq aq s aq d ammonium aq aq aq barium aq aq s aq aq aq s s d calcium aq aq ss aq aq s s d copper (II) aq aq s s n aq s iron (II) aq aq s aq n s s aq s iron (III) aq aq n aq s s d lead aq s s s s aq s s s magnesium aq aq s aq d mercury (I) s s s n s aq s s si mercury (II) aq s s s aq s d si potassium aq aq aq silver s s s n s s sodium aq aq aq zinc aq aq s Legend s = solid aq = aqueous d = decomposes n = not isolated
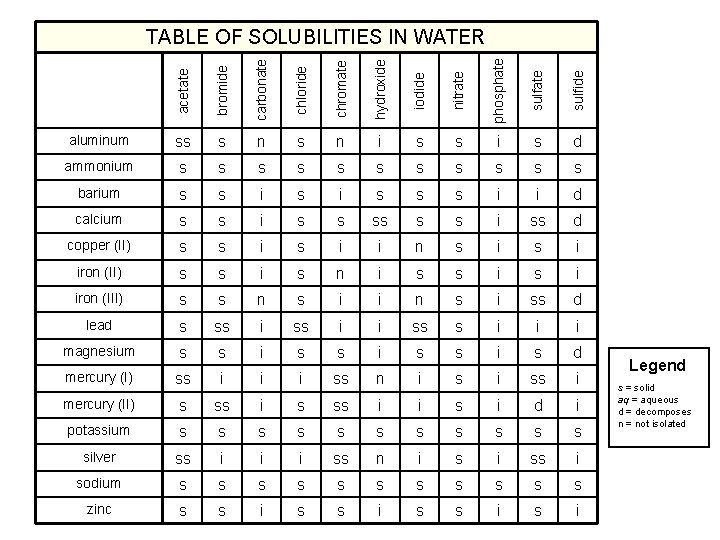
acetate bromide carbonate chloride chromate hydroxide iodide nitrate phosphate sulfide TABLE OF SOLUBILITIES IN WATER aluminum ss s n i s s i s d ammonium s s s barium s s i s s s i i d calcium s s i ss d copper (II) s s i i n s i iron (II) s s i s n i s s i iron (III) s s n s i i n s i ss d lead s ss i i ss s i i i magnesium s s i s d mercury (I) ss i i i ss n i ss i mercury (II) s ss i i s i d i potassium s s silver ss i i i ss n i ss i sodium s s s zinc s s i Legend s = solid aq = aqueous d = decomposes n = not isolated
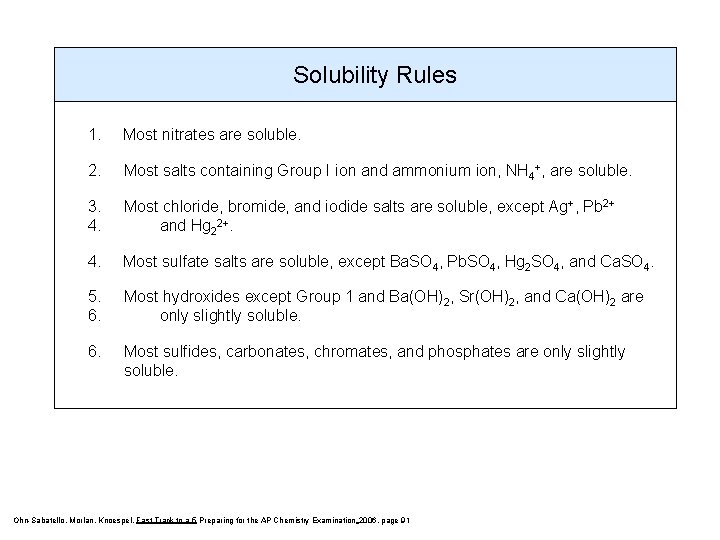
Solubility Rules 1. Most nitrates are soluble. 2. Most salts containing Group I ion and ammonium ion, NH 4+, are soluble. 3. 4. Most chloride, bromide, and iodide salts are soluble, except Ag+, Pb 2+ and Hg 22+. 4. Most sulfate salts are soluble, except Ba. SO 4, Pb. SO 4, Hg 2 SO 4, and Ca. SO 4. 5. 6. Most hydroxides except Group 1 and Ba(OH)2, Sr(OH)2, and Ca(OH)2 are only slightly soluble. 6. Most sulfides, carbonates, chromates, and phosphates are only slightly soluble. Ohn-Sabatello, Morlan, Knoespel, Fast Track to a 5 Preparing for the AP Chemistry Examination 2006, page 91
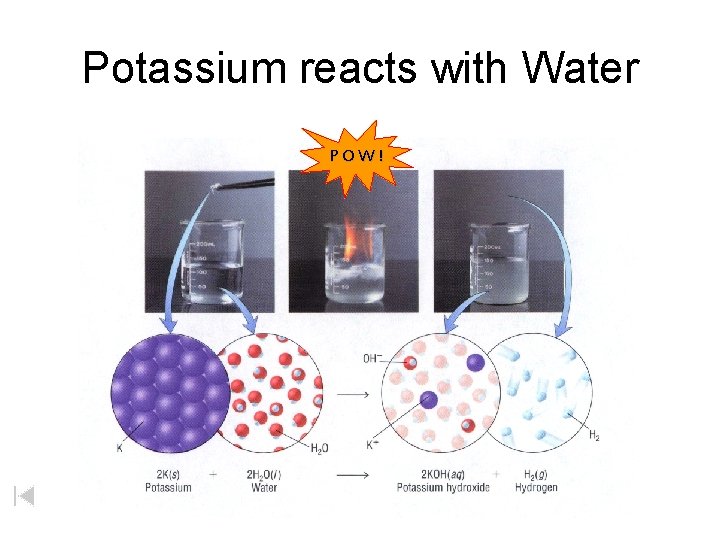
Potassium reacts with Water POW!
