Chapter 2 Atoms Molecules and Ions Chemistry 1411






- Slides: 39

Chapter 2: Atoms, Molecules, and Ions Chemistry 1411 Joanna Sabey 1

Dalton’s Atomic Theory � � Elements are composed of extremely small particles called atoms. All atoms of a given element are identical, having the same size, mass and chemical properties. The atoms of one element are different from the atoms of all other elements. Compounds are composed of atoms of more than one element. In any compound, the ratio of the numbers of atoms of any two of the elements present is either an integer or a simple fraction. A chemical reaction involves only the separation, combination, or rearrangement of atoms; it does not result in their creation or destruction. 2

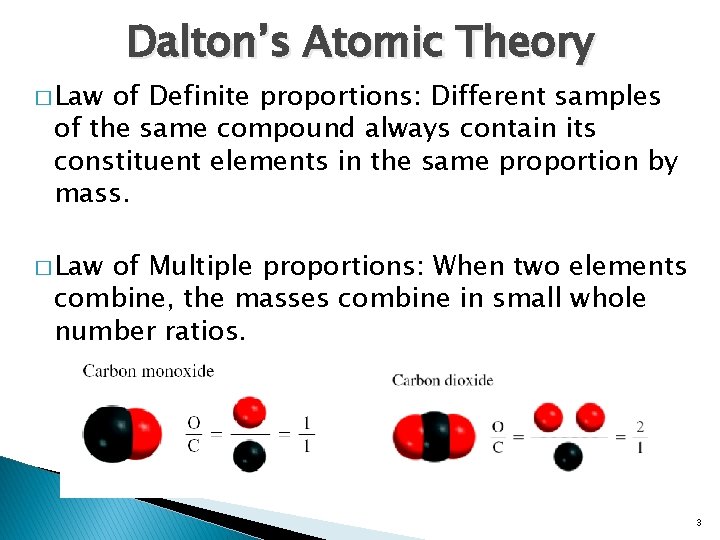
� Law Dalton’s Atomic Theory of Definite proportions: Different samples of the same compound always contain its constituent elements in the same proportion by mass. � Law of Multiple proportions: When two elements combine, the masses combine in small whole number ratios. 3

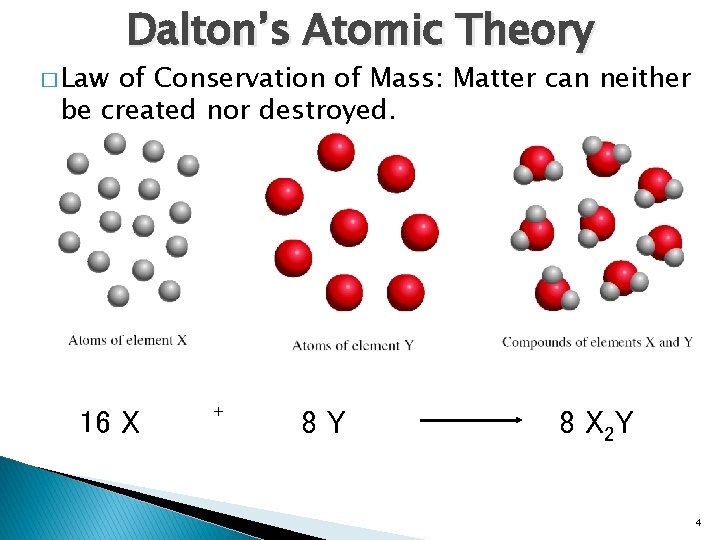
� Law Dalton’s Atomic Theory of Conservation of Mass: Matter can neither be created nor destroyed. 16 X + 8 Y 8 X 2 Y 4

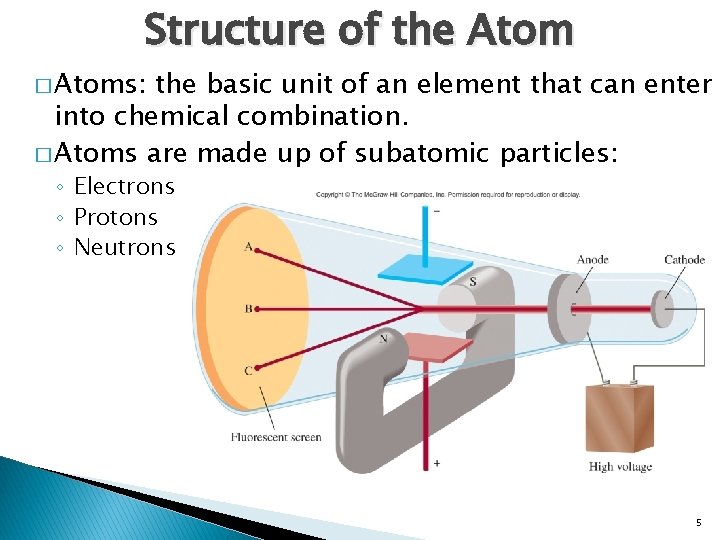
Structure of the Atom � Atoms: the basic unit of an element that can enter into chemical combination. � Atoms are made up of subatomic particles: ◦ Electrons ◦ Protons ◦ Neutrons 5

