Chapter 2 Atoms Molecules and Ions Chemistry 1061




























- Slides: 35

Chapter 2: Atoms, Molecules, and Ions Chemistry 1061: Principles of Chemistry I Andy Aspaas, Instructor

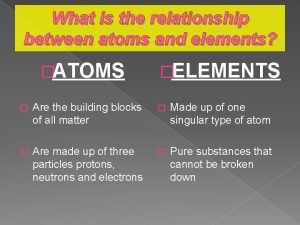
Atomic theory of matter • Dalton’s atomic theory – All matter composed of indivisible atoms • Atom: small particle, retains identity in reactions – Element: type of matter composed of only one kind of atom – Compound: type of matter composed of fixed proportion of 2 or more elements – Chemical reaction: rearrangement of atoms to give new chemical combinations

Deductions from Dalton’s Atomic Theory • Law of conservation of mass • Law of definite proportions – Example: 1. 00 g C reacted with oxygen – 2 compounds formed • 1. 3321 g O : 1. 00 g C • 2. 5542 g O : 1. 00 g C

Structure of the Atom • Dalton said atoms were indivisible, but experiments starting around 1900 showed that atoms themselves consist of particles – Nucleus: atom’s central core • Positively charged • Contains most of atom’s mass – Electron • Very light, negatively charged particles

Discovery of the electron • 1897, British physicist J. J. Thomson – Experiments showed atoms are not indivisible – Cathode ray tube – Calculated ratio of electron’s mass to its charge • 1909, American Robert Millikan – Charge on electron: 1. 602 x 10 -19 coulomb – Mass: 9. 109 x 10 -34 kg

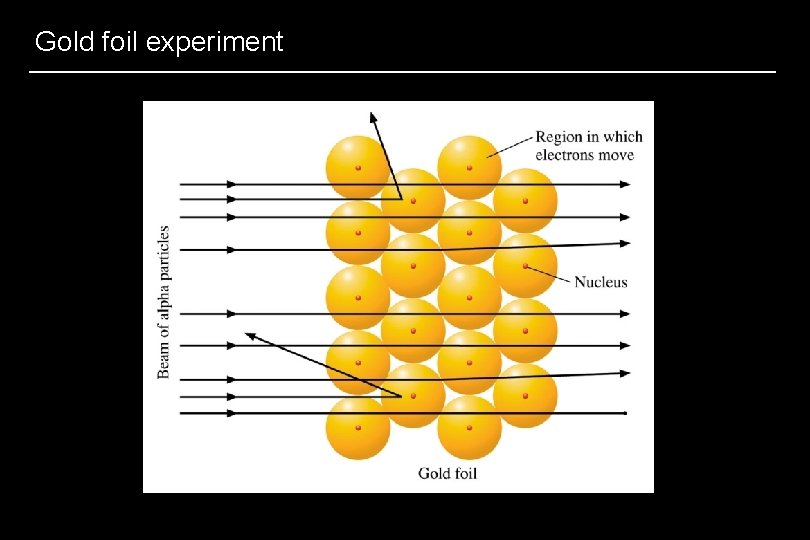
Nuclear model of the atom • 1911, British physicist Ernest Rutherford – Gold foil experiment – Most alpha particles pass straight through gold foil – Most of atom = empty space – If a golf ball was the nucleus, the atom would be about 3 miles in diameter

Gold foil experiment

Nuclear isotopes • Nucleus comprised of 2 kinds or particles – Protons • Have positive charge equal in magnitude to electron’s negative charge • More than 1800 times more massive than electron – Neutrons • Mass nearly identical to proton, no charge

Atomic number and mass number • Atomic number (Z): total number of protons in the nucleus of an atom – New element definition: substance whose atoms all have the same atomic number • Mass number (A): total number of protons and neutrons in a nucleus • Nuclide: atom characterized by certain mass number and certain atomic number • Nuclide symbol: Z subscript, A superscript, element

Isotopes • Isotopes: atoms whose nuclei have the same atomic number but different mass numbers • Some elements have only one naturally occurring isotope (sodium-23) • Some have several naturally occurring isotopes: – Oxygen contains the following isotopes • 99. 769% oxygen-16 • 0. 037% oxygen-17 • 0. 204% oxygen-18