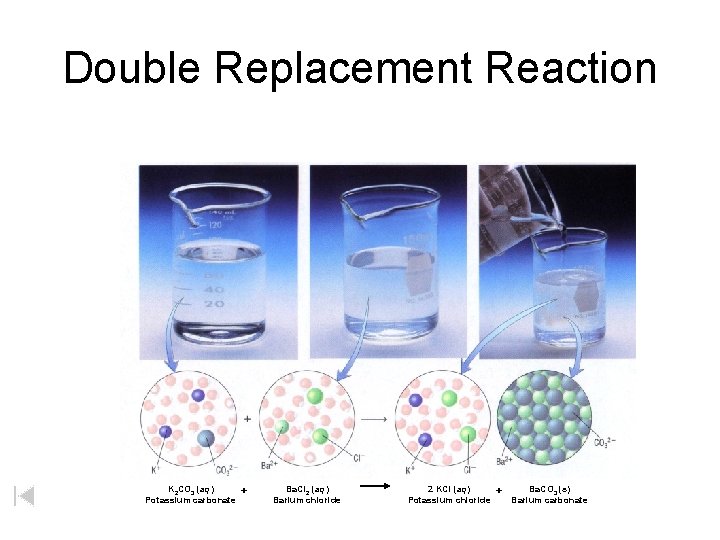
Double Replacement Reaction K 2 CO 3 (aq) Potassium carbonate + Ba. Cl 2 (aq) Barium chloride 2 KCl (aq) Potassium chloride + Ba. CO 3 (s) Barium carbonate
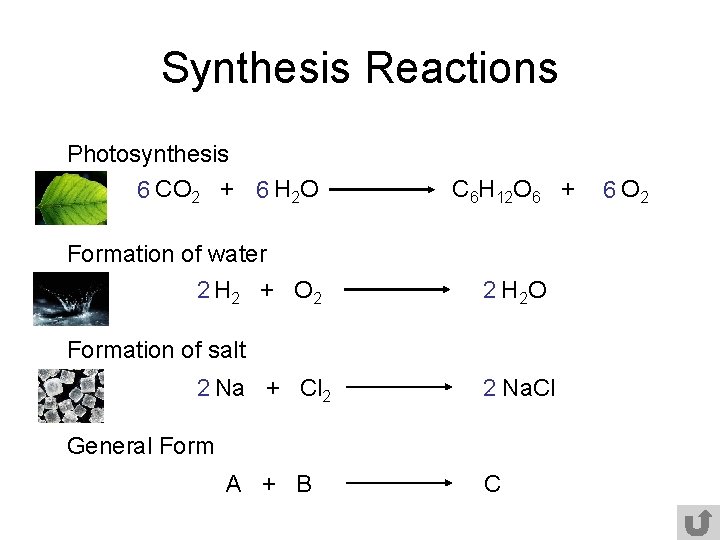
Synthesis Reactions Photosynthesis 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + Formation of water 2 H 2 + O 2 2 H 2 O Formation of salt 2 Na + Cl 2 2 Na. Cl General Form A + B C 6 O 2
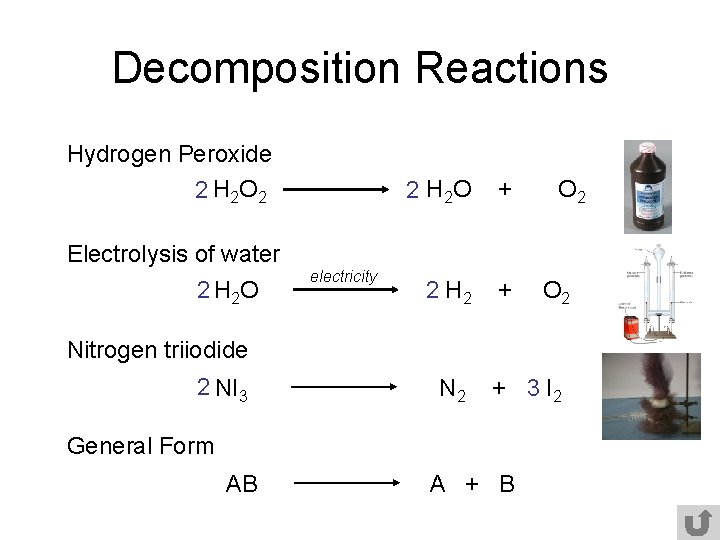
Decomposition Reactions Hydrogen Peroxide 2 H 2 O 2 2 H 2 O + 2 H 2 + O 2 Electrolysis of water 2 H 2 O electricity O 2 Nitrogen triiodide 2 NI 3 N 2 + 3 I 2 General Form AB A + B
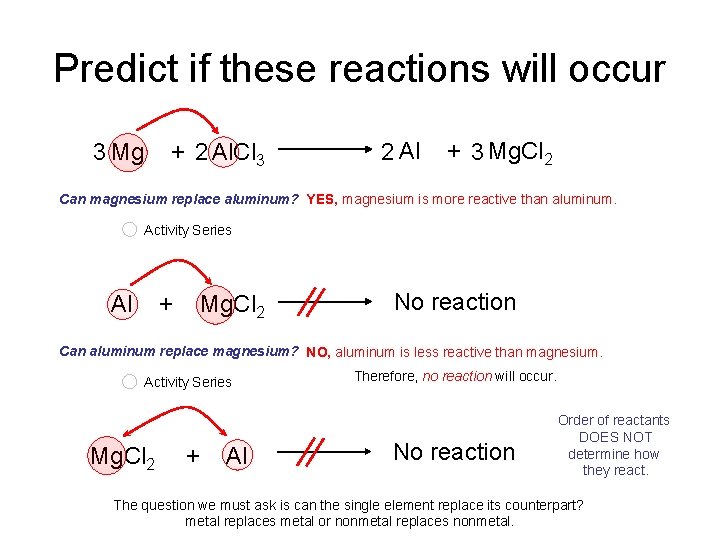
Predict if these reactions will occur 3 Mg + 2 Al. Cl 3 2 Al + 3 Mg. Cl 2 Can magnesium replace aluminum? YES, magnesium is more reactive than aluminum. Activity Series Al + Mg. Cl 2 No reaction Can aluminum replace magnesium? NO, aluminum is less reactive than magnesium. Activity Series