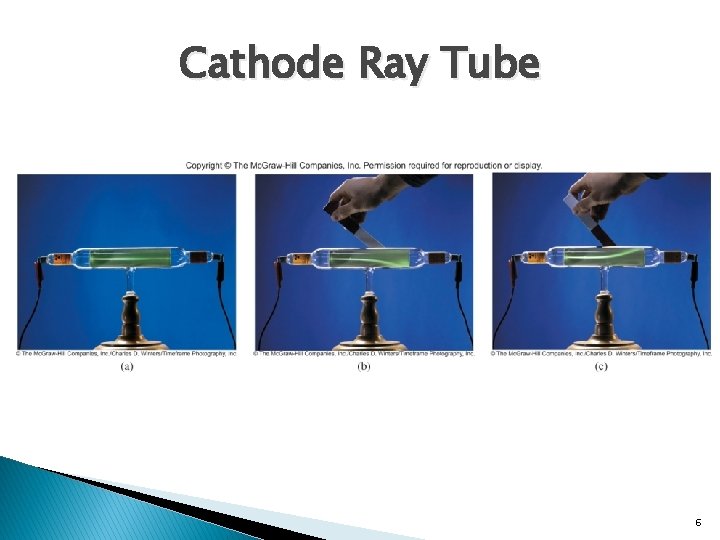
Cathode Ray Tube 6

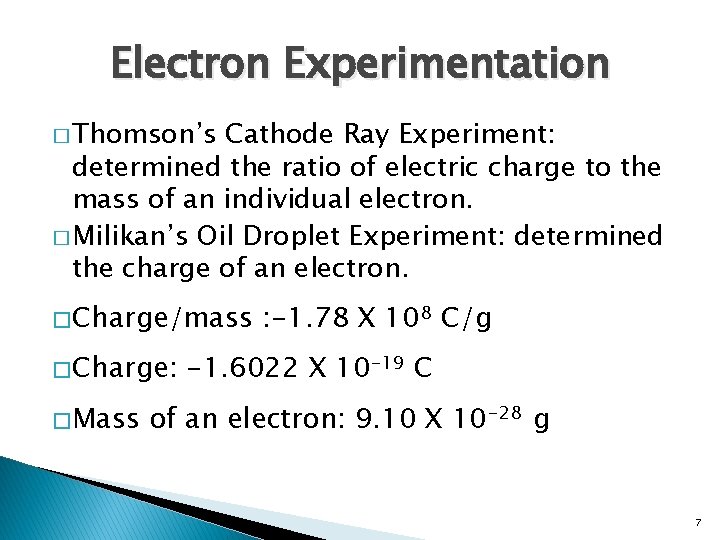
Electron Experimentation � Thomson’s Cathode Ray Experiment: determined the ratio of electric charge to the mass of an individual electron. � Milikan’s Oil Droplet Experiment: determined the charge of an electron. � Charge/mass � Charge: � Mass : -1. 78 X 108 C/g -1. 6022 X 10 -19 C of an electron: 9. 10 X 10 -28 g 7

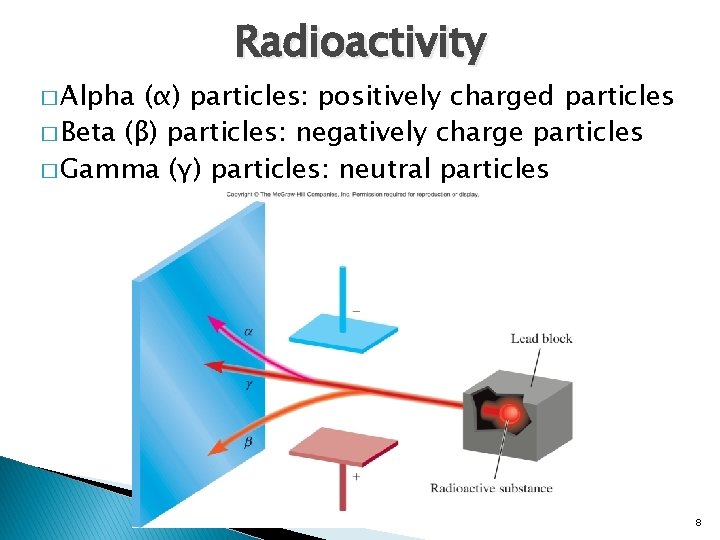
� Alpha Radioactivity (α) particles: positively charged particles � Beta (β) particles: negatively charge particles � Gamma (γ) particles: neutral particles 8

Thomson’s model 9

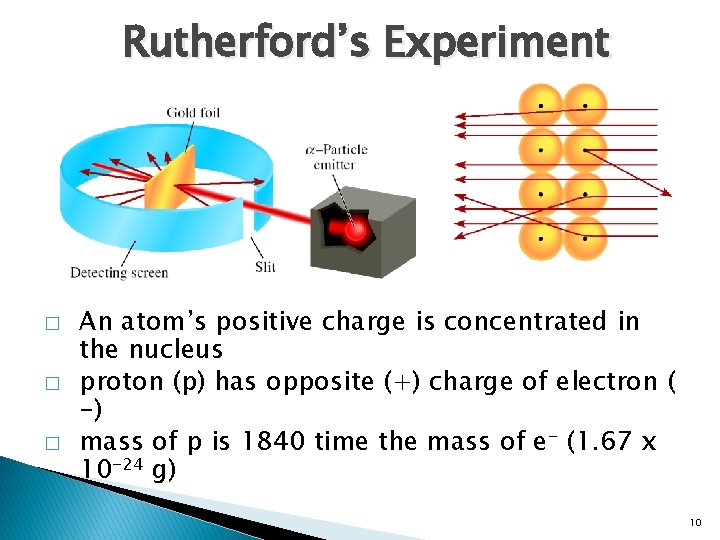
Rutherford’s Experiment � � � An atom’s positive charge is concentrated in the nucleus proton (p) has opposite (+) charge of electron ( -) mass of p is 1840 time the mass of e- (1. 67 x 10 -24 g) 10

