PROTEIN Oleh Dr Ir Ani Suryani DEA DEPARTEMEN














- Slides: 71

PROTEIN Oleh Dr. Ir. Ani Suryani, DEA DEPARTEMEN TEKNOLOGI INDUSTRI PERTANIAN FAKULTAS TEKNOLOGI PERTANIAN INSTITUT PERTANIAN BOGOR

Protein Homoprotein (hanya mengandung asam amino) Heteroprotein (asam amino dan senyawa non-protein) contoh : nukleoprotein, lipoprotein, fosfoprotein, glikoprotein, dll Protein berdasarkan konformasi atau organisasi tiga dimensi terdiri dari : - fibrous protein (contoh : kolagen, keratin, dll) - globular protein (contoh : actin, fibrinogen) • Struktur primer susunan asam amino dalam protein • Struktur sekunder dan tertier berhubungan dengan bentuk tiga dimensi • Struktur kuartener penyusunan geometrik diantara rantai polipeptida, dan rantai tersebut saling berikatan (ikatan non-kovalen)

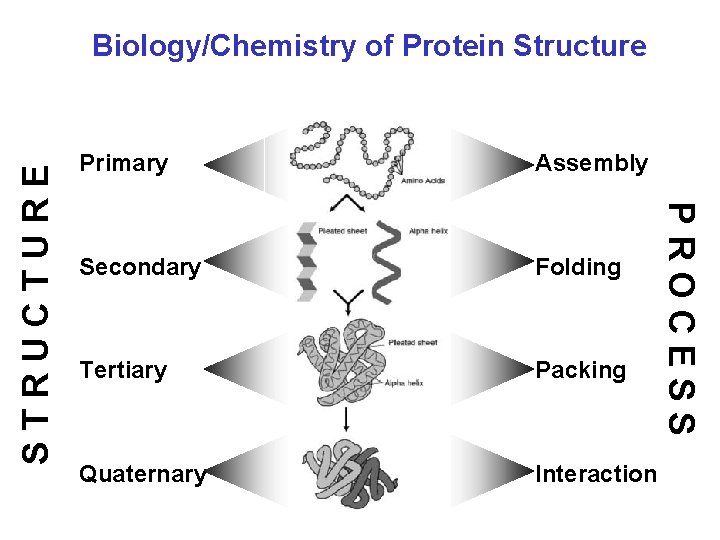
Primary Assembly Secondary Folding Tertiary Packing Quaternary Interaction PROCESS STRUCTURE Biology/Chemistry of Protein Structure

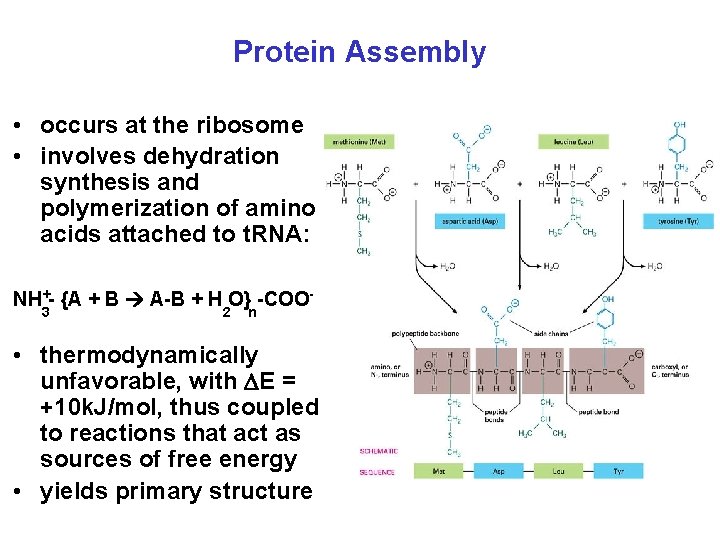
Protein Assembly • occurs at the ribosome • involves dehydration synthesis and polymerization of amino acids attached to t. RNA: NH +- {A + B A-B + H O} -COO 3 2 n • thermodynamically unfavorable, with E = +10 k. J/mol, thus coupled to reactions that act as sources of free energy • yields primary structure

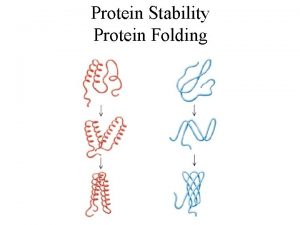
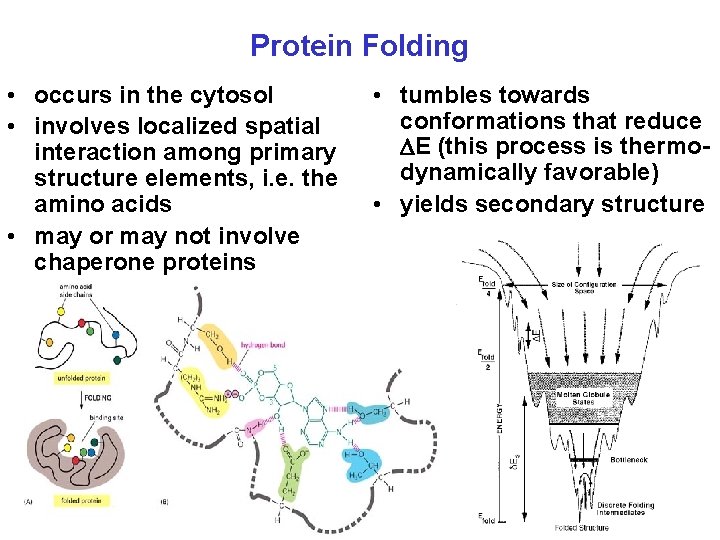
Protein Folding • occurs in the cytosol • involves localized spatial interaction among primary structure elements, i. e. the amino acids • may or may not involve chaperone proteins • tumbles towards conformations that reduce E (this process is thermodynamically favorable) • yields secondary structure

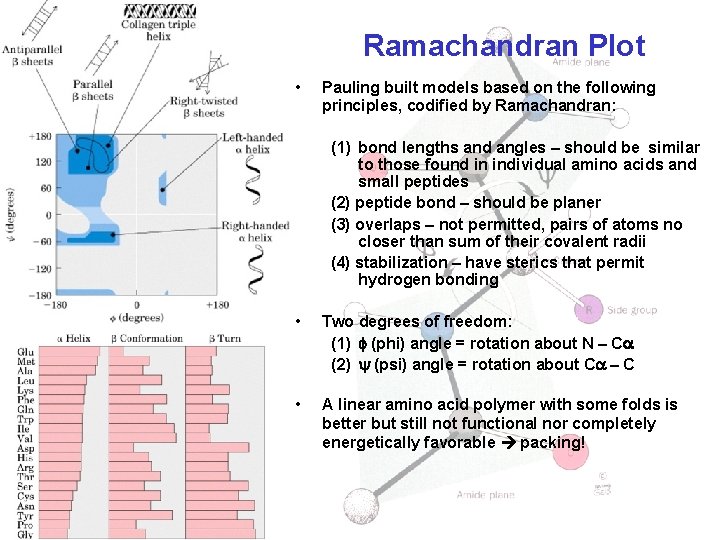
Ramachandran Plot • Pauling built models based on the following principles, codified by Ramachandran: (1) bond lengths and angles – should be similar to those found in individual amino acids and small peptides (2) peptide bond – should be planer (3) overlaps – not permitted, pairs of atoms no closer than sum of their covalent radii (4) stabilization – have sterics that permit hydrogen bonding • Two degrees of freedom: (1) (phi) angle = rotation about N – C (2) (psi) angle = rotation about C – C • A linear amino acid polymer with some folds is better but still not functional nor completely energetically favorable packing!

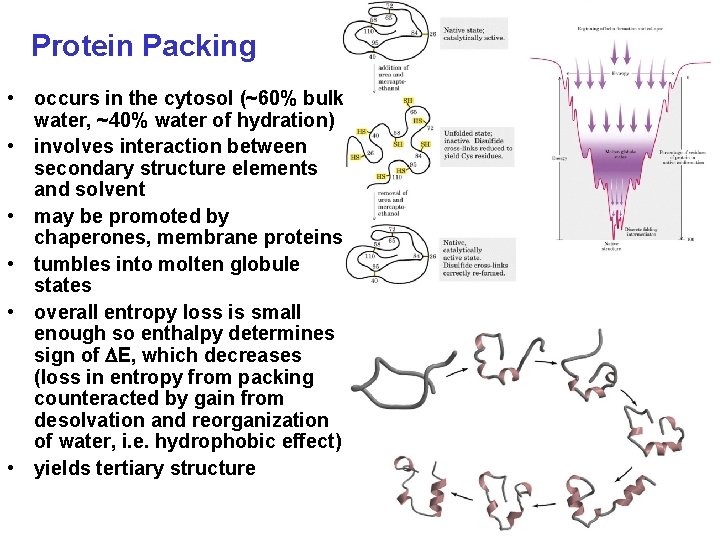
Protein Packing • occurs in the cytosol (~60% bulk water, ~40% water of hydration) • involves interaction between secondary structure elements and solvent • may be promoted by chaperones, membrane proteins • tumbles into molten globule states • overall entropy loss is small enough so enthalpy determines sign of E, which decreases (loss in entropy from packing counteracted by gain from desolvation and reorganization of water, i. e. hydrophobic effect) • yields tertiary structure