Mg. Cl 2 + Al Therefore, no reaction will occur. No reaction Order of reactants DOES NOT determine how they react. The question we must ask is can the single element replace its counterpart? metal replaces metal or nonmetal replaces nonmetal.
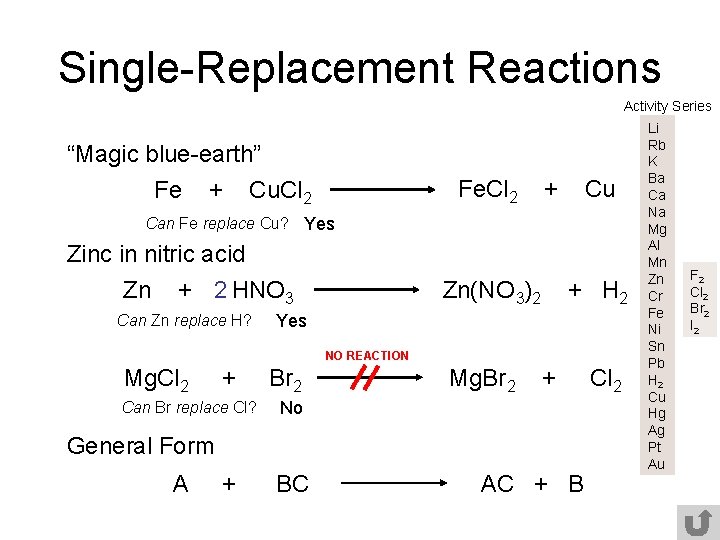
Single-Replacement Reactions Activity Series “Magic blue-earth” Fe + Cu. Cl 2 Fe. Cl 2 + Cu Can Fe replace Cu? Yes Zinc in nitric acid Zn + 2 HNO 3 Can Zn replace H? Zn(NO 3)2 + H 2 Yes NO REACTION Mg. Cl 2 + Can Br replace Cl? Br 2 Mg. Br 2 + No General Form A + BC AC + B Cl 2 Li Rb K Ba Ca Na Mg Al Mn Zn Cr Fe Ni Sn Pb H 2 Cu Hg Ag Pt Au F 2 Cl 2 Br 2 I 2
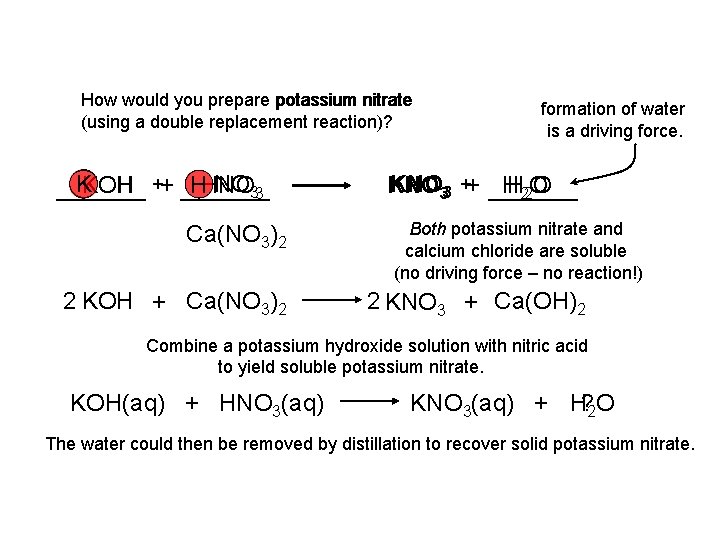
How would you prepare potassium nitrate (using a double replacement reaction)? KKOH NO 33 HHNO OH ++ _________ Ca(NO 3)2 2 KOH + Ca(NO 3)2 formation of water is a driving force. KNO 33 ++ _____ H 22 O O KNO H Both potassium nitrate and calcium chloride are soluble (no driving force – no reaction!) 