Chapter 2 Atoms Molecules and Ions AP Chemistry




































- Slides: 80

Chapter 2 Atoms, Molecules, and Ions AP Chemistry West Valley High School Mr. Mata

History Greeks n Democritus and Leucippus – “atomos” n Aristotle- elements (earth, water, fire, air) n Alchemy (tried to convert Pb Au) n 1660 - Robert Boyle- experimental definition of element. n Lavoisier- Father of modern chemistry n He wrote the book- used measurement n

Laws Conservation of Mass n Law of Definite Proportion- compounds have a constant composition by mass. n They react in specific ratios by mass. n Multiple Proportions- When two elements form more than one compound, the ratios of the masses can be reduced to small whole numbers. n

What? ! Water (H 2 O) has 16 g of O per 2 g of H or 8 g O per 1 g of H. n Hydrogen peroxide (H 2 O 2) has 32 g of O per 2 g of H or 16 g of O per 1 g of H. n If both H 2 O and H 2 O 2 form from reactants, they would have a 16 g/8 g ratio = 2/1 or a 2: 1 ratio. n Small whole number ratios. n

Dalton’s Atomic Theory 1. Elements are made up of atoms n 2. Atoms of each element are identical; atoms of different elements are different. n 3. Compounds are formed when atoms combine. Each compound has a specific number and kinds of atom. n 4. Chemical reactions are rearrangements of atoms. Atoms are not created nor destroyed. n

A Helpful Observation Gay-Lussac- under the same conditions of temperature and pressure, compounds always react in whole number ratios by volume. n Avogadro- interpreted that to mean: at the same temperature and pressure, equal volumes of gas contain the same number of particles n (called Avogadro’s hypothesis) n

Experiments to determine what an atom was n J. J. Thomson- used Cathode ray tubes

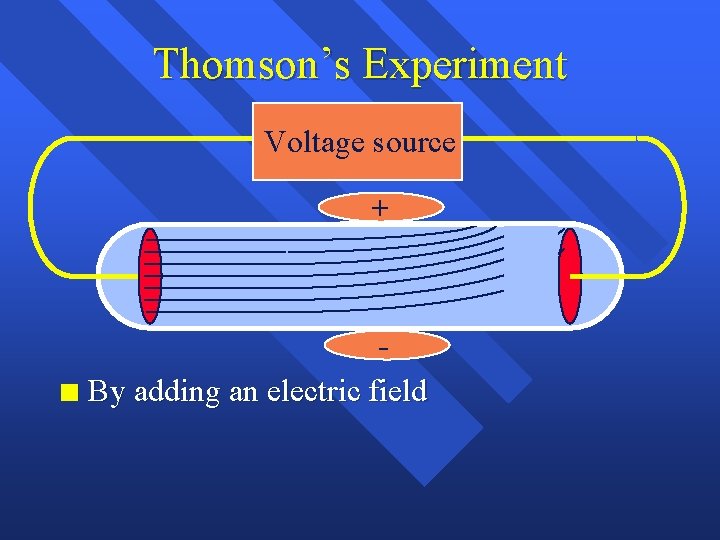
Thomson’s Experiment - Voltage source +

Thomson’s Experiment - Voltage source +

Thomson’s Experiment - Voltage source +

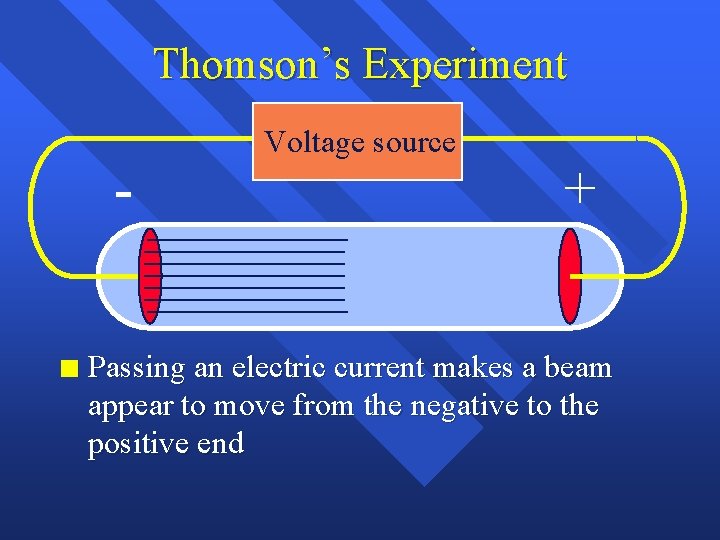
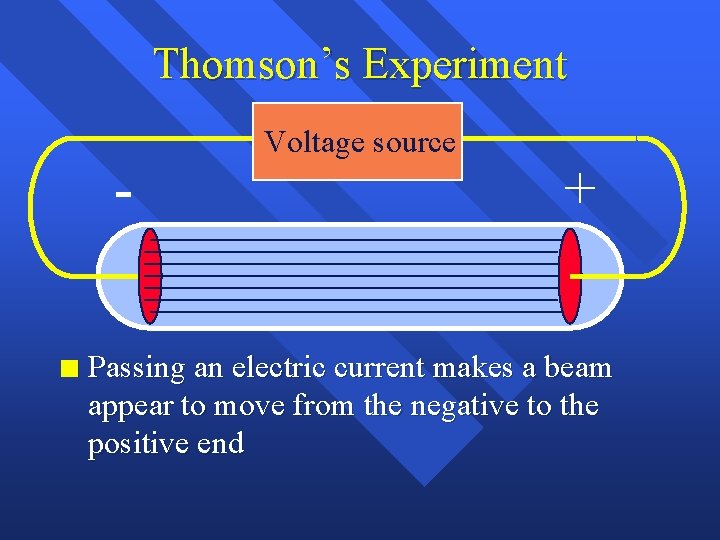
Thomson’s Experiment n Voltage source + Passing an electric current makes a beam appear to move from the negative to the positive end