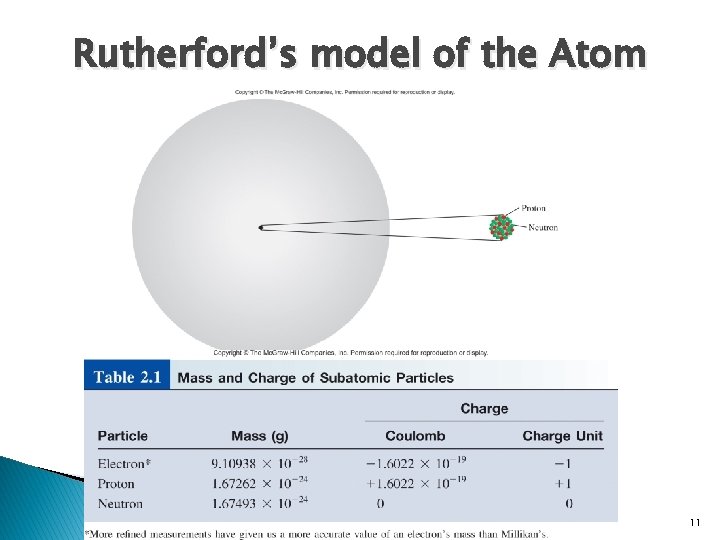
Rutherford’s model of the Atom 11

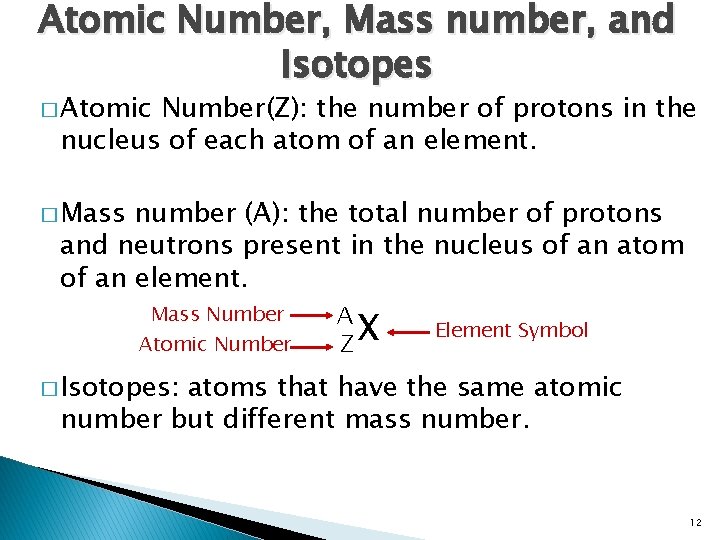
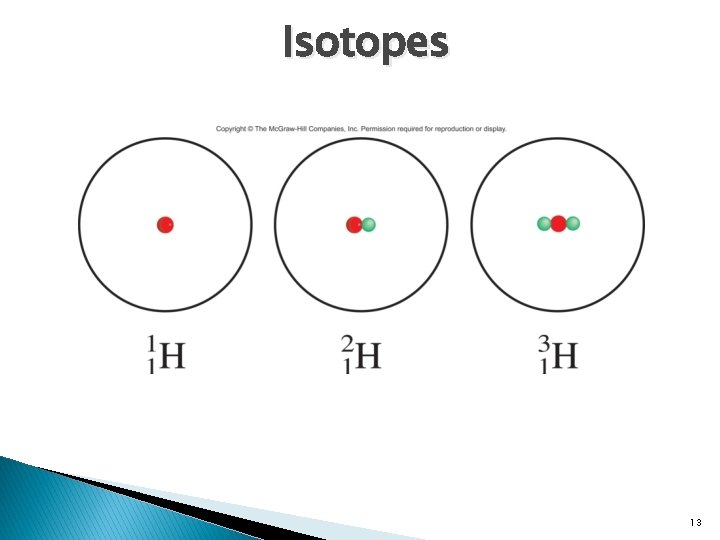
Atomic Number, Mass number, and Isotopes � Atomic Number(Z): the number of protons in the nucleus of each atom of an element. � Mass number (A): the total number of protons and neutrons present in the nucleus of an atom of an element. Mass Number Atomic Number A X Z Element Symbol � Isotopes: atoms that have the same atomic number but different mass number. 12

Isotopes 13

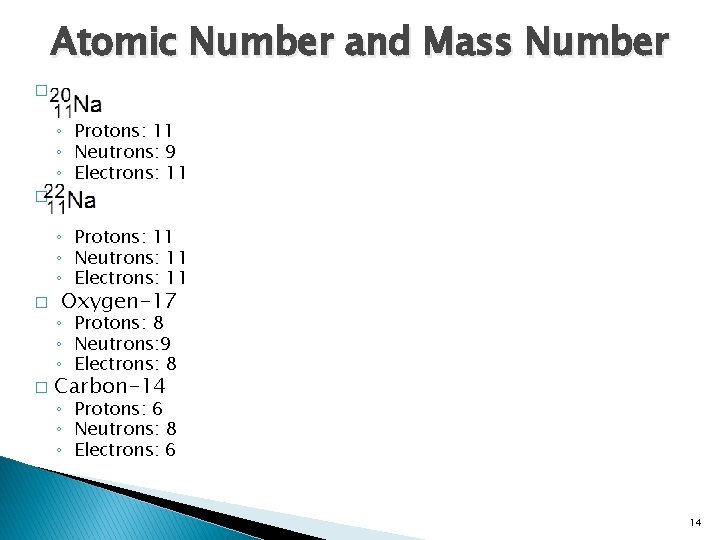
Atomic Number and Mass Number � ◦ Protons: 11 ◦ Neutrons: 9 ◦ Electrons: 11 � ◦ Protons: 11 ◦ Neutrons: 11 ◦ Electrons: 11 � � Oxygen-17 ◦ Protons: 8 ◦ Neutrons: 9 ◦ Electrons: 8 Carbon-14 ◦ Protons: 6 ◦ Neutrons: 8 ◦ Electrons: 6 14

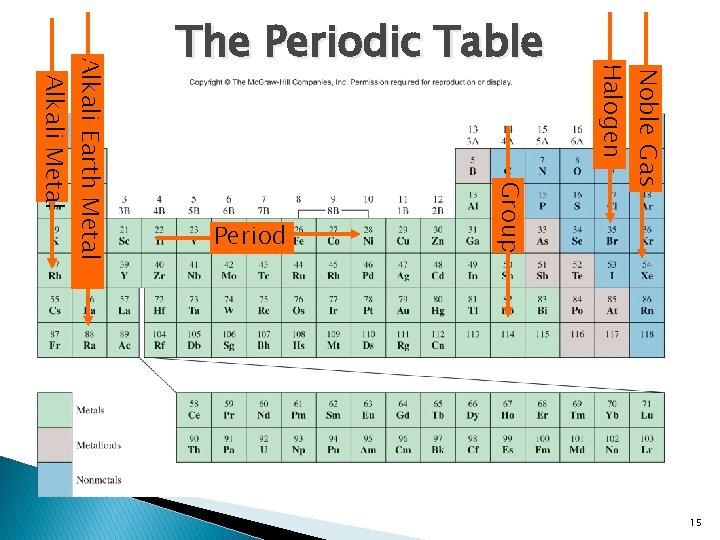
Noble Gas Halogen Group Period Alkali Earth Metal Alkali Metal The Periodic Table 15

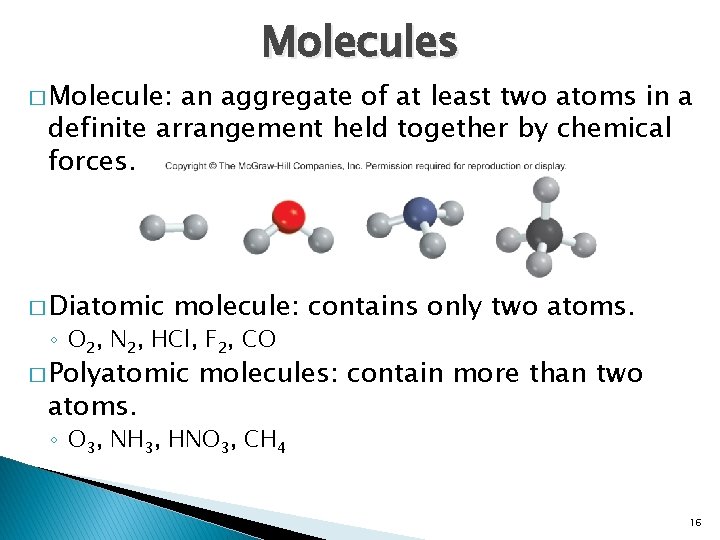
Molecules � Molecule: an aggregate of at least two atoms in a definite arrangement held together by chemical forces. � Diatomic molecule: contains only two atoms. ◦ O 2, N 2, HCl, F 2, CO � Polyatomic atoms. molecules: contain more than two ◦ O 3, NH 3, HNO 3, CH 4 16

