Chapter 2 Atoms Molecules and Ions AP Chemistry



















- Slides: 29

Chapter 2 Atoms, Molecules and Ions AP Chemistry Erik Ardieta 1

Law of Conservation of Mass Ø Discovered by Antoine Lavoisier ØThe father of modern chemistry Ø Mass is neither created nor destroyed Ø Combustion involves oxygen AP Chemistry Erik Ardieta 2

Other Fundamental Chemical Laws Law of Definite Proportion Ø A given compound always contains exactly the same proportion of elements by mass. Ø Carbon tetrachloride is always 1 atom carbon per 4 atoms chlorine. AP Chemistry Erik Ardieta 3

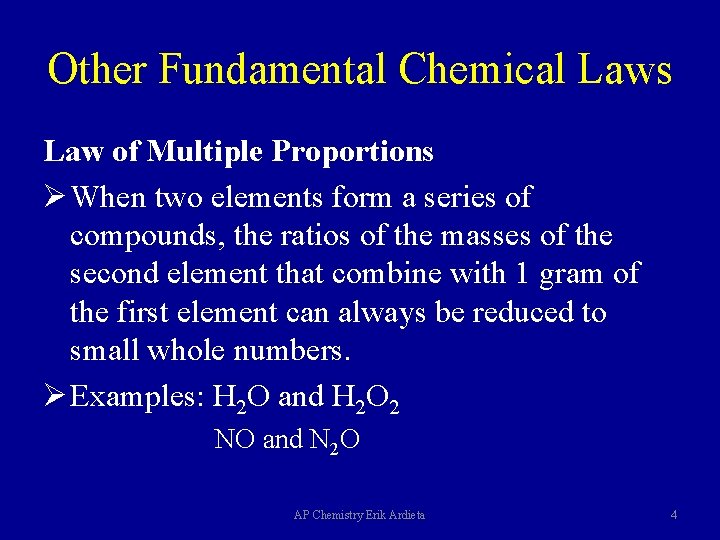
Other Fundamental Chemical Laws Law of Multiple Proportions Ø When two elements form a series of compounds, the ratios of the masses of the second element that combine with 1 gram of the first element can always be reduced to small whole numbers. Ø Examples: H 2 O and H 2 O 2 NO and N 2 O AP Chemistry Erik Ardieta 4

Dalton’s Atomic Theory (1808) 1. Each element is made up of tiny particles called atoms. 2. Atoms of each element are identical. Atoms of different elements are different. 3. Compounds are formed when atoms combine. Each compound has a specific number and kinds of atoms. 4. Chemical reactions are rearrangement of atoms. Atoms are not created or destroyed. AP Chemistry Erik Ardieta 5

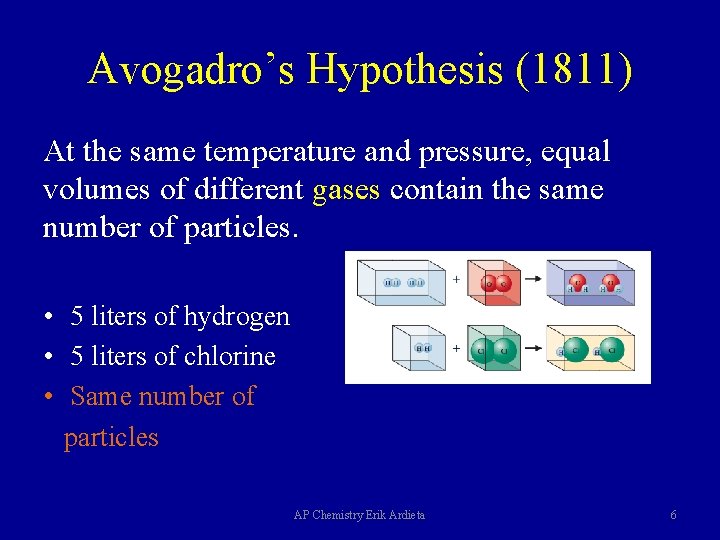
Avogadro’s Hypothesis (1811) At the same temperature and pressure, equal volumes of different gases contain the same number of particles. • 5 liters of hydrogen • 5 liters of chlorine • Same number of particles AP Chemistry Erik Ardieta 6

