AP CHEMISTRY CHAPTER 2 Atoms Molecules and Ions





















- Slides: 55

AP CHEMISTRY CHAPTER 2 Atoms, Molecules, and Ions

THE ATOMIC THEORY According to John Dalton's atomic theory, which he proposed in 1808, elements are composed of extremely small, indivisible particles called atoms. Dalton assumed that atoms were the smallest unit of an element that can enter into chemical combination. Atoms of the same element all have the same mass, and atoms of different elements have different masses. His theory explained three laws that were known at that time. The atomic theory consists of a set of postulates which can explain the law of definite proportions, the law of multiple proportions, and law of conservation of mass.

DALTON’S ATOMIC THEORY 1. 2. 3. 4. All matter is made of extremely small, indivisible particles called atoms. An atom is the smallest unit of an element that can enter into chemical combination. Atoms of the same element are identical in size, mass, and chemical properties. Atoms of one element are different from atoms of another element. Compounds are formed when atoms from two or more elements combine. Atoms combine in the ratio of small whole numbers. For a given compound, the number of atoms of one element that combine per atom of the other element is a fixed ratio. Atoms are the smallest units of chemical change. Chemical change involves the combination, separation, or rearrangement of atoms. Atoms are not destroyed or created in chemical processes.

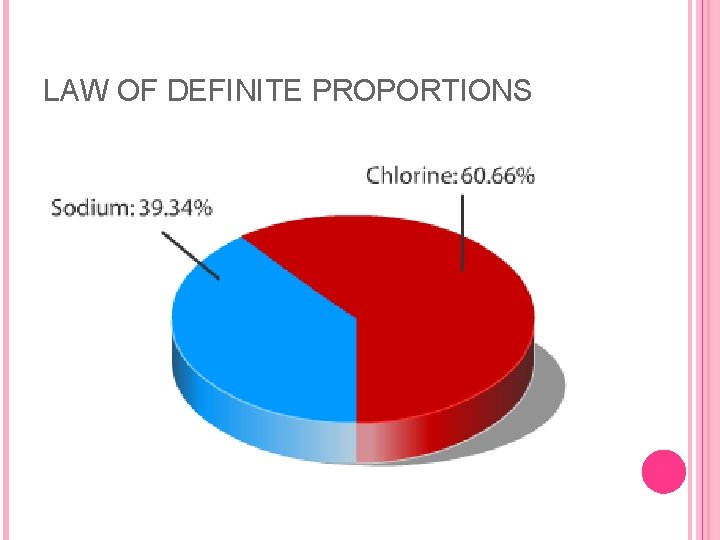
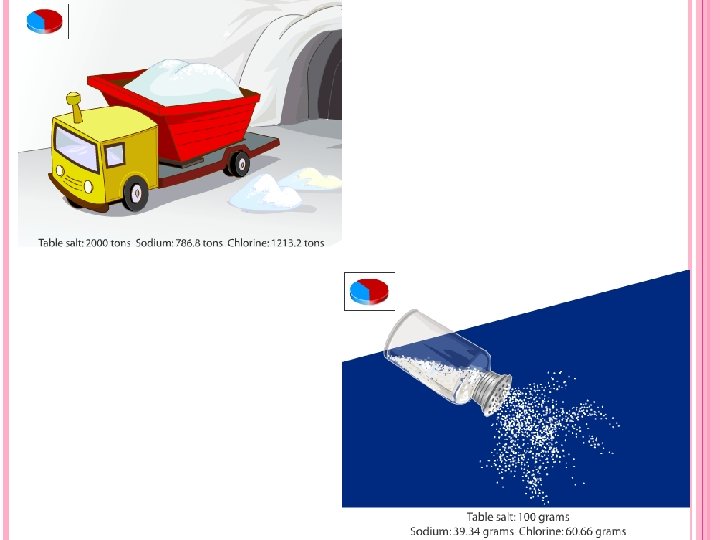
THREE LAWS Proust's law of definite proportions refers to the elemental constituents of compounds and states that all purified samples of a compound contain its constituent elements combined in the same proportions by mass. For example, all samples of the compound carbon monoxide, no matter what size, show that it is 43% carbon and 57% oxygen by mass. Dalton's proposal was that the smallest particle of carbon monoxide was a molecule consisting of one carbon atom and one oxygen atom. If all carbon atoms had the same mass, and all oxygen atoms had a mass about 1. 3 times greater than carbon atoms, then the composition of carbon monoxide would be exactly as given above.