Atomic weights • Dalton: an atom of a certain element has a characteristic mass • But, naturally occurring elements may be a mixture of isotopes – Isotope percentages are generally constant • Dalton actually calculated average atomic masses – They were relative to the mass of hydrogen, the smallest element

Atomic mass units and atomic weight • Carbon-12 isotope chosen arbitrarily as standard – 12 atomic mass units (amu) • Atomic weight: average atomic mass for a naturally occuring element, expressed in amu – Appears on periodic table along with atomic number

Calculating atomic weight • Fractional abundance: fraction of a total number of atoms, which consists of a particular isotope • Isotopic mass is not exactly equal to mass number – Neon-20, mass = 19. 992 amu, abund = 0. 9051 – Neon-21, mass = 20. 994 amu, abund = 0. 0027 – Neon-22, mass = 21. 991 amu, abund = 0. 0922 • Multiply isotopic mass by fractional abundance, and sum total to get atomic weight • Gives a “weighted average”

Periodic table of the elements • 1869, Dimitri Mendeleev (and J. Lothar Meyer, independently) – Arranged elements in order of atomic number – Placed elements in horizontal rows so that vertical columns of elements with similar properties formed

Periods • Period: elements in any one horizontal row of the periodic table – First has only 2 – 2 nd and 3 rd have 8 – 4 th and 5 th have 18 – 6 th has 32 – 7 th is incomplete

Groups • Group: elements in any one vertical column of the periodic table – Are numbered with Roman numeral and letter – (or just numbered with a number) • Main-group elements (labeled with A) • Transition elements (labeled with B) • Inner transition elements (shown below rest of table)

Groups • Elements in a single group have similar properties – For example: • IA: alkali metals - all react vigorously with water (except hydrogen) • VIIA: halogens - all react vigorously with sodium

Metals, Nonmetals, Metalloids • Metal: substances or mixtures that… – Have a characteristic shine – Generally are good conductors of heat and electricity – Are usually malleable (can be hammered into sheets) – Are usually ductile (can be drawn into wire) – Metallic elements are solid at room temp. , except for mercury

Metals, nonmetals, metalloids • Nonmetal: element that does not exhibit characteristics of a metal – Most are gases – Solid nonmetals are usually brittle and hard – Bromine is only liquid nonmetal • Metalloid (or semimetal) - both metallic and nonmetallic properties – Many are semiconductors (silicon, germanium) – Don’t conduct when pure, do when doped or at very high temperature

Chemical formulas • Chemical formula: atomic symbols with numeric subscripts that show relative proportions of atoms of different elements in a substance • Al 2 O 3: ratio of Al atoms to O atoms is 2 : 3 • No subscript: ratio of 1 (Na. Cl, 1: 1)

Molecular substances • Molecule: group of atoms that are chemically bonded together - tightly connected by attractive forces • Molecular substance: composed of all the same molecules • Molecular formula: shows number of different atoms of an element in a molecule • Structural formula: shows how atoms are bonded together, a line indicates a chemical bond

Molecular substances • Some elements are molecular substances – Cl 2, O 2, N 2, S 8 • Some exist as individual atoms – He, Ne • Some exist as a very large but indefinite number of atoms bonded together –C • Polymer: extremely large molecules made of small molecules repeatedly bonded together (monomers) – Natural (wool, cotton), synthetic (plastics, Nylon, polyester, Kevlar)

Ionic substances • Unlike molecular substances, ionic substances are formed from charged atoms or groups of atoms called ions – Metal atoms tend to lose electrons: result is a positive charge (cation) – Nonmetals tend to gain electrons: result is a negative charge (anion) • Sodium loses one electron to become Na+ • Calcium loses two electrons to become Ca 2+ • May consist of a group of bonded atoms that have a deficiency or surplus of electrons (SO 42+)