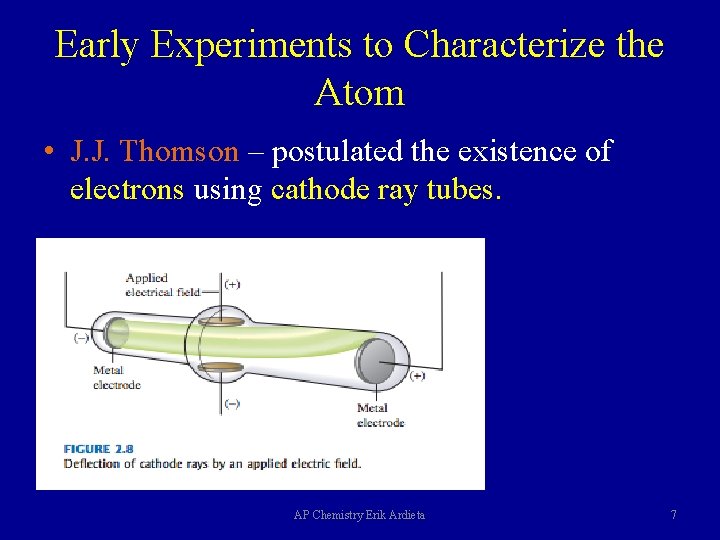
Early Experiments to Characterize the Atom • J. J. Thomson – postulated the existence of electrons using cathode ray tubes. AP Chemistry Erik Ardieta 7

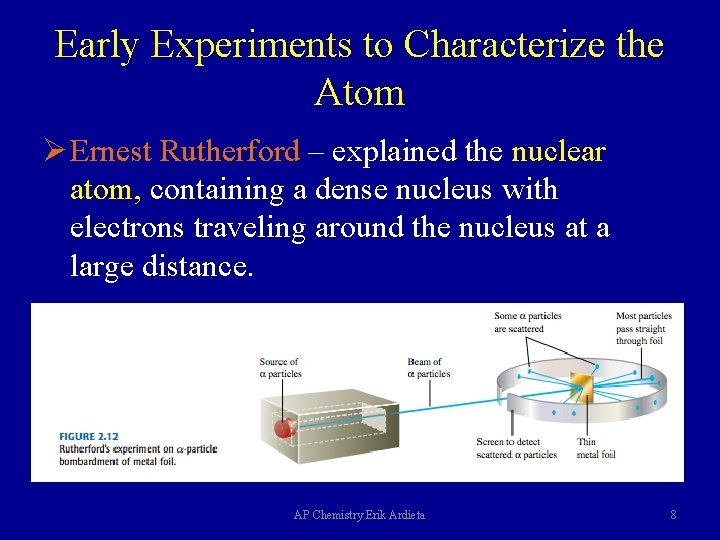
Early Experiments to Characterize the Atom Ø Ernest Rutherford – explained the nuclear atom, containing a dense nucleus with electrons traveling around the nucleus at a large distance. AP Chemistry Erik Ardieta 8

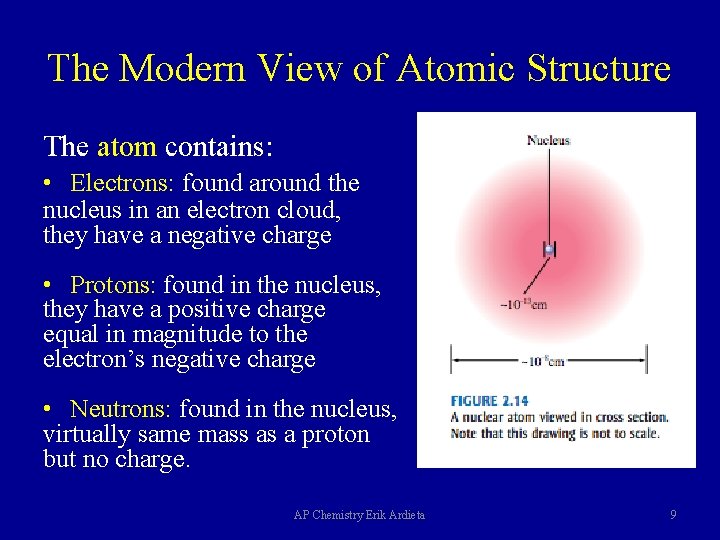
The Modern View of Atomic Structure The atom contains: • Electrons: found around the nucleus in an electron cloud, they have a negative charge • Protons: found in the nucleus, they have a positive charge equal in magnitude to the electron’s negative charge • Neutrons: found in the nucleus, virtually same mass as a proton but no charge. AP Chemistry Erik Ardieta 9