Thomson’s Experiment n Voltage source + Passing an electric current makes a beam appear to move from the negative to the positive end

Thomson’s Experiment n Voltage source + Passing an electric current makes a beam appear to move from the negative to the positive end

Thomson’s Experiment n Voltage source + Passing an electric current makes a beam appear to move from the negative to the positive end

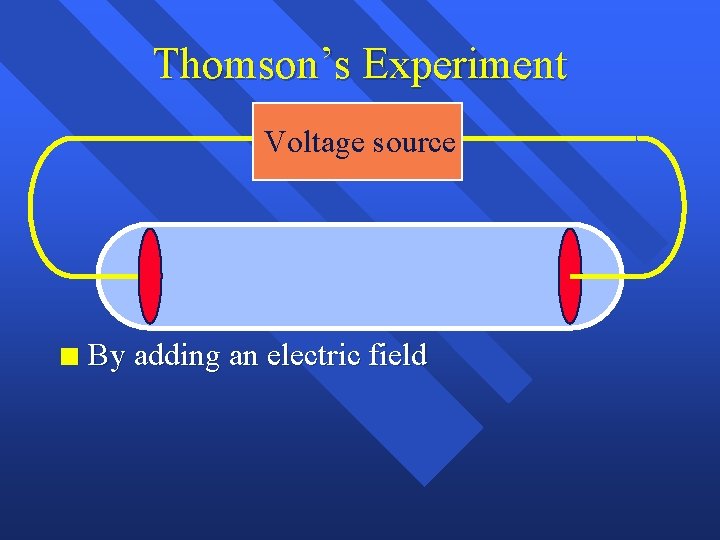
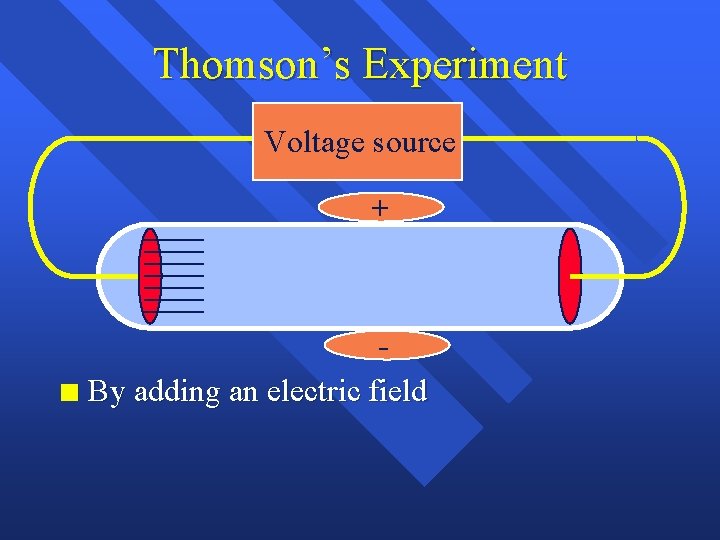
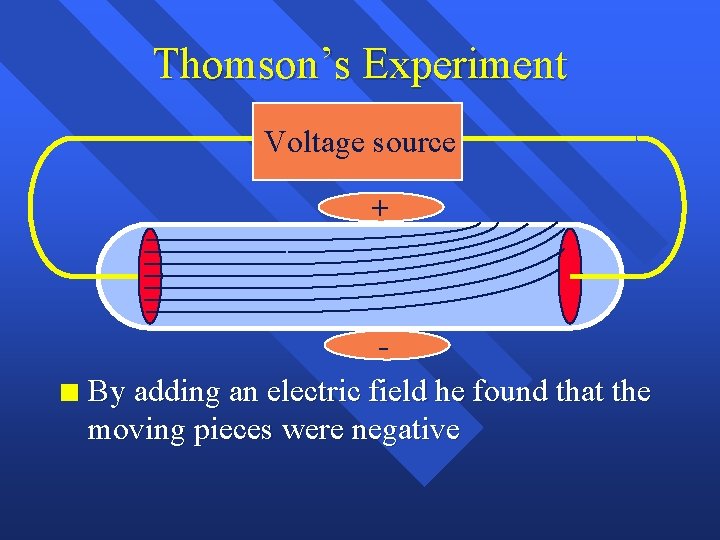
Thomson’s Experiment Voltage source n By adding an electric field

Thomson’s Experiment Voltage source + n By adding an electric field

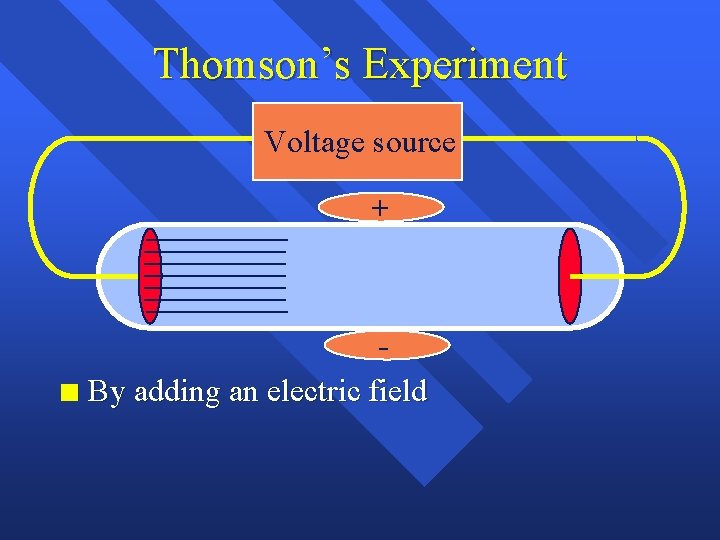
Thomson’s Experiment Voltage source + n By adding an electric field