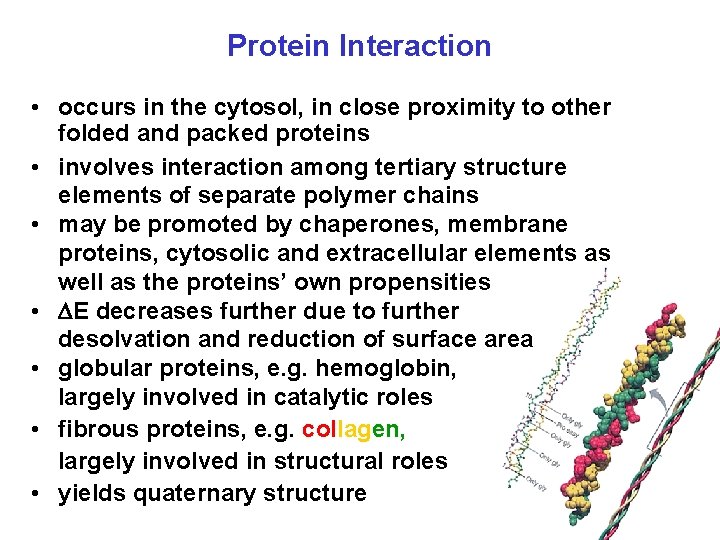
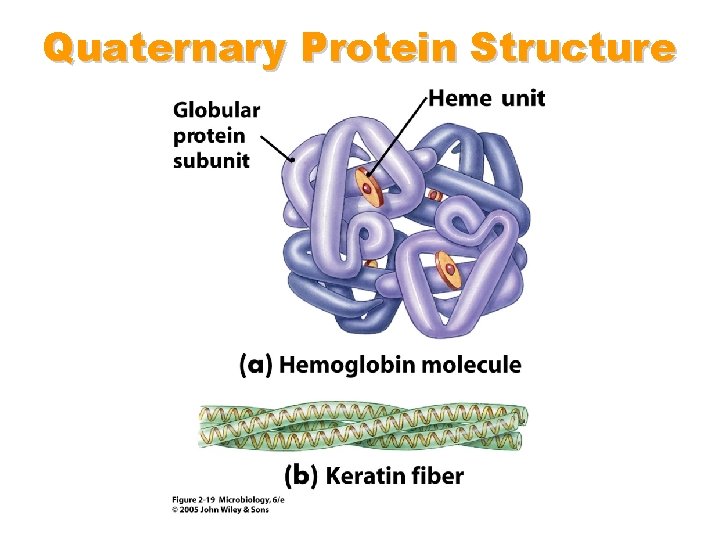
Protein Interaction • occurs in the cytosol, in close proximity to other folded and packed proteins • involves interaction among tertiary structure elements of separate polymer chains • may be promoted by chaperones, membrane proteins, cytosolic and extracellular elements as well as the proteins’ own propensities • E decreases further due to further desolvation and reduction of surface area • globular proteins, e. g. hemoglobin, largely involved in catalytic roles • fibrous proteins, e. g. collagen, largely involved in structural roles • yields quaternary structure

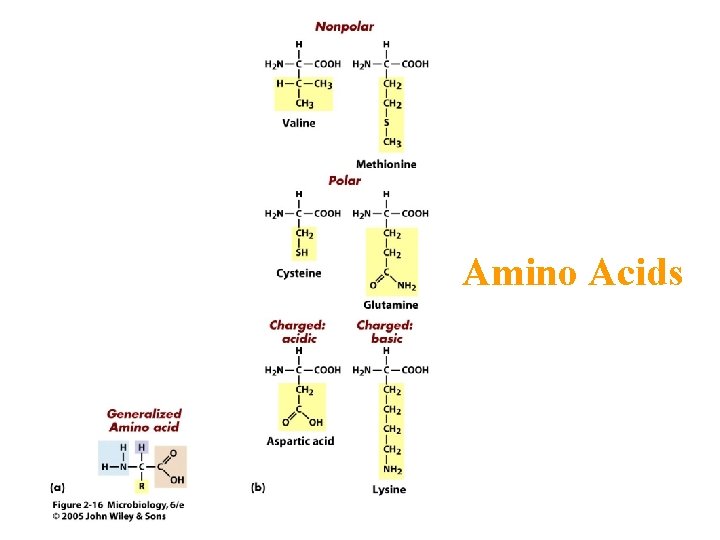
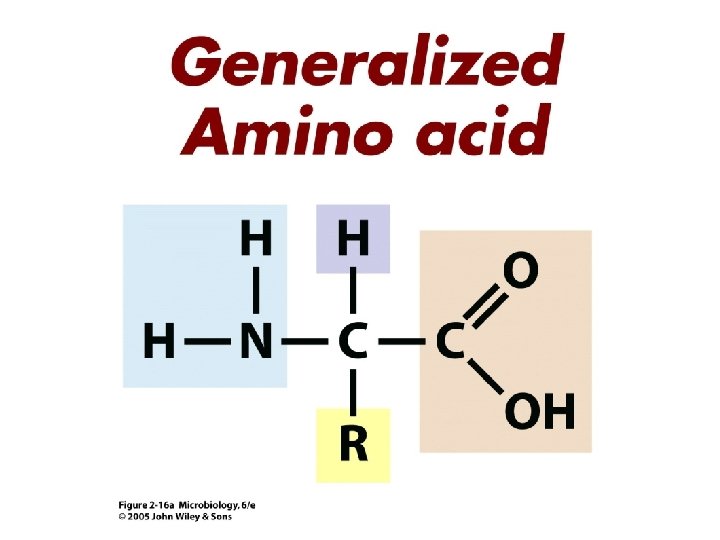
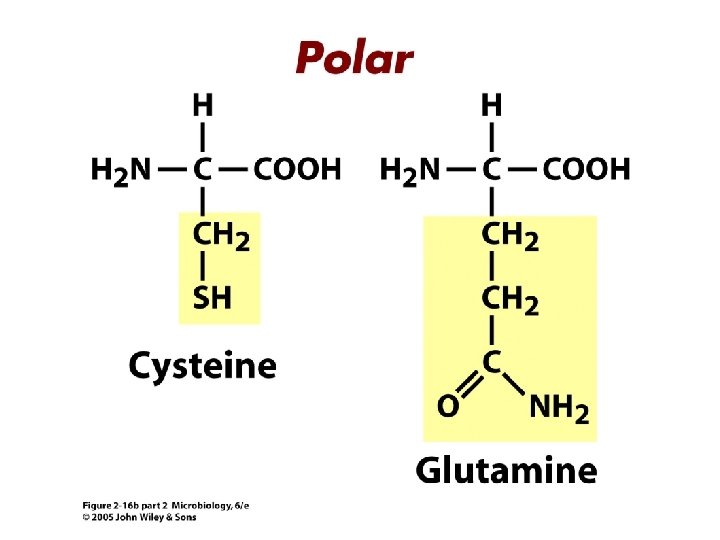
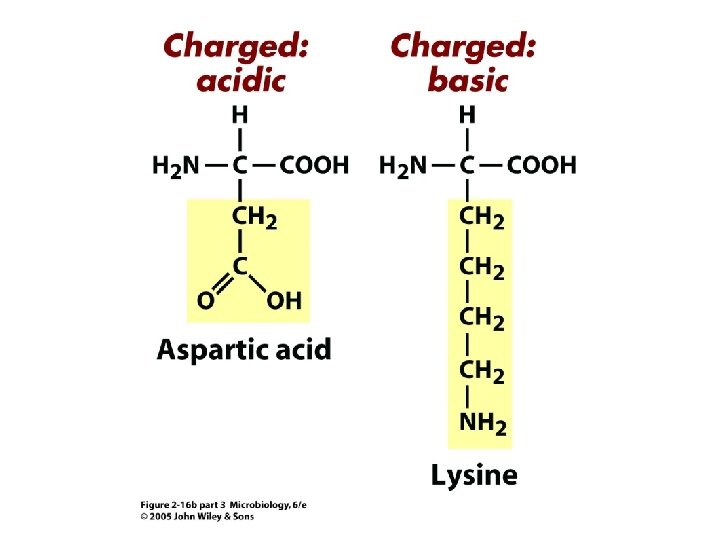
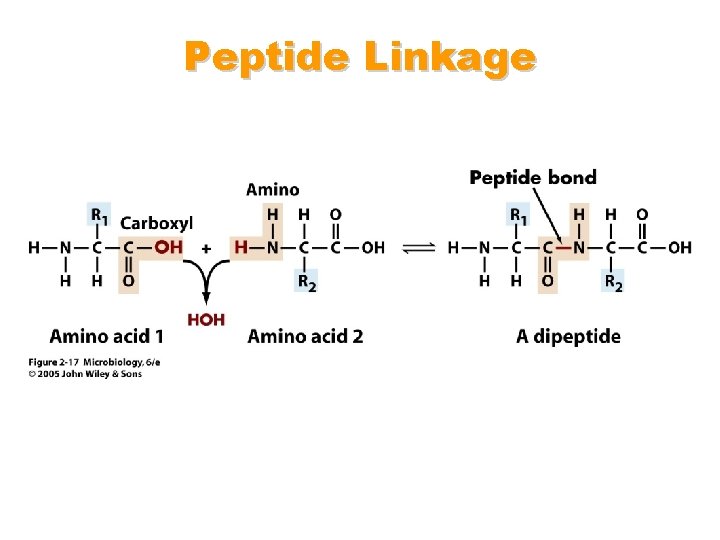
Proteins • Composed of building blocks called amino acids • Amino acids have at least one amino (-NH 2) group and one acidic carboxyl (-COOH) group • Each amino acid is distinguishable by a different chemical group (R group) • Peptide bonds: covalent bond that links an amino group of one amino acid to carboxyl group of another