Ionic compounds • Ionic compounds are composed of cations and anions • Ionic formula: given by smallest possible integer number of different ions in substance – Charges are omitted – Ionic compounds are uncharged as a whole – Ex. Na+ and Cl- = Na. Cl – Fe 3+ and SO 42 - = Fe 2(SO 4)3

Naming simple compounds • Chemical nomenclature: systematic naming of chemical compounds • Organic compounds: carbon-containing molecular substances • Inorganic compounds: composed of elements other than carbon (except CO, CO 2, cyanides, and carbonates)

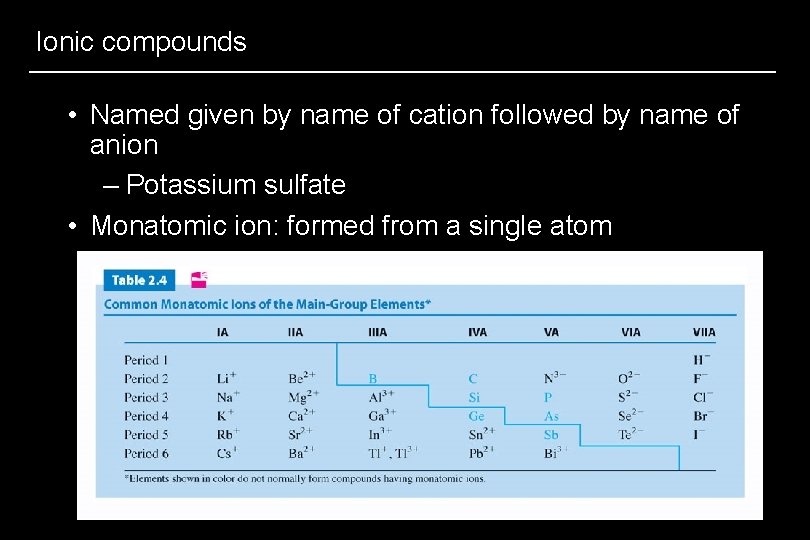
Ionic compounds • Named given by name of cation followed by name of anion – Potassium sulfate • Monatomic ion: formed from a single atom

Predicting a monatomic ion’s charge • Most main-group metallic ions have one monatomic cation with charge equal to group number – Aluminum, Group IIIA, Al 3+ • Many high-atomic-number metals have a cation equal to group number AND one equal to the group number minus 2 – Thallium, Group IIIA, Tl 3+, Tl+ • Most transition elements can have several monatomic cations. +2 is very common • A nonmetal main-group ion forms an anion – Charge = 8 - group # – Oxygen, Group VIA, O 2 -

Naming monatomic ions • Monatomic cations are named after element if there’s only one possible cation for that element • If more than one possible cation, use charge as roman numeral after name – Fe 2+ = iron(II), Fe 3+ = iron(III) – Or use suffixes with latin name (-ous for lower charge, -ic for higher) • Cu 2+ = cuprous, Cu 3+ = cupric • Monatomic anions use -ide suffix (bromide, sulfide)

Polyatomic ions • Polyatomic ion: ion consisting of 2 or more atoms chemically bonded together, which carry a net electric charge • Oxoanions: central element + some oxygens – Suffixes indicate relative amount of oxygens – Fewer oxygens: -ite – More oxygens: -ate – Ex. SO 32 - is sulfite, SO 42 - is sulfate

Polyatomic anions • If more than 2 oxoanions, use prefixes – hypo- and per • Cl. O- hypochlorite ion • Cl. O 2 - chlorite ion • Cl. O 3 - chlorate ion • Cl. O 4 - perchlorate ion