Thomson’s Experiment Voltage source + n By adding an electric field

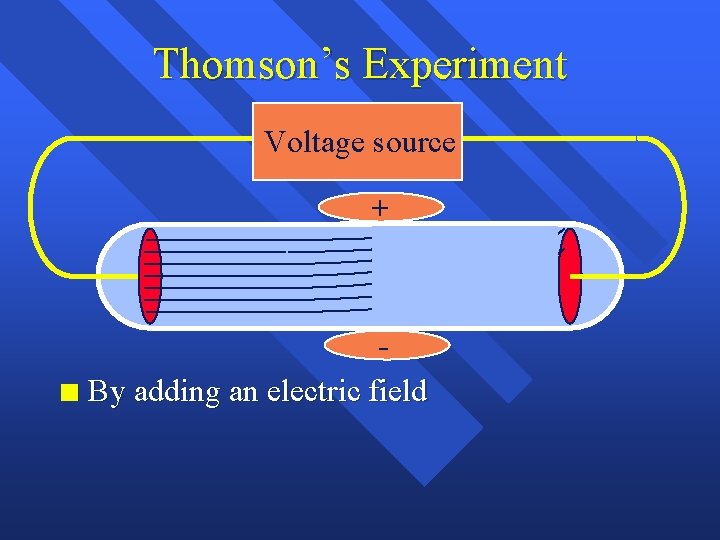
Thomson’s Experiment Voltage source + n By adding an electric field

Thomson’s Experiment Voltage source + n By adding an electric field

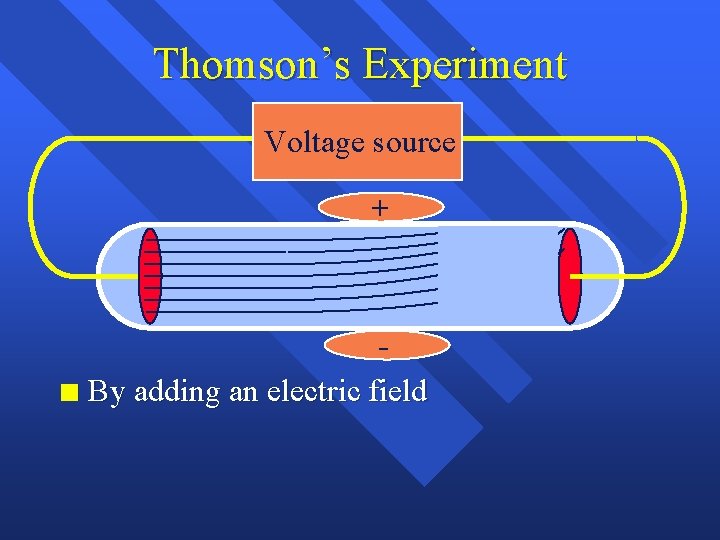
Thomson’s Experiment Voltage source + n By adding an electric field he found that the moving pieces were negative

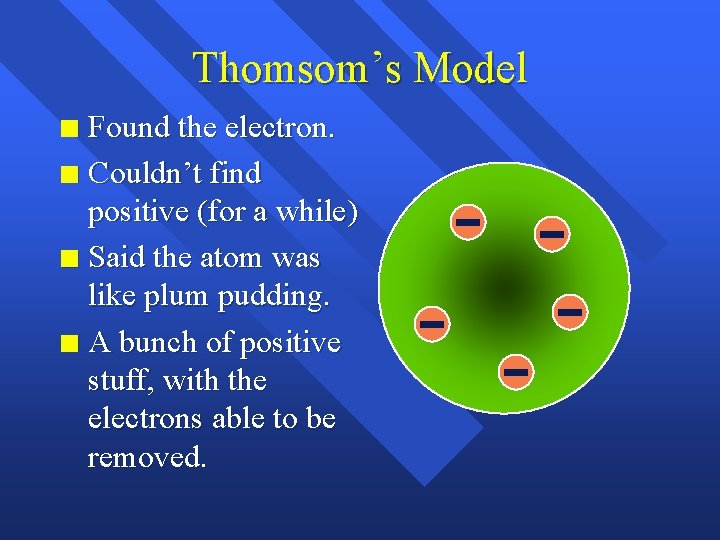
Thomsom’s Model Found the electron. n Couldn’t find positive (for a while) n Said the atom was like plum pudding. n A bunch of positive stuff, with the electrons able to be removed. n

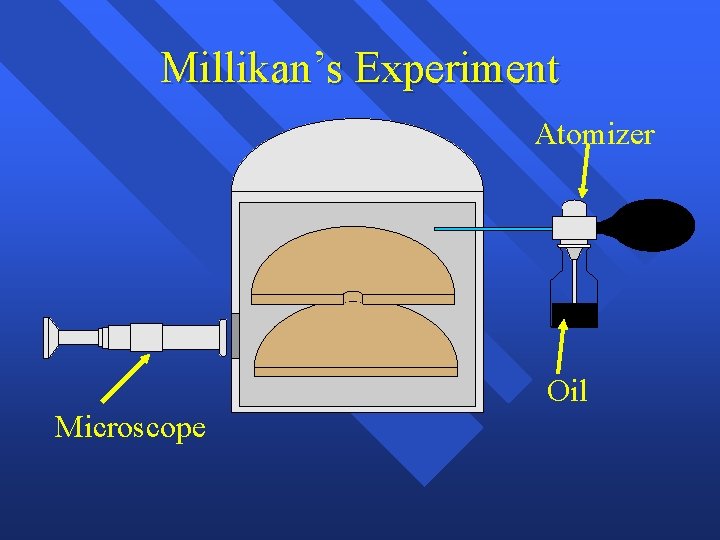
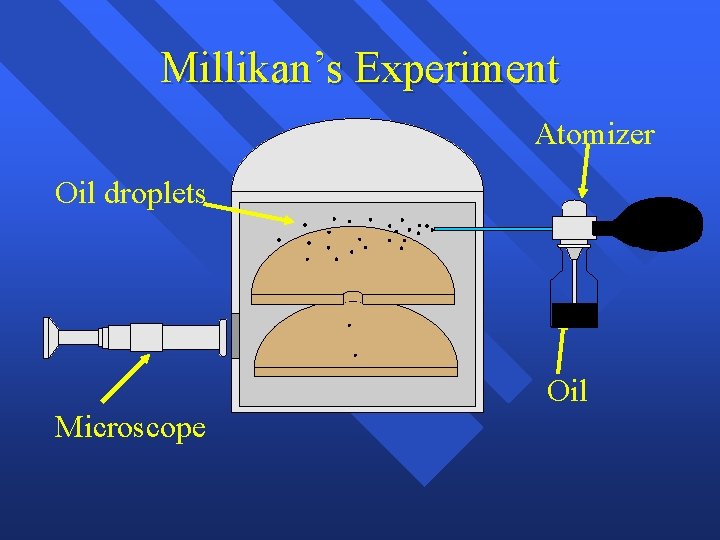
Millikan’s Experiment Atomizer + Oil Microscope