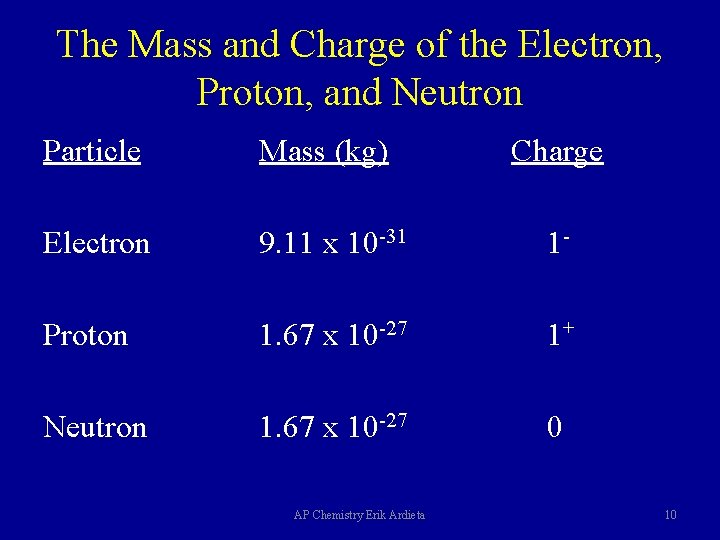
The Mass and Charge of the Electron, Proton, and Neutron Particle Mass (kg) Electron 9. 11 x 10 -31 1 - Proton 1. 67 x 10 -27 1+ Neutron 1. 67 x 10 -27 0 AP Chemistry Erik Ardieta Charge 10

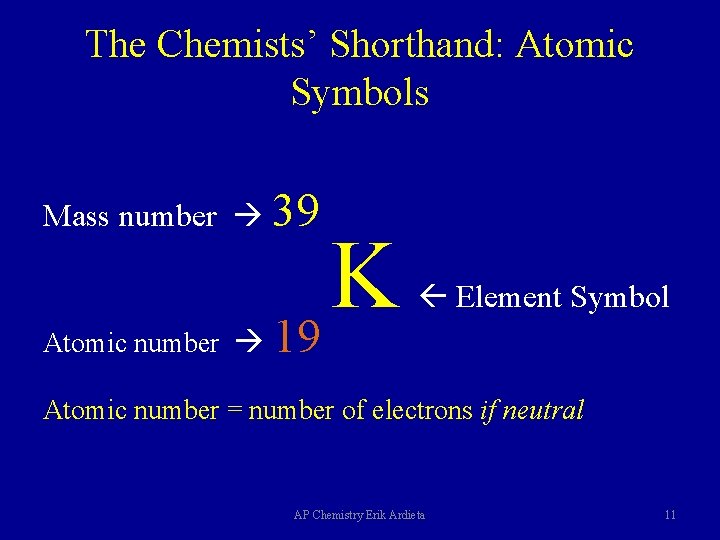
The Chemists’ Shorthand: Atomic Symbols Mass number 39 Atomic number K 19 Element Symbol Atomic number = number of electrons if neutral AP Chemistry Erik Ardieta 11

Chemical Bonds • The forces that hold atoms together in compounds. Covalent bonds result from atoms sharing electrons. • Molecule: a collection of covalently-bonded atoms. AP Chemistry Erik Ardieta 12

The Chemists’ Shorthand Formulas • Chemical Formula: • Symbols = types of atoms • Subscripts = relative numbers of atoms CO 2 • Structural Formula: • Individual bonds are shown by lines O=C=O AP Chemistry Erik Ardieta 13

Ions Cation: A positive ion Mg 2+, NH 4+ Anion: A negative ion Cl-, SO 42 Ionic Bonding: Force of attraction between oppositely charged ions. AP Chemistry Erik Ardieta 14

Periodic Table Elements classified by: Ø Properties Ø Atomic number Groups/Families (vertical) 1 A = alkali metals 2 A = alkaline earth metals 7 A = halogens 8 A = noble gases Periods (horizontal) AP Chemistry Erik Ardieta 15

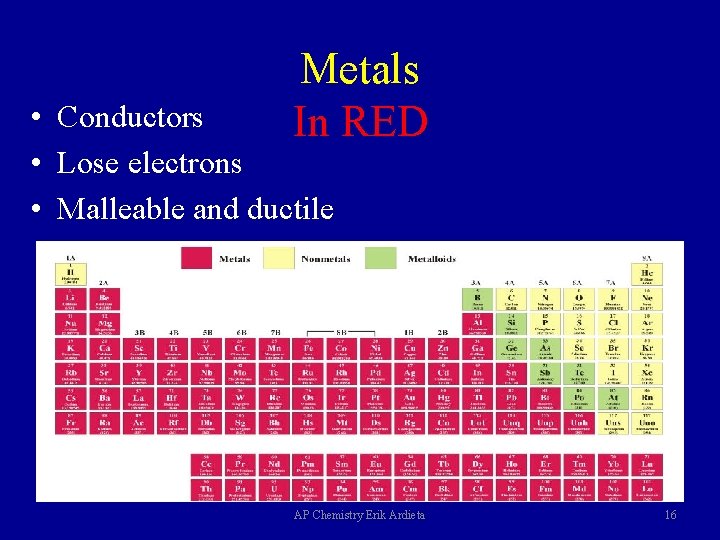
Metals In RED • Conductors • Lose electrons • Malleable and ductile AP Chemistry Erik Ardieta 16