Amino Acids





Peptide Linkage

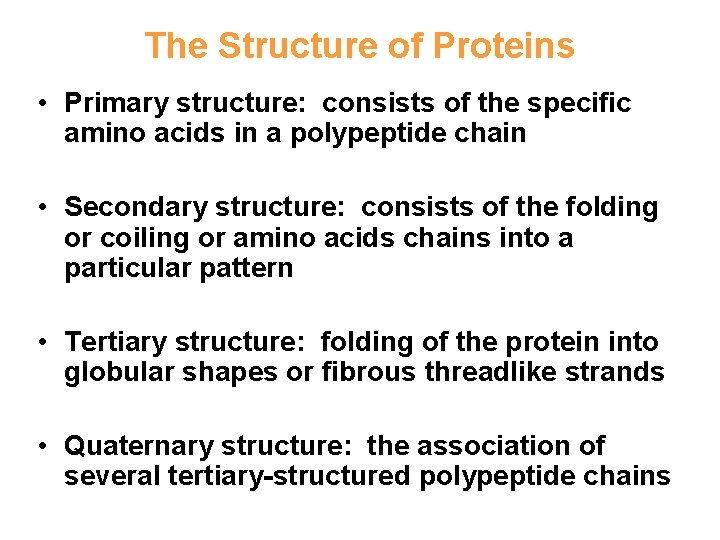
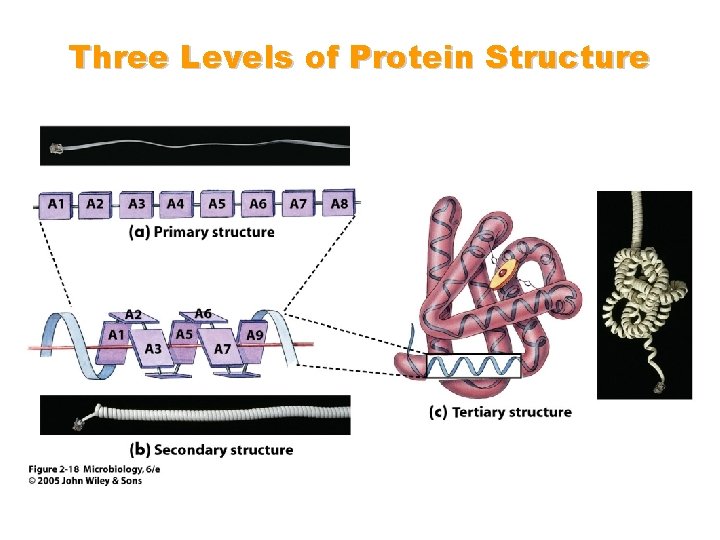
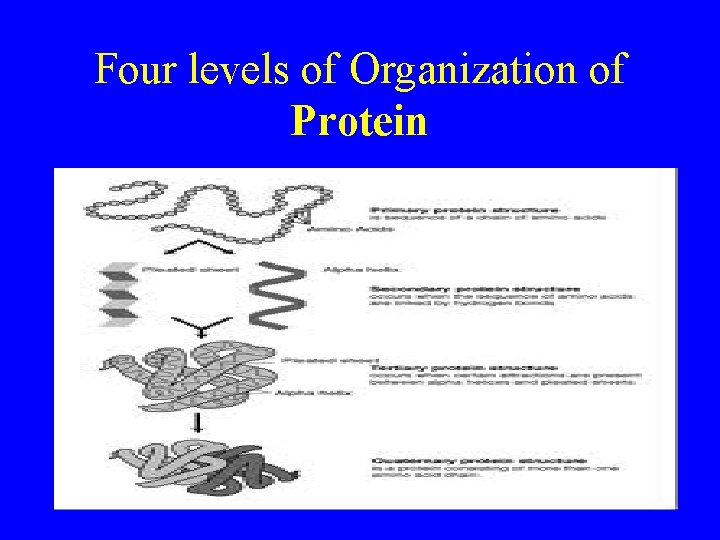
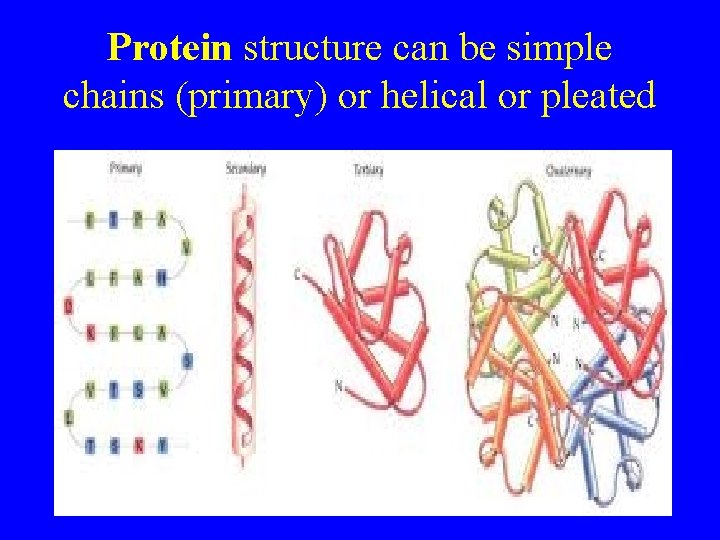
The Structure of Proteins • Primary structure: consists of the specific amino acids in a polypeptide chain • Secondary structure: consists of the folding or coiling or amino acids chains into a particular pattern • Tertiary structure: folding of the protein into globular shapes or fibrous threadlike strands • Quaternary structure: the association of several tertiary-structured polypeptide chains

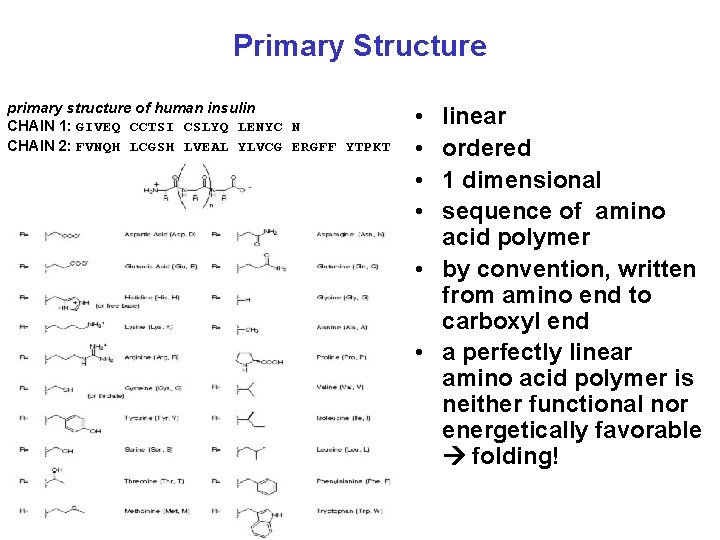
Primary Structure primary structure of human insulin CHAIN 1: GIVEQ CCTSI CSLYQ LENYC N CHAIN 2: FVNQH LCGSH LVEAL YLVCG ERGFF YTPKT • • linear ordered 1 dimensional sequence of amino acid polymer • by convention, written from amino end to carboxyl end • a perfectly linear amino acid polymer is neither functional nor energetically favorable folding!

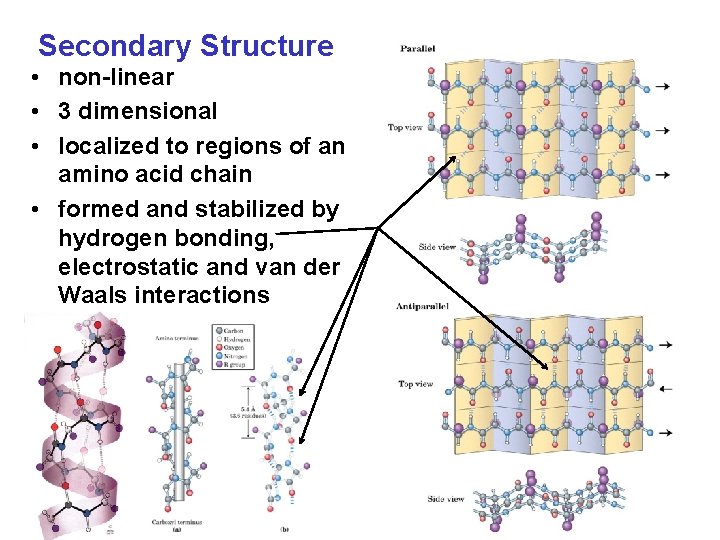
Secondary Structure • non-linear • 3 dimensional • localized to regions of an amino acid chain • formed and stabilized by hydrogen bonding, electrostatic and van der Waals interactions

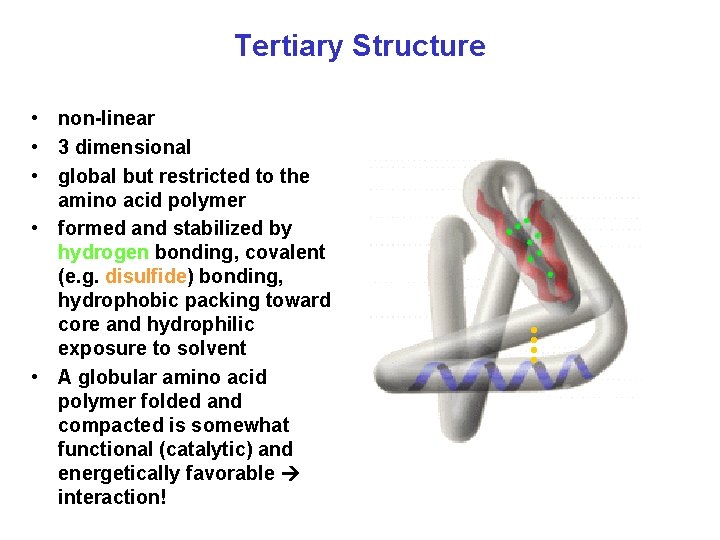
Tertiary Structure • non-linear • 3 dimensional • global but restricted to the amino acid polymer • formed and stabilized by hydrogen bonding, covalent (e. g. disulfide) bonding, hydrophobic packing toward core and hydrophilic exposure to solvent • A globular amino acid polymer folded and compacted is somewhat functional (catalytic) and energetically favorable interaction!

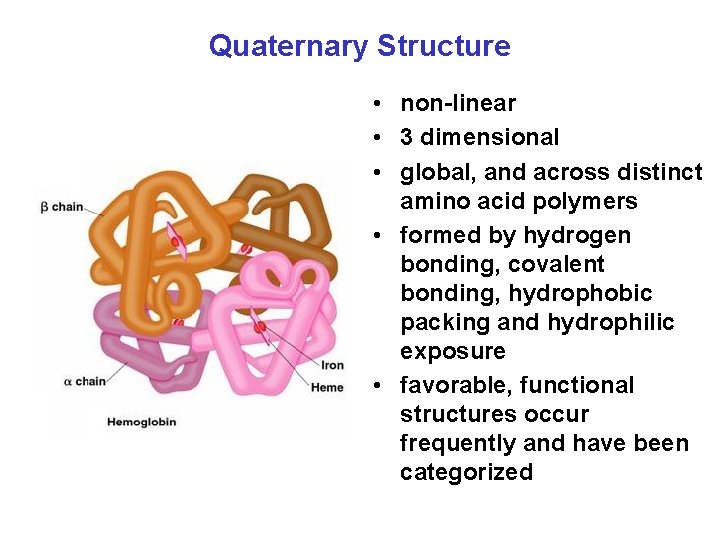
Quaternary Structure • non-linear • 3 dimensional • global, and across distinct amino acid polymers • formed by hydrogen bonding, covalent bonding, hydrophobic packing and hydrophilic exposure • favorable, functional structures occur frequently and have been categorized