LAW OF DEFINITE PROPORTIONS


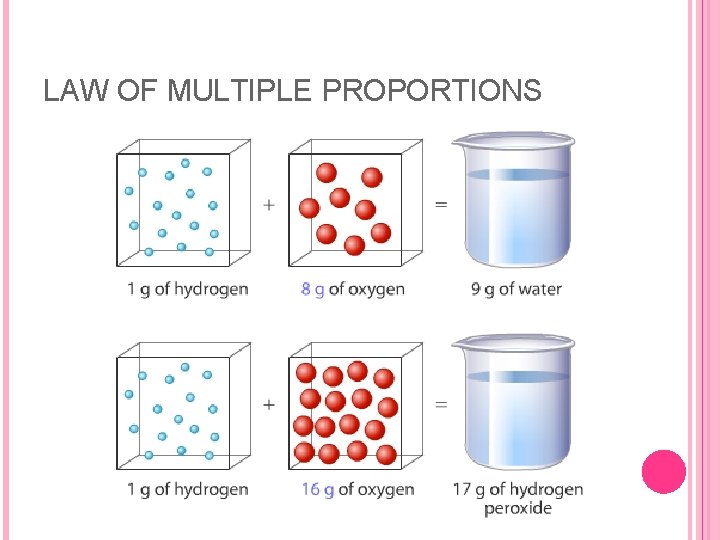
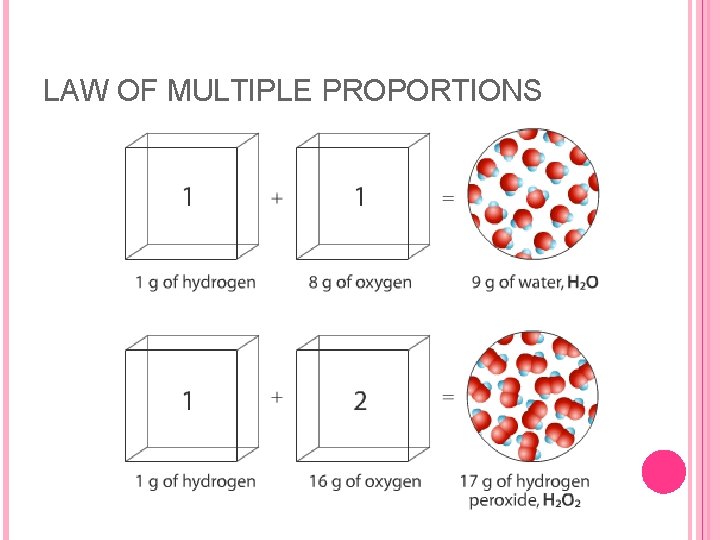
THREE LAWS While working on the atomic theory, Dalton discovered the law of multiple proportions: when two elements form more than one compound, the various masses of one element combining with a fixed mass of another element are related by small whole-number ratios.

LAW OF MULTIPLE PROPORTIONS This law can be applied to two compounds of nitrogen and oxygen; nitrogen monoxide, and nitrogen dioxide. The mass of oxygen combining with nitrogen in nitrogen dioxide was found to be twice as great as the mass of oxygen combining with nitrogen in nitrogen monoxide. Dalton proposed that the two compounds of N and O were the result of atoms of the two elements combining in different ratios to form two different molecules. When one atom of nitrogen combined with one atom of oxygen, nitrogen monoxide was formed. But when one atom of nitrogen combined with two atoms of oxygen, then nitrogen dioxide was the compound formed.

LAW OF MULTIPLE PROPORTIONS

LAW OF MULTIPLE PROPORTIONS

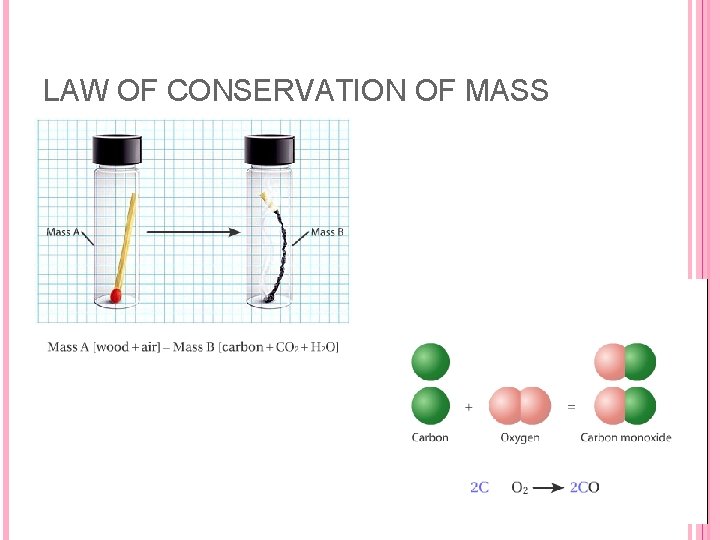
THREE LAWS The law of conservation of mass refers to an observation that has been made many times: there is no detectable gain or loss of mass during a chemical reaction. The law of conservation of mass is consistent with postulate 4. Atoms are neither created nor destroyed in a chemical reaction. Rather they are merely rearranged to form new combinations of atoms which we would recognize as new compounds. Mass is conserved in chemical reactions because atoms are conserved.