� Ion: Ions an atom or group of atoms that has a net positive or net negative charge. � Cation: An ion with a positive charge. � Anion: An ion with a negative charge. ◦ If a neutral atom loses one or more electrons it becomes a cation, metals. ◦ Na Na+ ◦ If a neutral atom gains one or more electrons it becomes an anion, nonmetals. ◦ Cl 17

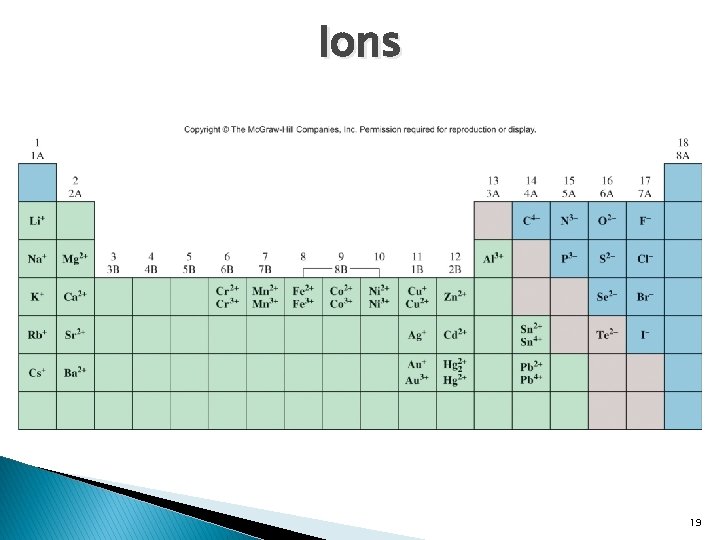
Ions � Monatomic ions: ions that contain only one atom. Cation or anion. ◦ Mg 2+, Fe 3+, S 2 -, N 3 - � Polyatomic one atom. ions: ions that contain more than ◦ OH- , CN- , NH 4+ 18

Ions 19

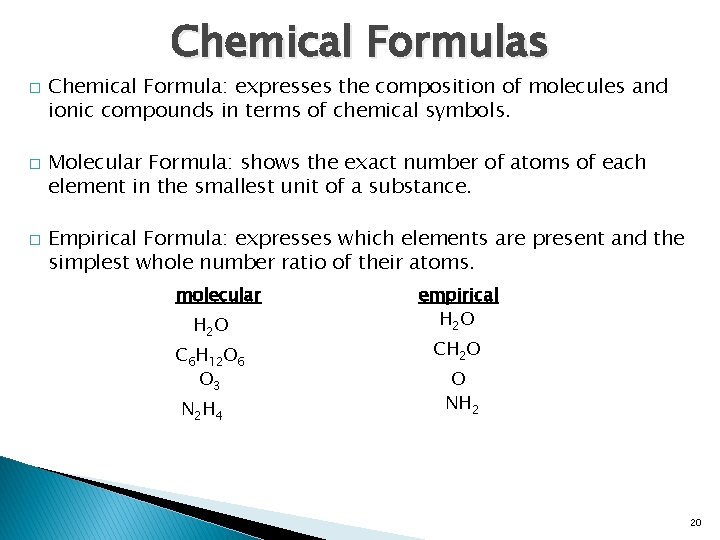
Chemical Formulas � � � Chemical Formula: expresses the composition of molecules and ionic compounds in terms of chemical symbols. Molecular Formula: shows the exact number of atoms of each element in the smallest unit of a substance. Empirical Formula: expresses which elements are present and the simplest whole number ratio of their atoms. molecular H 2 O C 6 H 12 O 6 O 3 N 2 H 4 empirical H 2 O CH 2 O O NH 2 20

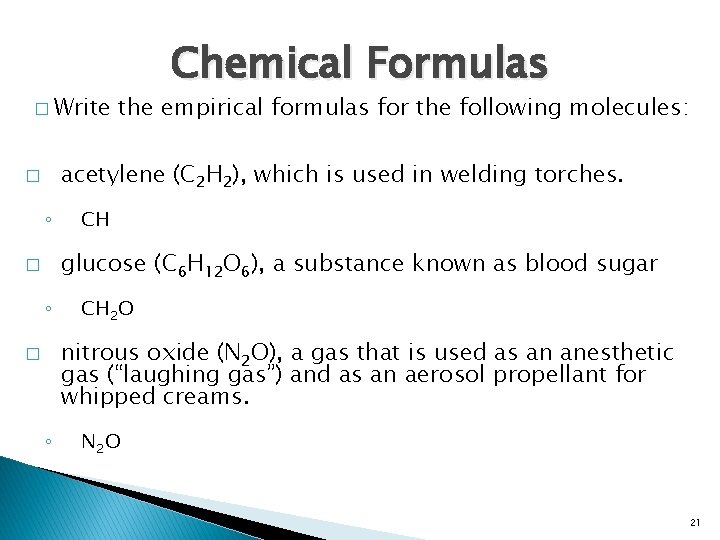
� Write Chemical Formulas the empirical formulas for the following molecules: acetylene (C 2 H 2), which is used in welding torches. � ◦ CH glucose (C 6 H 12 O 6), a substance known as blood sugar � ◦ CH 2 O nitrous oxide (N 2 O), a gas that is used as an anesthetic gas (“laughing gas”) and as an aerosol propellant for whipped creams. � ◦ N 2 O 21

� Ionic Compounds: consist of a combination of cations and anions. Metal combined to a nonmetal. � The sum of the charges on the cation(s) and anion(s) in each formula unit must equal zero. 22