Three Levels of Protein Structure

Quaternary Protein Structure

Classification of Proteins • Structural proteins: contribute to the threedimensional structure of cells, cell parts, and membranes • Enzymes: protein catalysts – substances that control the rate of chemical reactions in cells

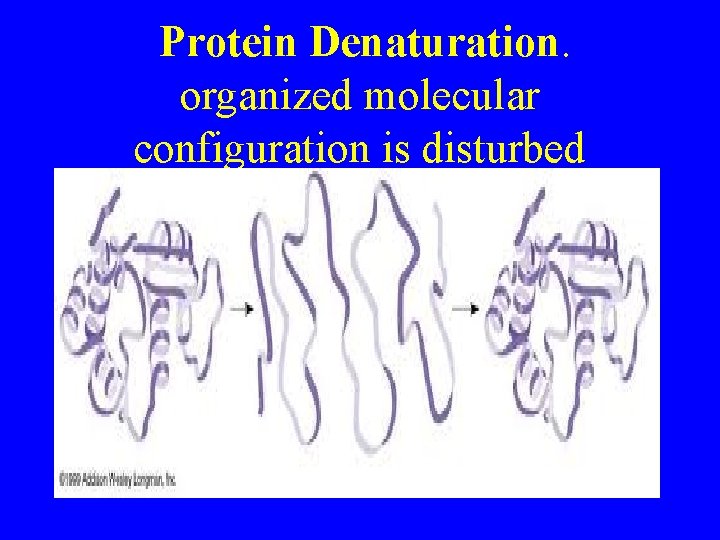
Protein Denaturation

Denaturasi Protein : Perubahan konfigurasi protein dari bentuk struktur sekunder dan tertier yang rapuh. Bentuk struktur primer tidak berubah Agen Penyebab Denaturasi : Agen Fisik : panas, dingin, perlakuan mekanis, tekanan hidrostatis Agen kimiawi : asam, basa, logam, pelarut organik, persenyawaan organik

Protein Denaturation. organized molecular configuration is disturbed


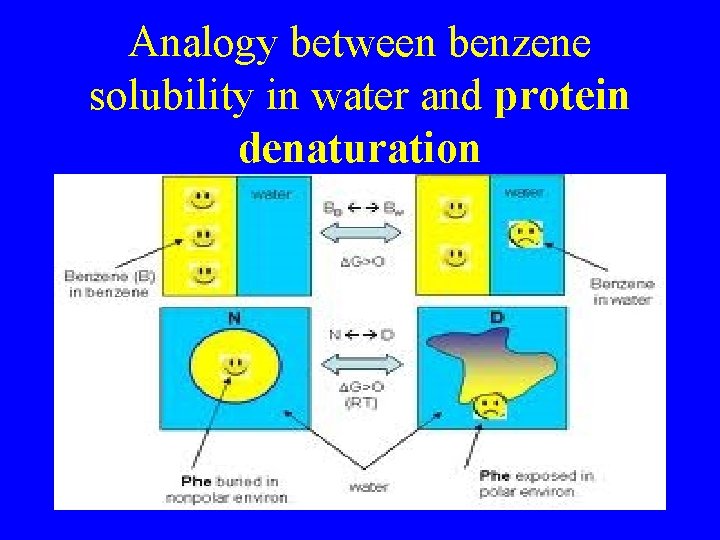
Analogy between benzene solubility in water and protein denaturation


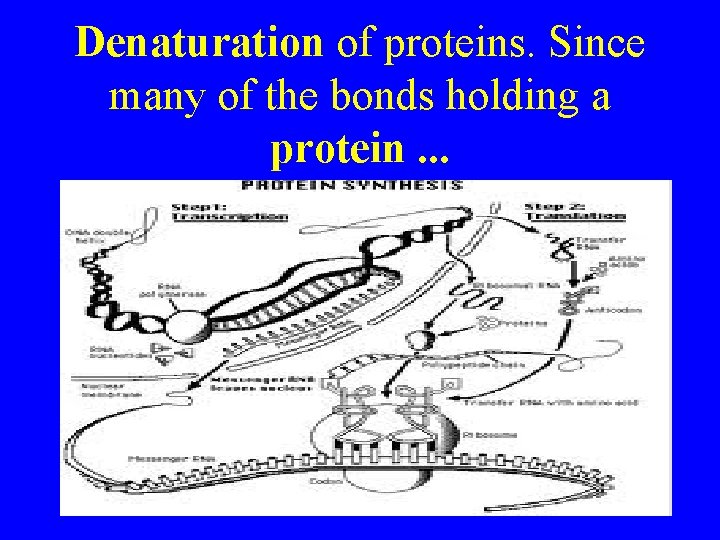
Denaturation of proteins. Since many of the bonds holding a protein. . .

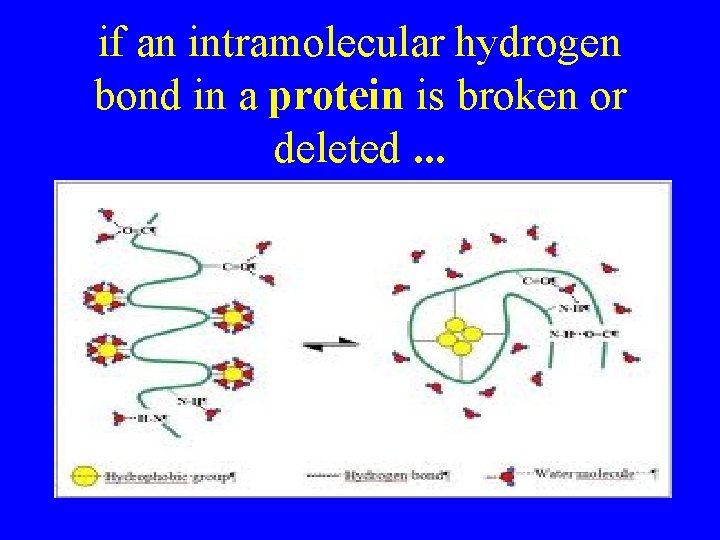
if an intramolecular hydrogen bond in a protein is broken or deleted. . .

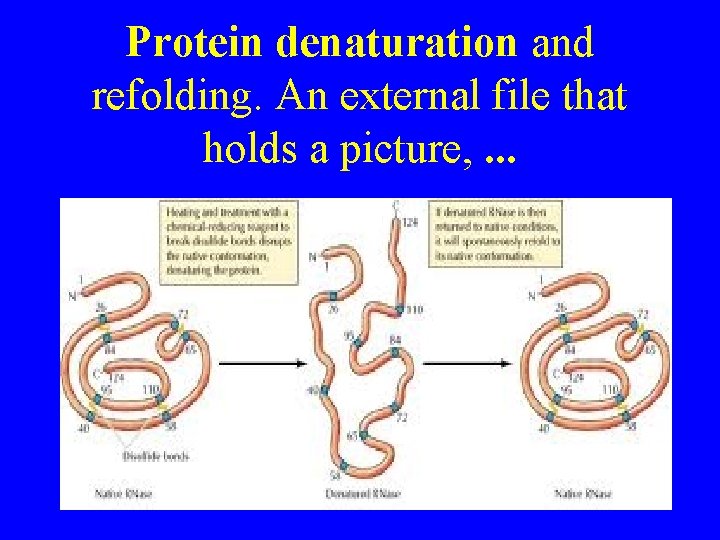
Protein denaturation and refolding. An external file that holds a picture, . . .

The zone of protein denaturation

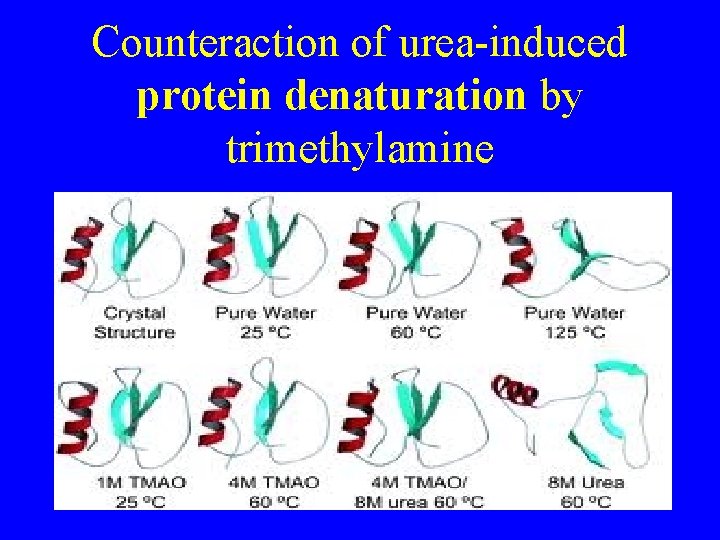
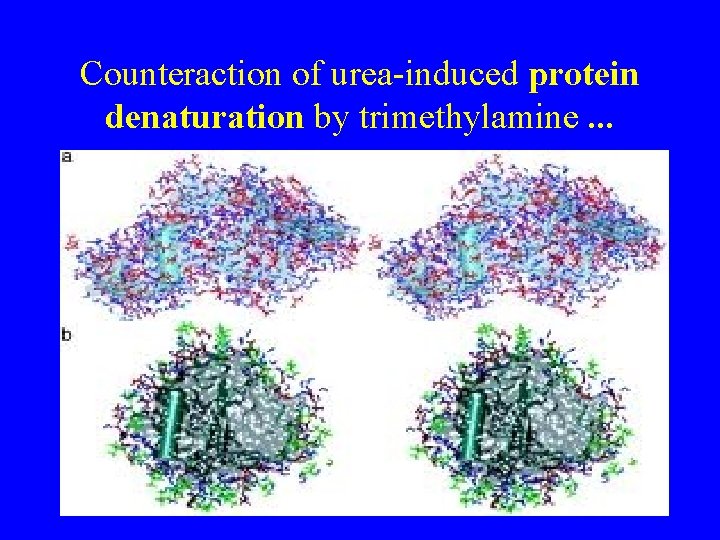
Counteraction of urea-induced protein denaturation by trimethylamine

function of protein being by shape, denaturation

Four levels of Organization of Protein

by water molecules, no further desiccation or denaturation occurs.

protein denaturation by coagulation (e. g. , acetone and methanol);

Counteraction of urea-induced protein denaturation by trimethylamine. . .