LAW OF CONSERVATION OF MASS

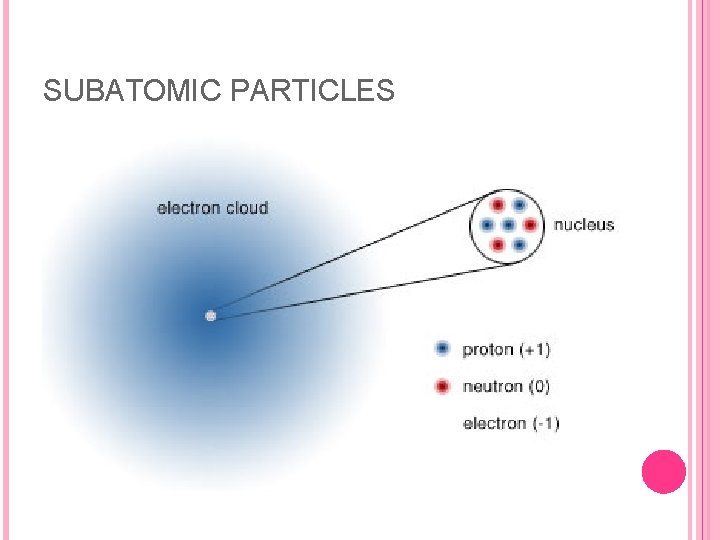
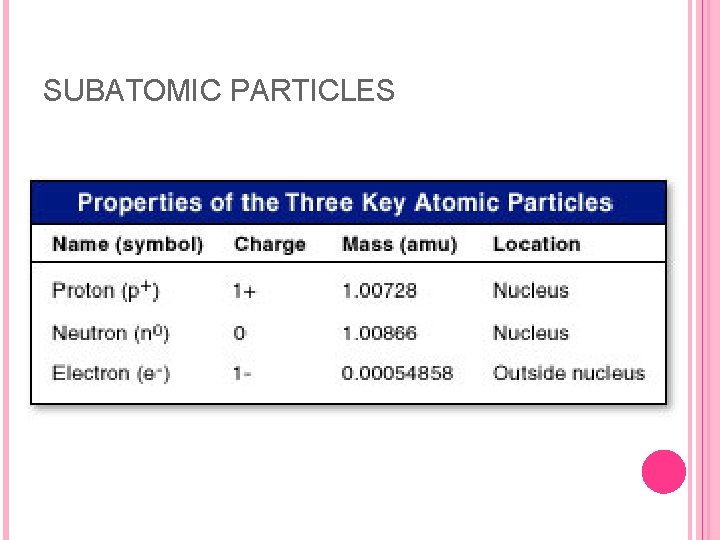
SUBATOMIC PARTICLES In the early 1900 s, scientists learned that all atoms are constructed from the same three subatomic particles: the electron, proton, and neutron. Whereas atoms themselves are electrically neutral they were found to consist of electrically charged particles. The electron has a single unit of negative charge; the proton has a single unit of positive charge; and the neutron has no charge. The proton and neutron have essentially the same mass, 1. 673 x 10– 24 g, and 1. 675 x 10– 24 g, respectively. The electron mass is much smaller: 9. 11 x 10– 28 g which is only 1/1836 the mass of a proton.

SUBATOMIC PARTICLES

SUBATOMIC PARTICLES

RUTHERFORD’S GOLD FOIL EXPERIMENT Rutherford's experiments on alpha particle scattering in 1910 led the way to the nuclear model of the atom. Alpha particles are ejected from certain radioactive elements with very high kinetic energies. Alpha particles have 2 units of positive charge, and a mass 4 times that of a proton. Being smaller than the atoms of most elements they can act as probes of the interior of atoms.

RUTHERFORD’S GOLD FOIL EXPERIMENT Rutherford's experiments lead to the following conclusions about the structure of atoms. Observations made by Rutherford when alpha particles were scattered by gold foil: 1. 2. 3. The majority of particles passed through the foil and were either undeflected or only slightly deflected (angle less than 2%). A few particles were deflected (scattered) by more than a few degrees. It was extremely rare, but 1 in 20, 000 alpha particles were deflected back toward the direction from which they had come.

RUTHERFORD’S GOLD FOIL EXPERIMENT Interpretations made by Rutherford about the nature of the atom: 1. 2. 3. Most of the atom must be empty space. The atom's positive charges are concentrated into a dense central core called the nucleus. The diameter of a typical atom is about 10, 000 times greater than the diameter of a typical nucleus.

Animation of Rutherford’s Gold Foil Experiment

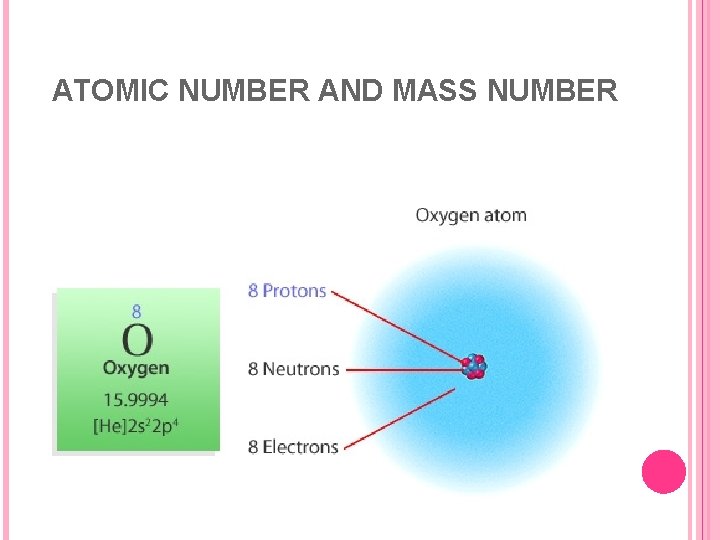
ATOMIC NUMBER AND MASS NUMBER An atom of one element is distinguished from an atom of another element by its number of protons. The atomic number, Z, of an element is the number of protons in the atomic nucleus. For example, the atomic number of oxygen is 8; therefore, all oxygen atoms contain eight protons and also eight electrons. You know this because atoms are neutral species and the positive charge must equal the negative charge.

ATOMIC NUMBER AND MASS NUMBER The total mass of an atom is determined almost entirely by the number of protons and neutrons. In many cases the mass of the electrons can be neglected because it is so much smaller. The mass number, A, is the same as the total number of neutrons and protons present in the nucleus of an atom. The number of neutrons in an atom is A – Z.