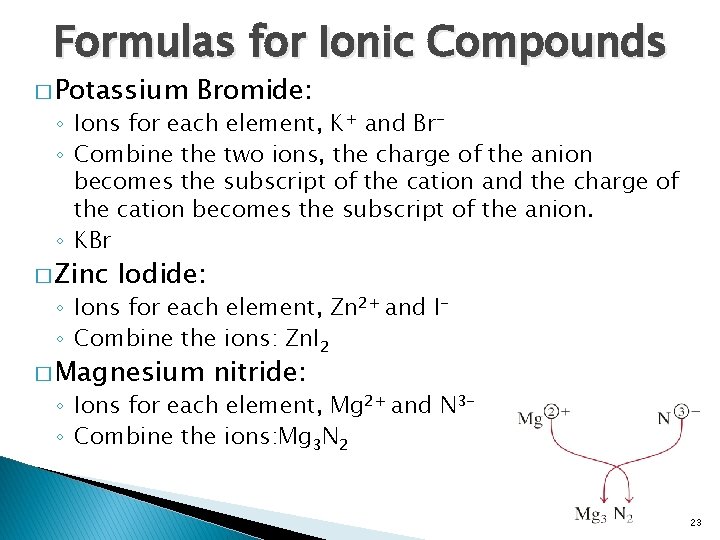
Formulas for Ionic Compounds � Potassium Bromide: ◦ Ions for each element, K+ and Br◦ Combine the two ions, the charge of the anion becomes the subscript of the cation and the charge of the cation becomes the subscript of the anion. ◦ KBr � Zinc Iodide: ◦ Ions for each element, Zn 2+ and I◦ Combine the ions: Zn. I 2 � Magnesium nitride: ◦ Ions for each element, Mg 2+ and N 3◦ Combine the ions: Mg 3 N 2 23

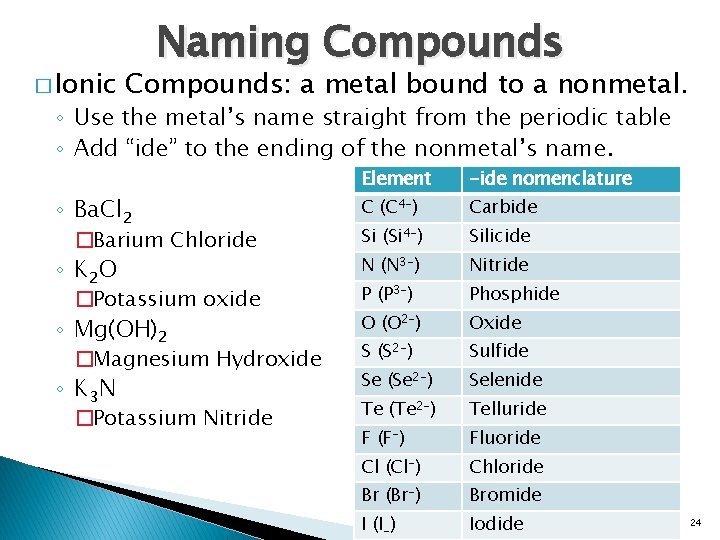
� Ionic Naming Compounds: a metal bound to a nonmetal. ◦ Use the metal’s name straight from the periodic table ◦ Add “ide” to the ending of the nonmetal’s name. Element -ide nomenclature C (C 4 -) Carbide �Barium Chloride Si (Si 4 -) Silicide ◦ K 2 O N (N 3 -) Nitride �Potassium oxide P (P 3 -) Phosphide ◦ Mg(OH)2 O (O 2 -) Oxide �Magnesium Hydroxide S (S 2 -) Sulfide ◦ K 3 N Se (Se 2 -) Selenide �Potassium Nitride Te (Te 2 -) Telluride F (F-) Fluoride Cl (Cl-) Chloride Br (Br-) Bromide I (I-) Iodide ◦ Ba. Cl 2 24

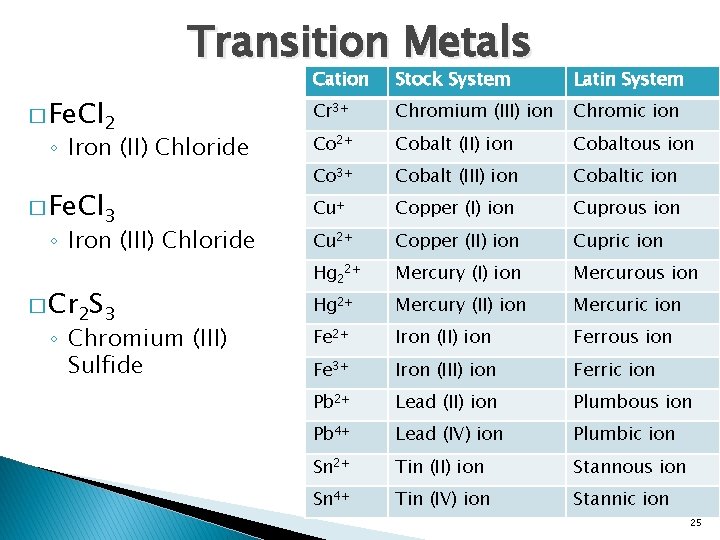
Transition Metals � Fe. Cl 2 ◦ Iron (II) Chloride � Fe. Cl 3 ◦ Iron (III) Chloride � Cr 2 S 3 ◦ Chromium (III) Sulfide Cation Stock System Latin System Cr 3+ Chromium (III) ion Chromic ion Co 2+ Cobalt (II) ion Cobaltous ion Co 3+ Cobalt (III) ion Cobaltic ion Cu+ Copper (I) ion Cuprous ion Cu 2+ Copper (II) ion Cupric ion Hg 22+ Mercury (I) ion Mercurous ion Hg 2+ Mercury (II) ion Mercuric ion Fe 2+ Iron (II) ion Ferrous ion Fe 3+ Iron (III) ion Ferric ion Pb 2+ Lead (II) ion Plumbous ion Pb 4+ Lead (IV) ion Plumbic ion Sn 2+ Tin (II) ion Stannous ion Sn 4+ Tin (IV) ion Stannic ion 25