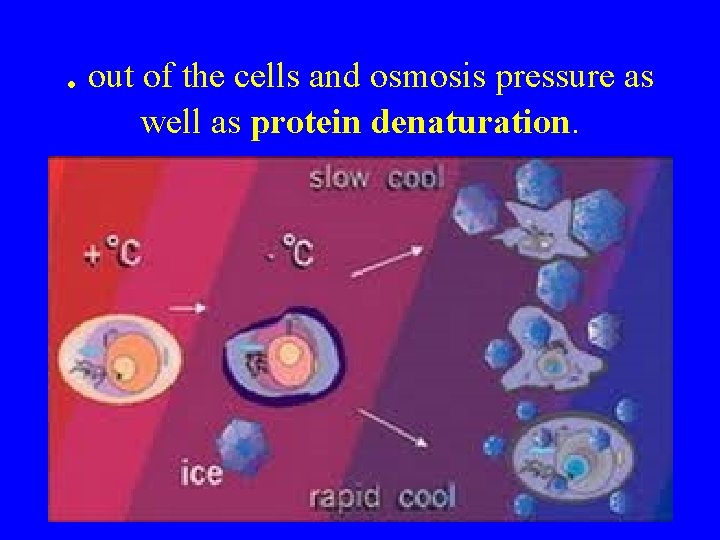
. out of the cells and osmosis pressure as well as protein denaturation.

DENATURATION OF PROTEIN:

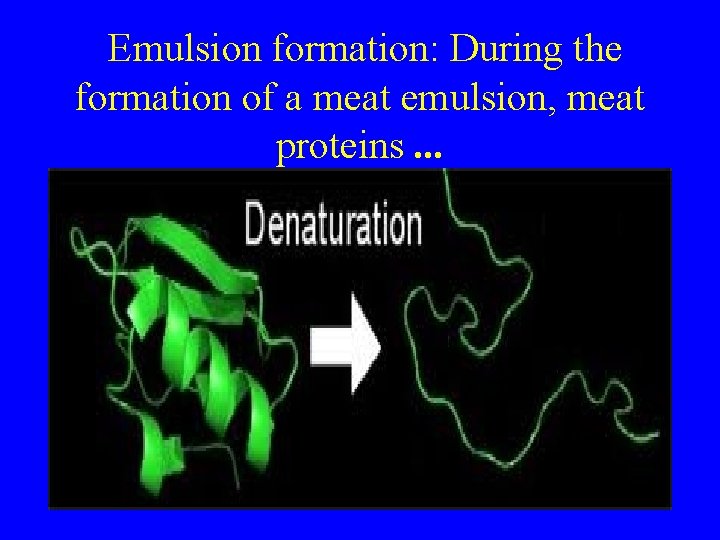
Emulsion formation: During the formation of a meat emulsion, meat proteins. . .

Protein structure can be simple chains (primary) or helical or pleated . . .

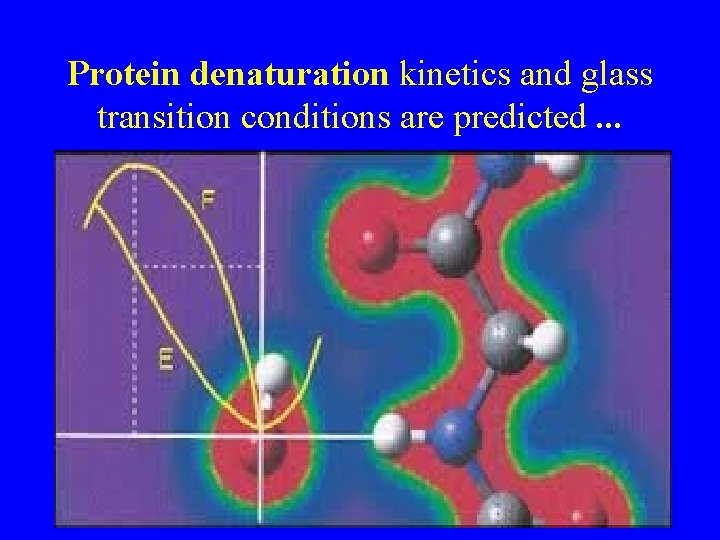
Protein denaturation kinetics and glass transition conditions are predicted. . .

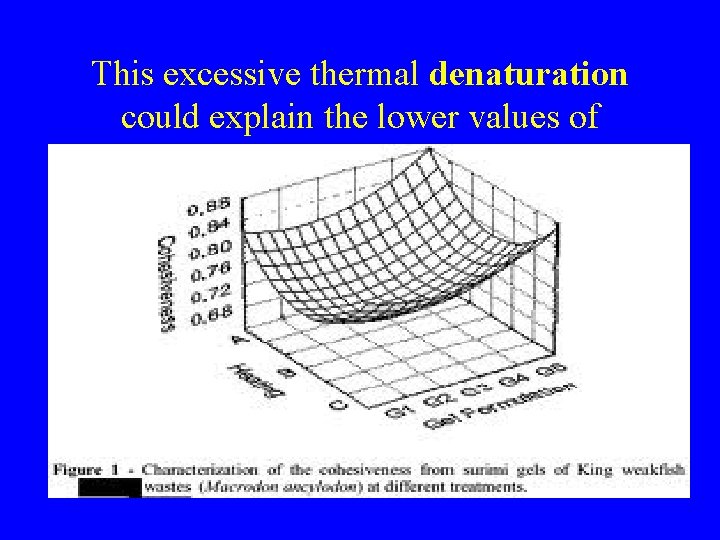
This excessive thermal denaturation could explain the lower values of

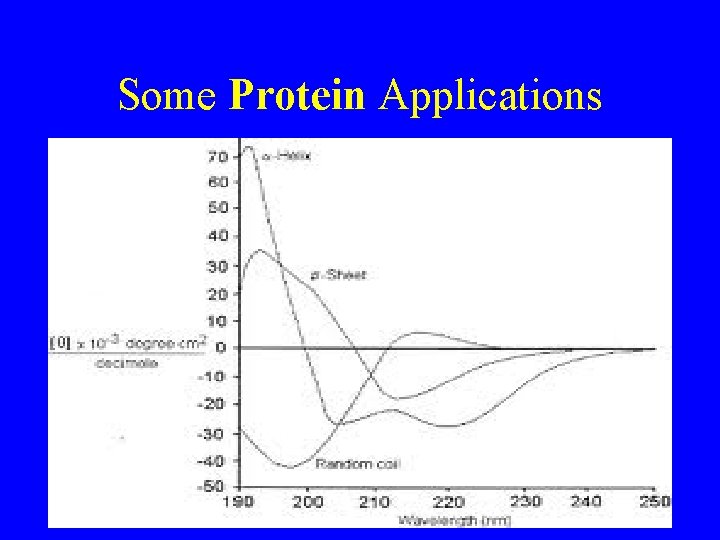
Some Protein Applications

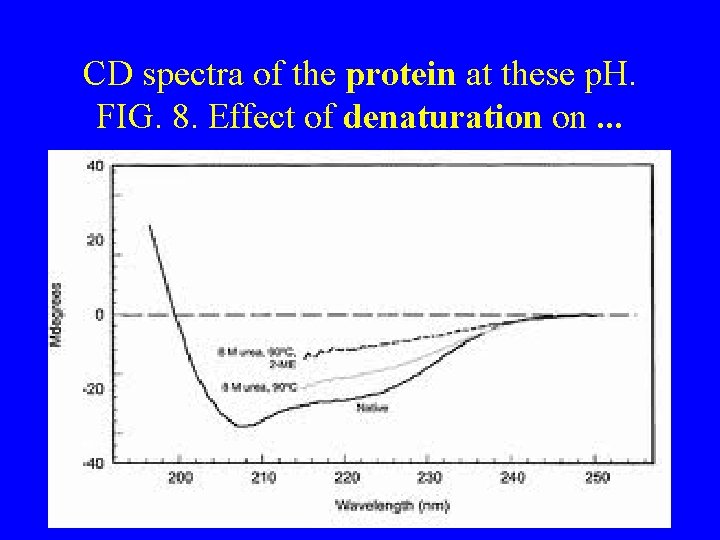
CD spectra of the protein at these p. H. FIG. 8. Effect of denaturation on. . .

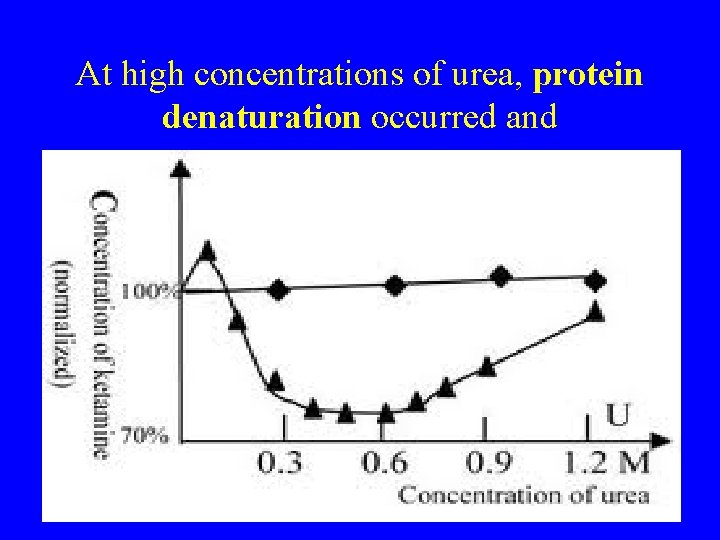
At high concentrations of urea, protein denaturation occurred and

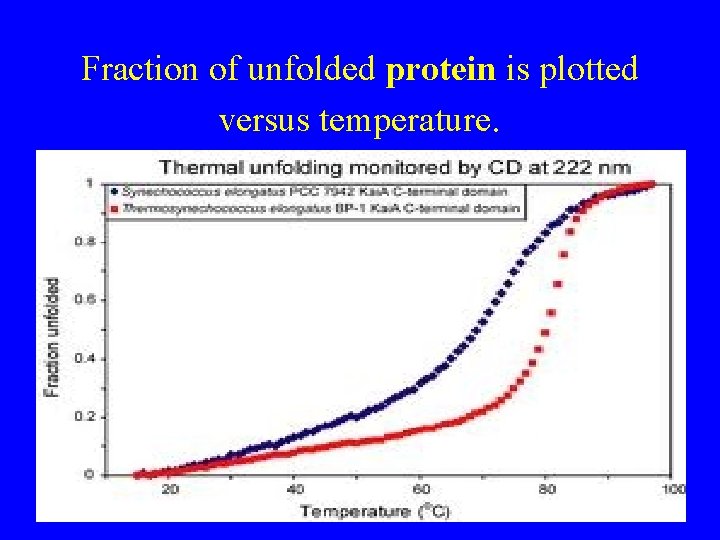
Fraction of unfolded protein is plotted versus temperature.