ATOMIC NUMBER AND MASS NUMBER

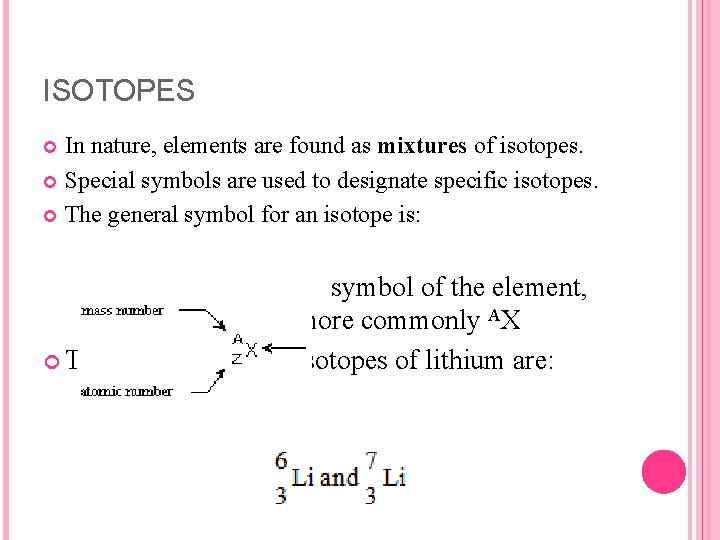
ISOTOPES Atoms of a given element that differ in the number of neutrons, and consequently in mass are called isotopes. For example, there are two atoms of the element lithium, one with a mass number of 6 and another with a mass number of 7. Isotopes can be referred to by their mass numbers as in lithium 6 (pronounced lithium six) and lithium 7. The different mass numbers are the result of different numbers of neutrons per atom.

ISOTOPES An atom of the isotope lithium 6 contains three protons, three electrons, and three neutrons, whereas an atom of lithium 7 contains three protons, three electrons and four neutrons. Both atoms contain three protons, and so both are isotopes of the element lithium.

ISOTOPES In nature, elements are found as mixtures of isotopes. Special symbols are used to designate specific isotopes. The general symbol for an isotope is: symbol of the element, or more commonly AX The symbols for the isotopes of lithium are:

ISOTOPES Isotopes of an element have similar chemical properties, and form the same types of compounds. The chemical properties of an element depend on the number of protons and electrons in an atom, not the number of neutrons.

THE PERIODIC TABLE The periodic table is a chart in which elements having similar chemical and physical properties are grouped together. The vertical columns are called groups, and the horizontal rows are called periods. Elements can be categorized into three main categories: Metals metals are good conductors of heat and electricity, they are usually ductile and can be pounded into sheets or stretched into wires. Nonmetals nonmetals are usually poor conductors of heat and electricity. Metalloids metalloids have properties that are intermediate between metals and nonmetals.


THE PERIODIC TABLE Groups of elements are frequently referred to by their periodic table group member (Group 1, Group 2, etc. ). For historic reasons, however, some groups have been given special names. Group 1 alkali metals Group 2 alkaline earth metals Group 17 – halogens Group 18 noble gases (or rare gases)

COMPOUNDS

MOLECULES Compounds are produced by the combination of atoms of different elements. Compounds fall into two general types depending on how their atoms are held together: molecular and ionic. For a given compound, the number of atoms of one element that combine per atom of the other element is a fixed ratio. A molecule is a discrete particle composed of atoms that are bonded together. A molecule is the smallest particle that has the properties of the compound. Diatomic molecules contain two atoms, while polyatomic molecules contain more than two atoms.


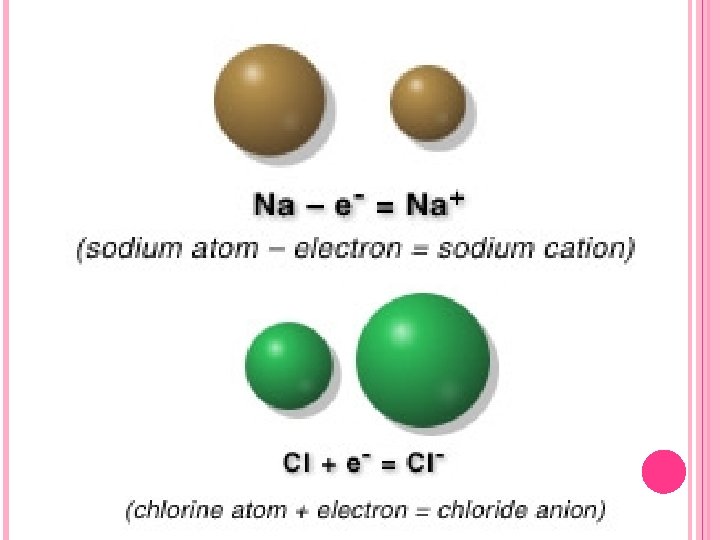
IONS Atoms are electrically neutral because they have equal numbers of protons and electrons. However, an atom can acquire a charge by gaining or losing electrons. These processes do not change the identity of an atom because the atomic number (the number of protons) is unaffected. A change in the number of electrons an atom has will alter its electrical charge. Any atom or group of atoms having a net electrical charge is called an ion. Certain atoms (typically metal elements) always tend to lose electrons (1 or 2, some atoms lose 3 electrons). These form positive ions which are called cations.