Millikan’s Experiment Atomizer Oil droplets + Oil Microscope

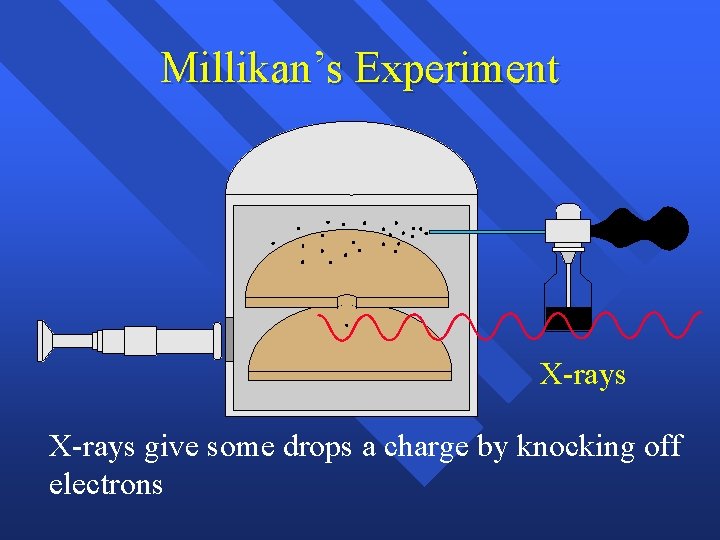
Millikan’s Experiment X-rays give some drops a charge by knocking off electrons

Millikan’s Experiment +

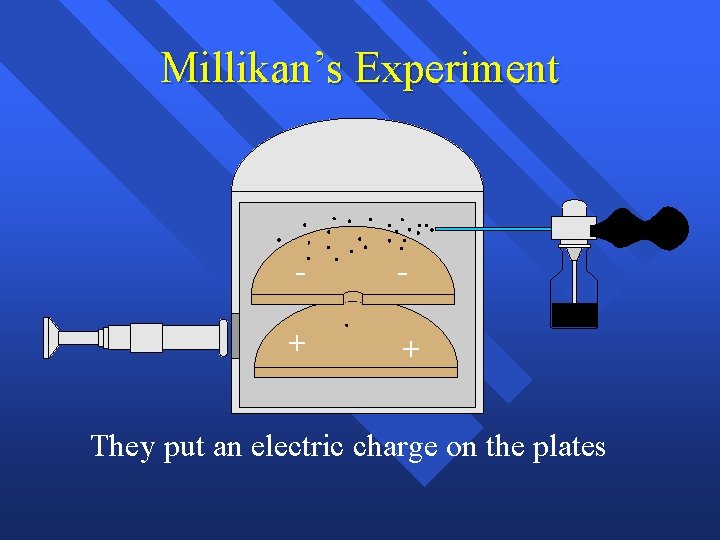
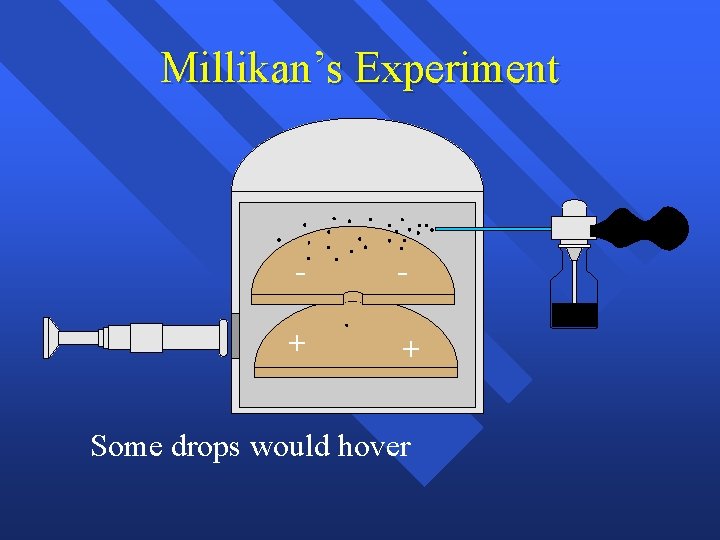
Millikan’s Experiment - - + + They put an electric charge on the plates