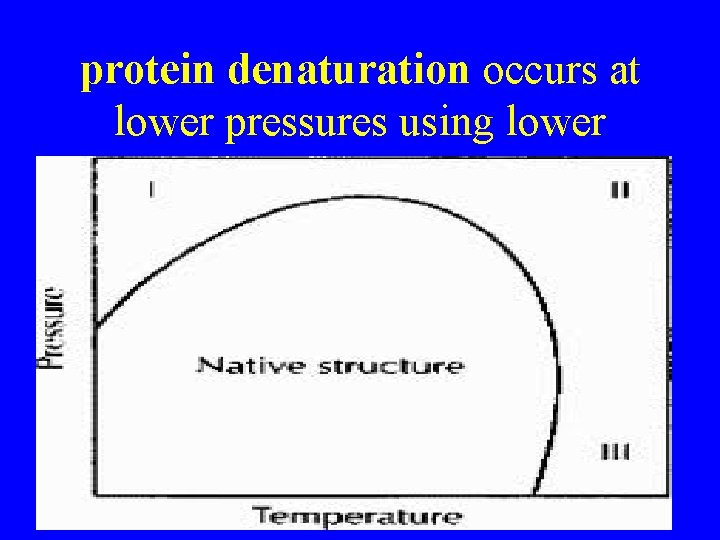
protein denaturation occurs at lower pressures using lower

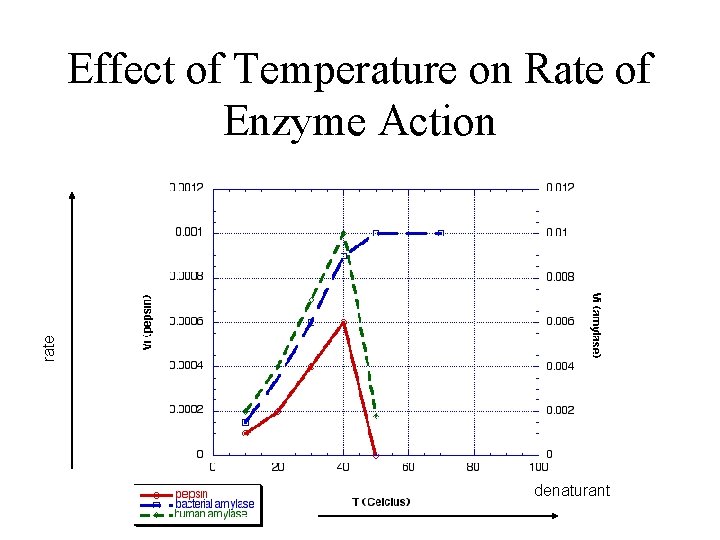
rate Effect of Temperature on Rate of Enzyme Action denaturant

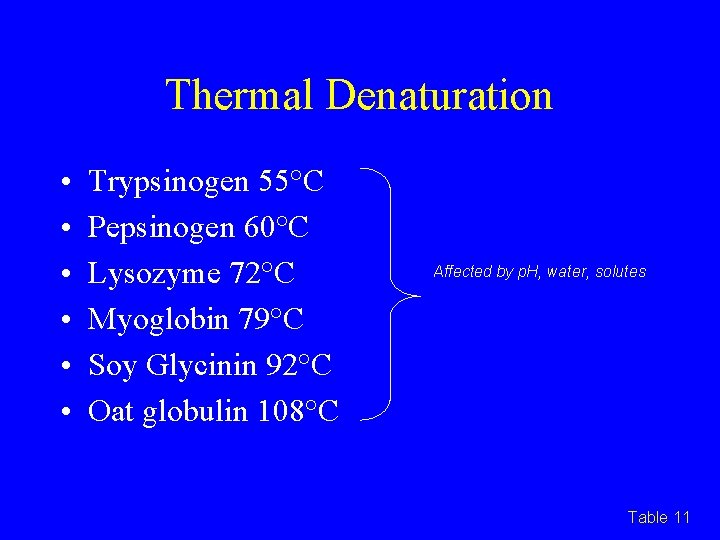
Thermal Denaturation • • • Trypsinogen 55°C Pepsinogen 60°C Lysozyme 72°C Myoglobin 79°C Soy Glycinin 92°C Oat globulin 108°C Affected by p. H, water, solutes Table 11

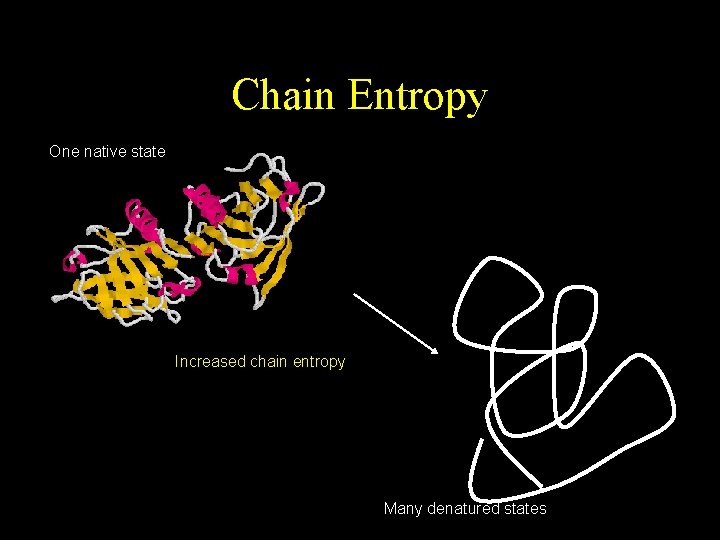
Chain Entropy One native state Increased chain entropy Many denatured states

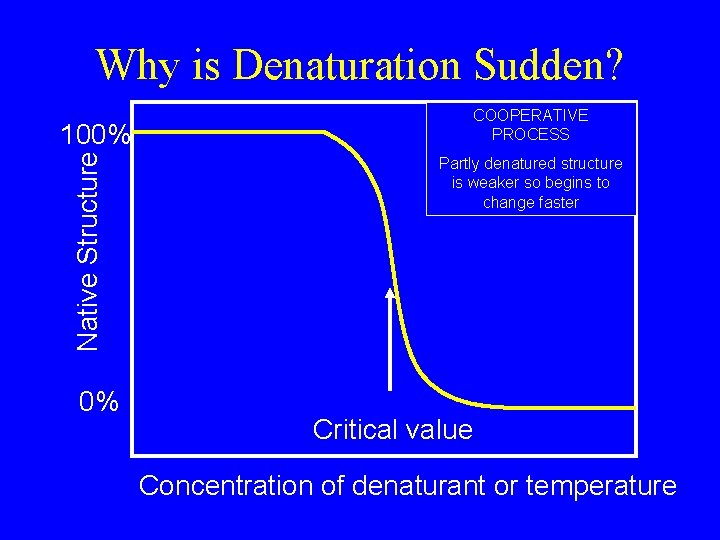
Why is Denaturation Sudden? Native Structure 100% 0% COOPERATIVE PROCESS Partly denatured structure is weaker so begins to change faster Critical value Concentration of denaturant or temperature

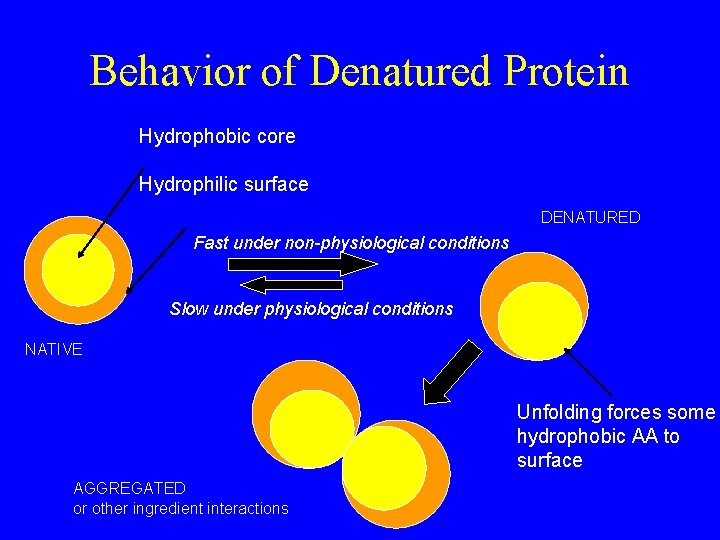
Behavior of Denatured Protein Hydrophobic core Hydrophilic surface DENATURED Fast under non-physiological conditions Slow under physiological conditions NATIVE Unfolding forces some hydrophobic AA to surface AGGREGATED or other ingredient interactions