IONS Others atoms (typically those of nonmetal elements) tend to gain electrons (1 or 2, some atoms gain 3) forming negative ions called anions. Take for example, the magnesium atom, which has 12 protons in the nucleus and 12 electrons outside the nucleus. It can lose two of its electrons. The result is a particle called the magnesium ion which has 12 protons and 10 electrons. Its net charge is +2 because in the ion the number of positive charges outnumber the negative charges by two. The symbol for the magnesium ion is Mg 2+. The superscript (2+) represents the net charge of the ion. A few elements form ions with 3+ charges. Note that the cation is smaller than the atom.

IONS Negative ions are formed when atoms gain extra electrons. For example, the oxygen atom which has 8 protons and 8 electrons can gain two electrons. The result is an oxygen ion which has 8 protons and 10 electrons. The net charge of the ion is – 2, because there are two more negative charges than positive charges. The symbol for this ion is O 2–. The O 2– ion is called the oxide ion. The names of monatomic anions end in -ide. Note that the anion is larger than the atom.


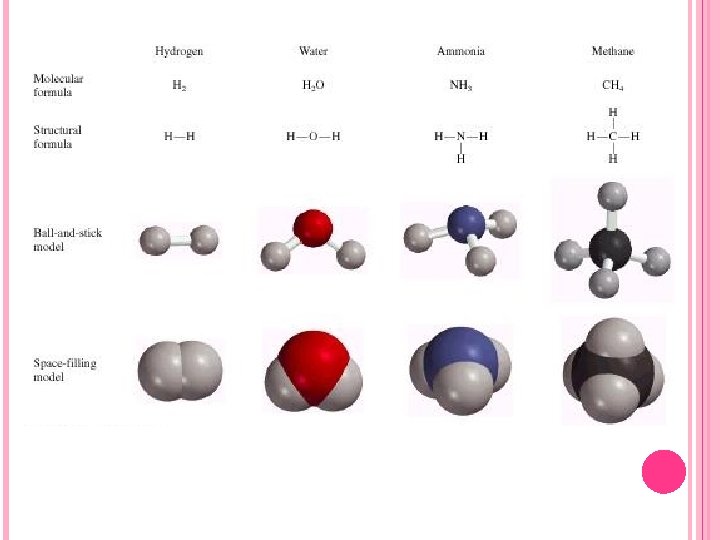
MOLECULAR FORMULAS A molecule of a compound is formed by the combination of atoms of different elements. It is the smallest unit of a compound that has properties of the compound. The molecular formula indicates the numbers of atoms of each element in a molecule of the compound. The formula for ethylene glycol is C 2 H 6 O 2 because there are two carbon atoms, six hydrogen atoms, and two oxygen atoms in a molecule of the compound. All molecules are not necessarily compounds, since some elements exist as molecules. H 2, N 2, O 2, F 2, Cl 2, Br 2, and I 2 are molecular formulas just as CH 4, NH 3, and C 2 H 6 O 2 are molecular formulas. Figure 2. 12 of the text shows some molecular models of several molecular compounds.

EMPIRICAL FORMULAS The empirical formula of a compound gives the simplest whole number ratio of the various kinds of atoms of the elements making up the compound. They are written by reducing the subscripts in molecular formulas to the smallest whole number ratio. Benzene, for example, has the molecular formula C 6 H 6. Dividing 6 by 6 gives the simplest ratio. The empirical formula is just CH, the simplest ratio of C atoms to H atoms being 1 : 1.

EMPIRICAL FORMULAS The empirical formula of many compounds is the same as the molecular formula. For ammonia (NH 3) and methane (CH 4), for example, the ratios of subscripts cannot be reduced and still remain whole numbers. Empirical formulas are the only thing that can be determined using the mass ratios of elements in a compound. More information (such as formula weight) is necessary to determine molecular formulas.

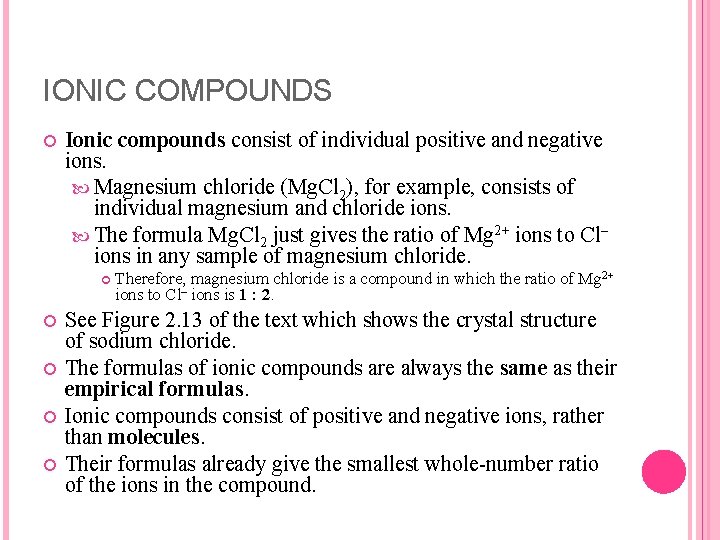
IONIC COMPOUNDS Ionic compounds consist of individual positive and negative ions. Magnesium chloride (Mg. Cl 2), for example, consists of individual magnesium and chloride ions. The formula Mg. Cl 2 just gives the ratio of Mg 2+ ions to Cl– ions in any sample of magnesium chloride. Therefore, magnesium chloride is a compound in which the ratio of Mg 2+ ions to Cl– ions is 1 : 2. See Figure 2. 13 of the text which shows the crystal structure of sodium chloride. The formulas of ionic compounds are always the same as their empirical formulas. Ionic compounds consist of positive and negative ions, rather than molecules. Their formulas already give the smallest whole number ratio of the ions in the compound.