Millikan’s Experiment - - + + Some drops would hover

Millikan’s Experiment - - - - + + + +

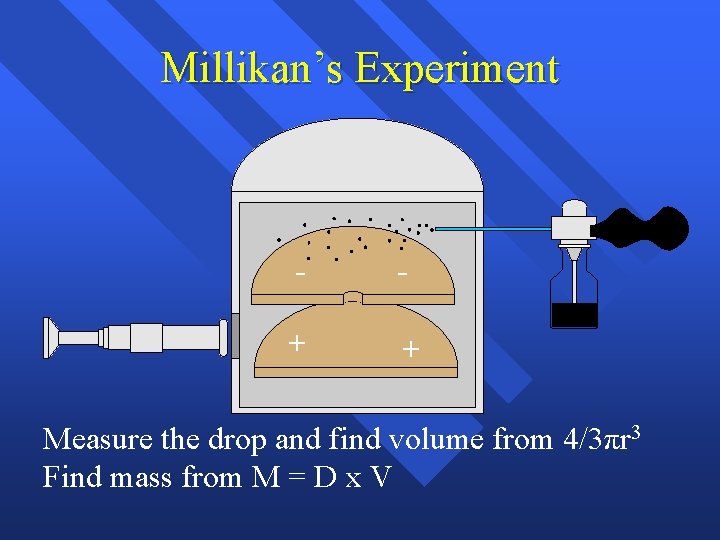
Millikan’s Experiment - - + + Measure the drop and find volume from 4/3πr 3 Find mass from M = D x V

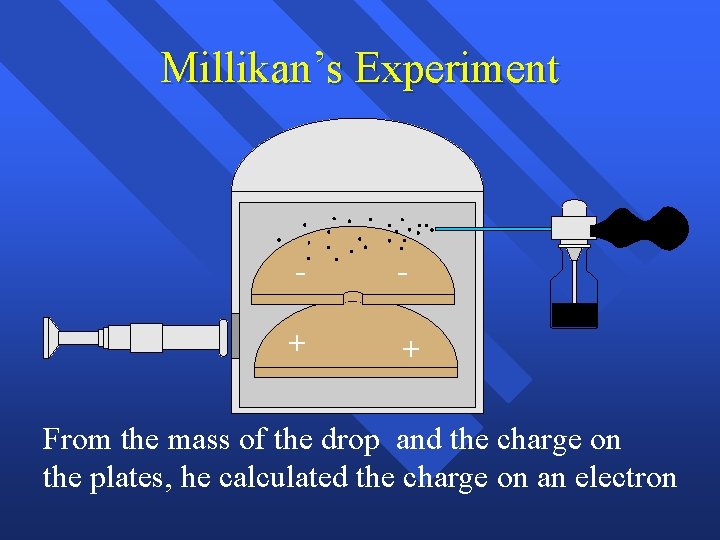
Millikan’s Experiment - - + + From the mass of the drop and the charge on the plates, he calculated the charge on an electron

Radioactivity Discovered by accident n Henri Bequerel n Three types – alpha- helium nucleus (+2 charge, large mass) – beta- high speed electron – gamma- high energy light n

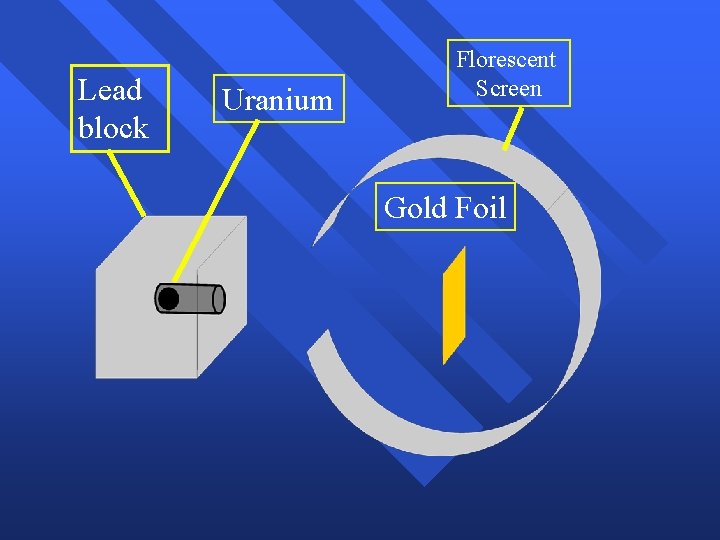
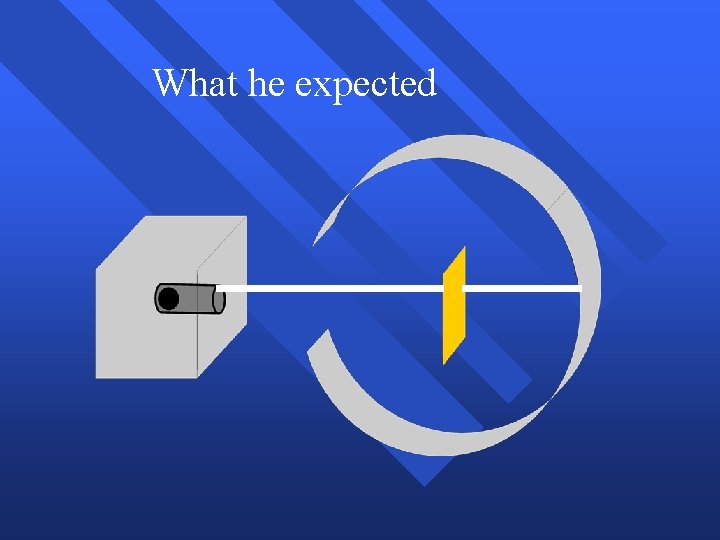
Rutherford’s Experiment Used uranium to produce alpha particles. n Aimed alpha particles at gold foil by drilling hole in lead block. n Since the mass is evenly distributed in gold atoms alpha particles should go straight through. n Used gold foil because it could be made atoms thin. n