Types of Denaturation • • • Temperature Organic solvents Surface p. H Shear

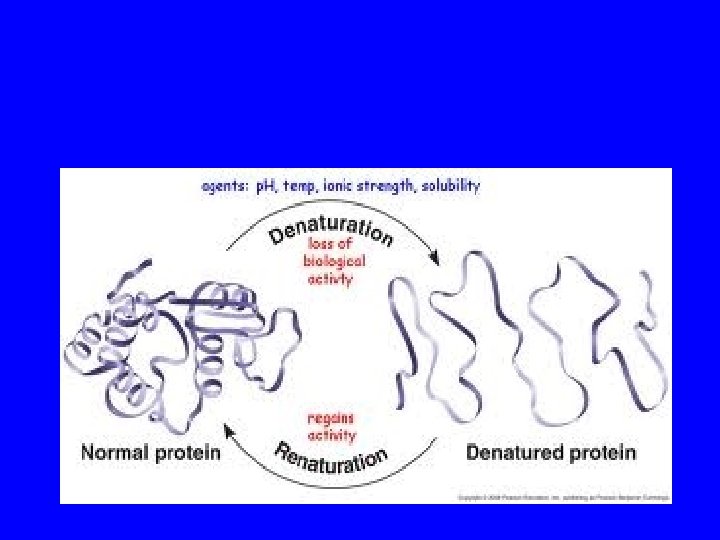
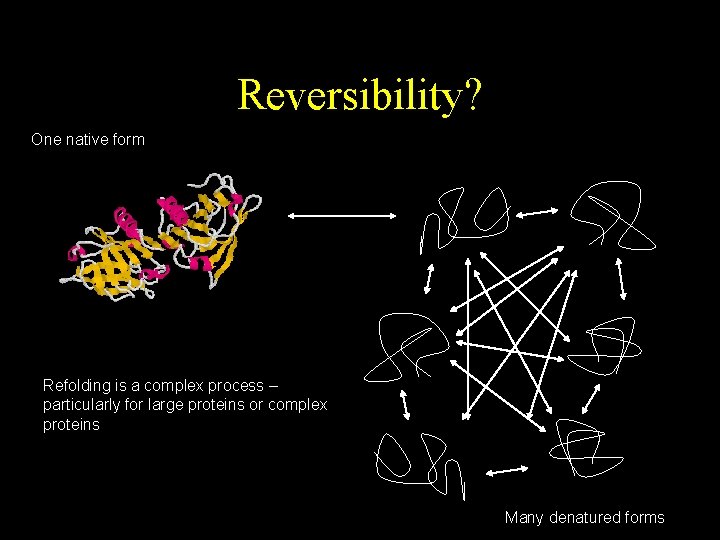
Reversibility? One native form Refolding is a complex process – particularly for large proteins or complex proteins Many denatured forms

Denaturation • The conversion of a biologically functional molecule into a non-functional form • There are many denatured states but one native state • Proteins can regenerate to their native state but slowly • Denatured proteins have a greater tendency to aggregate.

Pengaruh denaturasi : • Penurunan kelarutan • Mengubah kapasitas pengikatan air • Kehilangan aktivitas biologis (enzim, bahan imunologi) • Meningkatkan kemampuan bahan untuk dihidrolisis oleh protease • Meningkatkan viskositas intrinsik • Tidak dapat dikristalisasi

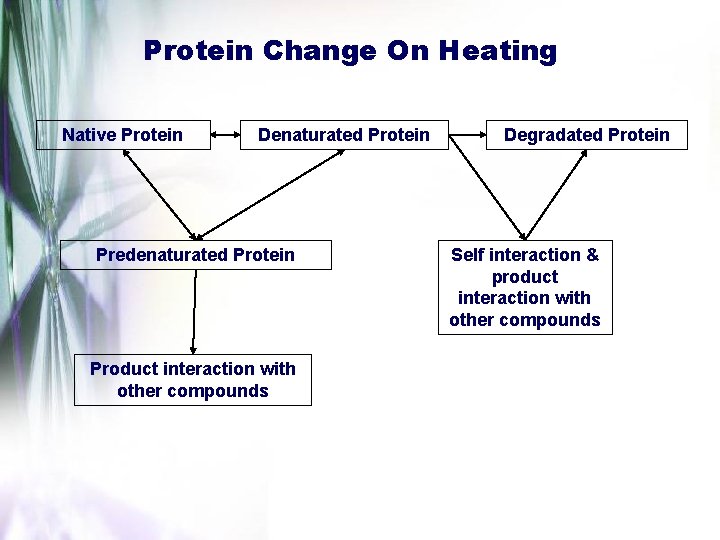
Protein Change On Heating Native Protein Denaturated Protein Predenaturated Protein Product interaction with other compounds Degradated Protein Self interaction & product interaction with other compounds

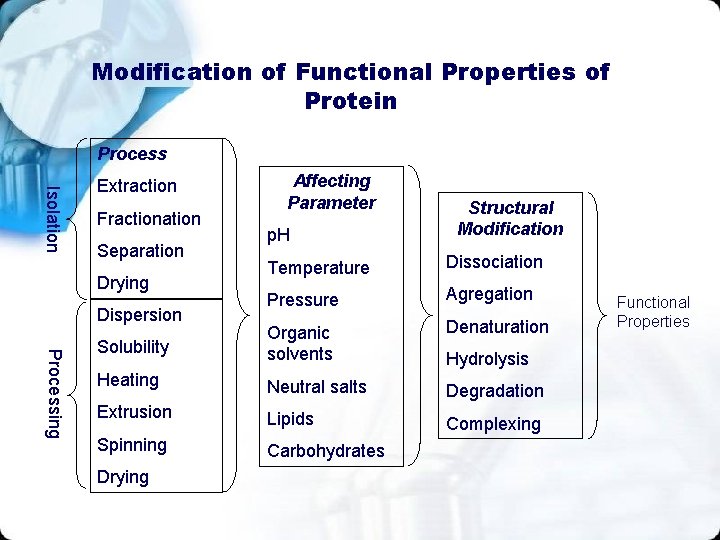
Modification of Functional Properties of Protein Process Isolation Extraction Fractionation Separation Affecting Parameter p. H Structural Modification Temperature Dissociation Pressure Agregation Denaturation Solubility Organic solvents Heating Neutral salts Degradation Extrusion Lipids Complexing Spinning Carbohydrates Drying Dispersion Processing Drying Hydrolysis Functional Properties

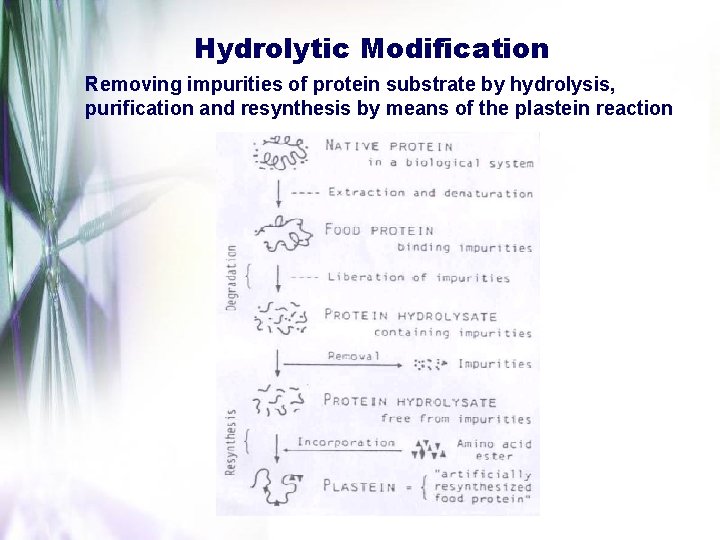
Hydrolytic Modification Removing impurities of protein substrate by hydrolysis, purification and resynthesis by means of the plastein reaction

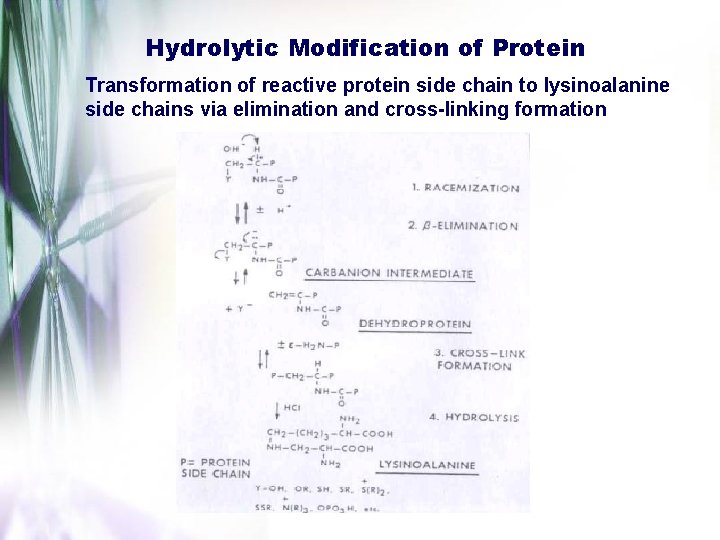
Hydrolytic Modification of Protein Transformation of reactive protein side chain to lysinoalanine side chains via elimination and cross-linking formation

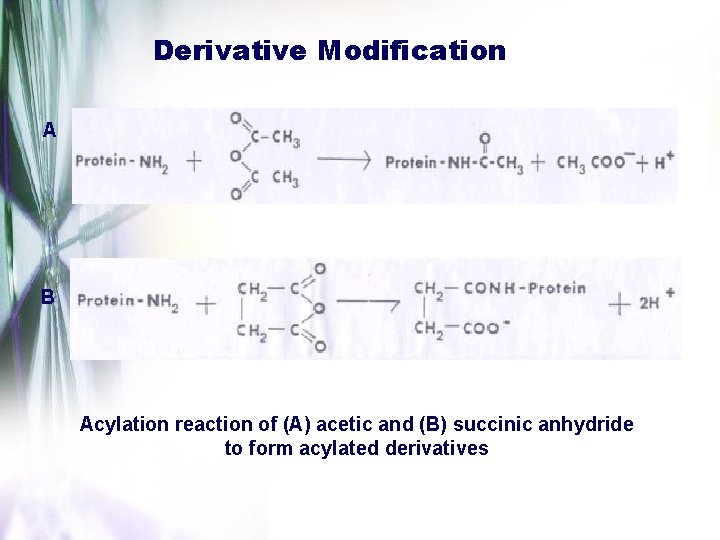
Derivative Modification A B Acylation reaction of (A) acetic and (B) succinic anhydride to form acylated derivatives

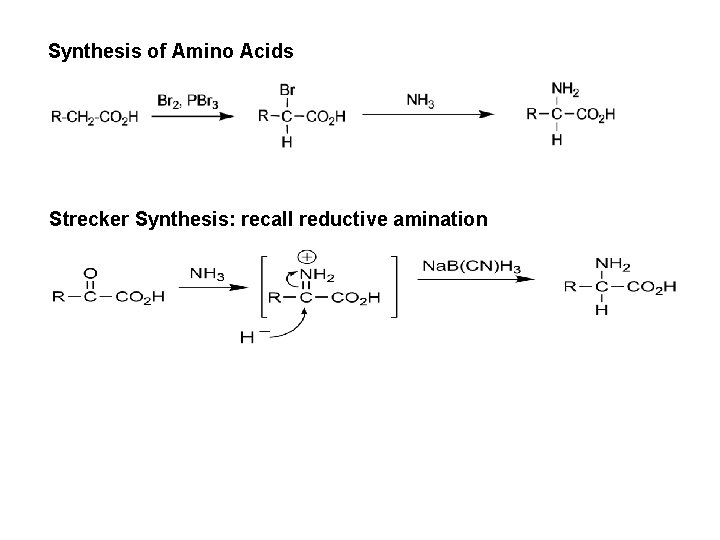
Synthesis of Amino Acids Strecker Synthesis: recall reductive amination