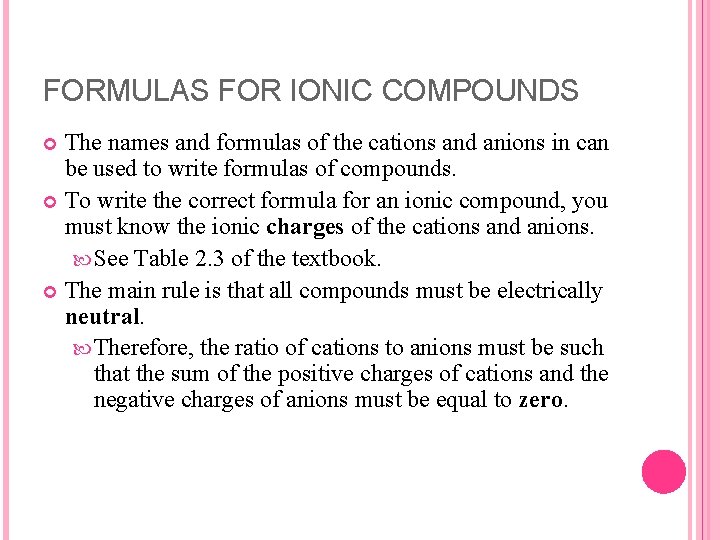
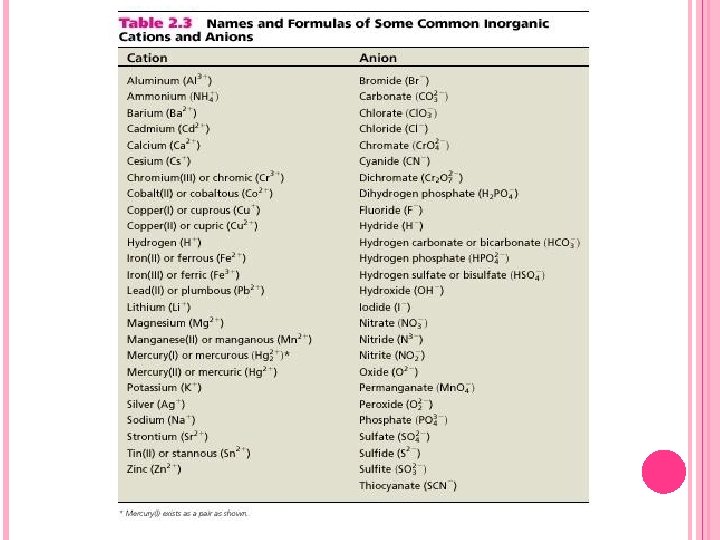
FORMULAS FOR IONIC COMPOUNDS The names and formulas of the cations and anions in can be used to write formulas of compounds. To write the correct formula for an ionic compound, you must know the ionic charges of the cations and anions. See Table 2. 3 of the textbook. The main rule is that all compounds must be electrically neutral. Therefore, the ratio of cations to anions must be such that the sum of the positive charges of cations and the negative charges of anions must be equal to zero.

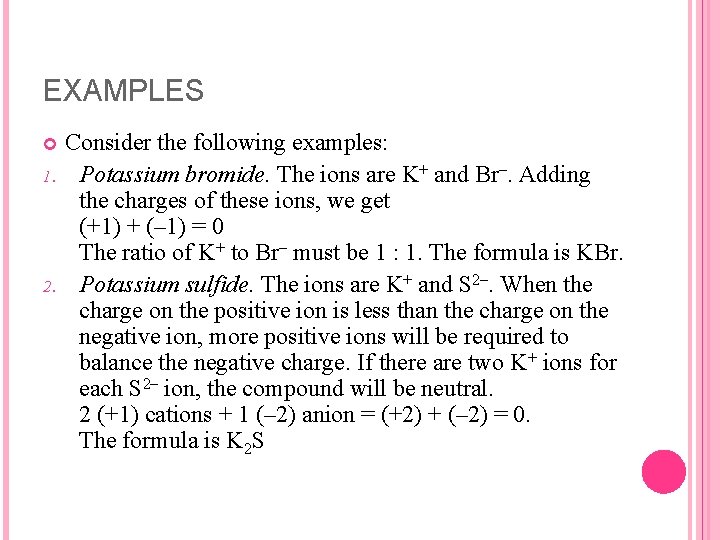
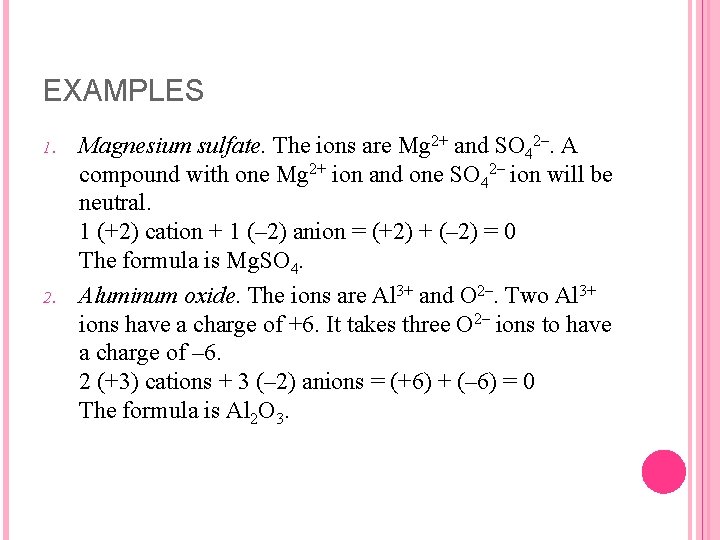
EXAMPLES 1. 2. Consider the following examples: Potassium bromide. The ions are K+ and Br–. Adding the charges of these ions, we get (+1) + (– 1) = 0 The ratio of K+ to Br– must be 1 : 1. The formula is KBr. Potassium sulfide. The ions are K+ and S 2–. When the charge on the positive ion is less than the charge on the negative ion, more positive ions will be required to balance the negative charge. If there are two K+ ions for each S 2– ion, the compound will be neutral. 2 (+1) cations + 1 (– 2) anion = (+2) + (– 2) = 0. The formula is K 2 S