Lead block Uranium Florescent Screen Gold Foil

What he expected

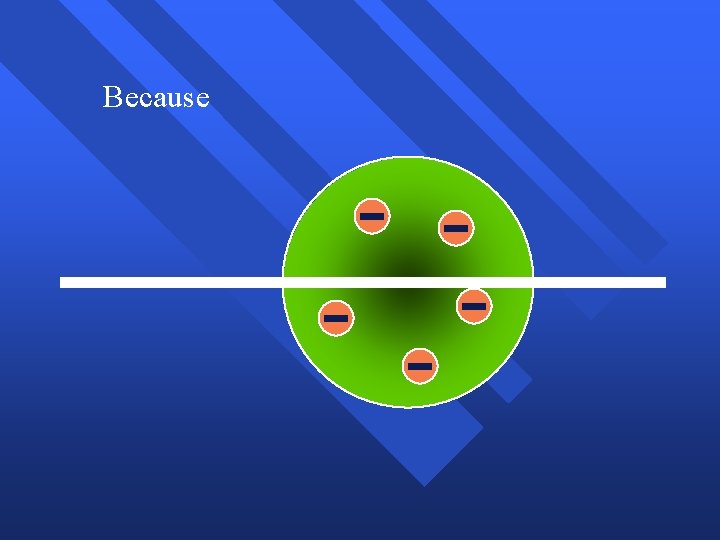
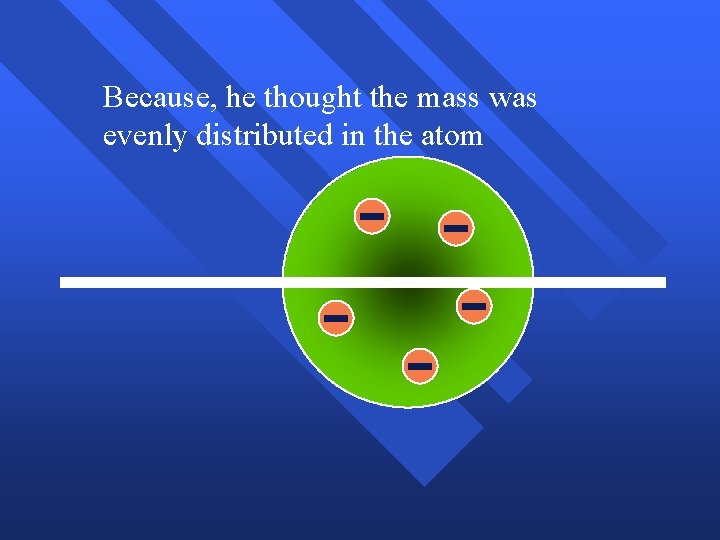
Because

Because, he thought the mass was evenly distributed in the atom

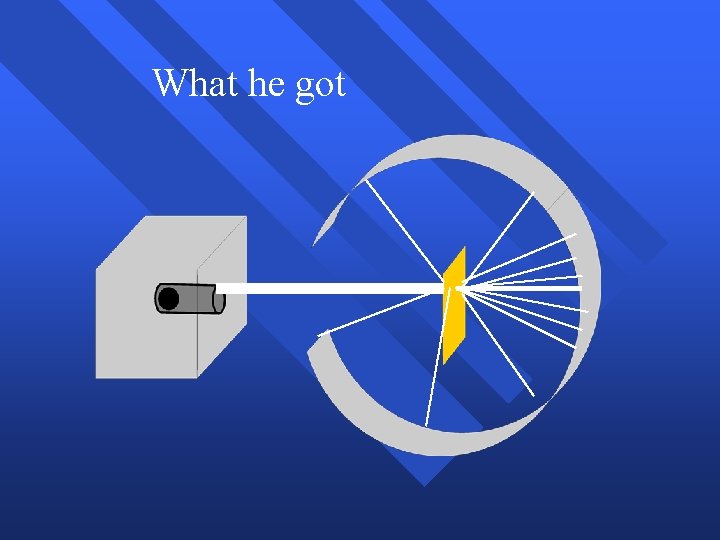
What he got

How he explained it Atom is mostly empty n Small dense, positive piece center n Alpha particles are deflected by it if they get close enough n at +


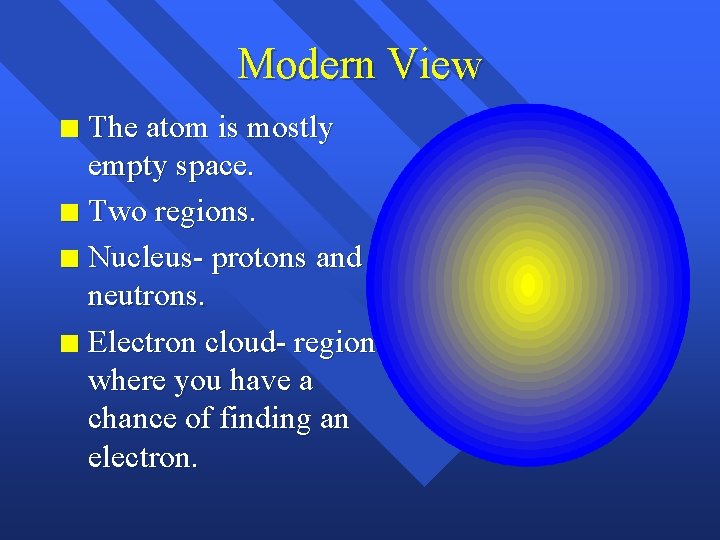
Modern View The atom is mostly empty space. n Two regions. n Nucleus- protons and neutrons. n Electron cloud- region where you have a chance of finding an electron. n