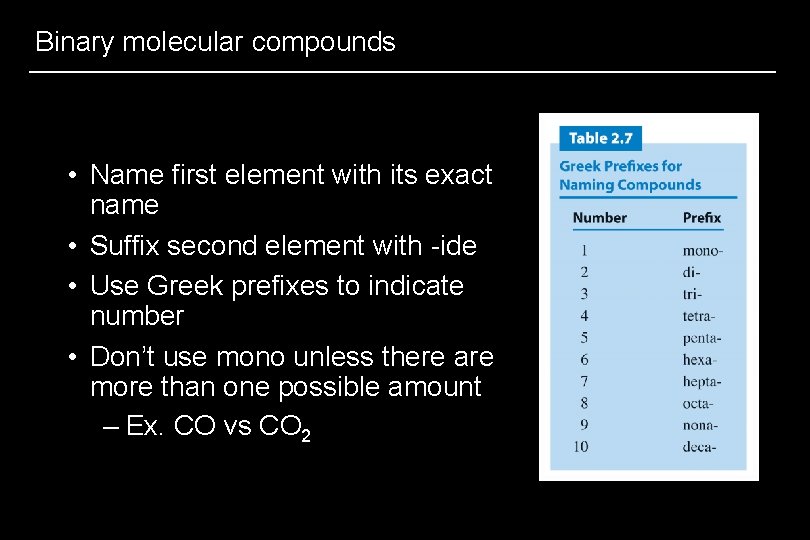
Binary molecular compounds • Name first element with its exact name • Suffix second element with -ide • Use Greek prefixes to indicate number • Don’t use mono unless there are more than one possible amount – Ex. CO vs CO 2

Acids and corresponding anions • Acids have H+ as the cation • Oxoacids contain an oxoanion – -ate becomes -ic – -ite becomes -ous – End name with “acid” • Binary compounds of hydrogen and nonmetal – Prefix hydro– Suffix -ic acid

Hydrates • Simply indicate how many water molecules are present by using the Greek numerical prefix and hydrate at the end • Mg. SO 4 • 7 H 2 O = magnesium sulfate heptahydrate

Writing chemical reactions • Reactant ----> Product • Arrow means “reacts to form” or “yields” • Useful to indicate state or phase – (g) = gas, (l) = liquid, (s) = solid, – (aq) = aqueous solution • Use a coefficient to indicate relative number of particles involved • ∆ over arrow means heat is applied • A compound written over the arrow is usually a catalyst

Balancing chemical equations • Mass must be conserved so use coefficients to make sure the same number of each atom occurs on each side of the equation • Start by balancing atoms for elements that occur in only one substance on each side – Ex: H 3 PO 3 ---> H 3 PO 4 + PH 3 – Start by balancing oxygen – Practice!
 Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms ions and molecules
Atoms ions and molecules Atoms ions and molecules
Atoms ions and molecules Collision theory states that
Collision theory states that Nfpa 1061 certifications
Nfpa 1061 certifications Positive ions and negative ions table
Positive ions and negative ions table Relationship between atoms and molecules
Relationship between atoms and molecules Mixture of compounds diagram
Mixture of compounds diagram 3bacl2 counting atoms
3bacl2 counting atoms Interacting molecules or ions
Interacting molecules or ions Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules How does a positive ion form
How does a positive ion form Atoms or ions are considered isoelectronic if
Atoms or ions are considered isoelectronic if Why do atoms combine to form molecules
Why do atoms combine to form molecules Compared to atoms of metals, atoms of nonmetals generally
Compared to atoms of metals, atoms of nonmetals generally Identifying polyatomic ions
Identifying polyatomic ions Spectator ions equation example
Spectator ions equation example Chemsheets shapes of molecules
Chemsheets shapes of molecules Chemistry molecules
Chemistry molecules Examples of ab2e type molecules
Examples of ab2e type molecules Shapes of molecules a level chemistry
Shapes of molecules a level chemistry Shapes of molecules a level chemistry
Shapes of molecules a level chemistry Ap chemistry electronic structure of atoms
Ap chemistry electronic structure of atoms Chapter 6 chemistry in biology
Chapter 6 chemistry in biology Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Modern chemistry chapter 13 review answers
Modern chemistry chapter 13 review answers Chapter 6 ions charged particles in solution
Chapter 6 ions charged particles in solution Ib organic chemistry functional groups
Ib organic chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Chapter 3 molecules of life
Chapter 3 molecules of life Chapter 4 section 2 the structure of atoms
Chapter 4 section 2 the structure of atoms Arrangement of electrons in atoms chapter 4 test
Arrangement of electrons in atoms chapter 4 test