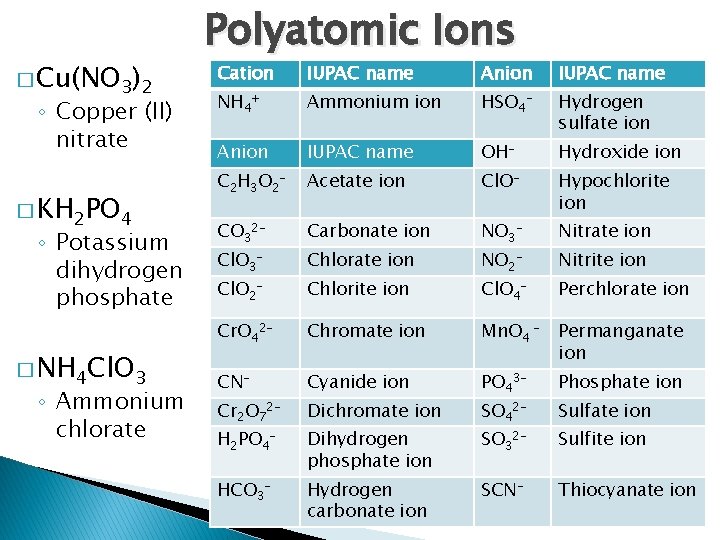
� Cu(NO 3)2 ◦ Copper (II) nitrate � KH 2 PO 4 ◦ Potassium dihydrogen phosphate � NH 4 Cl. O 3 ◦ Ammonium chlorate Polyatomic Ions Cation IUPAC name Anion IUPAC name NH 4+ Ammonium ion HSO 4 - Hydrogen sulfate ion Anion IUPAC name OH- Hydroxide ion C 2 H 3 O 2 - Acetate ion Cl. O- Hypochlorite ion CO 32 - Carbonate ion NO 3 - Nitrate ion Cl. O 3 - Chlorate ion NO 2 - Nitrite ion Cl. O 2 - Chlorite ion Cl. O 4 - Perchlorate ion Cr. O 42 - Chromate ion Mn. O 4 - Permanganate ion CN- Cyanide ion Phosphate ion Cr 2 O 72 - Dichromate ion PO 43 - H 2 PO 4 - Dihydrogen phosphate ion HCO 3 - Hydrogen carbonate ion SO 42 - Sulfate ion SO 32 - Sulfite ion SCN- Thiocyanate ion 26

Ionic Compounds � Write the chemical formula for the following compounds: � Mercury (II) nitrite ◦ Hg(NO 2)2 � Potassium ◦ KF � Cesium ◦ Cs 2 S � Calcium fluoride sulfide phosphate ◦ Ca 3(PO 4)2 � Iron (II) acetate ◦ Fe(C 2 H 3 O 2)2 27

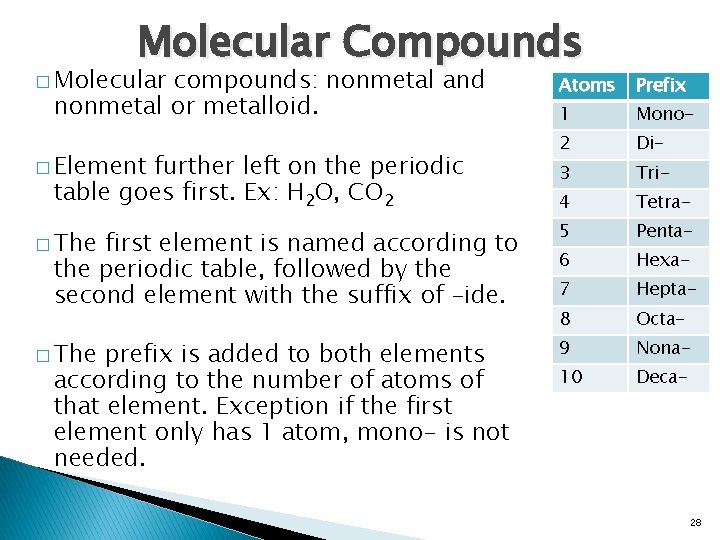
Molecular Compounds � Molecular compounds: nonmetal and nonmetal or metalloid. � Element further left on the periodic table goes first. Ex: H 2 O, CO 2 � The first element is named according to the periodic table, followed by the second element with the suffix of –ide. � The prefix is added to both elements according to the number of atoms of that element. Exception if the first element only has 1 atom, mono- is not needed. Atoms Prefix 1 Mono- 2 Di- 3 Tri- 4 Tetra- 5 Penta- 6 Hexa- 7 Hepta- 8 Octa- 9 Nona- 10 Deca- 28

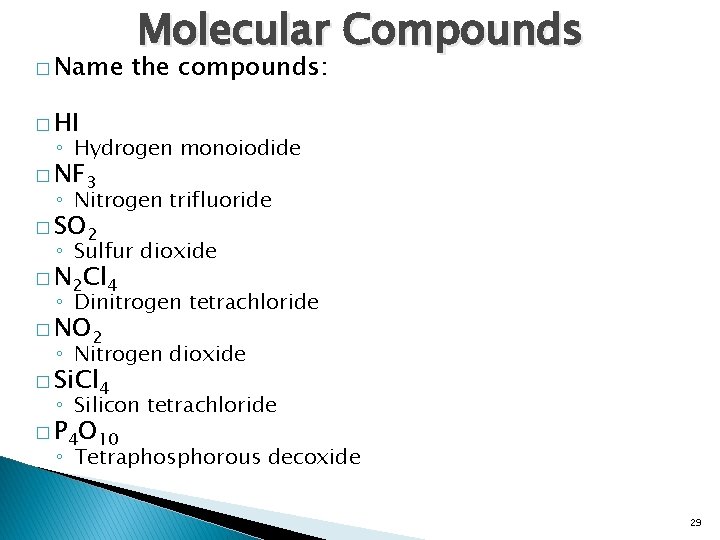
� Name Molecular Compounds the compounds: � HI ◦ Hydrogen monoiodide � NF 3 ◦ Nitrogen trifluoride � SO 2 ◦ Sulfur dioxide � N 2 Cl 4 ◦ Dinitrogen tetrachloride � NO 2 ◦ Nitrogen dioxide � Si. Cl 4 ◦ Silicon tetrachloride � P 4 O 10 ◦ Tetraphosphorous decoxide 29

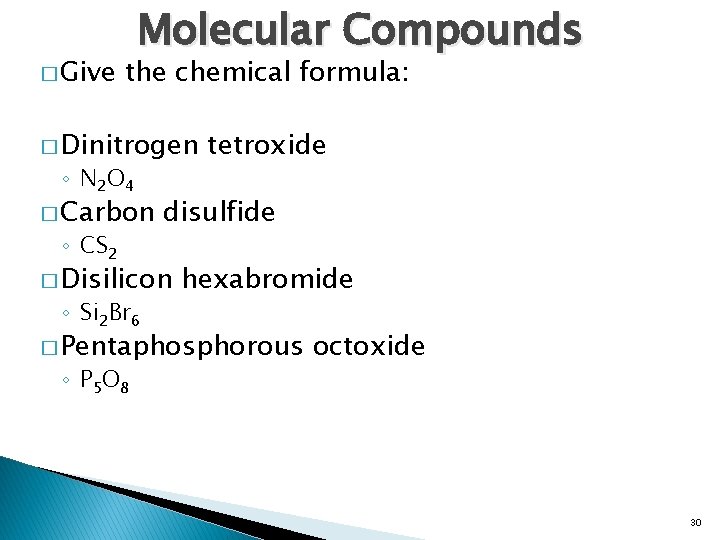
� Give Molecular Compounds the chemical formula: � Dinitrogen ◦ N 2 O 4 � Carbon ◦ CS 2 disulfide � Disilicon ◦ Si 2 Br 6 tetroxide hexabromide � Pentaphosphorous ◦ P 5 O 8 octoxide 30