EXAMPLES 1. 2. Magnesium sulfate. The ions are Mg 2+ and SO 42–. A compound with one Mg 2+ ion and one SO 42– ion will be neutral. 1 (+2) cation + 1 (– 2) anion = (+2) + (– 2) = 0 The formula is Mg. SO 4. Aluminum oxide. The ions are Al 3+ and O 2–. Two Al 3+ ions have a charge of +6. It takes three O 2– ions to have a charge of – 6. 2 (+3) cations + 3 (– 2) anions = (+6) + (– 6) = 0 The formula is Al 2 O 3.


CHEMICAL NAMES Nomenclature refers to the naming of substances. Chemical nomenclature and formulas form the basis of the language of chemistry. In the textbook, inorganic compounds are divided into four categories: ionic compounds, molecular compounds, acids and bases, and hydrates. Binary compounds are those with only two elements and are the easiest to name.

CHEMICAL NAMES Learning chemical nomenclature is like learning a new language. First, you build up a vocabulary of words, and then you learn rules for putting words together meaningfully. To learn a vocabulary requires strict memorization, and so treat the names of cations and anions as you would words in a new language. Table 2. 3 in the textbook has 25 cations and 26 anions. If you learn their names and formulas, you will be able to name 650 possible chemical compounds!

NAMING IONIC COMPOUNDS In binary ionic compounds, the metal ion is positively charged and is named first. The name of a simple cation is the same as the name of the element. The negative ion name appears second, and is derived from the name of the nonmetallic element in which an -ide ending replaces the usual ending of the element name. The following formulas and names illustrate naming binary ionic compounds: Na. I _______________ Ca. F 2 _______________ Sn. O _______________

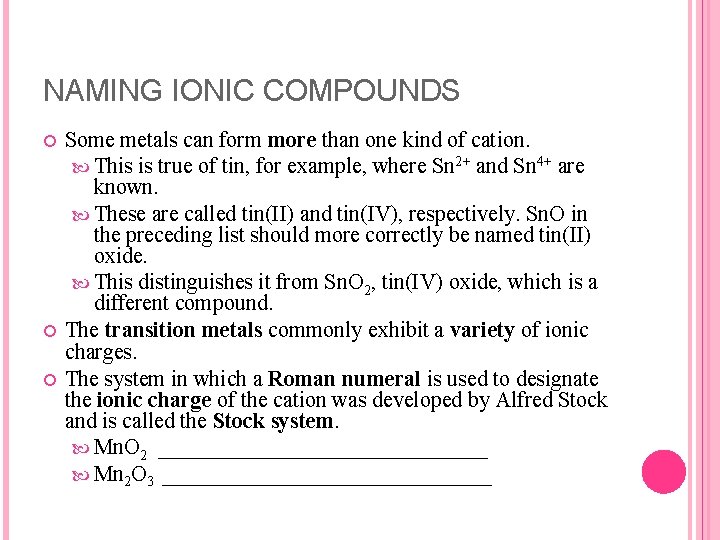
NAMING IONIC COMPOUNDS Some metals can form more than one kind of cation. This is true of tin, for example, where Sn 2+ and Sn 4+ are known. These are called tin(II) and tin(IV), respectively. Sn. O in the preceding list should more correctly be named tin(II) oxide. This distinguishes it from Sn. O 2, tin(IV) oxide, which is a different compound. The transition metals commonly exhibit a variety of ionic charges. The system in which a Roman numeral is used to designate the ionic charge of the cation was developed by Alfred Stock and is called the Stock system. Mn. O 2 _______________ Mn 2 O 3 _______________

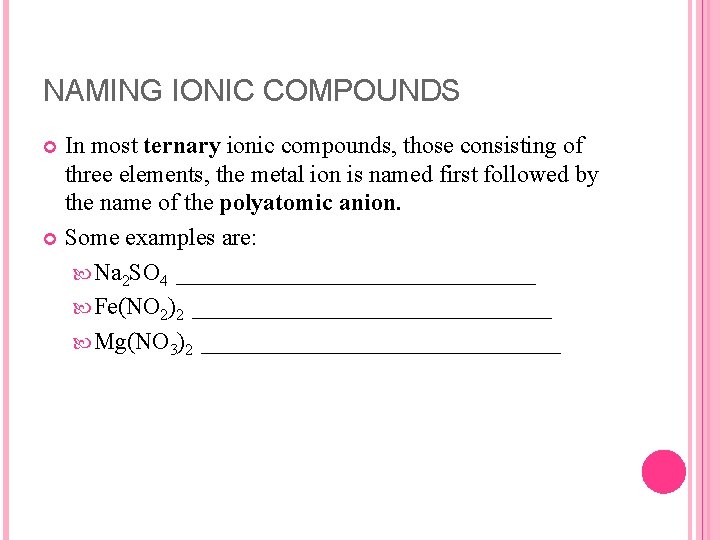
NAMING IONIC COMPOUNDS In most ternary ionic compounds, those consisting of three elements, the metal ion is named first followed by the name of the polyatomic anion. Some examples are: Na 2 SO 4 _______________ Fe(NO 2)2 _______________ Mg(NO 3)2 _______________