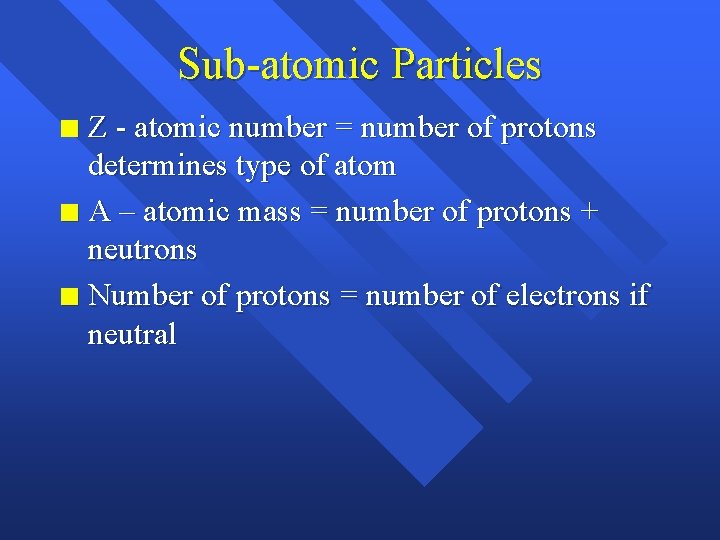
Sub-atomic Particles Z - atomic number = number of protons determines type of atom n A – atomic mass = number of protons + neutrons n Number of protons = number of electrons if neutral n

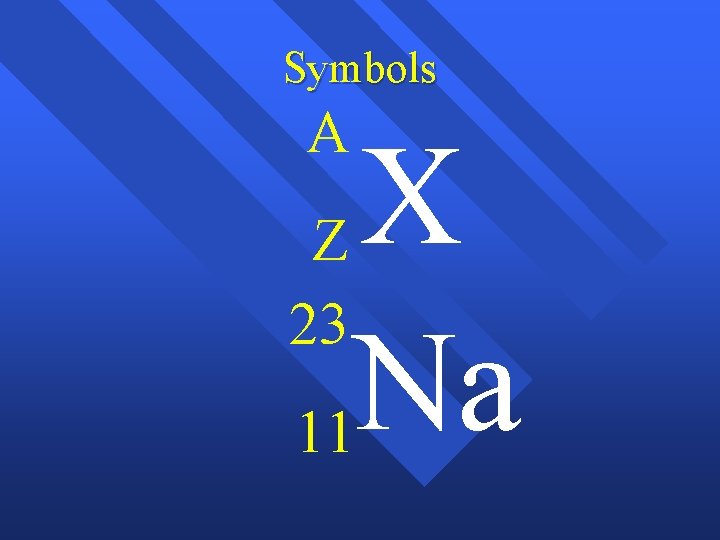
Symbols A X Z 23 Na 11

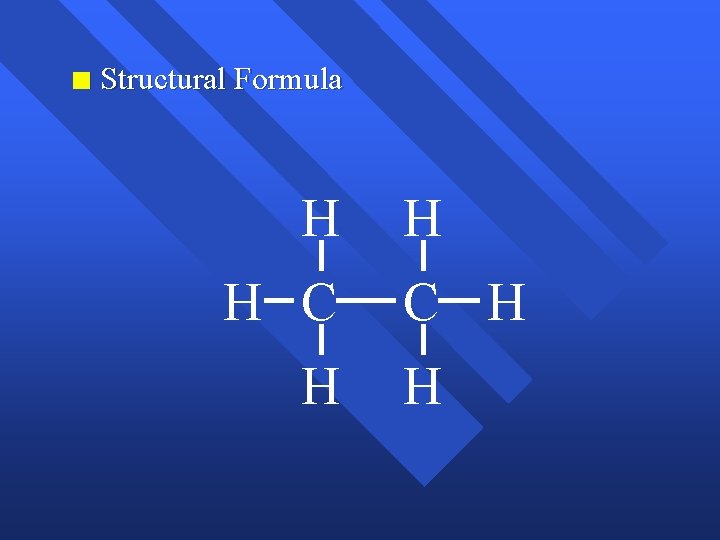
Chemical Bonds The forces that hold atoms together. n Covalent bonding - sharing electrons. n makes molecules. n Chemical formula- the number and type of atoms in a molecule. n C 2 H 6 - 2 carbon atoms, 6 hydrogen atoms, n n Structural formula shows the connections, but not necessarily the shape.

n Structural Formula H H C H H

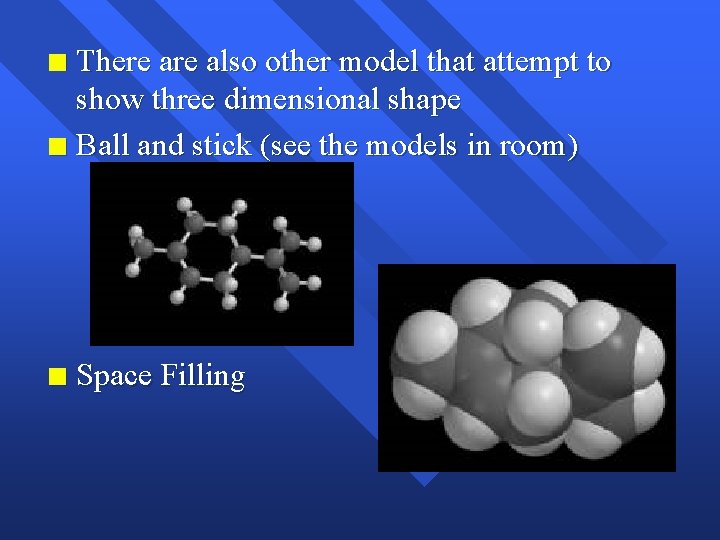
There also other model that attempt to show three dimensional shape n Ball and stick (see the models in room) n n Space Filling

Ions Atoms or groups of atoms with a charge. n Cations- positive ions - get by losing electrons(s). n Anions- negative ions - get by gaining electron(s). n Ionic bonding- held together by the opposite charges. n Ionic solids are called salts. n

Polyatomic Ions Groups of atoms that have a charge. n Yes, you have to memorize them. n List on page 65 of Zumdahl (9 th edition). n

