atoms molecules and compounds atoms Atoms make up





- Slides: 10

atoms, molecules, and compounds

atoms • Atoms make up all the different elements • Although atoms make up everything, atoms usually DO NOT exist on their own. Na - Sodium Fe - Iron Both are atoms of different elements but would not usually be found in nature on their own!

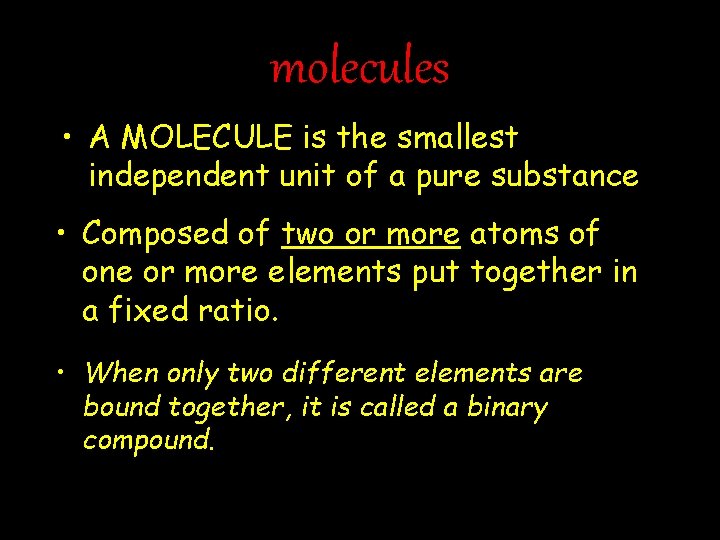
molecules • A MOLECULE is the smallest independent unit of a pure substance • Composed of two or more atoms of one or more elements put together in a fixed ratio. • When only two different elements are bound together, it is called a binary compound.

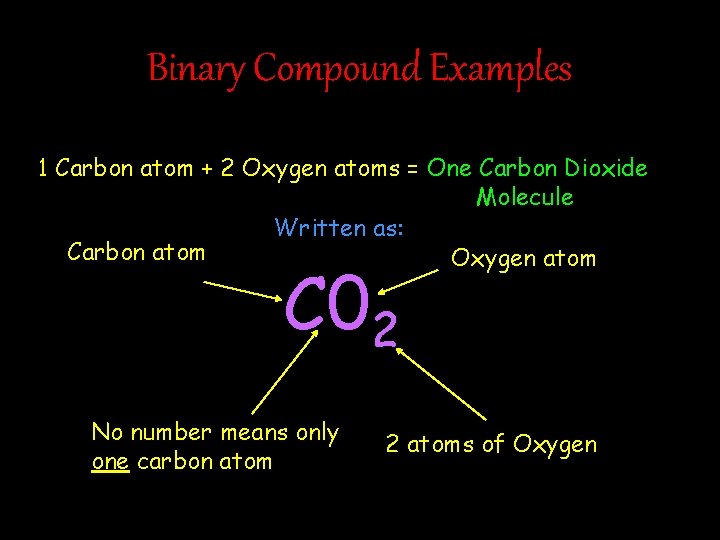
Binary Compound Examples 1 Carbon atom + 2 Oxygen atoms = One Carbon Dioxide Molecule Written as: Carbon atom Oxygen atom C 02 No number means only one carbon atom 2 atoms of Oxygen

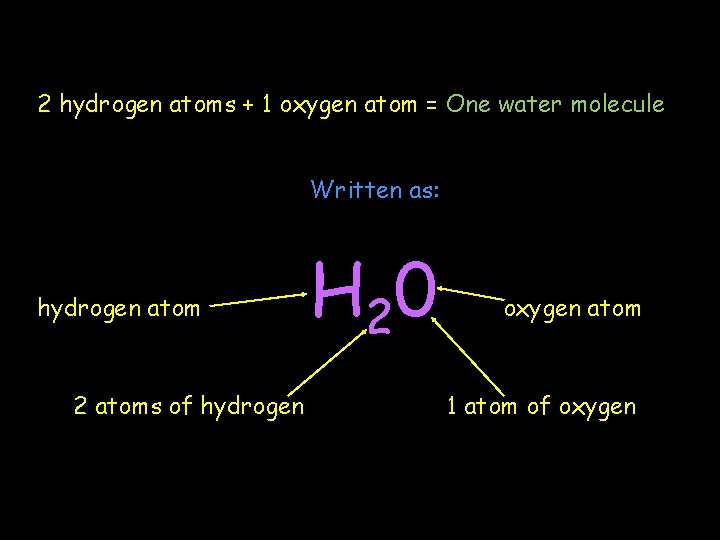
2 hydrogen atoms + 1 oxygen atom = One water molecule Written as: hydrogen atom 2 atoms of hydrogen H 2 0 oxygen atom 1 atom of oxygen

• Molecules stay linked together even if the substance is melted, boiled, or frozen

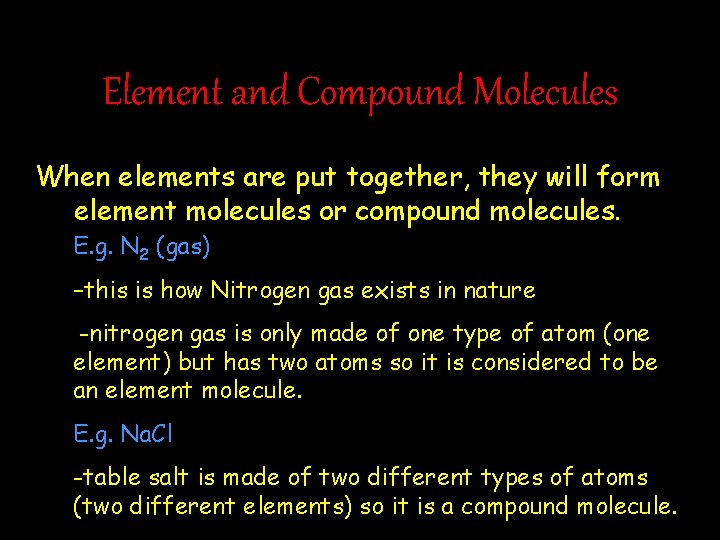
Element and Compound Molecules When elements are put together, they will form element molecules or compound molecules. E. g. N 2 (gas) –this is how Nitrogen gas exists in nature -nitrogen gas is only made of one type of atom (one element) but has two atoms so it is considered to be an element molecule. E. g. Na. Cl -table salt is made of two different types of atoms (two different elements) so it is a compound molecule.

How to do the worksheet For each of the following chemicals, identify four things: a. Element or Compound b. Number of elements and their names c. Number of atoms and number of each d. Is it a molecule or not? (molecule/not a molecule)

1. N 2 (nitrogen gas)a. Element (only made of one type of element) b. 1 Element; nitrogen c. 2 atoms; 2 nitrogen d. Molecule (two or more atoms) Two atoms of the same element bound together is called a diatomic molecule. 2. Na. Cl (salt) a. Compound (made of two different elements) b. 2 elements: Sodium, Chlorine c. 2 atoms; 1 Sodium, 1 Chlorine d. Molecule

3. Cu. SO 4 (Copper (II) Sulphate) a. Compound (made of three elements) b. 3 Element; Copper, Sulfur, Oxygen c. 6 atoms; 1 Copper, 1 Sulfur, 4 Oxygen d. Molecule (two or more atoms)