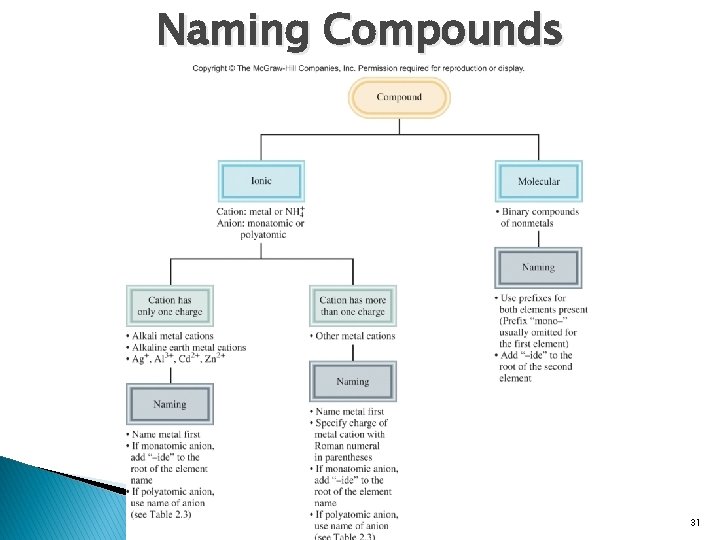
Naming Compounds 31

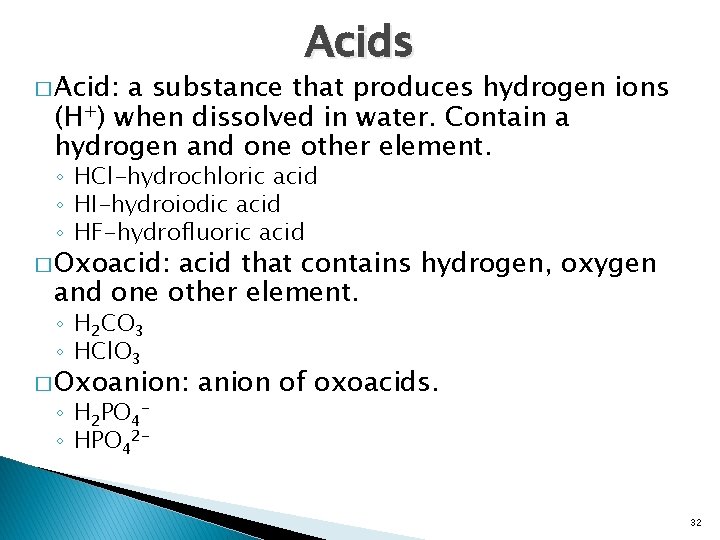
� Acid: Acids a substance that produces hydrogen ions (H+) when dissolved in water. Contain a hydrogen and one other element. ◦ HCl-hydrochloric acid ◦ HI-hydroiodic acid ◦ HF-hydrofluoric acid � Oxoacid: acid that contains hydrogen, oxygen and one other element. ◦ H 2 CO 3 ◦ HCl. O 3 � Oxoanion: ◦ H 2 PO 4◦ HPO 42 - anion of oxoacids. 32

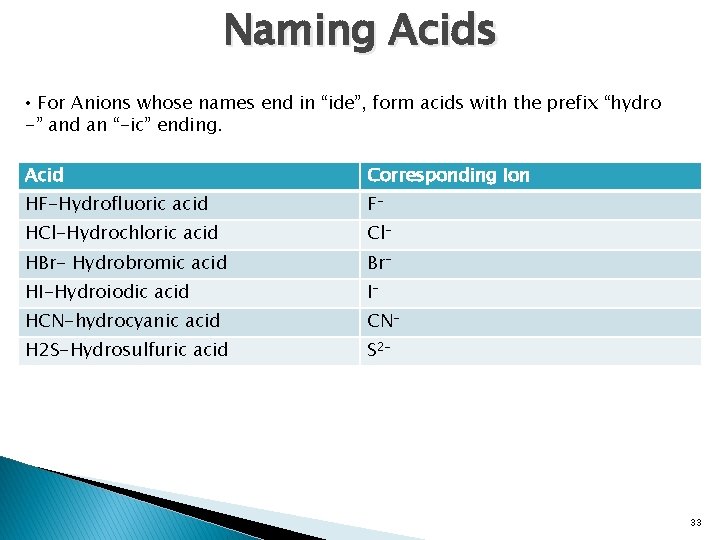
Naming Acids • For Anions whose names end in “ide”, form acids with the prefix “hydro -” and an “-ic” ending. Acid Corresponding Ion HF-Hydrofluoric acid F- HCl-Hydrochloric acid Cl- HBr- Hydrobromic acid Br- HI-Hydroiodic acid I- HCN-hydrocyanic acid CN- H 2 S-Hydrosulfuric acid S 2 - 33