Periodic Table

Metals Conductors n Lose electrons n Malleable and ductile n

Nonmetals Brittle n Gain electrons n Covalent bonds n

Semi-metals or Metalloids

Alkali Metals

Alkaline Earth Metals

Halogens

Transition metals

Noble Gases

Inner Transition Metals

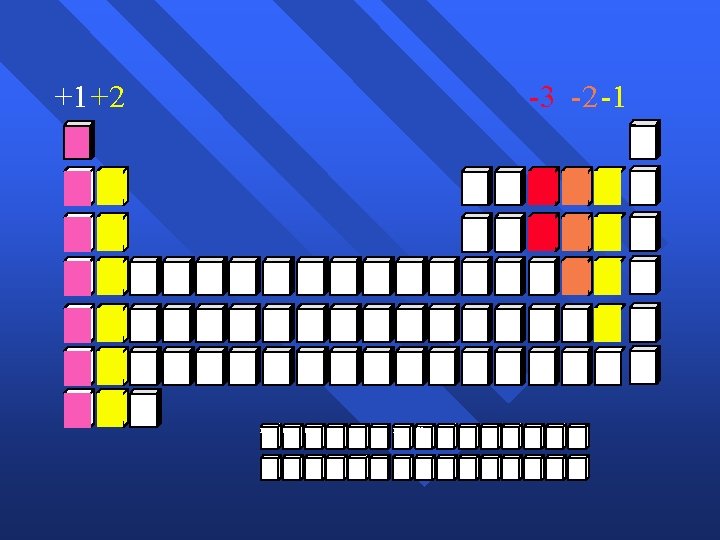
+1+2 -3 -2 -1

Naming compounds Two types. n Ionic - metal and non metal or polyatomics. n Covalent- we will just learn the rules for 2 non-metals. n

Ionic compounds If the cation is monoatomic- Name the metal (cation) just write the name. n If the cation is polyatomic- name it n If the anion is monoatomic- name it but change the ending to -ide n If the anion is poly atomic- just name it. n practice n

Covalent compounds Two words, with prefixes. n Prefixes tell you how many. n mono, di, tri, tetra, penta, hexa, hepta, octa, nona, deca (know these first ten prefixes). n First element whole name with the appropriate prefix, except mono. n Second element, -ide ending with appropriate prefix. n Practice n

Ionic Compounds Have to know what ions they form n off table, polyatomic, or figure it out n Ca. S calcium sulfide n K 2 S potassium sulfide n n Al. PO 4 K 2 SO 4 n Fe. S n Co. I 3 n aluminum phosphate potassium sulfate iron sulfide cobalt iodide

Ionic Compounds n Fe 2(C 2 O 4) iron oxalate n Mg. O magnesium oxide n Mn. O manganese oxide n KMn. O 4 potassium permanganate n NH 4 NO 3 ammonium nitrate n Hg 2 Cl 2 mercury chloride n Cr 2 O 3 chromium oxide

Ionic Compounds KCl. O 4 n Na. Cl. O 3 n YBr. O 2 n Cr. Cl. O 2 n potassium perchlorate sodium chlorate yttrium bromite chromium chlorite

Naming Covalent Compounds CO 2 n CO n CCl 4 n N 2 O 4 n Xe. F 6 n N 4 O 4 n P 2 O 10 n carbon dioxide carbon monoxide carbon tetrachloride dinitrogen tetraoxide xenon hexafloride tetranitrogen tetraoxide diphosphorous decaoxide

Writing Formulas Two sets of rules, ionic and covalent. n To decide which to use, decide what the first word is. n If is a metal or polyatomic use ionic. n If it is a non-metal use covalent. n

Ionic Formulas Charges must add up to zero. n get charges from table, name of metal ion, or memorized from the list. n use parenthesis to indicate multiple polyatomics. n

Ionic Formulas Sodium nitride n sodium- Na is always +1 n nitride - ide tells you it comes from the table n nitride is N-3 n

Ionic Formulas Sodium nitride n sodium- Na is always +1 n nitride - ide tells you it comes from the table n nitride is N-3 n doesn’t add up to zero n +1 Na -3 N

Ionic Formulas Sodium nitride n sodium- Na is always +1 n nitride - ide tells you it comes from the table n nitride is N-3 n doesn’t add up to zero n Need 3 Na n +1 Na -3 N Na 3 N

Ionic Compounds Sodium sulfite n calcium iodide n Lead (II) oxide n Lead (IV) oxide n Mercury (I) sulfide n Barium chromate n Na 2 SO 3 Ca. I 2 Pb. O 2 Hg 2 S Ba. Cr. O 4

Covalent compounds The name tells you how to write the formula n Sulfur dioxide SO 2 n diflourine monoxide F 2 O n nitrogen trichloride NCl 3 n diphosphorus pentoxide P 2 O 5 n

More Names and formulas

Acids Substances that produce H+ ions when dissolved in water n All acids begin with H n Two types of acids n Oxyacids n non oxyacids n

Naming acids If the formula has oxygen in it n write the name of the anion, but change – ate to -ic acid – ite to -ous acid n Watch out for sulfuric and sulfurous n HCl. O 4 perchloric acid n H 2 SO 3 sulfurous acid n HNO 2 nitrous acid n

Naming acids If the acid doesn’t have oxygen n add the prefix hydron change the suffix -ide to -ic acid n HCl hydrochloric acid n H 2 S hydrosulfuric acid n HCN hydrocyanic acid n

Formulas for acids Backwards from names n If it has hydro- in the name; no oxygen. n anion ends in –ide. n No hydro, anion ends in -ate or –ite. n Write anion and add enough H to balance the charges. n

Hydrates Some salts trap water crystals when they form crystals. n these are hydrates. n Both the name and the formula needs to indicate how many water molecules are trapped. n In the name we add the word hydrate with a prefix that tells us how many water molecules. n

Hydrates In the formula you put a dot and then write the number of molecules. n Calcium chloride dihydrate = Ca. Cl 2· 2 H 2 O n Chromium (III) nitrate hexahydrate = Cr(NO 3)3· 6 H 2 O n