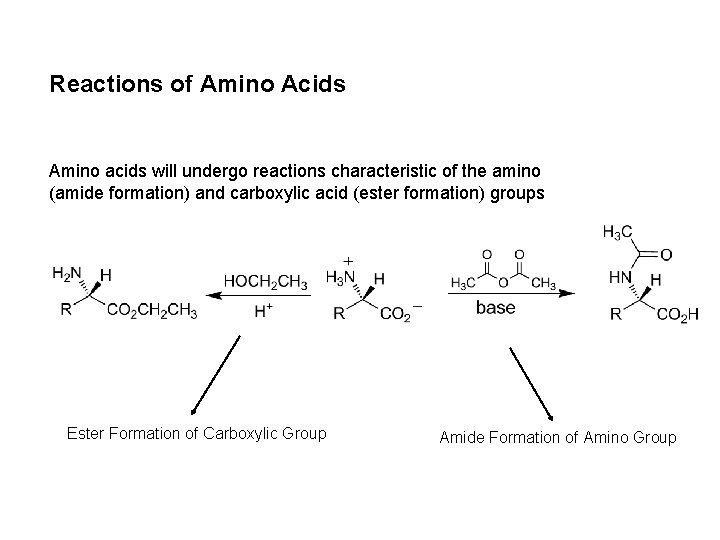
Reactions of Amino Acids Amino acids will undergo reactions characteristic of the amino (amide formation) and carboxylic acid (ester formation) groups Ester Formation of Carboxylic Group Amide Formation of Amino Group

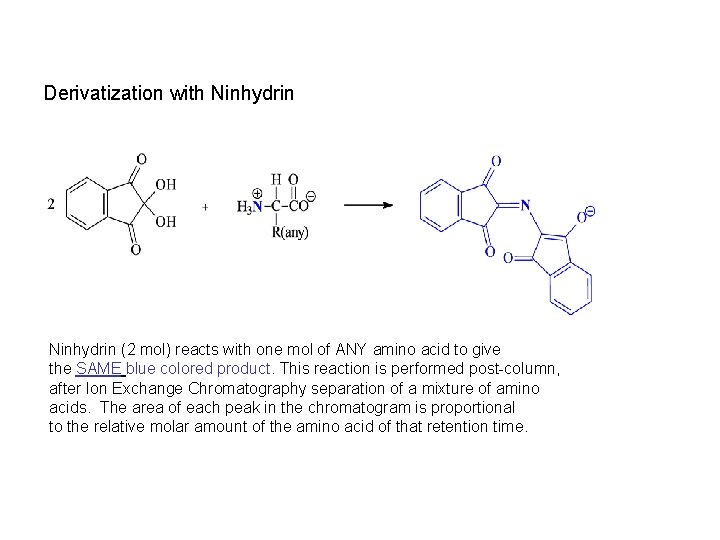
Derivatization with Ninhydrin (2 mol) reacts with one mol of ANY amino acid to give the SAME blue colored product. This reaction is performed post-column, after Ion Exchange Chromatography separation of a mixture of amino acids. The area of each peak in the chromatogram is proportional to the relative molar amount of the amino acid of that retention time.

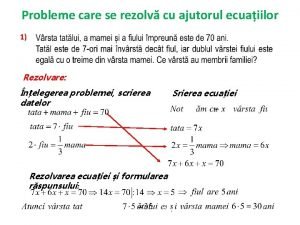
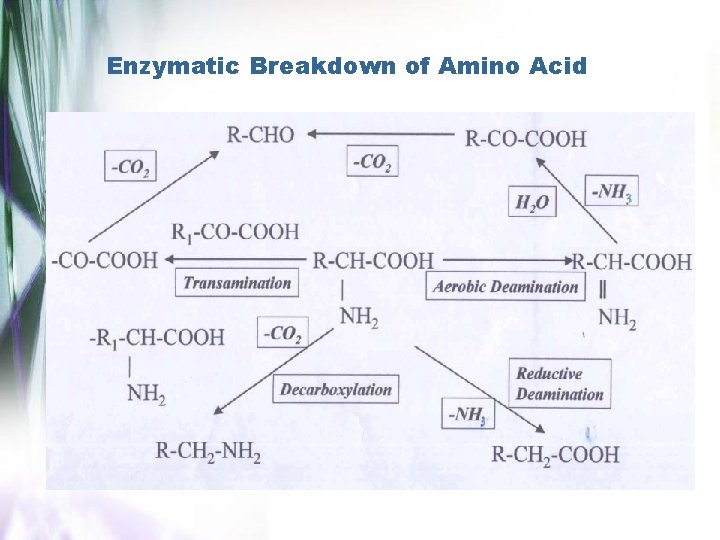
Reaksi Deaminasi dan Dekarboksilasi 1. Secara kimiawi : dekarboksilasi (degradasi strecker) Pemecahan asam amino α- dengan gugus karbonil dan bahan pengoksidasi lainnya, menghasilkan evolusi CO 2, aldehid, amino, dan senyawa lain. Mekanisme reaksi : R-CH-COOH NH 2 Oxidizing agents -CO 2 ½ O 2 R-CH=O -NH 3 Agen yang dapat menyebabkan degradasi asam amino : bahan organik & anorganik

2. Secara Enzimatis Sumber utama dekarboksilasi adalah kontaminasi (spoilage) mikroorganisme (genera Achromobacter, Micrococcus, Staphylococcus, Sarcina, Pseudomonas, dll) yang menghasilkan enzim tertentu untuk asam amino tertentu Contoh : kontaminasi (spoilage) produk perikanan oleh mikroorganisme, flavor khas dari produk susu

Enzymatic Breakdown of Amino Acid

 De ani si ani cadeti in hau
De ani si ani cadeti in hau Ani woda ani dropsy nie smakuja tak jak koksy
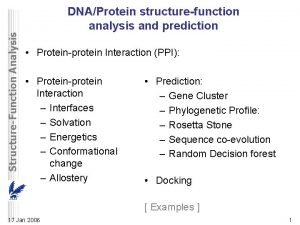
Ani woda ani dropsy nie smakuja tak jak koksy Protein-protein docking
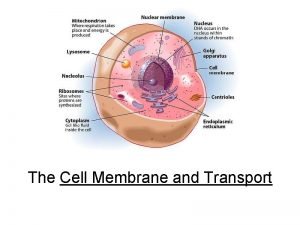
Protein-protein docking Carrier vs channel proteins
Carrier vs channel proteins Erd persediaan barang
Erd persediaan barang Kalkulasi biaya proses adalah
Kalkulasi biaya proses adalah Penambahan bahan pada departemen lanjutan
Penambahan bahan pada departemen lanjutan Metode alokasi aljabar
Metode alokasi aljabar Perbedaan biro dan departemen
Perbedaan biro dan departemen Program kerja bidang keilmuan bem
Program kerja bidang keilmuan bem Penentuan biaya proses
Penentuan biaya proses Metode harga pokok proses 2 departemen
Metode harga pokok proses 2 departemen Anggaran biaya overhead pabrik
Anggaran biaya overhead pabrik Departemen
Departemen Contoh data mart pada departemen penjualan
Contoh data mart pada departemen penjualan Organigrama ani
Organigrama ani Dr ani binti ahmad
Dr ani binti ahmad Pentru constructia unei autostrazi sunt necesari 3 ani
Pentru constructia unei autostrazi sunt necesari 3 ani ścieżka brzóz ania z zielonego wzgórza
ścieżka brzóz ania z zielonego wzgórza Trust boundary
Trust boundary Příčestí minulé
Příčestí minulé Organigrama ani
Organigrama ani Czym jest poezja która nie ocala narodów ani ludzi
Czym jest poezja która nie ocala narodów ani ludzi Oratoriul haleluia text in l romana
Oratoriul haleluia text in l romana Ani shehigian
Ani shehigian Dr bobby urologi
Dr bobby urologi Plica salpingopharyngeus
Plica salpingopharyngeus Ani kast
Ani kast Organigrama ani
Organigrama ani Agbala igbo
Agbala igbo Organigrama ani
Organigrama ani Pembagian 20 sifat wajib allah
Pembagian 20 sifat wajib allah Nemohla být ani stvořena z prvků pozemských
Nemohla být ani stvořena z prvků pozemských Ani
Ani Organigrama ani
Organigrama ani Ani tam tutuşma
Ani tam tutuşma Ce este pilonul 2 de pensii
Ce este pilonul 2 de pensii Ani dönme merkezi
Ani dönme merkezi Ani social
Ani social Musculus ani
Musculus ani Ani lo projekt
Ani lo projekt Organigrama con consejo de administracion
Organigrama con consejo de administracion Ani agi asi
Ani agi asi Levator ani syndrome
Levator ani syndrome Dom państwa hammondów
Dom państwa hammondów Arda deniz aksular
Arda deniz aksular Ani żadnej rzeczy która jego jest prezentacja
Ani żadnej rzeczy która jego jest prezentacja Historia papieru prezentacja
Historia papieru prezentacja Lp atresia ani 2020
Lp atresia ani 2020 Domande difficili
Domande difficili Lapte de crestere 1-3 ani
Lapte de crestere 1-3 ani Na západní frontě klid hlavní myšlenka
Na západní frontě klid hlavní myšlenka Suma wieku ani i oli wynosi 22 lata
Suma wieku ani i oli wynosi 22 lata Organigrama ani
Organigrama ani Organigrama ideam
Organigrama ideam Hiatus levatorius
Hiatus levatorius Ani marlina
Ani marlina Fascia rectoprostatica
Fascia rectoprostatica Ani nenkova
Ani nenkova Dea konstruieren
Dea konstruieren Dea strategic plan
Dea strategic plan Dea declension
Dea declension Diploma dea
Diploma dea Milica ilicic
Milica ilicic Norditropon
Norditropon Sistemas digitales
Sistemas digitales Dea lungul sau de-a lungul
Dea lungul sau de-a lungul Diversion investigator
Diversion investigator Dea strategic plan
Dea strategic plan Cea dea
Cea dea Komponen dfd
Komponen dfd Dangerous adverb
Dangerous adverb