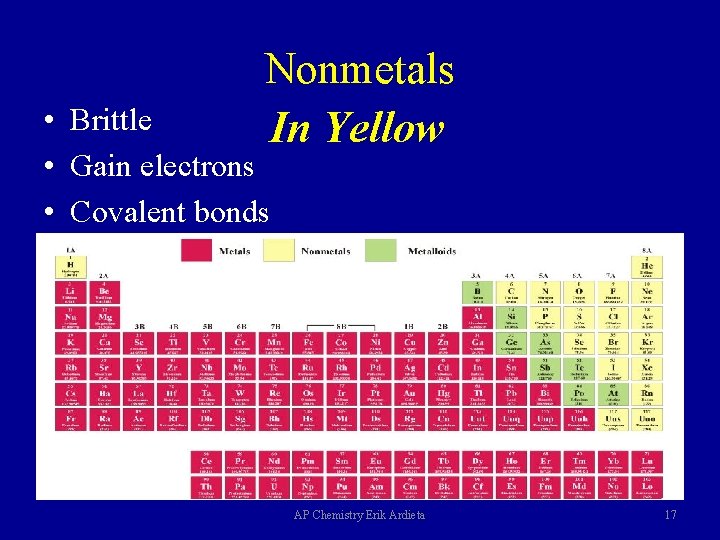
Nonmetals In Yellow • Brittle • Gain electrons • Covalent bonds AP Chemistry Erik Ardieta 17

Semi-metals or Metalloids In GREEN AP Chemistry Erik Ardieta 18

Naming Compounds Binary Ionic Compounds: 1. Cation first, then anion 2. Monatomic cation = name of the element Ca 2+ = calcium ion 3. Monatomic anion = root + -ide Cl- = chloride Ca. Cl 2 = calcium chloride AP Chemistry Erik Ardieta 19

Naming Compounds (continued) Binary Ionic Compounds (Type II): • Metal forms more than one cation (iron, lead, copper, cobalt, tin, chromium…) • Use Roman numeral in name Pb. Cl 2 Pb 2+ is cation Pb. Cl 2 = lead (II) chloride AP Chemistry Erik Ardieta 20

Naming Compounds (continued) Binary Covalent compounds (Type III): • Compounds between two nonmetals • First element in the formula is named first. • Second element is named as if it were an anion. • Use prefixes (mono-, di-, tri-, tetra-, pent-…) • Only use mono- for the second element P 2 O 5 = diphosphorus pentoxide AP Chemistry Erik Ardieta 21

Practice Ca. S N 2 O 4 Xe. F 6 Al. PO 4 P 2 O 10 Mn. O CCl 4 Mg. O K 2 S Fe(C 2 O 4) N 4 O 4 Cr(Cl. O)6 AP Chemistry Erik Ardieta 22

Practice 1. 2. 3. 4. 5. 6. Krypton hexafluoride Cobalt (III) iodide Ammonium nitrate Iron (II) sulfide Oxygen trichloride Difluorine monoxide 7. Nitrogen dioxide 8. Potassium nitrite 9. Tin (IV) nitride 10. Potassium sulfate 11. Barium chromate 12. Strontium oxide AP Chemistry Erik Ardieta 23

Polyatomic Ions Memorize these: Acetate Hypochlorite Chlorate Perchlorate Bicarbonate Nitrite Nitrate Hydroxide Carbonate Chromate Dichromate Sulfite Sulfate AP Chemistry Erik Ardieta Phosphite Phosphate Ammonium 24

Acidic Compounds • Substances that produce H+ ions when dissolved in water. • All acids begin with H. • Two types of acids: – Oxyacids – Non-oxyacids (binary acids) AP Chemistry Erik Ardieta 25

Naming Oxyacids • Write the name of the anion, but change – ate to –ic acid – ite to –ous acid • • Watch out for sulfuric and sulfurous H 2 Cr. O 4 HCl. O 4 HNO 2 AP Chemistry Erik Ardieta 26

Naming Binary Acids • • • If the acid doesn’t have oxygen Add the prefix hydro. Change the suffix –ide to –ic acid HCl H 2 S HCN AP Chemistry Erik Ardieta 27

Acid Formula Writing • • • Hydrofluoric acid Dichromic acid Carbonic acid Hydrophosphoric acid Perchloric acid Phosphorous acid AP Chemistry Erik Ardieta 28

AP Chemistry Erik Ardieta 29