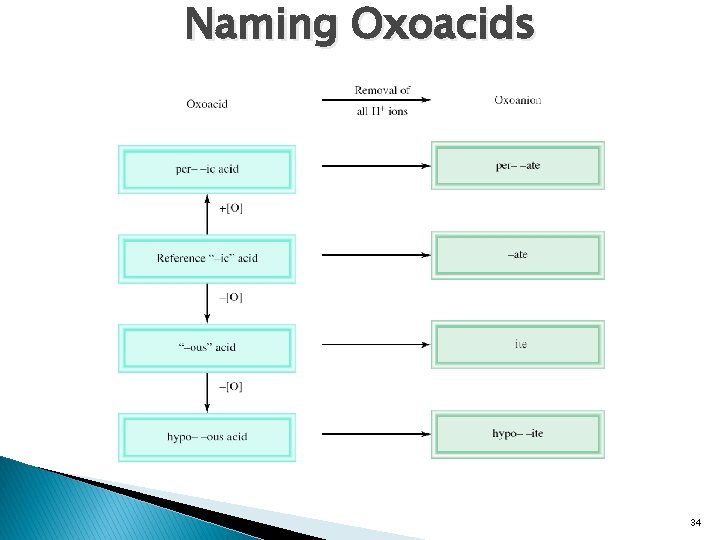
Naming Oxoacids 34

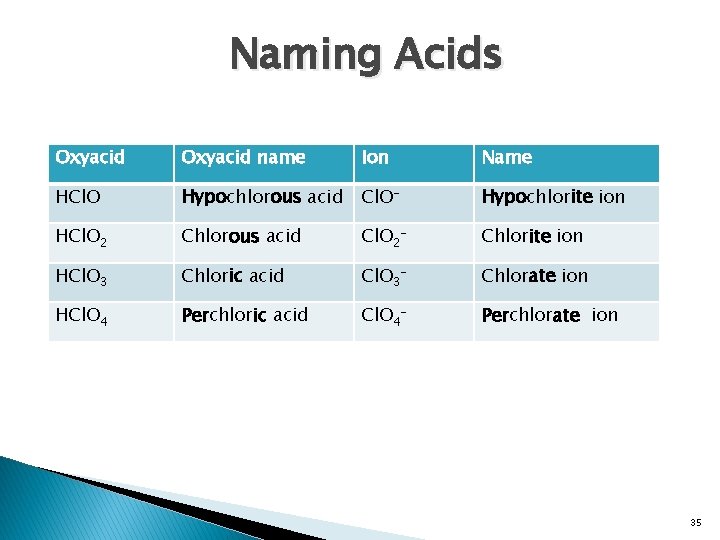
Naming Acids Oxyacid name Ion Name HCl. O Hypochlorous acid Cl. O- Hypochlorite ion HCl. O 2 Chlorous acid Cl. O 2 - Chlorite ion HCl. O 3 Chloric acid Cl. O 3 - Chlorate ion HCl. O 4 Perchloric acid Cl. O 4 - Perchlorate ion 35

� Name Naming Acids the following compounds: � H 3 PO 4 ◦ Phosphoric Acid � HSO 4 - ◦ Hydrogen sulfate � Write the formula for the following compounds: � Carbonic Acid ◦ H 2 CO 3 � Phosphoric ◦ H 3 PO 4 Acid 36

Naming Bases � Base: A substance the yields hydroxide(OH-) ions when dissolved in water. � Na. OH ◦ Sodium hydroxide � KOH ◦ Potassium hydroxide � Magnesium ◦ Mg(OH)2 � Ammonia ◦ NH 4 OH hydroxide 37

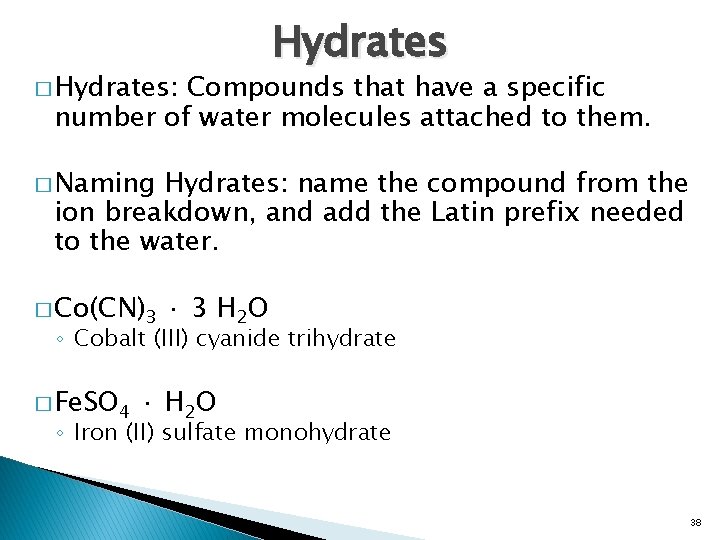
� Hydrates: Hydrates Compounds that have a specific number of water molecules attached to them. � Naming Hydrates: name the compound from the ion breakdown, and add the Latin prefix needed to the water. � Co(CN)3 · 3 H 2 O ◦ Cobalt (III) cyanide trihydrate � Fe. SO 4 · H 2 O ◦ Iron (II) sulfate monohydrate 38

� Ba. Cl 2 · 2 H 2 O Hydrates ◦ Barium chloride dihydrate � Li. Cl · H 2 O ◦ Lithium chloride monohydrate � Magnesium sulfate heptahydrate ◦ Mg. SO 4 · 7 H 2 O � Strontium nitrate tetrahydrate ◦ Sr(NO 3)2 · 4 H 2 O 39
 Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Ion chapter 11
Ion chapter 11 Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms ions and molecules
Atoms ions and molecules Atoms ions and molecules
Atoms ions and molecules States that atoms ions and molecules must collide to react
States that atoms ions and molecules must collide to react 1411 atm withdrawal meaning
1411 atm withdrawal meaning What do the roman numerals in a cation's name indicate?
What do the roman numerals in a cation's name indicate? Relationship between atoms and molecules
Relationship between atoms and molecules Compound substance
Compound substance How can you count atoms and molecules
How can you count atoms and molecules Interacting molecules or ions
Interacting molecules or ions Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Magnesium and fluorine ionic compound
Magnesium and fluorine ionic compound Atoms or ions are considered isoelectronic if
Atoms or ions are considered isoelectronic if Why do atoms combine to form molecules
Why do atoms combine to form molecules Periodic table of elements regents
Periodic table of elements regents What is a polyatomic ion definition
What is a polyatomic ion definition Spectator ions equation example
Spectator ions equation example Structure types chemsheets
Structure types chemsheets Chemistry molecules
Chemistry molecules Bond angles
Bond angles Shapes of molecules a level chemistry
Shapes of molecules a level chemistry A level chemistry shapes of molecules
A level chemistry shapes of molecules Ap chemistry electronic structure of atoms
Ap chemistry electronic structure of atoms Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Modern chemistry chapter 13
Modern chemistry chapter 13 Ions charged particles in solution
Ions charged particles in solution Functional groups ib chemistry
Functional groups ib chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Chapter 3 molecules of life
Chapter 3 molecules of life Chapter 4 section 2 the structure of atoms answer key
Chapter 4 section 2 the structure of atoms answer key Chapter 4 arrangement of electrons in atoms
Chapter 4 arrangement of electrons in atoms Stable electron configurations are likely to contain
Stable electron configurations are likely to contain Chapter 6 electronic structure of atoms answers
Chapter 6 electronic structure of atoms answers Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Chapter 6 electronic structure of atoms
Chapter 6 electronic structure of atoms