2 KNO 3 + Ca(OH)2 Combine a potassium hydroxide solution with nitric acid to yield soluble potassium nitrate. KOH(aq) + HNO 3(aq) KNO 3(aq) + H? 2 O The water could then be removed by distillation to recover solid potassium nitrate.
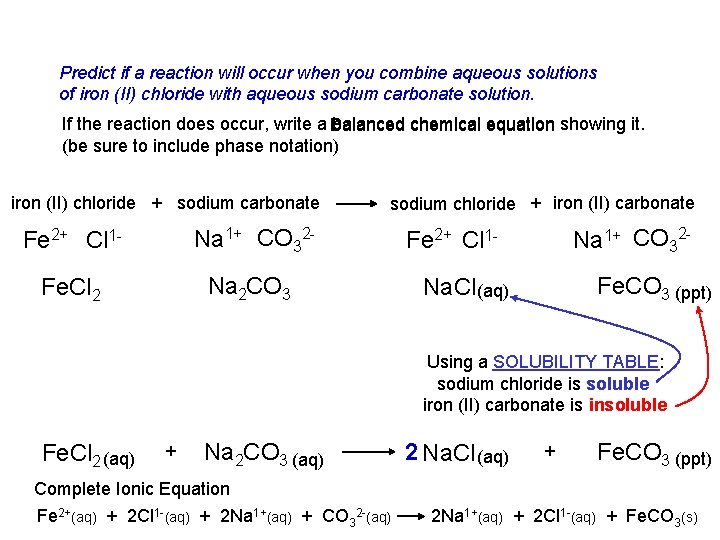
Predict if a reaction will occur when you combine aqueous solutions of iron (II) chloride with aqueous sodium carbonate solution. If the reaction does occur, write a Balanced balanced chemical equation showing it. (be sure to include phase notation) iron (II) chloride + sodium carbonate Fe 2+ Cl 1 - Na 1+ CO 32 - Fe. Cl 2 Na 2 CO 3 sodium chloride + iron (II) carbonate Na 1+ CO 32 - Fe 2+ Cl 1 - Fe. CO 3 (ppt) Na. Cl (aq) Using a SOLUBILITY TABLE: sodium chloride is soluble iron (II) carbonate is insoluble Fe. Cl 2 (aq) + Na 2 CO 3 (aq) Complete Ionic Equation Fe 2+(aq) + 2 Cl 1 -(aq) + 2 Na 1+(aq) + CO 32 -(aq) 2 Na. Cl (aq) + Fe. CO 3 (ppt) 2 Na 1+(aq) + 2 Cl 1 -(aq) + Fe. CO 3(s)
- Slides: 23