NAMING IONIC COMPOUNDS These polyatomic ions are examples of oxoanions. They contain a central atom, such as sulfur or nitrogen, that is bonded to several oxygen atoms. Some elements form several oxoanions that differ only in the number of oxygen atoms. In the case of an element that can have two oxoanions, the name of the anion with more oxygen atoms ends in -ate, and the name of the anion with less oxygen atoms ends in ite. See nitrate and nitrite in the preceding examples. An exception involves the ammonium ion (NH 4+) which is a polyatomic cation. NH 4 Cl _______________


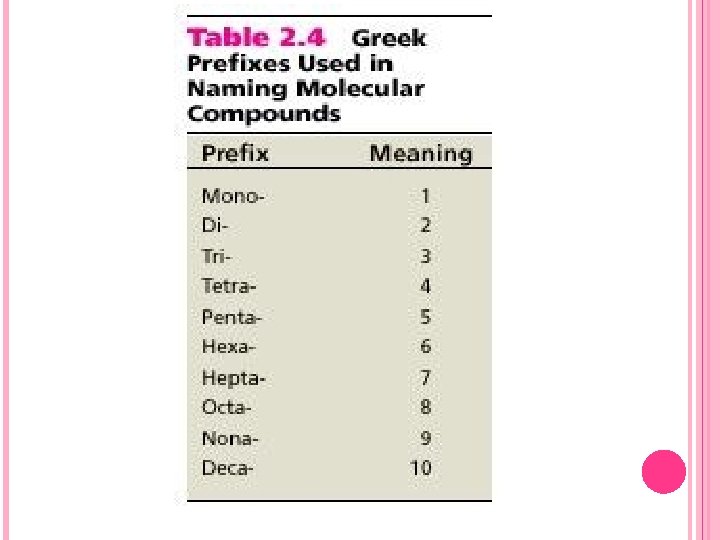
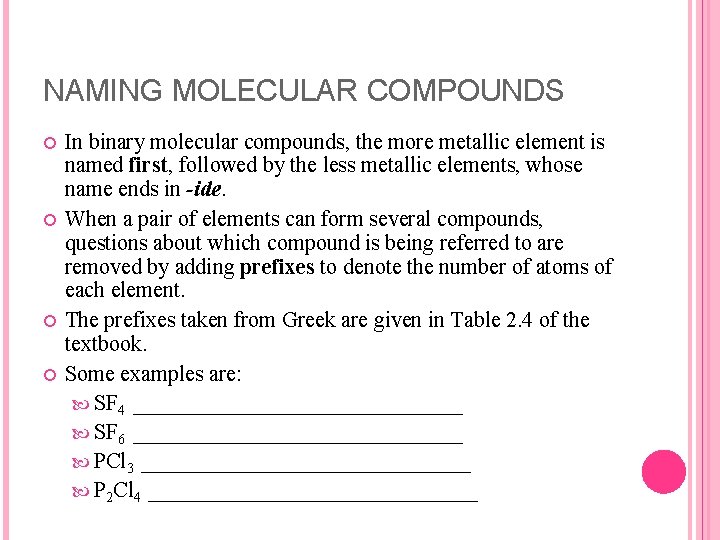
NAMING MOLECULAR COMPOUNDS In binary molecular compounds, the more metallic element is named first, followed by the less metallic elements, whose name ends in -ide. When a pair of elements can form several compounds, questions about which compound is being referred to are removed by adding prefixes to denote the number of atoms of each element. The prefixes taken from Greek are given in Table 2. 4 of the textbook. Some examples are: SF 4 _______________ SF 6 _______________ PCl 3 _______________ P 2 Cl 4 _______________

NAMING ACIDS AND BASES Binary acids are compounds of hydrogen and a nonmetal. Many hydrogen compounds have completely different properties when they are in the pure gaseous or liquid state than when they are dissolved in water. In the gaseous state, HCl and H 2 S, for instance, are molecular compounds called hydrogen chloride and hydrogen sulfide, respectively, whereas water solutions of these compounds contain hydrogen ions (H+) and anions and have slightly different names. Acids are substances that release H+ ions when they dissolve in water. In naming binary acids, the word hydrogen is replaced by hydro-, and the ide ending of the anion name is replaced with -ic acid. For example: HCl (g) _______________ HCl (aq) _______________ H 2 S (g) _______________ H 2 S (aq) _______________

NAMING ACIDS The oxoacids are compounds containing hydrogen and oxoanions. The -ate ending of the anion name gets changed to -ic acid. For oxoanions with fewer oxygen atoms, the -ite ending of the anion name is changed to -ous acid for the oxyacid. The acids of nitrate and sulfate are: HNO 3 _______________ H 2 SO 4 _______________ HNO 2 _______________ H 2 SO 3 _______________

NAMING BASES Bases are substances that release hydroxide ions (OH–) in aqueous solution. The metal hydroxides are named as if they were binary compounds and employ the -ide ending. Li. OH _______________ Ca(OH)2 _______________